The “Colors” of Moringa: Biotechnological Approaches
Abstract
1. Introduction
Moringa Genus
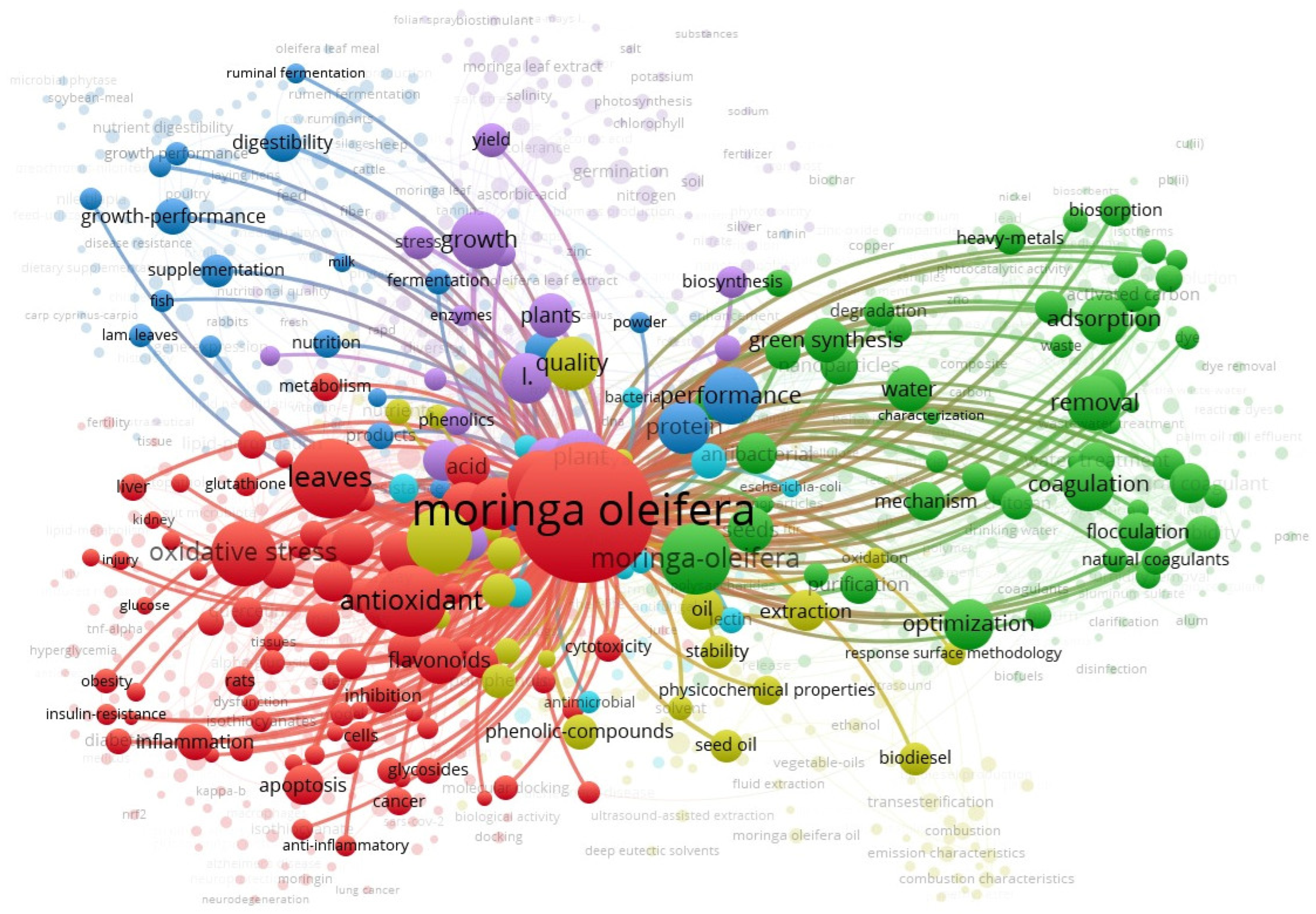
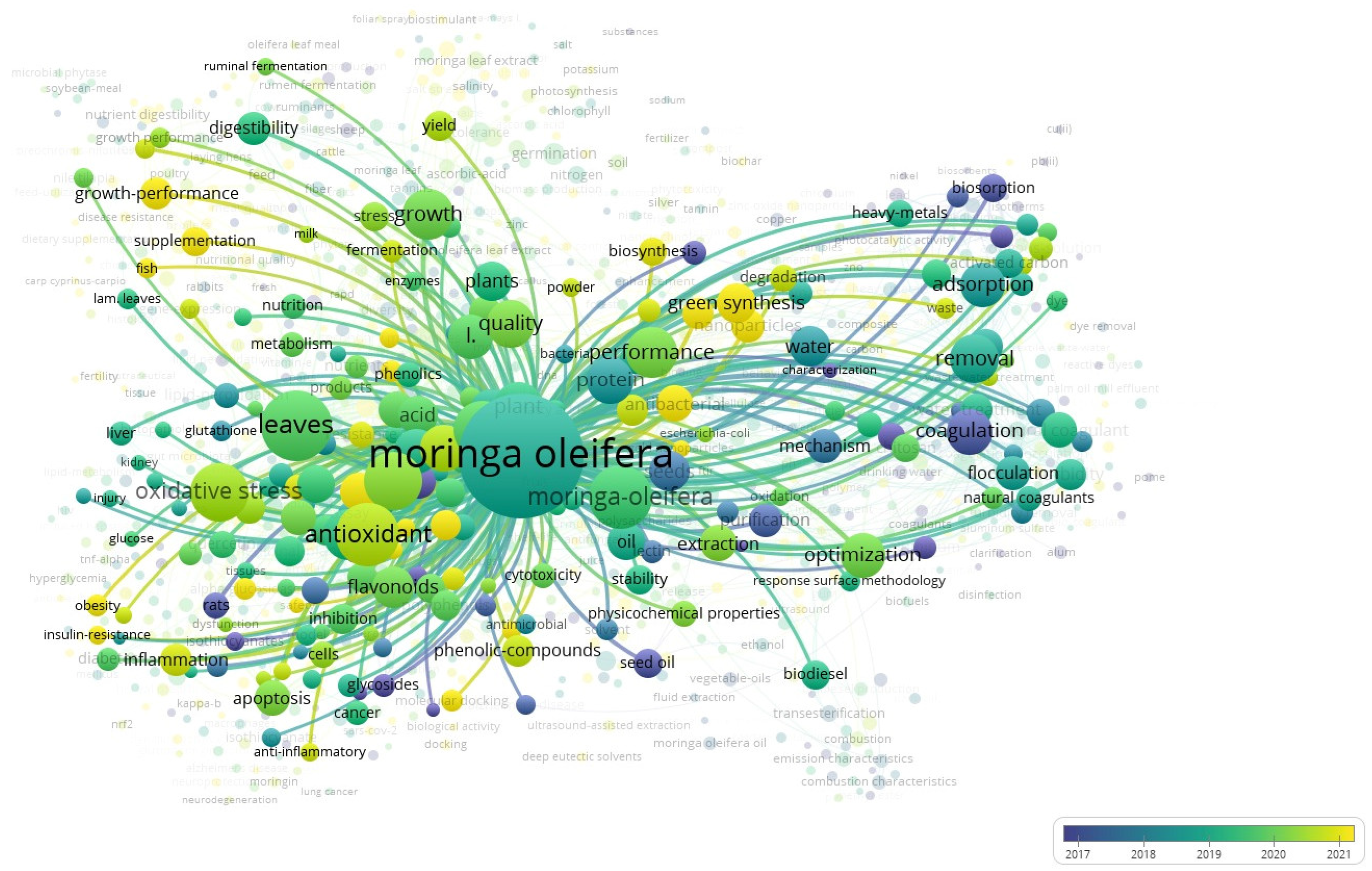
2. Current Status of Moringa oleifera in the Global Literature over Time
3. Current Application of Moringa in White Biotechnology
3.1. Biofuels and Bioenergy Industries
3.2. Bioprocess Applied to the Extraction of Moringa Phytochemicals
3.3. Food Industry
4. Current Application of Moringa in Green Biotechnology
4.1. Agriculture Industry
4.2. Green Nano-Industry
4.3. Water Treatment Industry
5. Current Application of Moringa in Red Biotechnology
5.1. Pharmaceutical Industry
5.2. Biomedical Industry
5.3. Cosmetic Industry
6. Current Application of Moringa in Blue Biotechnology
7. Current Application of Moringa in Yellow Biotechnology
8. Current Application of Moringa in Brown Biotechnology
9. Current Application of Moringa in Violet Biotechnology
10. Current Application of Gold Biotechnology in Moringa Research
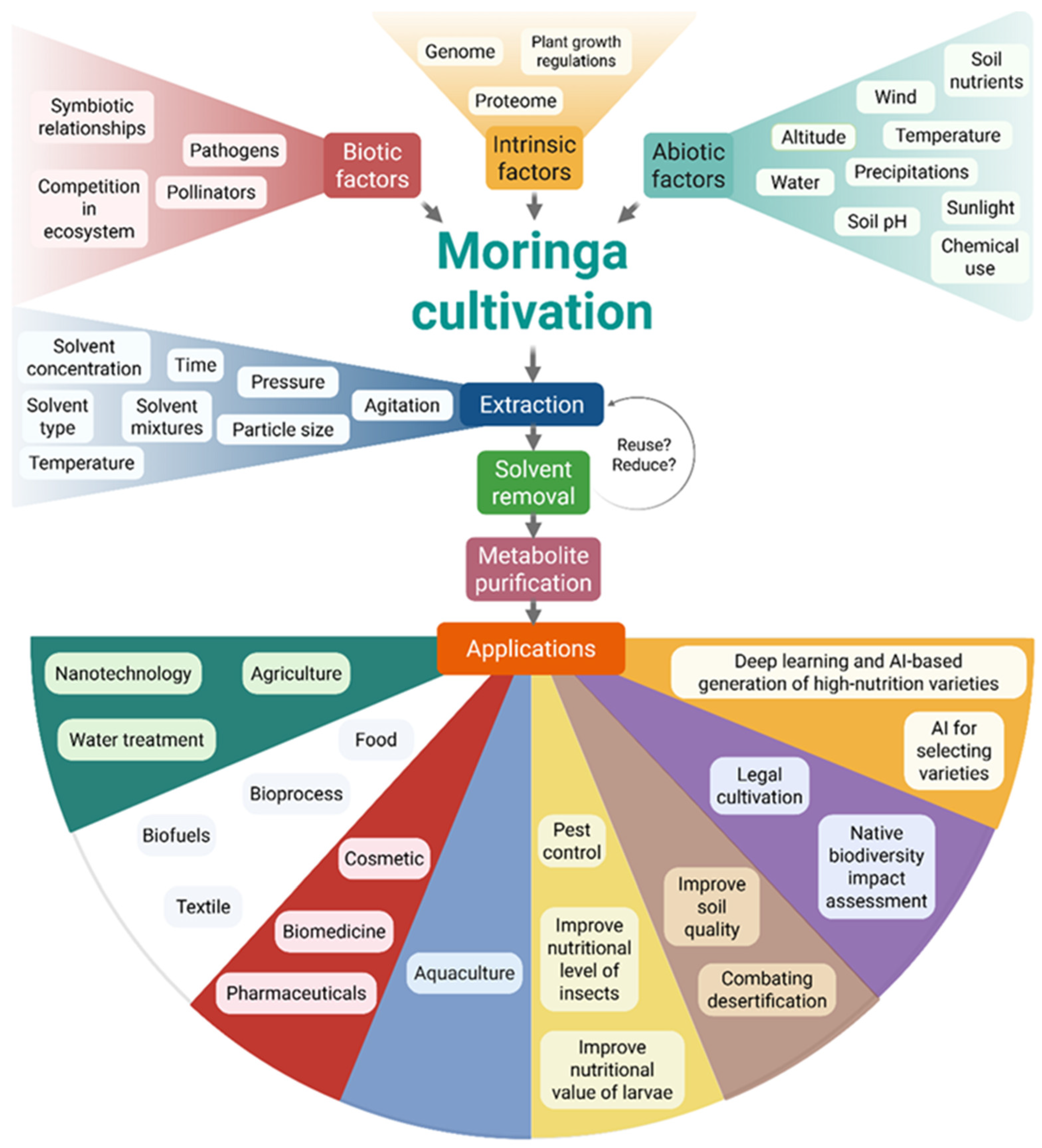
11. Biotechnological Challenges
12. Future Directions for Unlocking the MO Potential
12.1. Agrigenomics and Breeding
12.2. Standardization for Clinical Use
12.3. Green Nanotechnology
12.4. Policy and Sustainability
12.5. Integration Across Disciplines
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billiones, R. Biotechnology—Diverse as the colours of the rainbow. Med. Writ. 2023, 32, 6–7. [Google Scholar] [CrossRef]
- El Bilali, H.; Dan Guimbo, I.; Nanema, R.K.; Falalou, H.; Kiebre, Z.; Rokka, V.M.; Tietiambou, S.R.F.; Nanema, J.; Dambo, L.; Grazioli, F.; et al. Research on Moringa (Moringa oleifera Lam.) in Africa. Plants 2024, 13, 1613. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Ibrahim, M.D.; Kntayya, S.B. Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rana, H.K.; Tshabalala, T.; Kumar, R.; Gupta, A.; Ndhlala, A.R.; Pandey, A.K. Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam: An overview. S. Afr. J. Bot. 2020, 129, 209–220. [Google Scholar] [CrossRef]
- Boopathi, N.; Abubakar, B. Botanical Descriptions of Moringa spp. In The Moringa Genome; Springer International Publishing: Cham, Switzerland, 2021; pp. 11–20. [Google Scholar]
- Hamada, F.; Sabah, S.S.; Mahdy, E.M.; El-Raouf, H.S.A.; El-Taher, A.M.; El-Leel, O.F.; Althobaiti, A.T.; Ghareeb, M.A.; Randhir, R.; Randhir, T.O. Genetic, phytochemical and morphological identification and genetic diversity of selected Moringa species. Sci. Rep. 2024, 14, 30476. [Google Scholar] [CrossRef]
- El-Haddad, A.E.; El-Deeb, E.M.; Koheil, M.A.; El-Khalik, S.M.A.; El-Hefnawy, H.M. Nitrogenous phytoconstituents of genus Moringa: Spectrophotometrical and pharmacological characteristics. Med. Chem. Res. 2019, 28, 1591–1600. [Google Scholar] [CrossRef]
- Ayyanar, M.; Krupa, J.; Jenipher, C.; Amalraj, S.; Gurav, S.S. Phytochemical composition, in vitro antioxidant and antibacterial activity of Moringa conaniensis Nimmo leaves. Vegetos 2024, 37, 1377–1388. [Google Scholar] [CrossRef]
- Letsara, R.; Murielle, M.S.B.; Baovola, R.; Christian, M.S.; Fabrice, R.-T.J.E.; Ngbolua, K.-T.-N.J.-P.; Baholy, R.R. Unveiling the nutritional treasures of Moringa drouhardii, Moringa hildebrandtii (endemic to Madagascar), and Moringa oleifera leaves. Pharm. Pharmacol. Int. J. 2025, 13, 7–19. [Google Scholar] [CrossRef]
- Ojeda-López, J.; Marczuk-Rojas, J.P.; Polushkina, O.A.; Purucker, D.; Salinas, M.; Carretero-Paulet, L. Evolutionary analysis of the Moringa oleifera genome reveals a recent burst of plastid to nucleus gene duplications. Sci. Rep. 2020, 10, 17646. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, Y.; Zhang, J.; Yang, C.; Yan, L.; Wang, X.; Shi, C.; Xie, J.; Dai, T.; Peng, L.; et al. High quality reference genome of drumstick tree (Moringa oleifera Lam.), a potential perennial crop. Sci. China Life Sci. 2015, 58, 627–638. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, H.; Liu, M.; Liao, X.; Sahu, S.K.; Fu, Y.; Song, B.; Cheng, S.; Kariba, R.; Muthemba, S. The draft genomes of five agriculturally important African orphan crops. GigaScience 2019, 8, giy152. [Google Scholar] [CrossRef]
