The Impact of Short-Term Drought on the Photosynthetic Characteristics and Yield of Peanuts Grown in Saline Alkali Soil
Abstract
1. Introduction
2. Results
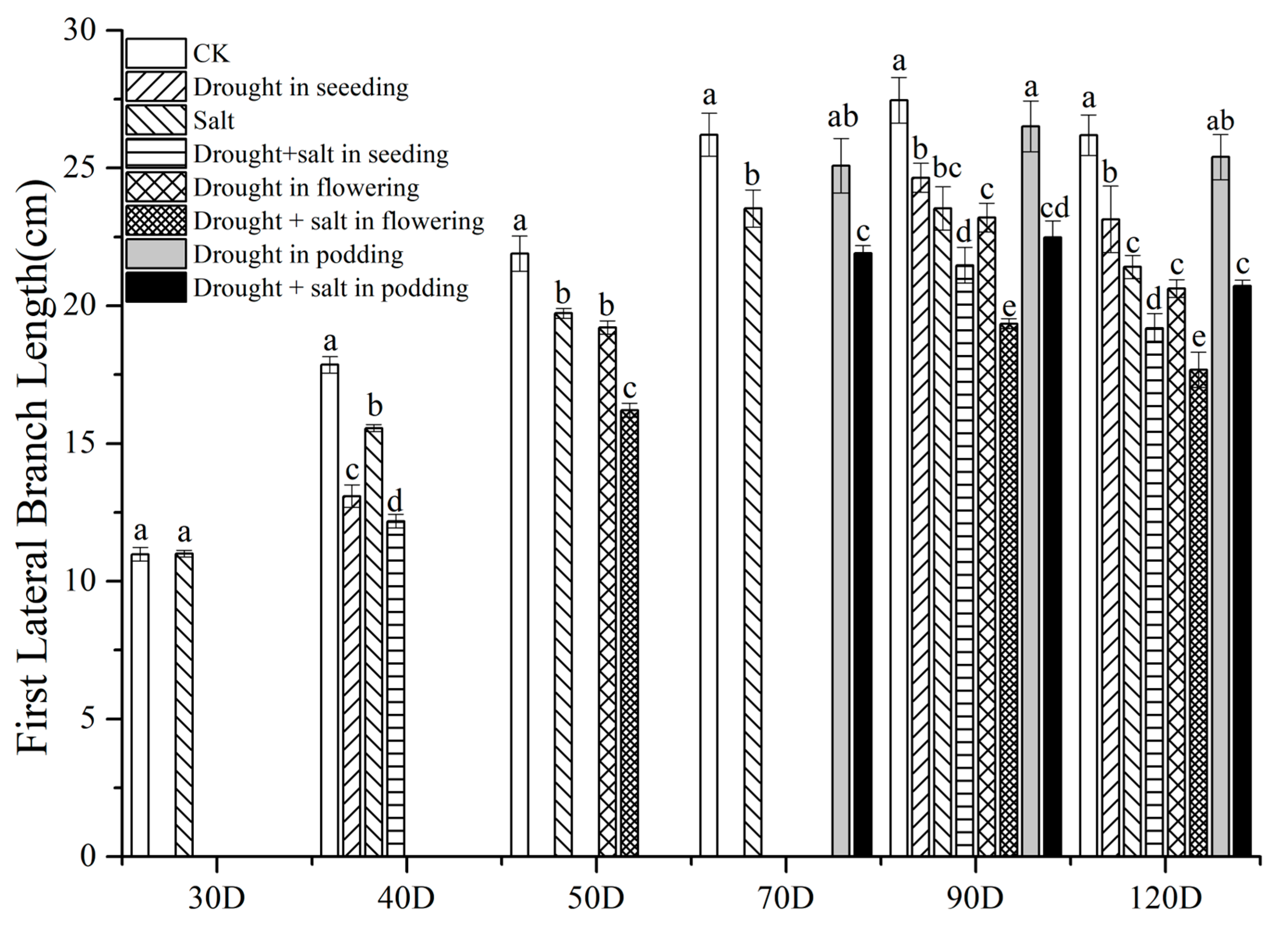
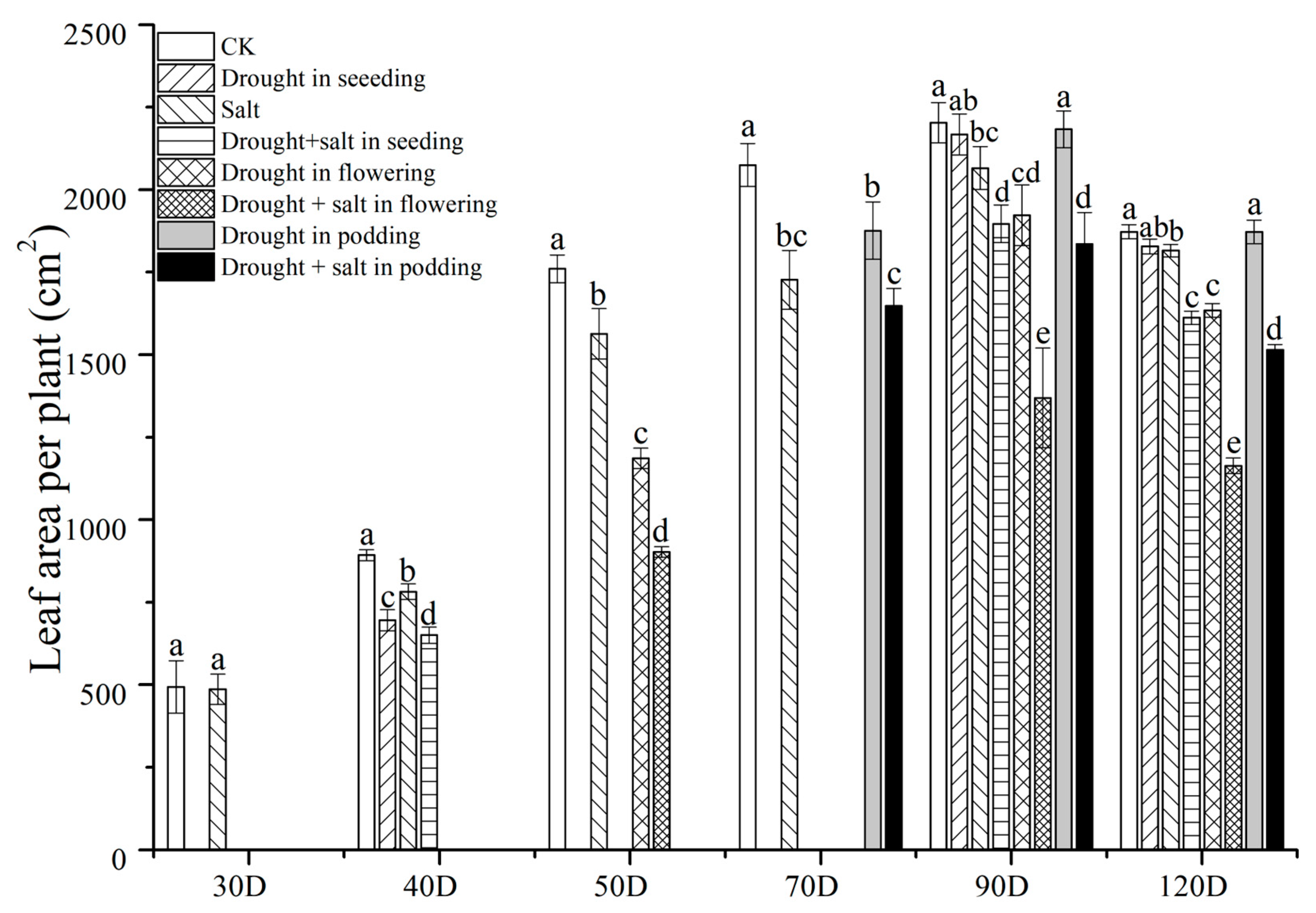
2.1. The Impact of Drought at Different Stages on the Agronomic Traits of Peanuts
2.2. Effects of Drought at Different Stages on Photosynthetic Characteristics and SPAD Values of Peanut Leaves
2.3. The Impact of Drought at Different Stages on the Accumulation and Distribution of Dry Matter in Peanuts
2.4. The Impact of Drought at Different Stages on Peanut Yield
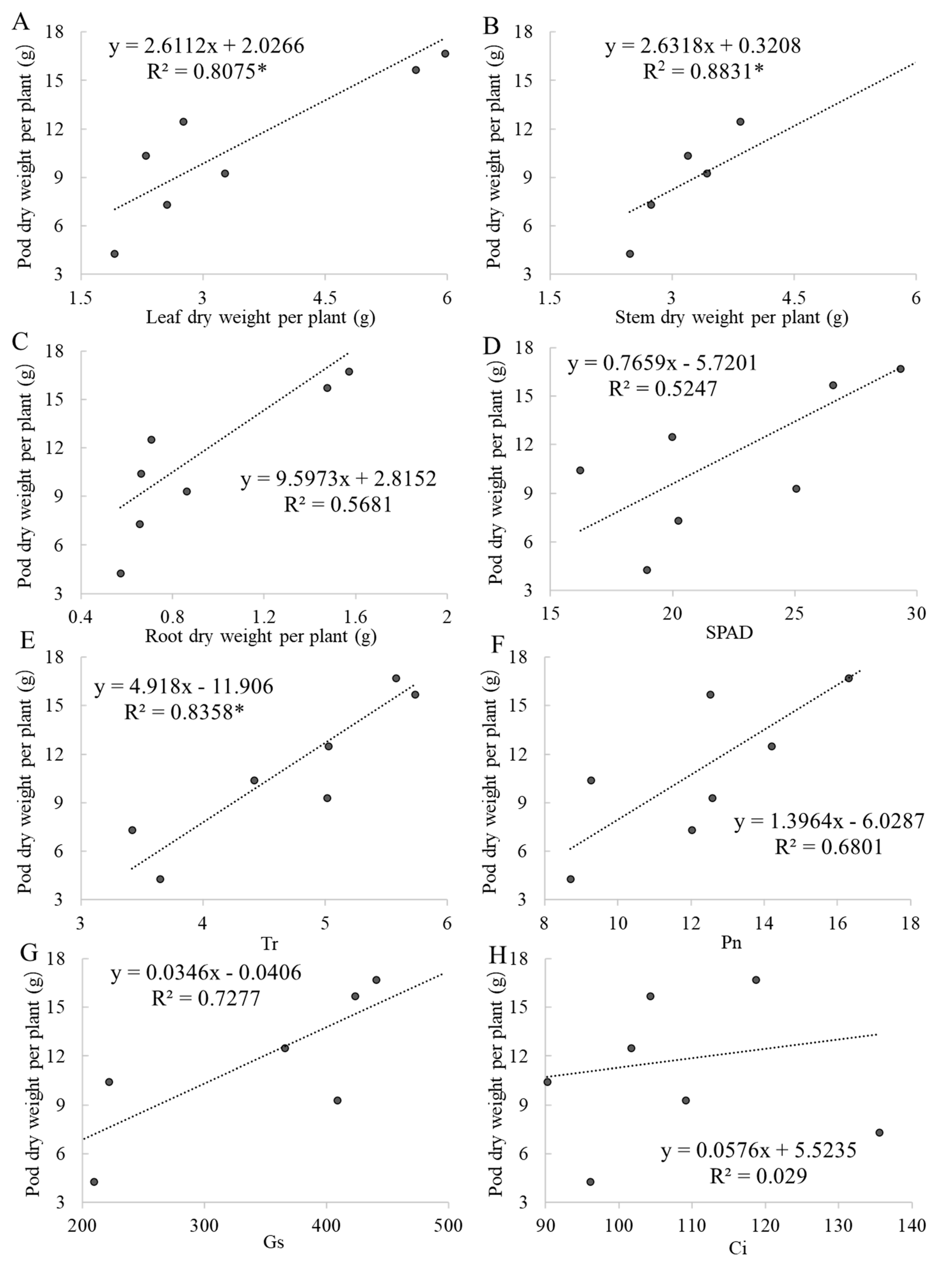
2.5. Correlations Between Yield and Other Indicators
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Measurement Items and Methods
4.2.1. Sample Collection
4.2.2. Determination of Net Photosynthetic Rate and SPAD Value
4.2.3. Pod Yield and Yield Composition Factors
4.3. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.W.H.; Bello, F.D.; Dole Al, J.I.; Lep, J. Niche overlap reveals the effects of competition, disturbance and contrasting assembly processes in experimental grassland communities. J. Ecol. 2011, 99, 788–796. [Google Scholar] [CrossRef]
- Fajardo, A.; Mcintire, E.J.B. Under strong niche overlap conspecifics do not compete but help each other to survive: Facilitation at the intraspecific level. J. Ecol. 2011, 99, 642–650. [Google Scholar] [CrossRef]
- Abd El-RheemKh, M.; Safi-Naz, S. Effect of soil salinity on growth, yield and nutrient balance of peanut plants. Int. J. Chemtech. Res. 2015, 8, 564–568. [Google Scholar]
- Qin, L.; Li, L.; Bi, C.; Zhang, Y.; Wan, S.; Meng, J.; Meng, Q.; Li, X. Damaging mechanisms of chilling- and salt stress to Arachis hypogaea L. leaves. Photosynthetica 2011, 49, 37–42. [Google Scholar] [CrossRef]
- Singh, A.L.; Hariprasanna, K.; Chaudhari, V.; Gor, H.K.; Chikani, B.M. Identification of groundnut (Arachis hypogaea L.) cultivars tolerant of soil salinity. J. Plant Nutr. 2010, 33, 1761–1776. [Google Scholar] [CrossRef]
- Meena, H.N.; Meena, M.; Yadav, R.S. Comparative performance of seed types on yield potential of peanut (Arachis hypogaea L.) under saline irrigation. Field Crops Res. 2016, 196, 305–310. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Dai, X.; Zhang, G.; Ci, D.; Ding, H.; Tian, J. Effect of exogenous calcium application on absorption and distribution of nutrient elements in peanut under salt stress. J. Appl. Ecol. 2018, 29, 9. [Google Scholar]
