Exogenous Melatonin Application Improves Shade Tolerance and Growth Performance of Soybean Under Maize–Soybean Intercropping Systems
Abstract
1. Introduction
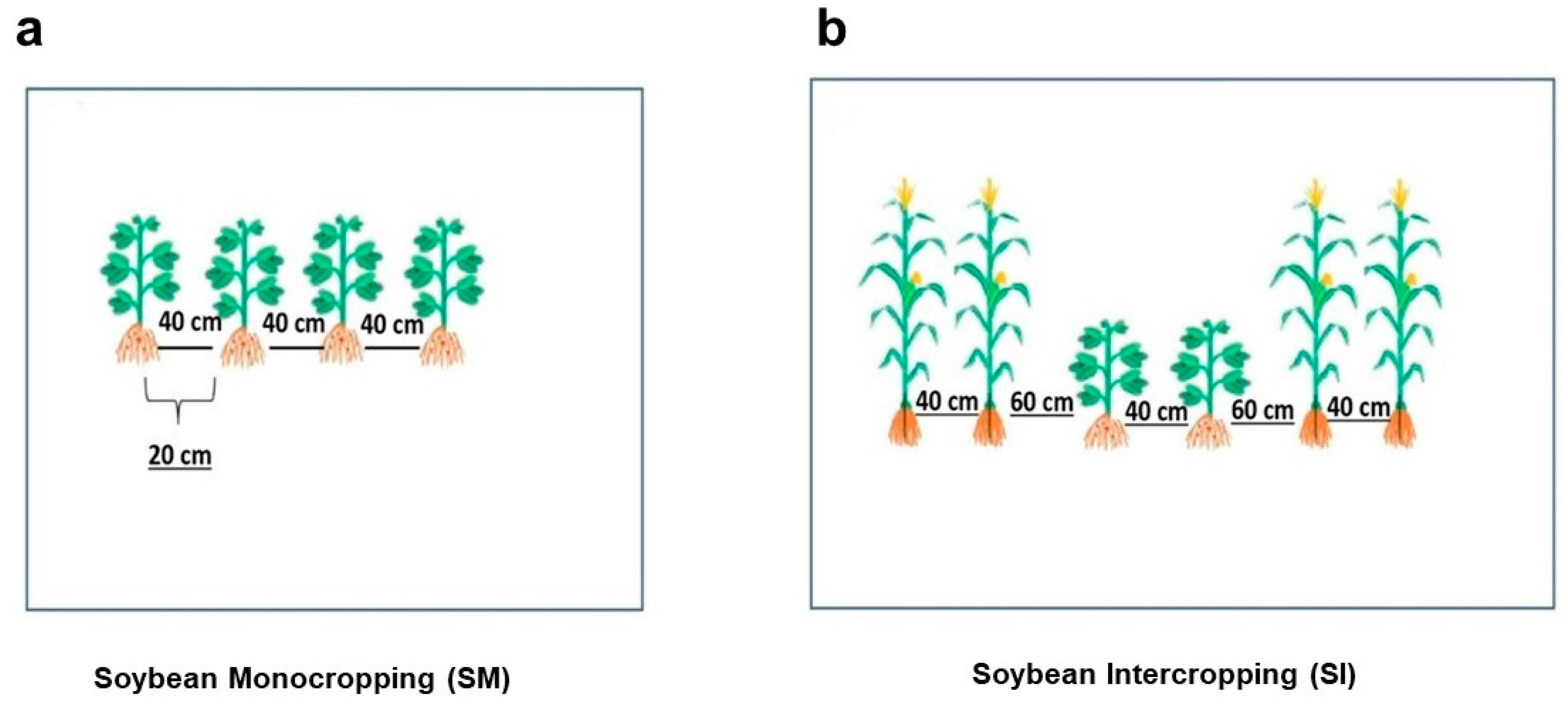
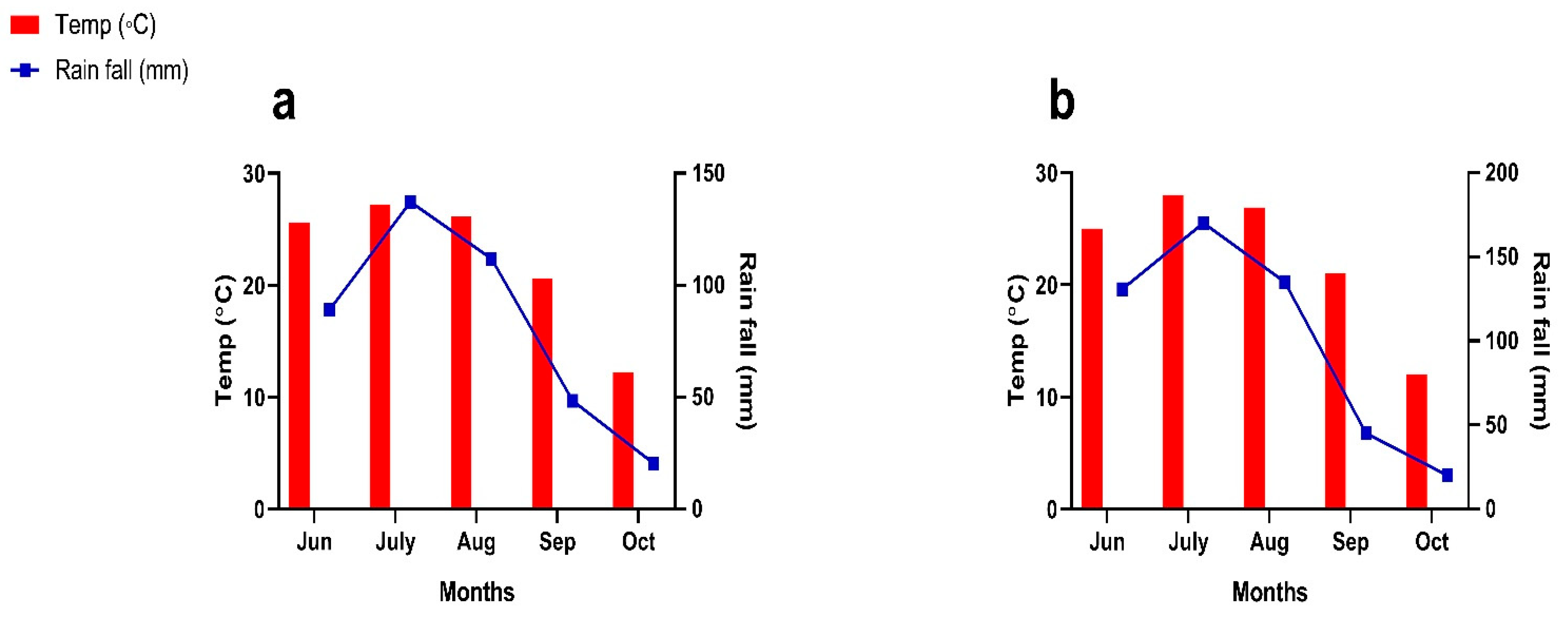
2. Materials and Methods
2.1. Data Collection and Analysis
2.1.1. Growth Indices and Yield Assessment
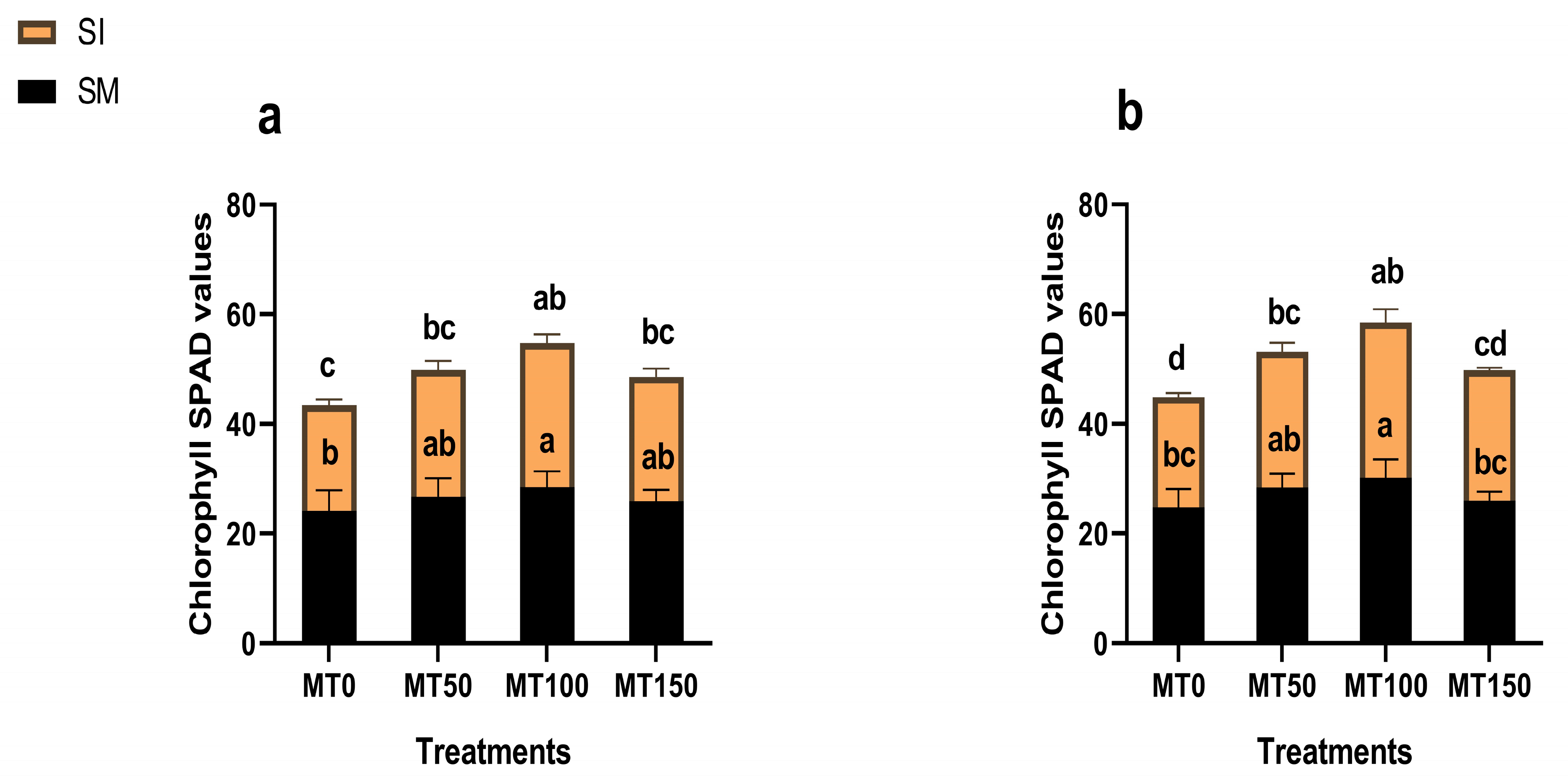
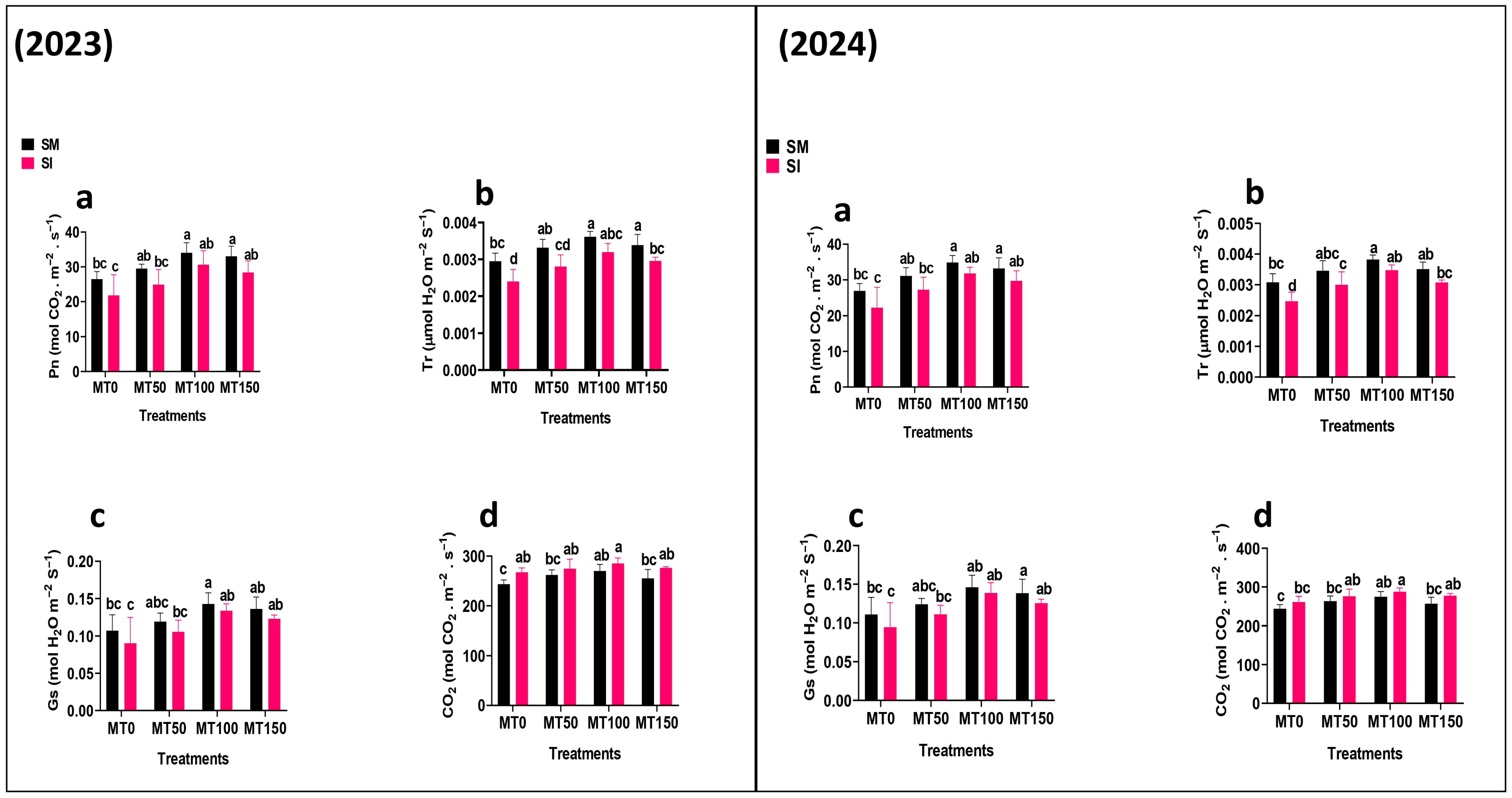
2.1.2. Chlorophyll SPAD and Photosynthesis
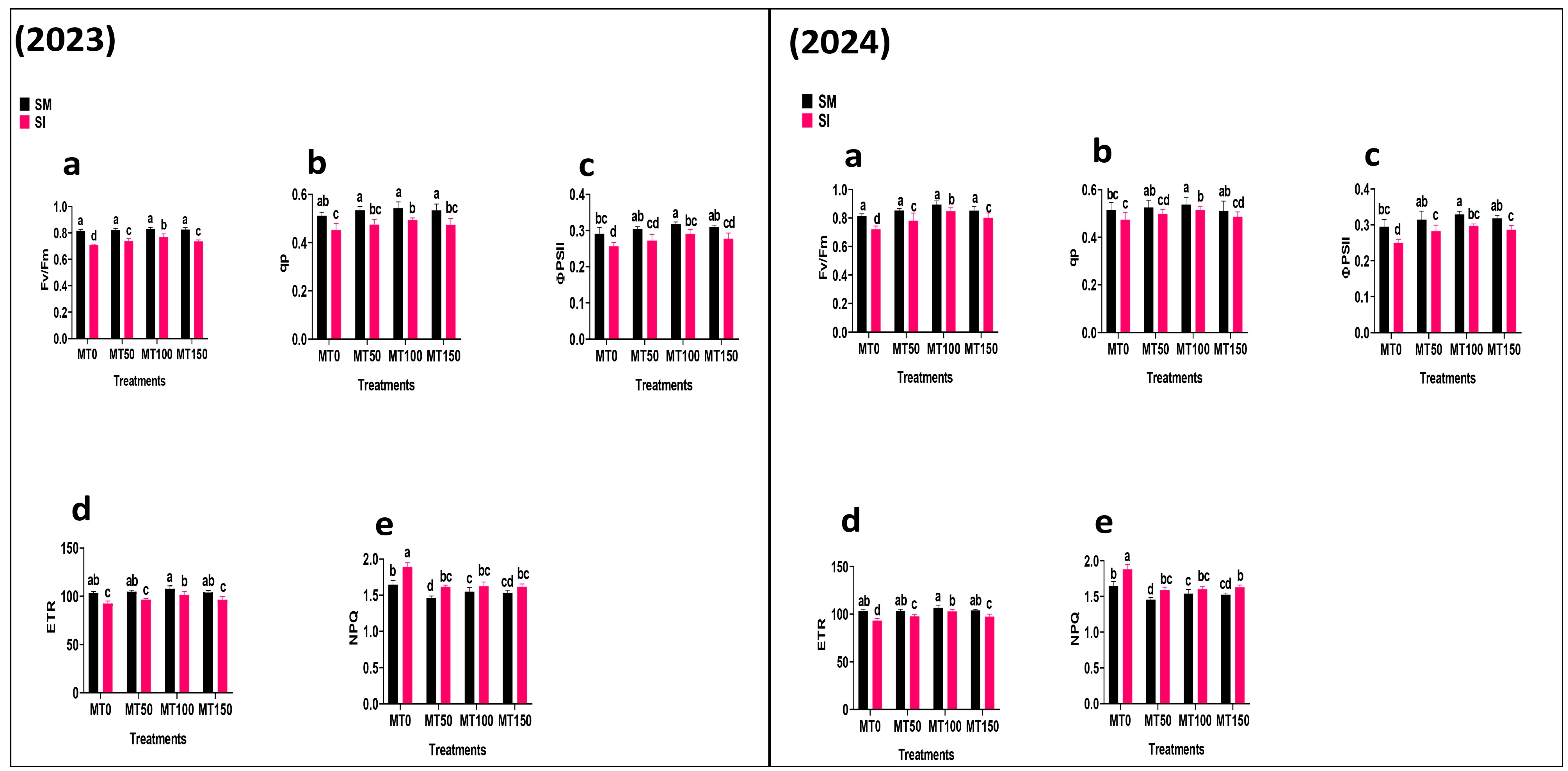
2.1.3. Chlorophyll Fluorescence
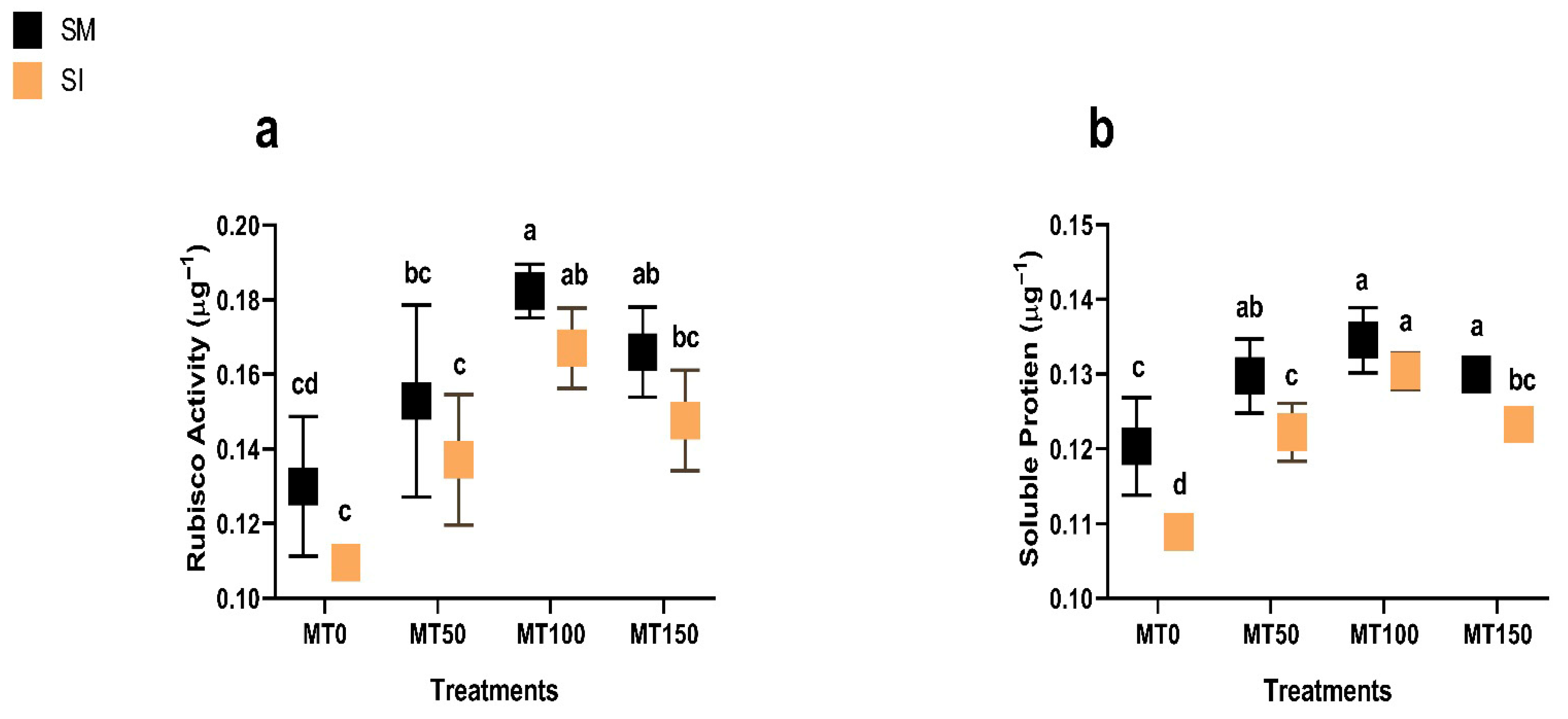
2.1.4. Rubisco Activity
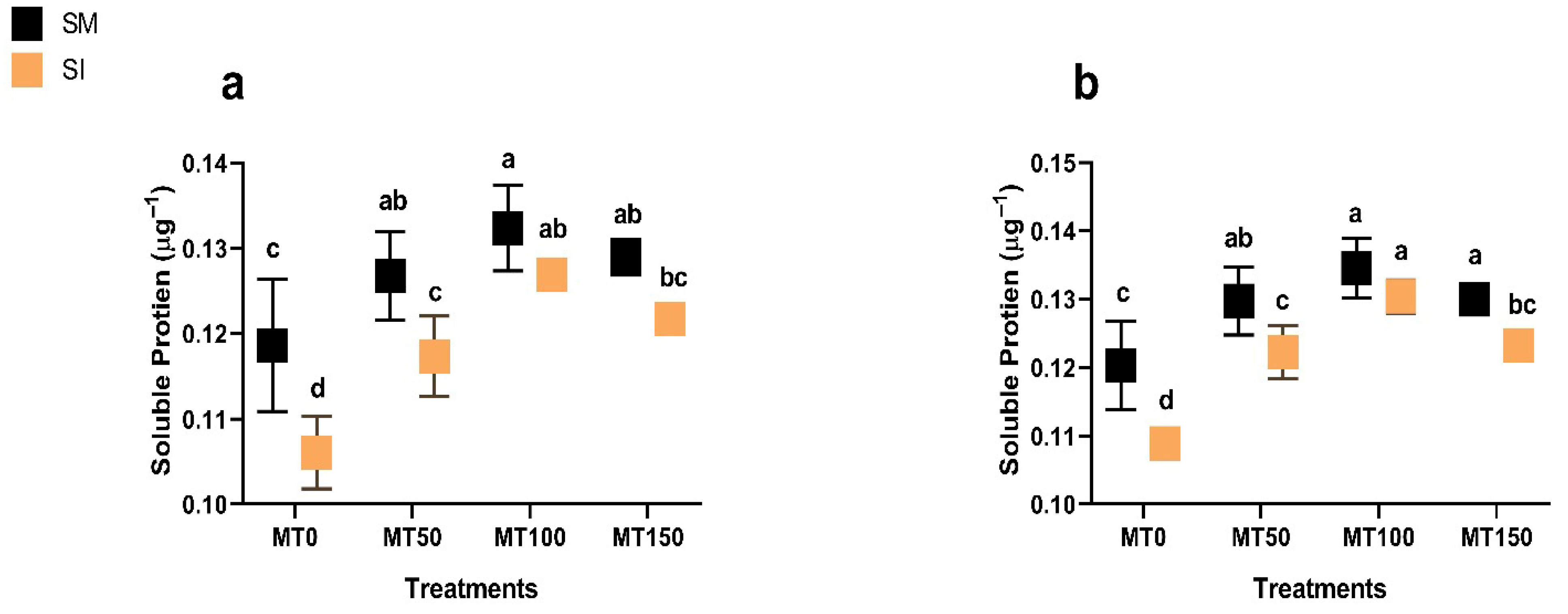
2.1.5. Total Soluble Protein
2.1.6. Land Equivalent Ratio (LER)
2.1.7. Data Analysis
3. Results
3.1. Growth Indices
3.2. Yield Indices and Biomass Dry Matter
3.3. Chlorophyll Content and Photosynthetic Activities
3.4. Chlorophyll Fluorescence
3.5. Rubisco Activity and Soluble Protein
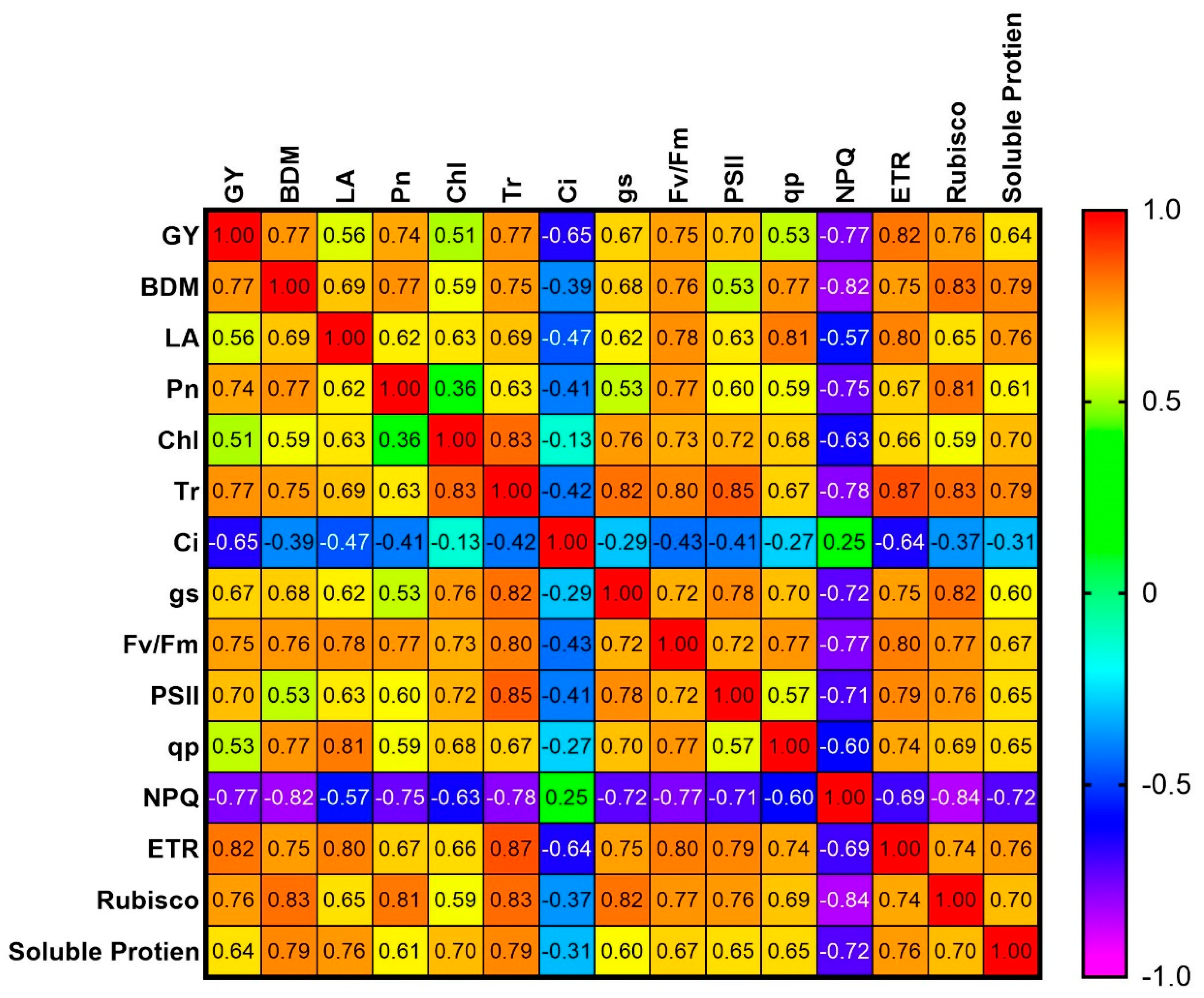
3.6. Correlation
4. Discussion
4.1. Impact of Melatonin on the Growth and Yield of Soybean
4.2. Effects on Chlorophyll Content and Photosynthetic Activities
4.3. Impact on the Chlorophyll Fluorescence
4.4. Impact on the Rubisco Activity and Protein Content
4.5. Correlation Between Physiological Traits and Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghorbel, M.; Brini, F.; Brestic, M.; Landi, M. Interplay between Low Light and Hormone-Mediated Signaling Pathways in Shade Avoidance Regulation in Plants. Plant Stress 2023, 9, 100178. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.Y.; Zhou, F.J.; Gitari, H.; Zhou, X.B.; Tabl, K.M.; Hasan, M.E.; Ali, H.; Waqas, M.M.; Ali, I.; et al. Nitrogen Fertilization Coupled with Foliar Application of Iron and Molybdenum Improves Shade Tolerance of Soybean under Maize-Soybean Intercropping. Front. Plant Sci. 2022, 13, 1014640. [Google Scholar] [CrossRef]
- Corwin, K.A.; Corr, C.A.; Burkhardt, J.; Fischer, E.V. Smoke-Driven Changes in Photosynthetically Active Radiation During the U.S. Agricultural Growing Season. J. Geophys. Res. Atmos. 2022, 127, e2022JD037446. [Google Scholar] [CrossRef]