- Tena, G. Sequencing forgotten crops. Nat. Plants 2019, 5, 5. [Google Scholar] [CrossRef]
- Neale, D.B.; Martínez-García, P.J.; De La Torre, A.R.; Montanari, S.; Wei, X.-X. Novel insights into tree biology and genome evolution as revealed through genomics. Annu. Rev. Plant Biol. 2017, 68, 457–483. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.P.; VanBuren, R. Building near-complete plant genomes. Curr. Opin. Plant Biol. 2020, 54, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Alavilli, H.; Poli, Y.; Verma, K.S.; Kumar, V.; Gupta, S.; Chaudhary, V.; Jyoti, A.; Sahi, S.V.; Kothari, S.L.; Jain, A. Miracle tree Moringa oleifera: Status of the genetic diversity, breeding, in vitro propagation, and a cogent source of commercial functional food and non-food products. Plants 2022, 11, 3132. [Google Scholar] [CrossRef] [PubMed]
- Heux, S.; Meynial-Salles, I.; O’Donohue, M.J.; Dumon, C. White biotechnology: State of the art strategies for the development of biocatalysts for biorefining. Biotechnol. Adv. 2025, 33, 1653–1670. [Google Scholar] [CrossRef]
- Soliman, N.; Moustafa, A.; Aboud, A.; Halim, K. Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J. Mater. Res. Technol. 2019, 8, 1798–1808. [Google Scholar] [CrossRef]
- Gharsallah, K.; Rezig, L.; Msaada, K.; Chalh, A.; Soltani, T. Chemical composition and profile characterization of Moringa oleifera seed oil. S. Afr. J. Bot. 2021, 137, 475–482. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The potential of Moringa oleifera in food formulation: A promising source of functional compounds with health-promoting properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]
- Aleman-Ramirez, J.; Okoye, P.U.; Saldaña-Trinidad, S.; Torres-Arellano, S.; Sebastian, P. The role of Moringa oleifera in the development of alternative biofuels, under the concept of an integral one-tree biorefinery: A minireview. Biofuels Bioprod. Biorefining 2025, 19, 942–962. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Ajav, E.A. Process optimization of mechanical oil expression from Moringa (Moringa oleifera) seeds. Ind. Crops Prod. 2016, 90, 142–151. [Google Scholar] [CrossRef]
- Oladipo, B.; Betiku, E. Process optimization of solvent extraction of seed oil from Moringa oleifera: An appraisal of quantitative and qualitative process variables on oil quality using D-optimal design. Biocatal. Agric. Biotechnol. 2019, 20, 101187. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Yang, R.; Liu, X.; Yang, Q.; Qin, X. The application of ultrasound and microwave to increase oil extraction from Moringa oleifera seeds. Ind. Crops Prod. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Rajesh, Y.; Khan, N.M.; Shaikh, A.R.; Mane, V.S.; Daware, G.; Dabhade, G. Investigation of geranium oil extraction performance by using soxhlet extraction. Mater. Today Proc. 2023, 72, 2610–2617. [Google Scholar] [CrossRef]
- Nuchdang, S.; Phruetthinan, N.; Paleeleam, P.; Domrongpokkaphan, V.; Chuetor, S.; Chirathivat, P.; Phalakornkule, C. Soxhlet, microwave-assisted, and room temperature liquid extraction of oil and bioactive compounds from palm kernel cake using isopropanol as solvent. Ind. Crops Prod. 2022, 176, 114379. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Kotoka, F. Kinetics and thermodynamic studies on Moringa oleifera oil extraction for biodiesel production via transesterification. Biofuels 2022, 13, 341–349. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterization of Moringa oleifera leaves oil. Sep. Purif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Díaz, Y.; Tabío, D.; Rondón, M.; Piloto-Rodríguez, R.; Fernández, E. Phenomenological model for the prediction of Moringa oleifera extracted oil using a laboratory Soxhlet apparatus. Grasas Y Aceites 2021, 72, e422. [Google Scholar] [CrossRef]