- Foad, M.; Ismail, A.M. Responses of Photosynthesis, Chlorophyll Fluorescence and ROS-Scavenging Systems to Salt Stress during Seedling and Reproductive Stages in Rice. Ann. Bot. 2007, 1161–1173. [Google Scholar]
- Sakoda, K.; Taniyoshi, K.; Yamori, W.; Tanaka, Y. Drought stress reduces crop carbon gain due to delayed photosynthetic induction under fluctuating light conditions. Physiol. Plant. 2022, 174, e13603. [Google Scholar] [CrossRef]
- Alam, H.; Khattak, J.Z.K.; Saleem, M.H.; Fahad, S.; Alkahtani, J. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 2020, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Dai, L.; Sun, H.; Chen, M.; Sun, Y. Effects of moderate soil salinity on osmotic adjustment and energy strategy in soybean under drought stress. Plant Physiol. Biochem. 2019, 139, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Lü, H.; Gao, L.; Wang, Y.; Shao, K. Dynamic responses of Haloxylon ammodendron to various degrees of simulated drought stress. Plant Physiol. Biochem. 2019, 139, 121–131. [Google Scholar]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.; Li, F. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Torres, R.O.; Henry, A. Yield stability of selected rice breeding lines and donors across conditions of mild to moderately severe drought stress. Field Crops Res. 2018, 220, 37–45. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Zhang, B.; Fu, R.; Kaiyong, G.; William, C. Programmed cell death in wheat (Triticum aestivum L.) endosperm cells is affected by drought stress. Protoplasma 2018, 255, 1039–1052. [Google Scholar] [CrossRef]
- Furlan, A.; Bianucci, E.; MarA, D.C.T.; Kleinert, A.; Valentine, A.; Castro, S. Dynamic responses of photosynthesis and the antioxidant system during a drought and rehydration cycle in peanut plants. Funct. Plant Biol. 2016, 43, 337. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Li, M.; Su, L.; Lian, S.; Zhang, B.; Li, X.; Ge, K.; Li, L. AhGLK1 affects chlorophyll biosynthesis and photosynthesis in peanut leaves during recovery from drought. Sci. Rep. 2018, 8, 2250. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Parida, A.; Das, A. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Ehdaie, B.; Layne, A.P.; Waines, J.G. Root system plasticity to drought influences grain yield in bread wheat. Euphytica 2012, 186, 219–232. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J. Cell Signaling during Cold, Drought, and Salt Stress. Plant Cell 2002, 14, S165. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Cui, L.; Lv, S.; Bian, J.; Wang, M.; Song, W.; Nie, X. Comprehensive evaluating of wild and cultivated emmer wheat (Triticum turgidum L.) genotypes response to salt stress. Plant Growth Regul. 2018, 2, 261–273. [Google Scholar] [CrossRef]
- Kumar, D.; Kushwaha, S.K.; Delvento, C.; Liatukas, I.; Vivekanand, V.; Svensson, J.T.; Henriksson, T.; Brazauskas, G.; Chawade, A. Affordable Phenotyping of Winter Wheat under Field and Controlled Conditions for Drought Tolerance. Agronomy 2020, 10, 882. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Sun, L.; Huang, B.; Ding, C.; Gu, Y.; Liao, J.; Hu, C.; Zhang, Z.; Yuan, S. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Zhao, F.; Qin, P. Protective effect of exogenous polyamines on root tonoplast function against salt stress in barley seedlings. Plant Growth Regul. 2004, 42, 97–103. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, L.; Yu, Z. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 2006, 49, 157–165. [Google Scholar] [CrossRef]
- Yahyazadeh, M.; Meinen, R.; Nsch, R.; Abouzeid, S.; Selmar, D. Impact of drought and salt stress on the biosynthesis of alkaloids in Chelidonium majus L. Phytochemistry 2018, 152, 204–212. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Liu, Q.; Zhang, J.; Zhang, G.; Li, G. Biochar Enhances the Resistance of Legumes and Soil Microbes to Extreme Short-Term Drought. Plants 2023, 12, 4155. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.; Lian, W.; Wang, Y.; Liu, M.; Zhang, Y.; Wang, X.; Chen, G. Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice. Int. J. Mol. Sci. 2024, 25, 1185. [Google Scholar] [CrossRef] [PubMed]
- Hemasundar, A.; Seher, Y.; Monika, S.; Bahar, A.S.; Muhammad, A. Salt and drought stress-mitigating approaches in sugar beet (Beta vulgaris L.) to improve its performance and yield. Planta 2023, 258, 30. [Google Scholar]
- Ors, S.; Suarez, D.L. Spinach biomass yield and physiological response to interactive salinity and water stress. Agric. Water Manag. 2017, 190, 31–41. [Google Scholar] [CrossRef]