- Delarue, M.; Behnamed, M.; Fragkostefanakis, S. The role of epigenetics in tomato stress adaptation. New Crops. 2024, 2, 100044. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Response of Growth, Photosynthetic Electron Transfer, and Chloroplast Ultrastructure to Different LED Light Combination in Green Onion (Allium fistulosum L.). Physiol. Plant. 2021, 172, 1662–1672. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, P.; Sun, W.; Zhang, T. Plant Cell Walls: Emerging Targets of Stomata Engineering to Improve Photosynthesis and Water Use Efficiency. New Crops. 2024, 1, 100021. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.Y.; Zhu, S.G.; Khan, A.; Tao, X.P.; Huang, G.F.; Liu, H.Y.; Zhang, W.; Tao, H.Y.; Gong, D.S.; et al. Plant Facilitation Improves Carbon Production Efficiency While Reducing Nitrogen Input in Semiarid Agroecosystem. Catena 2023, 230, 107247. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, P.; Zhang, X.; Du, Q.; Zheng, B.; Yang, H.; Luo, K.; Lin, P.; Li, Y.; Pu, T.; et al. Maize-Legume Intercropping Achieves Yield Advantages by Improving Leaf Functions and Dry Matter Partition. BMC Plant Biol. 2023, 23, 438. [Google Scholar] [CrossRef] [PubMed]
- Dimande, P.; Arrobas, M.; Rodrigues, M.Â. Intercropped Maize and Cowpea Increased the Land Equivalent Ratio and Enhanced Crop Access to More Nitrogen and Phosphorus Compared to Cultivation as Sole Crops. Sustainability 2024, 16, 1440. [Google Scholar] [CrossRef]
- Jahan, M.S.; Li, G.; Xie, D.; Farag, R.; Hasan, M.M.; Alabdallah, N.M.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Zeeshan, M.; Nasar, J.; et al. Melatonin Mitigates Salt-Induced Growth Inhibition Through the Regulation of Carbohydrate and Nitrogen Metabolism in Tomato Seedlings. J. Soil Sci. Plant Nutr. 2023, 23, 4290–4308. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Kaźmierczak, A. Melatonin—This Is Important to Know. Sci. Total Environ. 2024, 919, 170871. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Karumannil, S.; Khan, T.A.; Kappachery, S.; Gururani, M.A. Impact of Exogenous Melatonin Application on Photosynthetic Machinery under Abiotic Stress Conditions. Plants 2023, 12, 2948. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin-Mediated Development and Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2023, 14, 1100827. [Google Scholar] [CrossRef]
- Shahani, A.A.A.; Abbas, A.; Hameed, R.; Iqbal, A.; Chen, S.; Liu, Q.; Liu, Y.; Zhang, D.; Zhu, R.; Fayyaz, A.; et al. Melatonin in Plants: A Pleiotropic Molecule for Abiotic Stresses and Pathogens Infection. Sci. Hortic. 2023, 322, 112387. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin Affects the Photosynthetic Performance of Pepper (Capsicum annuum L.) Seedlings under Cold Stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef]