- Ojewumi, M.; Oyekunle, D.; Emetere, M.; Olanipekun, O. Optimization of Oil from Moringa oleifera seed using Soxhlet Extraction method. Korean J. Food Health Converg. 2019, 5, 11–25. [Google Scholar]
- Nebolisa, N.M.; Umeyor, C.E.; Ekpunobi, U.E.; Umeyor, I.C.; Okoye, F.B. Profiling the effects of microwave-assisted and soxhlet extraction techniques on the physicochemical attributes of Moringa oleifera seed oil and proteins. Oil Crop Sci. 2023, 8, 16–26. [Google Scholar] [CrossRef]
- Sukarni, S.; Anis, S.; Aminullah, A.Y.; Assidiq, M.A.; Mufti, N.; Abdullah, T.A.T.; Johari, A. Moringa oleifera Seeds Potential as Biofuel via Thermal Conversion Method Based on Morphological and Chemical Content Evaluation. E3S Web Conf. 2024, 473, 8. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Osman, M.E. Second Generation Biofuel Production from Moringa oleifera Pod Husks Utilizing Cellulases of A New Decaying Fungus; Cladosporium halotolerans MDP OP903200. Egypt. J. Bot. 2024, 64, 341–357. [Google Scholar] [CrossRef]
- Del Carmen Razola-Díaz, M.; De-Montijo-Prieto, S.; Aznar-Ramos, M.J.; Martín-García, B.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Guerra-Hernández, E.; García-Villanova, B.; Verardo, V.; Gómez-Caravaca, A. Integrated biotechnological process based on submerged fermentation and sonotrode extraction as a valuable strategy to obtain phenolic enriched extracts from moringa leaves. Food Res. Int. 2024, 201, 115602. [Google Scholar] [CrossRef] [PubMed]
- Gunalan, S.; Thangaiah, A.; Rathnasamy, V.K.; Janaki, J.; Thiyagarajan, A.; Kuppusamy, S.; Arunachalam, L. Microwave-assisted extraction of biomolecules from moringa (Moringa oleifera Lam.) leaves var. PKM 1: A optimization study by response surface methodology (RSM). Kuwait J. Sci. 2023, 50, 339–344. [Google Scholar] [CrossRef]
- Gunalan, S.; Thangaiah, A.; Janaki, J.; Thiyagarajan, A.; Kuppusamy, S.; Arunachalam, L. Optimization of Microwave-Assisted Extraction Method for Increased Extraction Yield and Total Phenol Content from Moringa Leaves (Moringa oleifera Lam.) var. PKM 1. Adv. Agric. 2022, 2022, 7370886. [Google Scholar] [CrossRef]
- Simon, S.; K, S.; Joseph, J.; George, D. Optimization of extraction parameters of bioactive components from Moringa oleifera leaves using Taguchi method. Biomass Convers. Biorefinery 2022, 13, 11973–11982. [Google Scholar] [CrossRef]
- Kessler, J.; Martins, I.M.; Manrique, Y.A.; Rodrigues, A.E.; Barreiro, M.F.; Dias, M.M. Advancements in conventional and supercritical CO2 extraction of Moringa oleifera bioactives for cosmetic applications: A review. J. Supercrit. Fluids 2024, 214, 106388. [Google Scholar] [CrossRef]
- Saidu, A.; Abdulrahman, A.; Imam, Z.I. Effect of processing methods on the proximate and phytochemical constituents of Moringa oleifera (Lamarck, 1785) leaves. Sci. World J. 2023, 18, 272–275. [Google Scholar] [CrossRef]
- Stanley, O.O.; Emmanuel, K.A.; Jenyo-Oni, A. Phytochemical Screening of Moringa oleifera Leaf Extracts under Different Solvents. Int. J. Aquac. Fish. Sci. 2024, 10, 66–72. [Google Scholar] [CrossRef]
- Gandji, K.; Salako, V.; Fandohan, A.; Assogbadjo, A.; Kakaï, R.G. Factors Determining the Use and Cultivation of Moringa oleifera Lam. in the Republic of Benin. Econ. Bot. 2018, 72, 332–345. [Google Scholar] [CrossRef]
- Kume, T.; Iwai, C.B.; Shimamura, T.; Haruta, S. Growth and Mineral Uptake of Moringa oleifera Lam. in Low-Permeability Soils at Different Salinity Levels. Int. J. Environ. Rural. Dev. 2023, 14, 35–41. [Google Scholar] [CrossRef]
