The Transcription Factor Basic Pentacysteine 5, RsBPC5, Enhances Lead Stress Tolerance in Raphanus sativus
Abstract
1. Introduction
2. Results
2.1. Identification of BPC Family in R. sativus
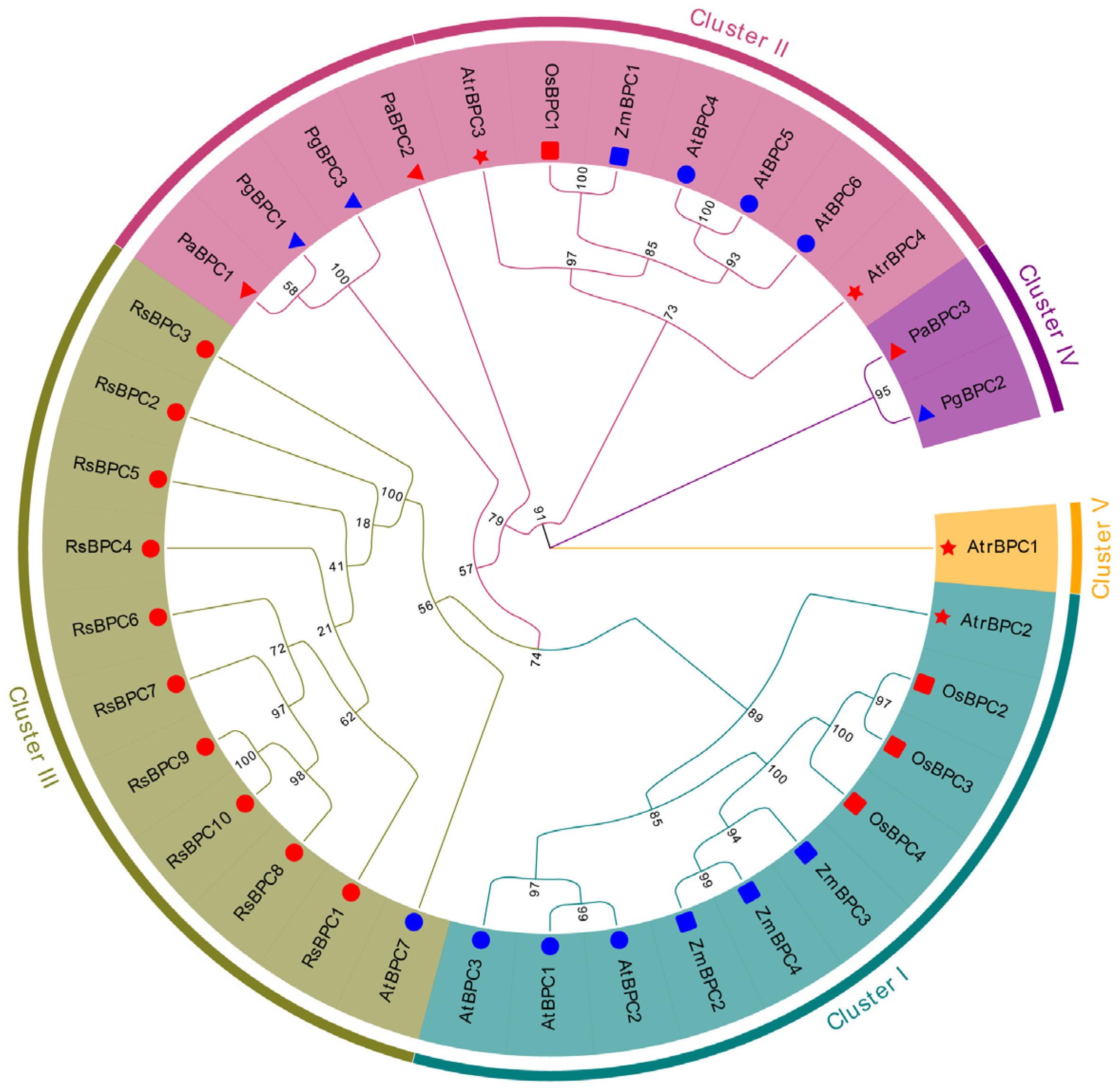
2.2. Phylogenetic Analysis of BPC Family
2.3. Protein and Gene Structure Analysis of RsBPCs
2.4. RsBPC5 Was Obviously Downregulated by Pb Stress
2.5. Functional Analysis of RsBPC5
2.6. RsBPC5 Positively Regulates the Tolerance of Radish to Pb Stress
2.7. Transient Overexpression of RsBPC5 Decreases the Pb Accumulation in Roots of R. sativus
2.8. Transient Overexpression of RsBPC5 Enhances the Antioxidant Activity in Roots of R. sativus
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Characterization of BPCs in Radish
4.3. Real-Time RT-qPCR Analysis
4.4. Subcellular Localization
4.5. Transcriptional Activity of RsBPC5
4.6. Transformation of Hairy Roots in R. sativus
4.7. Determination of Chlorophyll Content in Leaves of R. sativus
4.8. Determination of Oxidative Damage Index
4.9. Determination of the Activities of Antioxidant Enzymes
4.10. Determination of Pb Content
4.11. Principal Coordinates Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Kong, N.; Liu, Q.; Wang, M.; Lv, K.; Zeng, H.; Chen, W.; Luo, J.; Lou, H.; Song, L.; et al. Ti3C2Tx MXene nanosheets enhance the tolerance of Torreya grandis to Pb stress. J. Hazard. Mater. 2022, 445, 130647. [Google Scholar] [CrossRef] [PubMed]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of heavy metals and recent advances in their removal: A review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; M.M.S., C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Levin, R.; Vieira, C.L.Z.; Rosenbaum, M.H.; Bischoff, K.; Mordarski, D.C.; Brown, M.J. The urban lead (Pb) burden in humans, animals and the natural environment. Environ. Res. 2021, 193, 110377. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead toxicity in cereals: Mechanistic insight into toxicity, mode of action, and management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Wu, Y.; Mu, M.; Jiang, J.; Nie, W.; Zhao, S.; Cui, G.; Yin, X. Genome-wide identification and characterization of NAC transcription factor family members in Trifolium pratense and expression analysis under lead stress. BMC Genom. 2024, 25, 128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Chen, P.; Song, Y.; Liu, X.; Xiao, L.; Bu, C.; Liu, P.; Zhao, L.; Ingvarsson, P.K.; Wu, H.X.; El-Kassaby, Y.A.; et al. LncRNA PMAT–PtoMYB46 module represses PtoMATE and PtoARF2 promoting Pb2+ uptake and plant growth in poplar. J. Hazard. Mater. 2022, 433, 128769. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, F.; Hou, F.; Sun, W.; Zheng, Q.; Zhang, X.; Ma, L.; Fu, J.; He, X.; Peng, H.; et al. Transcription factors responding to Pb stress in maize. Genes 2017, 8, 231. [Google Scholar] [CrossRef]
- Yan, J.; Liu, Y.; Yang, L.; He, H.; Huang, Y.; Fang, L.; Scheller, H.V.; Jiang, M.; Zhang, A. Cell wall beta-1,4-galactan regulated by the BPC1/BPC2-GALS1 module aggravates salt sensitivity in Arabidopsis thaliana. Mol. Plant 2021, 14, 411–425. [Google Scholar] [CrossRef]
- Sahu, A.; Singh, R.; Verma, P.K. Plant BBR/BPC transcription factors: Unlocking multilayered regulation in development, stress and immunity. Planta 2023, 258, 31. [Google Scholar] [CrossRef]