- Nasar, J.; Ahmad, M.; Gitari, H.; Tang, L.; Chou, X. Maize/soybeans intercropping increases nutrient uptake, crop yield and modifies soil physio-chemical characteristics and enzymatic activities in a subtropical humid region based in Southwest China. BMC Plant Biol. 2024, 24, 434. [Google Scholar] [CrossRef]
- Hassan, M.U.; Mahmood, A.; Awan, M.I.; Maqbool, R.; Aamer, M.; Alhaithloul, H.A.S.; Huang, G.; Skalicky, M.; Brestic, M.; Pandey, S.; et al. Melatonin-Induced Protection Against Plant Abiotic Stress: Mechanisms and Prospects. Front. Plant Sci. 2022, 13, 902694. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Melatonin: A Promising Candidate for Maintaining Food Security under the Threat of Phytopathogens. Plant Physiol. Biochem. 2023, 198, 107691. [Google Scholar] [CrossRef] [PubMed]
- Seleim, M.F.; Ahmad, A.; Alotaib, M.; Al-Saif, A.M.; Ranjan, S.; Sow, S. High-Throughput Metabolomics for Studying Plant Stress Physiology under a Changing Climate. In High Throughput Plant Metabolomics; Chen, J., Ed.; CAB International: Wallingford, UK, 2025; Volume 11. [Google Scholar] [CrossRef]
- Nasar, J.; Zhao, C.J.; Khan, R.; Gul, H.; Gitari, H.; Shao, Z.; Abbas, G.; Haider, I.; Iqbal, Z.; Ahmed, W.; et al. Maize-Soybean Intercropping at Optimal N Fertilization Increases the N Uptake, N Yield and N Use Efficiency of Maize Crop by Regulating the N Assimilatory Enzymes. Front. Plant Sci. 2023, 13, 1077948. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Enhancing the Light Reactions of Photosynthesis: Strategies, Controversies, and Perspectives. Mol. Plant 2023, 16, 4–22. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Buckseth, T.; Zinta, R.; Saraswati, A.; Singh, R.K.; Rawat, S.; Dua, V.K.; Chakrabarti, S.K. Transcriptome Analysis of Potato Shoots, Roots and Stolons under Nitrogen Stress. Sci. Rep. 2020, 10, 1152. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Zeeshan, M.; Zhou, X.B. Melatonin and KNO3 Application Improves Growth, Physiological and Biochemical Characteristics of Maize Seedlings under Waterlogging Stress Conditions. Biology 2022, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the Defense Mechanisms during Plant Oxidative Stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Estrada, C.A.; Sánchez, E.; Flores-Córdova, M.A.; Pérez-Álvarez, S.; Noperi-Mosqueda, L.C.; Chávez-Mendoza, C. Photosynthetic Efficiency in Green Bean Plants through the Application of Omeprazole and Melatonin at Low Doses. Int. J. Plant Biol. 2023, 14, 864–878. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.H.; Li, M.Y.; Zhang, W.; Rehman, M.M.U.; Wang, B.Z.; Ullah, F.; Cheng, Z.G.; Zhu, L.; Zhang, J.L.; et al. Yield Loss of Inferior Crop Species and Its Physiological Mechanism in a Semiarid Cereal-Legume Intercropping System. Eur. J. Agron. 2024, 152, 127032. [Google Scholar] [CrossRef]