- Aslam, M.; Basra, S.; Hafeez, M.; Khan, S.; Irshad, S.; Iqbal, S.; Saqqid, M.S.; Akram, M. Inorganic fertilization improves quality and biomass of Moringa oleifera L. Agrofor. Syst. 2019, 94, 975–983. [Google Scholar] [CrossRef]
- Atteya, A.; Albalawi, A.; El-Serafy, R.; Albalawi, K.; Bayomy, H.; Genaidy, E. Response of Moringa oleifera Seeds and Fixed Oil Production to Vermicompost and NPK Fertilizers under Calcareous Soil Conditions. Plants 2021, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Neto, J.; Bonfim, B.; Difante, G.; Bezerra, J.; Lista, F.; Gurgel, A.; Bezerra, M. Growth and Biomass Production of Moringa Cultivated in Semiarid Region as Responses to Row Spacing and Cuts. Trop. Anim. Sci. J. 2021, 44, 183–187. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.; Ortolá, M.; García-Mares, F.; Soriano, M.D. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods 2020, 10, 31. [Google Scholar] [CrossRef]
- Gebrezihar, T.A.; Desta, Z.; Hagos, H. Assessing Factors Affecting Moringa Production at North-western Zone of Tigray, Ethiopia. Agric. Sci. 2020, 2, 17. [Google Scholar] [CrossRef]
- Kumssa, D.; Kumssa, D.; Kumssa, D.; Joy, E.; Young, S.; Odee, D.; Ander, E.; Magare, C.; Gitu, J.; Broadley, M. Challenges and opportunities for Moringa growers in southern Ethiopia and Kenya. PLoS ONE 2017, 12, e0187651. [Google Scholar] [CrossRef]
- Orisawayi, A.O.; Koziol, K.; Hao, S.; Tiwari, S.; Rahatekar, S. Development of hybrid electrospun alginate-pulverized moringa composites. RSC Adv. 2024, 14, 8502–8512. [Google Scholar] [CrossRef]
- Beluci, N.; Homem, N.; Amorim, M.; Bergamasco, R.; Vieira, A. Biopolymer extracted from Moringa oleifera Lam. in conjunction with graphene oxide to modify membrane surfaces. Environ. Technol. 2020, 41, 3069–3080. [Google Scholar] [CrossRef]
- Chitra, R.; Homem, N.; Amorim, M.; Bergamasco, R.; Vieira, A. Study on novel biopolymer electrolyte Moringa oleifera gum with ammonium nitrate. Polym. Bull. 2021, 79, 3555–3572. [Google Scholar] [CrossRef]
- Shivanna, S.K.; Naik, N.L.; Nataraj, B.H.; Rao, P.S. Moringa marvel: Navigating therapeutic insights and safety features for future functional foods. J. Food Meas. Charact. 2024, 18, 4940–4971. [Google Scholar] [CrossRef]
- Oyeyinka, A.; Oyeyinka, S. Moringa oleifera as a food fortificant: Recent trends and prospects. J. Saudi Soc. Agric. Sci. 2016, 17, 127–136. [Google Scholar] [CrossRef]
- Cao, J.; Shi, T.; Wang, H.; Zhu, F.; Wang, J.; Wang, Y.; Cao, F.; Su, E. Moringa oleifera leaf protein: Extraction, characteristics and applications. J. Food Compos. Anal. 2023, 119, 105234. [Google Scholar] [CrossRef]
- Kumar, M.; Selvasekaran, P.; Kapoor, S.; Barbhai, M.D.; Lorenzo, J.; Saurabh, V.; Potkule, J.; Changan, S.; Kelish, A.E.; Selim, S.; et al. Moringa oleifera Lam. seed proteins: Extraction, preparation of protein hydrolysates, bioactivities, functional food properties, and industrial application. Food Hydrocoll. 2022, 131, 107791. [Google Scholar] [CrossRef]
- Kashyap, P.; Kumar, S.; Riar, C.; Jindal, N.; Baniwal, P.; Guiné, R.; Correia, P.; Mehra, R.; Kumar, H. Recent Advances in Drumstick (Moringa oleifera) Leaves Bioactive Compounds: Composition, Health Benefits, Bioaccessibility, and Dietary Applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef]