- Zhao, C.; Cheng, X.; Wang, Y.; Wang, M. Effects of drought stress on peanut growth during different growth stages and compensatory effect after water recovery. Chin. J. Oil Crop Sci. 2012, 3, 12–23. [Google Scholar]
- Thangthong, N.; Jogloy, S.; Pensuk, V.; Kesmala, T.; Vorasoot, N. Distribution patterns of peanut roots under different durations of early season drought stress. Field Crops Res. 2016, 198, 40–49. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Craig, K. Root distribution pattern and their contribution in photosynthesis and biomass in jerusalem artichokeunder drought conditions. Pak. J. Bot. 2018, 50, 879–886. [Google Scholar]
- Senapati, N.; Stratonovitch, P.; Paul, M.J.; Semenov, M.A. Drought tolerance during reproductive development is important for increasing wheat yield potential under climate change in Europe. J. Exp. Bot. 2019, 70, 2549–2560. [Google Scholar] [CrossRef]
- Lan, Y.; Chawade, A.; Kuktaite, R.; Johansson, E. Climate Change Impact on Wheat Performance—Effects on Vigour, Plant Traits and Yield from Early and Late Drought Stress in Diverse Lines. Int. J. Mol. Sci. 2022, 23, 3333. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Z.; Kang, T.; Dai, L.; Song, W. Rooting traits of peanut genotypes differing in drought tolerance under drought stress. Int. J. Plant Prod. 2017, 11, 349–360. [Google Scholar]
- Zhang, G.; Liu, Q.; Zhang, Z.; Ci, D.; Zhang, J.; Xu, Y.; Guo, Q.; Xu, M.; He, K. Effect of Reducing Nitrogen Fertilization and Adding Organic Fertilizer on Net Photosynthetic Rate, Root Nodules and Yield in Peanut. Plants 2023, 12, 2902. [Google Scholar] [CrossRef] [PubMed]
- Ci, D.; Qin, F.; Tang, Z.; Zhang, G.; Zhang, J.; Si, T.; Yang, J.; Xu, Y.; Yu, T.; Xu, M.; et al. Arbuscular Mycorrhizal Fungi Restored the Saline–Alkali Soil and Promoted the Growth of Peanut Roots. Plants 2023, 12, 3426. [Google Scholar] [CrossRef] [PubMed]

| Growth Stage | Treatment | Pn | Ci | Gs | Tr | SPAD |
|---|---|---|---|---|---|---|
| (umol·m−2·s−1) | (umol·mol−1) | (umol·m−2·s−1) | (mmol·m−2·s−1) | |||
| 30D | CK | 16.91 ± 0.55 a | 261.8 ± 5.26 a | 393.34 ± 19.02 a | 5.94 ± 0.19 a | 46.28 ± 1.53 a |
| S | 13.47 ± 0.83 b | 226.77 ± 10.49 b | 273.2 ± 29.56 b | 6.15 ± 0.15 a | 39.06 ± 1.92 b | |