- Malshe, K.; Ghavale, S.; Wankhede, S. Yield Assessment of Intercropping of Bitter Gourd (Momordica charantia) in Coconut (Cocos nucifera). Int. J. Res. Agron. 2024, 7, 201–202. [Google Scholar] [CrossRef]
- Raza, M.A.; Yasin, H.S.; Gul, H.; Qin, R.; Mohi Ud Din, A.; Bin Khalid, M.H.; Hussain, S.; Gitari, H.; Saeed, A.; Wang, J.; et al. Maize/Soybean Strip Intercropping Produces Higher Crop Yields and Saves Water under Semi-Arid Conditions. Front. Plant Sci. 2022, 13, 1006720. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, F.; Zhang, S.; Zhang, Q.; Jiang, G.; Gao, B.; Cao, G.; Islam, M.U.I.; Cao, Z.; Zhao, X. Potential Crop Yield Gains under Intensive Soybean/Maize Intercropping in China. Plant Soil 2023, 506, 275–290. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, L.; Liu, R.; Wang, W.; Yu, R.; Zhou, T.; Ahmad, I.; Raza, A.; Jiang, S.; Xu, M.; et al. Shade-Tolerant Soybean Reduces Yield Loss by Regulating Its Canopy Structure and Stem Characteristics in the Maize–Soybean Strip Intercropping System. Front. Plant Sci. 2022, 13, 848893. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhou, T.; Jing, S.; Liu, R.; Gao, Y.; Deng, C.; Ye, W.; Luo, Z.; Raza, A.; et al. Quantifying the Effects of Plant Density on Soybean Lodging Resistance and Growth Dynamics in Maize-Soybean Strip Intercropping. Front. Plant Sci. 2023, 14, 1264378. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Hussain, S.; Shafiq, I.; Chattha, M.S.; Mumtaz, M.; Brestic, M.; Rastogi, A.; Chen, G.; Allakhverdiev, S.I.; Liu, W.; Yang, W. Effect of Ti Treatments on Growth, Photosynthesis, Phosphorus Uptake and Yield of Soybean (Glycine max L.) in Maize-Soybean Relay Strip Intercropping. Environ. Exp. Bot. 2021, 187, 104476. [Google Scholar] [CrossRef]
- Sabagh, A.E.L.; Mbarki, S.; Hossain, A.; Iqbal, M.A.; Islam, M.S.; Raza, A.; Llanes, A.; Reginato, M.; Rahman, M.A.; Mahboob, W.; et al. Potential Role of Plant Growth Regulators in Administering Crucial Processes Against Abiotic Stresses. Front. Agron. 2021, 3, 648694. [Google Scholar] [CrossRef]