- Falowo, A.; Mukumbo, F.; Idamokoro, E.; Lorenzo, J.; Afolayan, A.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.; Ngangyo-Heya, M.; Martínez-Ávila, G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, K.; Rezig, L.; Rajoka, M.; Mehwish, H.; Ali, M.; Chew, S.-C. Moringa oleifera: Processing, phytochemical composition, and industrial application. S. Afr. J. Bot. 2023, 160, 180–193. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, M.; Waghmare, R.; Suhag, R.; Gupta, O.; Lorenzo, J.; Prakash, S.; Radha; Rais, N.; Sampathrajan, V.; et al. Moringa (Moringa oleifera Lam.) polysaccharides: Extraction, characterization, bioactivities, and industrial application. Int. J. Biol. Macromol. 2022, 209, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Gull, T.; Nouman, W.; Olson, M. Industrial applications, toxicological impact and marketing trends of Moringa oleifera food products, a review. S. Afr. J. Bot. 2025, 176, 141–157. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Albarri, R.; Torun, M.; Şahin, S. Encapsulation of Moringa oleifera leaf extract in chitosan-coated alginate microbeads produced by ionic gelation. Food Biosci. 2022, 50, 102158. [Google Scholar] [CrossRef]
- Louisa, M.; Patintingan, C.G.H.; Wardhani, B.W. Moringa Oleifera Lam. in cardiometabolic disorders: A systematic review of recent studies and possible mechanism of actions. Front. Pharmacol. 2022, 13, 792794. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Gaweł-Bęben, K.; Rutka, A.; Blicharska, E.; Tatarczak-Michalewska, M.; Kulik-Siarek, K.; Kukula-Koch, W.; Malinowska, M.A.; Szopa, A. Moringa oleifera (drumstick tree)—Nutraceutical, cosmetological and medicinal importance: A review. Front. Pharmacol. 2024, 15, 1288382. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, O.; Famutimi, O.; Kadiri, F.A.; Kolapo, O.A.; Adewale, I. Studies on peroxidase from Moringa oleifera Lam leaves. Heliyon 2021, 7, e06032. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, O.; Adewale, I. Studies on latent and soluble polyphenol oxidase from Moringa oleifera Lam. leaves. Biocatal. Agric. Biotechnol. 2022, 45, 102515. [Google Scholar] [CrossRef]
- Barzan, G.; Sacco, A.; Giovannozzi, A.M.; Portesi, C.; Schiavone, C.; Salafranca, J.; Wrona, M.; Nerín, C.; Rossi, A.M. Development of innovative antioxidant food packaging systems based on natural extracts from food industry waste and Moringa oleifera leaves. Food Chem. 2024, 432, 137088. [Google Scholar] [CrossRef] [PubMed]
- Dubeni, Z.B.; Buwa-Komoreng, L.V.; Mthi, S. The Potential Application of Moringa oleifera Extracts as Natural Preservatives of Chicken Meat. Pharmacogn. Mag. 2024, 21, 09731296241288922. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. Int. J. Exp. Plant Biol. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Arif, Y.; Bajguz, A.; Hayat, S. Moringa oleifera Extract as a Natural Plant Biostimulant. J. Plant Growth Regul. 2022, 42, 1291–1306. [Google Scholar] [CrossRef]
- Oberoi, H.K.; Manchanda, P.; Kumar, A.; Umakanth, A.; Dhakad, A.K.; Kaur, M.; Kaur, H. Moringa Leaf Extract (MLE) Seed Priming Provides Early Seedling Protection to Biofuel Crop: Sweet Sorghum—Against Salinity. Sugar Technol. 2024, 26, 835–850. [Google Scholar] [CrossRef]
- Irshad, S.; Matloob, A.; Ghaffar, A.; Hussain, M.B.; Nadeem Tahir, M.H. Agronomic and biochemical aspects of moringa dried leaf extract mediated growth and yield improvements in soybean. N. Z. J. Crop Hortic. Sci. 2024, 1–20. [Google Scholar] [CrossRef]
- Banerjee, M.; Rajeswari, V.D. Green synthesis of selenium nanoparticles using leaf extract of Moringa oleifera, their biological applications, and effects on the growth of Phaseolus vulgaris-: Agricultural synthetic biotechnology for sustainable nutrition. Biocatal. Agric. Biotechnol. 2024, 55, 102978. [Google Scholar] [CrossRef]
- Alhudhaibi, A.M.; Ragab, S.M.; Sharaf, M.; Turoop, L.; Runo, S.; Nyanjom, S.; Haouala, F.; Hossain, A.S.; Alharbi, B.M.; Elkelish, A. Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress. Green Process. Synth. 2024, 13, 20240012. [Google Scholar] [CrossRef]
- Ngcobo, B.L.; Bertling, I.; Mbuyisa, S. Evaluating the efficacy of Moringa oleifera leaf extracts prepared using different solvents on growth, yield and quality of tomatoes and peppers. J. Hortic. Postharvest Res. 2024, 7, 389–406. [Google Scholar]