| 35D | CK | 24.8 ± 1.15 a | 240.12 ± 8.25 b | 414.68 ± 12.46 a | 7.05 ± 0.5 a | 40.5 ± 1.73 c |
| S | 20.08 ± 0.42 b | 232.2 ± 6.42 bc | 405.18 ± 12.06 a | 6.96 ± 0.18 a | 36.6 ± 1.13 d | |
| SD | 14.52 ± 1.01 c | 272.06 ± 9.4 a | 251.84 ± 6.45 b | 5.69 ± 0.6 b | 57.82 ± 3.76 a | |
| SDS | 11.36 ± 0.62 d | 227.96 ± 2.76 c | 237.82 ± 11.3 b | 7.1 ± 0.35 a | 46.32 ± 1.81 b | |
| 45D | CK | 25.82 ± 1.19 a | 193.8 ± 2.17 b | 466.46 ± 22.94 a | 8.02 ± 0.88 b | 36.48 ± 1.45 b |
| S | 18.8 ± 0.55 b | 189 ± 4.89 b | 283.15 ± 9.89 b | 9.04 ± 0.63 a | 33.3 ± 0.97 c | |
| FD | 13.05 ± 1.21 c | 257 ± 8.42 a | 191.38 ± 2.66 c | 6.2 ± 0.35 c | 44.18 ± 1.36 a | |
| FDS | 10.53 ± 1.22 d | 256.6 ± 5.13 a | 200.74 ± 16.76 c | 5.98 ± 0.61 c | 39.8 ± 2.51 b | |
| 65D | CK | 16.31 ± 0.64 a | 237.6 ± 10.92 a | 453.46 ± 23.75 a | 7.25 ± 0.88 a | 42.04 ± 1.11 a |
| S | 15.44 ± 0.87 a | 186.92 ± 4.97 b | 178.81 ± 1.36 b | 4.88 ± 0.38 b | 32.4 ± 1.4 b | |
| PD | 8.08 ± 1.03 b | 200.93 ± 14.58 b | 105.55 ± 5.47 c | 3.78 ± 0.17 c | 43.62 ± 2.22 a | |
| PDS | 2.95 ± 0.42 c | 251.79 ± 15.44 a | 75.09 ± 4.91 d | 2.83 ± 0.19 d | 41.7 ± 3.07 a | |
| 85D | CK | 17.72 ± 0.32 a | 174 ± 6.93 a | 244.19 ± 3.95 c | 4.65 ± 0.13 b | 43.32 ± 2.43 a |
| S | 10.33 ± 0.72 d | 166.96 ± 1.99 b | 243.49 ± 10.2 c | 5.05 ± 0.64 b | 31.38 ± 4.36 b | |
| SD | 16.3 ± 2.26 a | 162.02 ± 1.29 b | 370.72 ± 5.26 a | 6.55 ± 0.87 a | 42.6 ± 3.31 a | |
| SDS | 12.4 ± 0.45 bc | 111.81 ± 2.23 d | 262.37 ± 12.56 b | 5.09 ± 0.43 b | 33.18 ± 3.52 b | |
| FD | 13.82 ± 0.59 b | 148.28 ± 2.43 c | 266.63 ± 7.16 b | 5.35 ± 0.12 b | 43.12 ± 1.4 a | |
| FDS | 7.69 ± 0.19 e | 140.17 ± 3.48 c | 98.64 ± 1.06 e | 2.79 ± 0.18 d | 29.94 ± 3.47 b | |
| PD | 11.96 ± 0.37 c | 143.72 ± 3.98 c | 199.77 ± 9.37 d | 4.56 ± 0.09 c | 42.7 ± 1.52 a | |
| PDS | 8.75 ± 0.31 e | 103.78 ± 1.07 f | 76.78 ± 4.34 f | 2.09 ± 0.03 e | 28.64 ± 2.93 b | |
| 115D | CK | 16.3 ± 0.2 a | 118.7 ± 0.82 b | 440.59 ± 14.6 b | 5.58 ± 0.12 a | 29.33 ± 2.64 a |
| S | 12.63 ± 0.2 c | 117.7 ± 4.12 b | 494.44 ± 15.56 a | 5.73 ± 0.49 a | 26.84 ± 2.93 a | |
| SD | 14.2 ± 0.72 b | 101.67 ± 1.15 d | 365.68 ± 19.95 c | 5.03 ± 0.39 a | 19.98 ± 3.36 b | |
| SDS | 12.02 ± 0.46 c | 135.59 ± 1.89 a | 180.19 ± 7.96 e | 3.42 ± 0.23 c | 20.23 ± 2.52 b | |
| FD | 12.59 ± 0.79 c | 109.11 ± 1.17 c | 408.95 ± 14.42 b | 5.02 ± 0.39 a | 25.05 ± 2.87 ab | |