- Raza, M.A.; Zhiqi, W.; Yasin, H.S.; Gul, H.; Qin, R.; Rehman, S.U.; Mahmood, A.; Iqbal, Z.; Ahmed, Z.; Luo, S.; et al. Effect of Crop Combination on Yield Performance, Nutrient Uptake, and Land Use Advantage of Cereal/Legume Intercropping Systems. Field Crop. Res. 2023, 304, 109144. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.-Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Bin Khalid, M.H.; Zhou, X.-B.; et al. Nitrogen Fertilization Coupled with Iron Foliar Application Improves the Photosynthetic Characteristics, Photosynthetic Nitrogen Use Efficiency, and the Related Enzymes of Maize Crops under Different Planting Patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Crusciol, C.A.C.; Rodrigues, V.A.; Galeriani, T.M.; Portugal, J.R.; Bossolani, J.W.; Moretti, L.G.; Calonego, J.C.; Cantarella, H. Molybdenum Foliar Fertilization Improves Photosynthetic Metabolism and Grain Yields of Field-Grown Soybean and Maize. Front. Plant Sci. 2022, 13, 887682. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Jang, Y.H.; Kim, E.G.; Kim, N.; Kim, K.M. Exogenous Melatonin Induces Salt and Drought Stress Tolerance in Rice by Promoting Plant Growth and Defense System. Sci. Rep. 2024, 14, 1214. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.; Zeng, R.; Wang, X.; Huang, L.; Wang, L.; Wang, X.; Zhang, L. Shade Effects on Peanut Yield Associate with Physiological and Expressional Regulation on Photosynthesis and Sucrose Metabolism. Int. J. Mol. Sci. 2020, 21, 5284. [Google Scholar] [CrossRef]
- Gong, X.; Liu, C.; Dang, K.; Wang, H.; Du, W.; Qi, H.; Jiang, Y.; Feng, B. Mung Bean (Vigna radiata L.) Source Leaf Adaptation to Shading Stress Affects Not Only Photosynthetic Physiology Metabolism but Also Control of Key Gene Expression. Front. Plant Sci. 2022, 13, 753264. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-Mediated Photosynthetic Performance of Tomato Seedlings under High-Temperature Stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Muñoz-Jurado, A.; Escribano, B.M.; Caballero-Villarraso, J.; Galván, A.; Agüera, E.; Santamaría, A.; Túnez, I. Melatonin and Multiple Sclerosis: Antioxidant, Anti-Inflammatory and Immunomodulator Mechanism of Action. Inflammopharmacology 2022, 30, 1569–1596. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A.; et al. Shade Effect on Carbohydrates Dynamics and Stem Strength of Soybean Genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin Promotes Water-Stress Tolerance, Lateral Root Formation, and Seed Germination in Cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Sun, L.; Wang, T.; Miao, P.; Zhu, X.; Liu, S.; Song, F.; Mao, H.; Li, X. Melatonin Improves the Photosynthetic Carbon Assimilation and Antioxidant Capacity in Wheat Exposed to Nano-Zno Stress. Molecules 2017, 22, 1727. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.H.; Anjum, S. Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives. Sustainability 2021, 13, 294. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Z.; Zhang, Y.; Bai, L.; Hu, X.; Li, X.; Zhang, L.; Miao, Y.; Wang, Y. Melatonin Reduces Photoinhibition in Cucumber during Chilling by Regulating the Calvin-Benson Cycle. Sci. Hortic. 2022, 299, 111007. [Google Scholar] [CrossRef]
- Jahan, M.S.; Zhao, C.J.; Shi, L.B.; Liang, X.R.; Jabborova, D.; Nasar, J.; Zhou, X.B. Physiological Mechanism of Melatonin Attenuating to Osmotic Stress Tolerance in Soybean Seedlings. Front. Plant Sci. 2023, 14, 1193666. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous Melatonin Alleviates Oxidative Damages and Protects Photosystem II in Maize Seedlings under Drought Stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Lin, S.; Song, X.F.; Mao, H.T.; Li, S.Q.; Gan, J.Y.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y.; Su, Y.Q.; et al. Exogenous Melatonin Improved Photosynthetic Efficiency of Photosystem II by Reversible Phosphorylation of Thylakoid Proteins in Wheat under Osmotic Stress. Front. Plant Sci. 2022, 13, 966181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, H.; Wang, J.; Wang, Y.; Zhang, R. Melatonin Enhances Drought Tolerance by Regulating Leaf Stomatal Behavior, Carbon and Nitrogen Metabolism, and Related Gene Expression in Maize Plants. Front. Plant Sci. 2021, 12, 779382. [Google Scholar] [CrossRef]
- Hassan, M.U.; Ghareeb, R.Y.; Nawaz, M.; Mahmood, A.; Shah, A.N.; Abdel-Megeed, A.; Abdelsalam, N.R.; Hashem, M.; Alamri, S.; Thabit, M.A.; et al. Melatonin: A Vital Pro-Tectant for Crops against Heat Stress: Mechanisms and Prospects. Agronomy 2022, 12, 1116. [Google Scholar] [CrossRef]
- Cao, L.; Qin, B.; Gong, Z.; Zhang, Y. Melatonin Improves Nitrogen Metabolism during Grain Filling under Drought Stress. Physiol. Mol. Biol. Plants 2022, 28, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.D.; Zhang, J.Y.; Yang, Y.M.; Yu, D.Y.; Zhang, H.Y.; Zhang, D. Molecular mechanisms underlying plant responses to low phosphate stress and potential applications in crop improvement. New Crops. 2025, 2, 100064. [Google Scholar] [CrossRef]
| Year | Treatment | Plant Height (cm) | Leaf Area (cm2) | Stem Diameter (mm) | Stem Strength (N) | Internode Length (cm) | |