- Muniandi, S.K.; Ariff, F.F.M.; Pisar, M.M.; Harun, S.T.; Abdullah, M.Z.; Abdullah, F.; Hashim, S.N.A.M.; Bahari, S.N.S.; Saffie, N. Crop Improvement of Moringa oleifera L. through Genotype Screening for the Development of Clonal Propagation Techniques of High-Yielding Clones in Malaysia. Biology 2024, 13, 785. [Google Scholar] [CrossRef]
- Gautam, N.; Faroda, P.; Ameta, K.; Sharma, A.; Gupta, A.K. In vitro morphogenesis and micro-morpho-anatomical developments in Moringa concanensis Nimmo.: An endemic tree of Indian sub-continent. Curr. Plant Biol. 2024, 39, 100365. [Google Scholar] [CrossRef]
- Choudhary, R.; Kumari, A.; Kachhwaha, S.; Kothari, S.; Jain, R. Moringa oleifera: Biosynthesis strategies for enhanced metabolites and role in green nanoparticle synthesis. S. Afr. J. Bot. 2024, 170, 271–287. [Google Scholar] [CrossRef]
- Nagime, P.; Singh, S.; Chidrawar, V.; Rajput, A.; Syukri, D.M.; Marwan, N.; Shafi, S. Moringa oleifera: A plethora of bioactive reservoirs with tremendous opportunity for green synthesis of silver nanoparticles enabled with multifaceted applications. Nano-Struct. Nano-Objects 2024, 40, 101404. [Google Scholar] [CrossRef]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2017, 256, 296–304. [Google Scholar] [CrossRef]
- Mohammed, G.; Hawar, S. Green Biosynthesis of Silver Nanoparticles from Moringa oleifera Leaves and Its Antimicrobial and Cytotoxicity Activities. Int. J. Biomater. 2022, 19, 4136641. [Google Scholar] [CrossRef] [PubMed]
- Wahyudi, S.; Rizoputra, I.; Panatarani, C.; Faizal, F.; Bahtiar, A. Green Synthesis of Carbon Nanodots (CNDs) Moderated by Flavonoid Extracts from Moringa oleifera Leaves and Co-Doped Sulfur/Nitrogen (NS—CNDs—Fla) and Their Potential for Heavy Metals Sensing Application. J. Fluoresc. 2024. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Singh, R.; Ahmad, I.; Suliman, M.; Tripathi, S.C.; Rai, A.K.; Gupta, V.K. Lignocellulosic Moringa oleifera bark enabled biofabrication of MgO nanocatalyst: Application in developing temperature tolerance fungal cellulase cocktail. Ind. Crops Prod. 2024, 207, 117718. [Google Scholar] [CrossRef]
- Gutierrez Herrera, J.C.; Martínez Ovallos, C.A.; Agudelo-Castañeda, D.M.; Paternina-Arboleda, C.D. Exploring Moringa oleifera: Green Solutions for Sustainable Wastewater Treatment and Agricultural Advancement. Sustainability 2024, 16, 9433. [Google Scholar] [CrossRef]
- Simões, A.R.; de Souza, A.T.; Meurer, E.C.; Scaliante, M.H.N.O.; Cortelo, T.H. Moringa oleifera: Technological innovations and sustainable therapeutic potencials. Cad. Pedagógico 2025, 22, e13594. [Google Scholar] [CrossRef]
- Ahizi, A.E.; Njoku, C.N.; Onyelucheya, O.E.; Anusi, M.O.; Okonkwo, I.J.; Okoye, P.U.; Igwegbe, C.A. Optimization of Moringa oleifera cationic protein/zeolite adsorbent blend for synthetic turbid water treatment. Sustain. Water Resour. Manag. 2023, 9, 4. [Google Scholar] [CrossRef]
- Panigrahi, C.; Kamal, S.; Qin, J.; Ziemann, S.; Rahman, E.; House, M.; Dutcher, C.; Xiong, B. Removal of pristine and UV-weathered microplastics from water: Moringa oleifera seed protein as a natural coagulant. Environ. Eng. Sci. 2024, 41, 477–489. [Google Scholar] [CrossRef]
- Hemapriya, G.R.; Abinaya, R.; Dhinesh Kumar, S. Textile Effluent Treatment Using Moringa Oleifera. International journal of innovative research and development. Int. J. Innov. Res. Dev. 2015, 4, 385–390. [Google Scholar]
- Vilaseca, M.; López-Grimau, V.; Gutiérrez-Bouzán, C. Valorization of Waste Obtained from Oil Extraction in Moringa oleifera Seeds: Coagulation of Reactive Dyes in Textile Effluents. Materials 2014, 7, 6569–6584. [Google Scholar] [CrossRef]
- Worku, G.D.; Abate, S.N. Efficiency comparison of natural coagulants (Cactus pads and Moringa seeds) for treating textile wastewater (in the case of Kombolcha textile industry). Heliyon 2025, 11, e42379. [Google Scholar] [CrossRef]