| FDS | 8.71 ± 0.65 d | 96.11 ± 2.46 e | 209.63 ± 11.49 d | 3.65 ± 0.1 c | 18.96 ± 2.2 b | |
| PD | 12.52 ± 0.3 c | 104.33 ± 1.53 d | 423.32 ± 22.86 b | 5.74 ± 0.26 a | 26.58 ± 1.99 a | |
| PDS | 9.27 ± 0.4 d | 90.22 ± 1.35 f | 222.05 ± 11.03 d | 4.42 ± 0.4 b | 16.22 ± 3.49 b | |
| Analysis of variance (p value) | ||||||
| 35D | S × D | 0.038 * | <0.001 ** | 0.019 * | 0.796 | 0.006 ** |
| 45D | 0.022 * | 0.563 | 0.034 * | 0.024 * | 0.515 | |
| 65D | <0.001 ** | <0.001 ** | 0.01 ** | 0.001 ** | 0.003 ** | |
| Leaf | Stem + Petiole | Root | Pod | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth Stage | Treatment | Accumulation | Distribution | Accumulation | Distribution | Accumulation | Distribution | Accumulation | Distribution | Accumulation |
| (g·Plant−1) | Ratio (%) | (g·Plant−1) | Ratio (%) | (g·Plant−1) | Ratio (%) | (g·Plant−1) | Ratio (%) | (g·Plant−1) | ||
| 30D | CK | 0.84 ± 0.03 a | 45.16 | 0.76 ± 0.025 a | 40.86 | 0.26 ± 0.01 a | 13.99 | / | / | 1.86 ± 0.06 a |
| S | 0.54 ± 0.018 b | 44.63 | 0.51 ± 0.016 b | 42.15 | 0.16 ± 0.005 b | 13.22 | / | / | 1.22 ± 0.039 b | |
| 40D | CK | 1.55 ± 0.05 a | 46.69 | 1.46 ± 0.047 a | 43.98 | 0.31 ± 0.01 a | 9.34 | / | / | 3.31 ± 0.107 a |
| S | 1.02 ± 0.033 b | 44.93 | 1.04 ± 0.034 b | 45.81 | 0.21 ± 0.007 b | 9.25 | / | / | 2.28 ± 0.074 b | |
| SD | 0.93 ± 0.03 b | 42.66 | 1.02 ± 0.033 b | 46.79 | 0.23 ± 0.008 b | 10.55 | / | / | 2.18 ± 0.07 b | |
| SDS | 0.53 ± 0.017 c | 42.74 | 0.58 ± 0.019 c | 46.77 | 0.13 ± 0.004 c | 10.48 | / | / | 1.24 ± 0.04 c | |
| 50D | CK | 3.7 ± 0.12 a | 45.74 | 3.68 ± 0.119 a | 45.49 | 0.71 ± 0.023 a | 8.78 | / | / | 8.09 ± 0.262 a |
| S | 1.84 ± 0.06 b | 44.55 | 1.89 ± 0.061 b | 45.76 | 0.4 ± 0.013 c | 9.69 | / | / | 4.14 ± 0.134 b | |
| FD | 1.77 ± 0.057 b | 43.92 | 1.79 ± 0.058 b | 44.42 | 0.47 ± 0.012 b | 11.66 | / | / | 3.93 ± 0.127 b | |
| FDS | 1.12 ± 0.036 c | 46.26 | 1.11 ± 0.036 c | 45.87 | 0.19 ± 0.006 d | 7.85 | / | / | 2.42 ± 0.078 c | |
| 70D | CK | 4.26 ± 0.138 a | 37.47 | 4.13 ± 0.134 a | 36.32 | 1.18 ± 0.025 a | 10.38 | 1.8 ± 0.058 a | 15.83 | 11.36 ± 0.286 a |
| S | 2.24 ± 0.073 b | 32.65 | 3.11 ± 0.101 b | 45.33 | 0.58 ± 0.019 b | 8.45 | 0.93 ± 0.03 b | 13.56 | 6.87 ± 0.222 b | |
| PD | 4.12 ± 0.133 a | 37.87 | 3.99 ± 0.129 a | 36.67 | 1.11 ± 0.036 a | 10.2 | 1.66 ± 0.054 a | 15.26 | 10.87 ± 0.352 a | |