- Monfared, M.M.; Simon, M.K.; Meister, R.J.; Roig-Villanova, I.; Kooiker, M.; Colombo, L.; Fletcher, J.C.; Gasser, C.S. Overlapping and antagonistic activities of BASIC PENTACYSTEINE genes affect a range of developmental processes in Arabidopsis. Plant J. 2011, 66, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mohamed, D.; Dowhanik, S.; Petrella, R.; Gregis, V.; Li, J.; Wu, L.; Gazzarrini, S. Spatiotemporal Restriction of FUSCA3 Expression by Class I BPCs Promotes Ovule Development and Coordinates Embryo and Endosperm Growth. Plant Cell 2020, 32, 1886–1904. [Google Scholar] [CrossRef]
- Mu, Y.; Zou, M.; Sun, X.; He, B.; Xu, X.; Liu, Y.; Zhang, L.; Chi, W. BASIC PENTACYSTEINE proteins repress ABSCISIC ACID INSENSITIVE4 expression via direct recruitment of the Polycomb-Repressive Complex 2 in Arabidopsis root development. Plant Cell Physiol. 2017, 58, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, S.; Meng, D.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 is a key regulator of root growth and development. Physiol. Plant. 2023, 175, e13977. [Google Scholar] [CrossRef] [PubMed]
- Petrella, R.; Caselli, F.; Roig-Villanova, I.; Vignati, V.; Chiara, M.; Ezquer, I.; Tadini, L.; Kater, M.M.; Gregis, V. BPC transcription factors and a Polycomb Group protein confine the expression of the ovule identity gene SEEDSTICK in Arabidopsis. Plant J. 2020, 102, 582–599. [Google Scholar] [CrossRef]
- Zhao, H.; Wan, S.; Huang, Y.; Li, X.; Jiao, T.; Zhang, Z.; Ma, B.; Zhu, L.; Ma, F.; Li, M. The transcription factor MdBPC2 alters apple growth and promotes dwarfing by regulating auxin biosynthesis. Plant Cell 2024, 36, 585–604. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Yan, J. BPC1 and BPC2 positively regulates the waterlogging stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2025, 747, 151296. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Fang, L. BASIC PENTACYSTEINE2 negatively regulates osmotic stress tolerance by modulating LEA4-5 expression in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 168, 373–380. [Google Scholar] [CrossRef]
- Yan, J.; Liu, Y.; Yan, J.; Liu, Z.; Lou, H.; Wu, J. The salt-activated CBF1/CBF2/CBF3-GALS1 module fine-tunes galactan-induced salt hypersensitivity in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 1904–1917. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Feng, Y.; Xue, Y.; Wang, Y.; Zhao, M.; Chen, M.; Chen, C.; Su, W.; Chen, R. Genome-wide identification of flowering Chinese cabbage BPC family genes and BcBPC9 functional analysis in Cd stress tolerance. Plant Stress 2023, 10, 100220. [Google Scholar] [CrossRef]
- Singh, B.K. Radish (Raphanus sativus L.): Breeding for higher yield, better quality and wider adaptability. In Advances in Plant Breeding Strategies: Vegetable Crops: Volume 8: Bulbs, Roots and Tubers; Springer International Publishing: Cham, Switzerland, 2021; pp. 275–304. [Google Scholar]
- Zhang, J.; He, W.; Smith, W.N.; Grant, B.B.; Ding, W.; Jiang, R.; Zou, G.; Chen, Y.; He, P. Exploring management strategies to improve yield and mitigate nitrate leaching in a typical radish field in northern China. J. Environ. Manag. 2021, 290, 112640. [Google Scholar] [CrossRef]