|---|---|---|---|---|---|---|---|
| MT | PP | ||||||
| 2023 | 0 | SM | 52.40 ± 3.5 bc | 251.58 ± 8.5 bc | 10.36 ± 0.3 bc | 354.84 ± 5.1 a | 10.85 ± 1.0 c |
| SI | 43.34 ± 2.1 c | 237.41 ± 8.5 d | 9.96 ± 0.4 d | 330.10 ± 3.5 c | 8.67 ± 1.1 d | ||
| 50 | SM | 60.36 ± 2.5 ab | 258.14 ± 7.4 ab | 10.70 ± 0.2 ab | 358.76 ± 7.0 a | 13.27 ± 1.6 ab | |
| SI | 53.33 ± 7.2 ab | 248.34 ± 6.2 bc | 10.10 ± 0.4 c | 336.43 ± 3.5 bc | 10.85 ± 1.1 c | ||
| 100 | SM | 61.73 ± 6.6 a | 262.77 ± 6.7 a | 11.23 ± 0.2 a | 361.46 ± 7.0 a | 14.13 ± 2.0 a | |
| SI | 57.23 ± 5.7 ab | 255.55 ± 4.1 b | 10.60 ± 0.5 ab | 341.40 ± 4.1 b | 12.23 ± 1.0 bc | ||
| 150 | SM | 59.27 ± 7.8 ab | 258.91 ± 4.0 ab | 10.76 ± 0.3 b | 360.70 ± 4.3 a | 13.10 ± 1.1 b | |
| SI | 53.77 ± 3.8 ab | 244.93 ± 5.0 c | 10.26 ± 0.3 cd | 334.50 ± 4.0 b | 11.63 ± 0.6 bc | ||
| Significance | MT | 0.009 ** | 0.011 * | 0.011 * | 0.039 * | 0.000 *** | |
| PP | 0.008 ** | 0.001 ** | 0.000 *** | 0.000 *** | 0.000 *** | ||
| MT × PP | 0.889 ns | 0.751 ns | 0.904 ns | 0.718 ns | 0.851 ns | ||
| 2024 | 0 | SM | 57.20 ± 5.9 ab | 256.18 ± 9.5 b | 10.36 ± 0.7 bc | 361.38 ± 3.1 b | 10.73 ± 0.7 c |
| SI | 48.61 ± 3.4 c | 238.17 ± 8.3 c | 9.96 ± 0.4 c | 333.10 ± 2.5 e | 8.56 ± 1.1 c | ||
| 50 | SM | 60.50 ± 6.1 ab | 259.97 ± 9.1 a | 10.70 ± 0.2 ab | 363.80 ± 3.5 ab | 12.96 ± 1.1 ab | |
| SI | 53.30 ± 2.6 bc | 253.34 ± 3.1 ab | 10.10 ± 0.3 bc | 338.43 ± 3.0 d | 10.63 ± 1.4 c | ||
| 100 | SM | 64.86 ± 6.0 a | 263.29 ± 8.1 a | 11.23 ± 0.2 a | 368.01 ± 4.1 a | 13.70 ± 1.1 a | |
| SI | 59.96 ± 5.0 ab | 257.75 ± 4.0 ab | 10.60 ± 0.4 ab | 344.86 ± 5.3 c | 11.53 ± 1.4 b | ||
| 150 | SM | 58.56 ± 5.2 bc | 255.67 ± 5.1 ab | 10.76 ± 0.2 b | 361.03 ± 2.5 b | 12.30 ± 1.1 ab | |
| SI | 54.15 ± 3.3 bc | 246.52 ± 6.0 bc | 10.26 ± 0.3 bc | 340.56 ± 4.1 cd | 10.93 ± 1.0 c | ||
| Significance | MT | 0.029 * | 0.021 * | 0.035 * | 0.004 ** | 0.002 ** | |
| PP | 0.006 ** | 0.003 ** | 0.005 ** | 0.000 *** | 0.000 *** | ||
| MT × PP | 0.865 ns | 0.426 ns | 0.957 ns | 0.319 ns | 0.872 ns | ||
| Year | Treatment | Grain Yield (kg ha−1) | Biomass Dry Matter (kg ha−1) | 1000-Grain Weight (g) | LER | |
|---|---|---|---|---|---|---|
| MT | PP | |||||
| 2023 | 0 | SM | 1973.74 ± 100.2 b | 2893.74 ± 195.3 ab | 204.78 ± 15.1 ab | |
| SI | 1623.00 ± 89.3 c | 2180.12 ± 140.0 d | 160.94 ± 13.6 c | 0.82 | ||
| 50 | SM | 2065.97 ± 138.2 ab | 2930.23 ± 173.4 ab | 210.15 ± 12.6 a | ||
| SI | 1760.12 ± 141.1 c | 2366.79 ± 246.0 cd | 186.08 ± 22.0 b | 0.85 | ||