| PDS | 1.97 ± 0.064 c | 32.89 | 2.64 ± 0.086 c | 44.07 | 0.52 ± 0.017 c | 8.68 | 0.86 ± 0.028 c | 14.36 | 5.99 ± 0.194 c | |
| 90D | CK | 5.98 ± 0.193 a | 19.32 | 6.71 ± 0.217 a | 21.67 | 1.57 ± 0.051 a | 5.07 | 16.7 ± 0.541 ab | 53.94 | 30.96 ± 1.002 a |
| S | 2.76 ± 0.089 cd | 13.94 | 3.84 ± 0.124 b | 19.39 | 0.71 ± 0.023 e | 3.59 | 12.49 ± 0.404 c | 63.08 | 19.79 ± 0.641 b | |
| SD | 5.6 ± 0.181 a | 17.93 | 6.24 ± 0.202 a | 19.99 | 0.99 ± 0.032 c | 3.17 | 18.39 ± 0.595 a | 58.9 | 31.23 ± 1.01 a | |
| SDS | 2.56 ± 0.083 d | 19.32 | 2.74 ± 0.089 d | 20.68 | 0.66 ± 0.021 f | 4.98 | 7.29 ± 0.236 e | 55.02 | 13.24 ± 0.429 d | |
| FD | 3.27 ± 0.106 b | 19.41 | 3.43 ± 0.111 c | 20.36 | 0.86 ± 0.028 d | 5.1 | 9.29 ± 0.301 d | 55.13 | 16.86 ± 0.545 c | |
| FDS | 1.92 ± 0.062 f | 20.78 | 2.48 ± 0.08 f | 26.84 | 0.57 ± 0.049 f | 6.17 | 4.27 ± 0.138 f | 46.21 | 9.24 ± 0.299 e | |
| PD | 5.62 ± 0.182 a | 19.44 | 6.31 ± 0.204 a | 21.83 | 1.28 ± 0.048 b | 4.43 | 15.7 ± 0.508 b | 54.31 | 28.9 ± 0.941 a | |
| PDS | 2.3 ± 0.074 e | 13.89 | 3.19 ± 0.103 c | 19.27 | 0.66 ± 0.021 ef | 3.99 | 10.4 ± 0.336 cd | 62.84 | 16.55 ± 0.540 c | |
| 120D | CK | 4.32 ± 0.14 a | 12.58 | 5.99 ± 0.194 a | 17.45 | 1.52 ± 0.049 a | 4.42 | 22.5 ± 1.005 a | 65.54 | 34.33 ± 1.329 a |
| S | 2 ± 0.065 c | 8.92 | 3.43 ± 0.111 c | 15.29 | 0.68 ± 0.022 e | 3.03 | 16.32 ± 0.532 b | 72.76 | 22.43 ± 0.729 b | |
| SD | 3.84 ± 0.124 b | 11.35 | 5.57 ± 0.18 b | 16.46 | 0.95 ± 0.031 c | 2.81 | 23.47 ± 0.766 a | 69.38 | 33.84 ± 1.1 a | |
| SDS | 1.75 ± 0.056 d | 11.67 | 2.45 ± 0.079 e | 16.33 | 0.63 ± 0.020 f | 4.2 | 10.17 ± 0.711 d | 67.8 | 15 ± 0.808 d | |
| FD | 2.36 ± 0.076 c | 12.83 | 3.06 ± 0.099 cd | 16.63 | 0.84 ± 0.027 d | 4.57 | 12.14 ± 0.396 c | 65.98 | 18.4 ± 0.598 c | |
| FDS | 1.18 ± 0.038 f | 10.76 | 2.22 ± 0.072 f | 20.24 | 0.65 ± 0.017 e | 5.93 | 6.92 ± 1.213 e | 63.08 | 10.87 ± 1.256 e | |
| PD | 4.06 ± 0.131 ab | 12.86 | 5.74 ± 0.186 ab | 18.19 | 1.23 ± 0.039 b | 3.9 | 20.53 ± 0.67 a | 65.05 | 31.56 ± 1.026 ab | |
| PDS | 1.26 ± 0.041 e | 6.79 | 2.82 ± 0.091 d | 15.2 | 0.64 ± 0.020 e | 3.45 | 13.83 ± 0.614 c | 74.56 | 18.55 ± 0.767 c | |
| 100-Pod Mass (g) | 100-Kernel Mass (g) | Kernel to Pod Rate (g) | Pod Mass per Plant (g) | Pod Amount per Plant | |