- Khan, R.S.; Khan, S.S.; Siddique, R. Radish (Raphanus sativus): Potential antioxidant role of bioactive compounds extracted from radish leaves-A review. Pak. J. Med. Health Sci. 2022, 16, 2–4. [Google Scholar] [CrossRef]
- Peng, H.; Gao, J.; Song, X. Identification of heavy metal-responsive genes in radish (Raphanus sativus L.) through RNA-Seq meta-analysis. Sci. Hortic. 2021, 288, 110402. [Google Scholar] [CrossRef]
- Massaccesi, L.; Meneghini, C.; Comaschi, T.; D’Amato, R.; Onofri, A.; Businelli, D. Ligands involved in Pb immobilization and transport in lettuce, radish, tomato and Italian ryegrass. J. Plant Nutr. Soil Sci. 2014, 177, 766–774. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Wang, Y.; Dong, J.; Zhang, X.; Wang, K.; Ying, J.; Li, C.; Liu, L. Melatonin-induced DNA demethylation of metal transporters and antioxidant genes alleviates lead stress in radish plants. Hortic. Res. 2021, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Halabicky, O.; Giang, C.; Miller, A.; Peterson, K. Lead exposure, glucocorticoids, and physiological stress across the life course: A systematic review. Environ. Pollut. 2024, 345, 123329. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, T.; Zhang, L.; Kang, M.; Zhang, Z.; Zheng, Z.; Sun, P.; Shrestha, N.; Liu, J.; Yang, Y. Genomic analyses of a “living fossil”: The endangered dove-tree. Mol. Ecol. Resour. 2020, 20, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; Tang, Z.; Song, J.-J.; Huang, X.-Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Liu, S.; Long, T.; Chen, Z.; Liu, J.; Cui, W.; Leng, H.; Xing, Y.; Rodriguez, L.G.; Gao, Y.; Yao, Y.; et al. Genome-wide identification of NRAMP family genes in Populus trichocarpa and their roles in transport of heavy metals. Tree Genet. Genomes 2023, 19, 51. [Google Scholar] [CrossRef]
- Castaings, L.; Alcon, C.; Kosuth, T.; Correia, D.; Curie, C. Manganese triggers phosphorylation-mediated endocytosis of the Arabidopsis metal transporter NRAMP1. Plant J. 2021, 106, 1328–1337. [Google Scholar] [CrossRef]
- Kanwal, F.; Riaz, A.; Ali, S.; Zhang, G. NRAMPs and manganese: Magic keys to reduce cadmium toxicity and accumulation in plants. Sci. Total Environ. 2024, 921, 171005. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-D.; Gao, W.; Wang, P.; Zhao, F.-J. OsNRAMP5 is a major transporter for lead uptake in rice. Environ. Sci. Technol. 2022, 56, 17481–17490. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, Y.; Zhang, W.; Dong, J.; Zhang, X.; Zhu, Y.; Wang, Y.; Liu, L.; Xu, L. RsNRAMP5, a major metal transporter, promotes cadmium influx and ROS accumulation in radish (Raphanus sativus L.). Plant Physiol. Biochem. 2025, 218, 109323. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Qin, A.; Zain, M.; Mushtaq, Z.; Mehmood, F.; Riaz, L.; Naveed, S.; Ansari, M.J.; Saeed, M.; Ahmad, I. Pb uptake, accumulation, and translocation in plants: Plant physiological, biochemical, and molecular response: A review. Heliyon 2024, 10, e27724. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, Z.; Liu, X.; Imran, S.; Peng, L.; Dai, R.; Deng, Y. Environmental materials for remediation of soils contaminated with lead and cadmium using maize (Zea mays L.) growth as a bioindicator. Environ. Sci. Pollut. Res. 2016, 23, 6168–6178. [Google Scholar] [CrossRef]