- Temesgen, S. Extraction and Application of Moringa oleifera Seed Kernel Starch for Warp Yarn Sizing in Textile Industry. Trends Text. Eng. Fash. Technol. 2019, 5, 614. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Arora, S. Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant. J. Food Biochem. 2021, 45, e13933. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, A.; Afifi, M.; Abu-Alghayth, M.; Alkadri, D. Moringa oleifera: Recent Insights for Its Biochemical and Medicinal Applications. J. Food Biochem. 2024, 2024, 1270903. [Google Scholar] [CrossRef]
- Jikah, A.N.; Edo, G. Moringa oleifera: A valuable insight into recent advances in medicinal uses and pharmacological activities. J. Sci. Food Agric. 2023, 103, 7343–7361. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Kotal, A.; Das, M. Synthesis of Metal Based Nano particles from Moringa oleifera and its Biomedical Applications: A Review. Inorg. Chem. Commun. 2023, 158, 111438. [Google Scholar] [CrossRef]
- Abd Rani, N.Z.; Kumolosasi, E.; Jasamai, M.; Jamal, J.A.; Lam, K.W.; Husain, K. In vitro anti-allergic activity of Moringa oleifera Lam. extracts and their isolated compounds. BMC Complement. Altern. Med. 2019, 19, 361. [Google Scholar] [CrossRef] [PubMed]
- Chan Sun, M.; Ruhomally, Z.B.; Boojhawon, R.; Neergheen-Bhujun, V.S. Consumption of Moringa oleifera Lam leaves lowers postprandial blood pressure. J. Am. Coll. Nutr. 2020, 39, 54–62. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Aftab, K.; Shaheen, F.; Gilani, A.-u.-H. Hypotensive constituents from the pods of Moringa oleifera. Planta Medica 1998, 64, 225–228. [Google Scholar] [CrossRef]
- Ma, K.; Wang, Y.; Wang, M.; Wang, Z.; Wang, X.; Ju, X.; He, R. Antihypertensive activity of the ACE–renin inhibitory peptide derived from Moringa oleifera protein. Food Funct. 2021, 12, 8994–9006. [Google Scholar] [CrossRef]
- Oboh, G.; Oluokun, O.O.; Oyeleye, S.I.; Ogunsuyi, O.B. Moringa seed-supplemented diets modulate ACE activity but not its gene expression in L-NAME-induced hypertensive rats. Biomarkers 2022, 27, 684–693. [Google Scholar] [CrossRef]
- Silveira, F.D.; Gomes, F.I.F.; do Val, D.R.; Freitas, H.C.; de Assis, E.L.; de Almeida, D.K.C.; Braz, H.L.B.; Barbosa, F.G.; Mafezoli, J.; da Silva, M.R. Biological and molecular docking evaluation of a benzylisothiocyanate semisynthetic derivative from Moringa oleifera in a pre-clinical study of temporomandibular joint pain. Front. Neurosci. 2022, 16, 742239. [Google Scholar] [CrossRef]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male Wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Hossain, A.; Begum, S. Effects of Moringa oleifera on working memory: An experimental study with memory-impaired Wistar rats tested in radial arm maze. BMC Res. Notes 2022, 15, 314. [Google Scholar] [CrossRef]
- Bin-Meferij, M.M.; El-Kott, A.F. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. Int. J. Clin. Exp. Med. 2015, 8, 12487. [Google Scholar] [PubMed]
- Sinha, M.; Das, D.K.; Bhattacharjee, S.; Majumdar, S.; Dey, S. Leaf extract of Moringa oleifera prevents ionizing radiation-induced oxidative stress in mice. J. Med. Food 2011, 14, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Z.; Wu, H.; Tang, S.; Zhang, Y.; Yang, B.; Yang, H.; Huang, L. Therapeutic effect of Moringa oleifera leaves on constipation mice based on pharmacodynamics and serum metabonomics. J. Ethnopharmacol. 2022, 282, 114644. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, A.; Saravia, A.; Rizzo, S.; Zabala, L.; De Leon, E.; Nave, F. Pharmacologie properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J. Ethnopharmacol. 1992, 36, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, N.; Basha, H.; Meresa, A.; Degu, S.; Girma, B.; Geleta, B. Diuretic activity of the aqueous crude extract and hot tea infusion of Moringa stenopetala (Baker f.) Cufod. Leaves in rats. J. Exp. Pharmacol. 2017, 9, 73–80. [Google Scholar] [CrossRef]