|---|---|---|---|---|---|
| CK | 158.81 ± 2.91 a | 95.93 ± 1.76 ab | 67.12 ± 1.23 ab | 22.5 ± 1.005 a | 12.35 ± 0.23 a |
| S | 128.27 ± 1.41 b | 69.05 ± 0.76 c | 59.81 ± 0.86 c | 16.32 ± 0.532 b | 11.42 ± 0.13 b |
| SD | 159.14 ± 1.05 a | 97.89 ± 0.64 a | 68.35 ± 0.45 a | 23.47 ± 0.766 a | 12.69 ± 0.18 a |
| SDS | 118.67 ± 2.21 c | 60.19 ± 1.12 e | 56.36 ± 1.05 d | 10.17 ± 0.711 d | 10.85 ± 0.2 c |
| FD | 161.56 ± 2.34 a | 96.6 ± 1.36 a | 68.76 ± 1.22 a | 12.14 ± 0.396 c | 8.34 ± 0.16 d |
| FDS | 109.54 ± 0.99 d | 56.3 ± 0.51 f | 57.11 ± 0.52 d | 6.92 ± 1.213 e | 5.76 ± 0.05 e |
| PD | 154.32 ± 2.44 a | 93.2 ± 1.46 b | 66.46 ± 1.05 b | 20.53 ± 0.67 a | 12.13 ± 0.19 ab |
| PDS | 123.83 ± 2.25 c | 64.27 ± 1.17 d | 57.67 ± 1.05 cd | 13.83 ± 0.614 c | 11.12 ± 0.2 bc |
| Test Index | Organic Matter (g·kg−1) | pH | Total Nitrogen (g·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) |
|---|---|---|---|---|---|
| Value | 15.2 ± 1.2 | 6.7 ± 0.2 | 1.6 ± 0.3 | 45.1 ± 2.1 | 102.5 ± 5.3 |
| Treatment | Non-Drought | Drought in 30D | Drought in 40D | Drought in 60D |
|---|---|---|---|---|
| Non-salt | CK | SD | FD | PD |
| Salt stress | S | SDS | FDS | PDS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Xu, Y.; Ding, H.; Guo, Q.; Ci, D.; Zhang, J.; Qin, F.; Xu, M.; Zhang, G. The Impact of Short-Term Drought on the Photosynthetic Characteristics and Yield of Peanuts Grown in Saline Alkali Soil. Plants 2024, 13, 2920. https://doi.org/10.3390/plants13202920
He K, Xu Y, Ding H, Guo Q, Ci D, Zhang J, Qin F, Xu M, Zhang G. The Impact of Short-Term Drought on the Photosynthetic Characteristics and Yield of Peanuts Grown in Saline Alkali Soil. Plants. 2024; 13(20):2920. https://doi.org/10.3390/plants13202920
Chicago/Turabian StyleHe, Kang, Yang Xu, Hong Ding, Qing Guo, Dunwei Ci, Jialei Zhang, Feifei Qin, Manlin Xu, and Guanchu Zhang. 2024. "The Impact of Short-Term Drought on the Photosynthetic Characteristics and Yield of Peanuts Grown in Saline Alkali Soil" Plants 13, no. 20: 2920. https://doi.org/10.3390/plants13202920
APA StyleHe, K., Xu, Y., Ding, H., Guo, Q., Ci, D., Zhang, J., Qin, F., Xu, M., & Zhang, G. (2024). The Impact of Short-Term Drought on the Photosynthetic Characteristics and Yield of Peanuts Grown in Saline Alkali Soil. Plants, 13(20), 2920. https://doi.org/10.3390/plants13202920