- Naghavi, F. Effects of lead and zinc on seed germination and seedling growth of soybean (Glycine max L). Int. J. Biosci. 2014, 4, 306–315. [Google Scholar]
- Wang, Y.; Xu, L.; Chen, Y.; Shen, H.; Gong, Y.; Limera, C.; Liu, L.; Wu, K. Transcriptome profiling of radish (Raphanus sativus L.) root and identification of genes involved in response to lead (Pb) stress with next generation sequencing. PLoS ONE 2013, 8, e66539. [Google Scholar] [CrossRef]
- Yu, H.-J.; Baek, S.; Lee, Y.-J.; Cho, A.; Mun, J.-H. The radish genome database (RadishGD): An integrated information resource for radish genomics. Database 2019, 2019, baz009. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2022, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Jin, J.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2019, 48, D1104–D1113. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.-H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Liu, X.; Wang, R.; Chen, W.; Suo, J.; Yan, J.; Wu, J. Agrobacterium-mediated transient expression in Torreya grandis cones: A simple and rapid tool for gene expression and functional gene assay. Sci. Hortic. 2024, 338, 113664. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, J.; Wang, T.; Chen, W.; Suo, J.; Yan, J.; Wu, J. TgLCYB1 regulated by TgWRKY22 enhances the tolerance of Torreya grandis to waterlogging stress. Int. J. Biol. Macromol. 2023, 253, 126702. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, R.; Zhong, H.; Yan, J.; Liu, Z.; Liu, Y.; Chen, W.; Wu, J.; Yan, J. Sugars will eventually be exported transporter 18 mediates glucose metabolism in Torreya grandis kernels. Int. J. Biol. Macromol. 2025, 310, 143529. [Google Scholar] [CrossRef]
- Yan, J.; Liu, Z.; Wang, T.; Wang, R.; Wang, S.; Chen, W.; Suo, J.; Yan, J.; Wu, J. TgLUT1 regulated by TgWRKY10 enhances the tolerance of Torreya grandis to drought stress. Plant Physiol. Biochem. 2024, 207, 108436. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Yan, J.; Chen, J.; Wang, L.; Yu, W.; Wu, J.; Yan, J. A Torreya grandis ARF transcription factor TgARF15 enhances drought stress tolerance. Environ. Exp. Bot. 2024, 222, 105761. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Wen, Y.; Kang, W.; Yu, F.; Liu, C.; Peng, Z.; Xu, D. The Transcription Factor Basic Pentacysteine 5, RsBPC5, Enhances Lead Stress Tolerance in Raphanus sativus. Plants 2025, 14, 2362. https://doi.org/10.3390/plants14152362
Xiao J, Wen Y, Kang W, Yu F, Liu C, Peng Z, Xu D. The Transcription Factor Basic Pentacysteine 5, RsBPC5, Enhances Lead Stress Tolerance in Raphanus sativus. Plants. 2025; 14(15):2362. https://doi.org/10.3390/plants14152362
Chicago/Turabian StyleXiao, Jian, Yongli Wen, Wenjing Kang, Fangzhou Yu, Chuan Liu, Zhenyu Peng, and Dianheng Xu. 2025. "The Transcription Factor Basic Pentacysteine 5, RsBPC5, Enhances Lead Stress Tolerance in Raphanus sativus" Plants 14, no. 15: 2362. https://doi.org/10.3390/plants14152362
APA StyleXiao, J., Wen, Y., Kang, W., Yu, F., Liu, C., Peng, Z., & Xu, D. (2025). The Transcription Factor Basic Pentacysteine 5, RsBPC5, Enhances Lead Stress Tolerance in Raphanus sativus. Plants, 14(15), 2362. https://doi.org/10.3390/plants14152362