| 100 | SM | 2240.30 ± 105.4 a | 3130.13 ± 173.5 a | 215.89 ± 16.7 a | ||
| SI | 2033.60 ± 75.0 b | 2736.79 ± 248.5 b | 199.08 ± 11.6 ab | 0.91 | ||
| 150 | SM | 2050.33 ± 96.4 b | 2661.97 ± 98.0 c | 202.89 ± 5.1 ab | ||
| SI | 1749.97 ± 52.2 c | 2276.82 ± 165.6 d | 183.23 ± 6.2 bc | 0.85 | ||
| Significance | MT | 0.000 *** | 0.002 ** | 0.048 * | ||
| PP | 0.000 *** | 0.000 *** | 0.000 *** | |||
| MT × PP | 0.681 ns | 0.393 ns | 0.356 ns | |||
| 2024 | 0 | SM | 1995.26 ± 90.2 c | 3003.60 ± 126.1 ab | 212.19 ± 16.5 b | |
| SI | 1664.18 ± 49.2 e | 2273.49 ± 169.1 d | 177.93 ± 27.5 c | 0.834 | ||
| 50 | SM | 2183.89 ± 104.7 b | 3180.49 ± 270.2 a | 221.60 ± 12.0 ab | ||
| SI | 1873.15 ± 37.3 d | 2556.97 ± 220.1 cd | 197.29 ± 16.0 bc | 0.86 | ||
| 100 | SM | 2367.04 ± 84.7 a | 3306.78 ± 227.4 a | 232.56 ± 14.1 a | ||
| SI | 2200.23 ± 60.8 b | 2953.45 ± 144.3 ab | 216.82 ± 6.0 ab | 0.93 | ||
| 150 | SM | 2110.23 ± 20.0 bc | 2673.19 ± 258.7 c | 221.78 ± 12.2 ab | ||
| SI | 1830.34 ± 62.4 d | 2366.82 ± 125.1 d | 198.37 ± 12.1 bc | 0.86 | ||
| Significance | MT | 0.000 *** | 0.000 *** | 0.036 * | ||
| PP | 0.000 *** | 0.000 *** | 0.001 ** | |||
| MT × PP | 0.209 ns | 0.425 ns | 0.785 ns | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, D.; Meng, Z.; Hu, S.; Nasar, J.; Shao, Z.; Zhang, X.; Amin, B.; Arif, M.; Gitari, H. Exogenous Melatonin Application Improves Shade Tolerance and Growth Performance of Soybean Under Maize–Soybean Intercropping Systems. Plants 2025, 14, 2359. https://doi.org/10.3390/plants14152359
Jia D, Meng Z, Hu S, Nasar J, Shao Z, Zhang X, Amin B, Arif M, Gitari H. Exogenous Melatonin Application Improves Shade Tolerance and Growth Performance of Soybean Under Maize–Soybean Intercropping Systems. Plants. 2025; 14(15):2359. https://doi.org/10.3390/plants14152359
Chicago/Turabian StyleJia, Dan, Ziqing Meng, Shiqiang Hu, Jamal Nasar, Zeqiang Shao, Xiuzhi Zhang, Bakht Amin, Muhammad Arif, and Harun Gitari. 2025. "Exogenous Melatonin Application Improves Shade Tolerance and Growth Performance of Soybean Under Maize–Soybean Intercropping Systems" Plants 14, no. 15: 2359. https://doi.org/10.3390/plants14152359
APA StyleJia, D., Meng, Z., Hu, S., Nasar, J., Shao, Z., Zhang, X., Amin, B., Arif, M., & Gitari, H. (2025). Exogenous Melatonin Application Improves Shade Tolerance and Growth Performance of Soybean Under Maize–Soybean Intercropping Systems. Plants, 14(15), 2359. https://doi.org/10.3390/plants14152359