- Buabeid, M.A.; Yaseen, H.S.; Asif, M.; Murtaza, G.; Arafa, E.-S.A. Anti-inflammatory and anti-angiogenic aattributes of Moringa oleifera Lam. And its nanoclay-based pectin-sericin films. Front. Pharmacol. 2022, 13, 890938. [Google Scholar] [CrossRef]
- Varadarajan, S.; Balaji, T.M. Assessing the in vitro antioxidant and anti-inflammatory activity of Moringa oleifera crude extract. J. Contemp. Dent. Pract. 2022, 23, 438. [Google Scholar]
- Sayed, A.M.E.; Omar, F.A.; Emam, M.M.A.-A.; Farag, M.A. UPLC-MS/MS and GC-MS based metabolites profiling of Moringa oleifera seed with its anti-Helicobacter pylori and anti-inflammatory activities. Nat. Prod. Res. 2022, 36, 6433–6438. [Google Scholar] [CrossRef]
- Wang, F.; Bao, Y.; Zhang, C.; Zhan, L.; Khan, W.; Siddiqua, S.; Ahmad, S.; Capanoglu, E.; Skalicka-Woźniak, K.; Zou, L. Bioactive components and anti-diabetic properties of Moringa oleifera Lam. Crit. Rev. Food Sci. Nutr. 2022, 62, 3873–3897. [Google Scholar] [CrossRef]
- de Siqueira Patriota, L.L.; Ramos, D.D.B.M.; Dos Santos, A.C.L.A.; Silva, Y.A.; e Silva, M.G.; Torres, D.J.L.; Procópio, T.F.; de Oliveira, A.M.; Coelho, L.C.B.B.; Pontual, E.V.; et al. Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice. Food Chem. Toxicol. 2020, 145, 111691. [Google Scholar] [CrossRef] [PubMed]
- Promkum, C.; Kupradinun, P.; Tuntipopipat, S.; Butryee, C. Nutritive evaluation and effect of Moringa oleifera pod on clastogenic potential in the mouse. Asian Pac. J. Cancer Prev. 2010, 11, 627–632. [Google Scholar] [PubMed]
- Shruthi, S.; Shenoy, K.B. Septilin: A versatile anticlastogenic, antigenotoxic, antioxidant and histoprotective herbo-mineral formulation on cisplatin-induced toxicity in mice. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2022, 874, 503441. [Google Scholar]
- Abo-Elsoud, R.A.E.A.; Ahmed Mohamed Abdelaziz, S.; Attia Abd Eldaim, M.; Hazzaa, S.M. Moringa oleifera alcoholic extract protected stomach from bisphenol A–induced gastric ulcer in rats via its anti-oxidant and anti-inflammatory activities. Environ. Sci. Pollut. Res. 2022, 29, 68830–68841. [Google Scholar] [CrossRef] [PubMed]
- Dalhoumi, W.; Guesmi, F.; Bouzidi, A.; Akermi, S.; Hfaiedh, N.; Saidi, I. Therapeutic strategies of Moringa oleifera Lam.(Moringaceae) for stomach and forestomach ulceration induced by HCl/EtOH in rat model. Saudi J. Biol. Sci. 2022, 29, 103284. [Google Scholar] [CrossRef]
- Tian, H.; Wen, Z.; Liu, Z.; Guo, Y.; Liu, G.; Sun, B. Comprehensive analysis of microbiome, metabolome and transcriptome revealed the mechanisms of Moringa oleifera polysaccharide on preventing ulcerative colitis. Int. J. Biol. Macromol. 2022, 222, 573–586. [Google Scholar] [CrossRef]
- Adeoye, A.O.; Falode, J.A.; Jeje, T.O.; Agbetuyi-Tayo, P.T.; Giwa, S.M.; Tijani, Y.O.; Akinola, D.E. Modulatory potential of citrus sinensis and moringa oleifera extracts and epiphytes on rat liver mitochondrial permeability transition pore. Curr. Drug Discov. Technol. 2022, 19, 73–82. [Google Scholar] [CrossRef]
- Alkhudhayri, D.A.; Osman, M.A.; Alshammari, G.M.; Al Maiman, S.A.; Yahya, M.A. Moringa peregrina leaf extracts produce anti-obesity, hypoglycemic, anti-hyperlipidemic, and hepatoprotective effects on high-fat diet fed rats. Saudi J. Biol. Sci. 2021, 28, 3333–3342. [Google Scholar] [CrossRef]
- Liao, M.; Sun, C.; Li, R.; Li, W.; Ge, Z.; Adu-Frimpong, M.; Xu, X.; Yu, J. Amelioration action of gastrodigenin rhamno-pyranoside from Moringa seeds on non-alcoholic fatty liver disease. Food Chem. 2022, 379, 132087. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.H.; Lee, C.-W.; Chou, J.-Y.; Murugan, M.; Shieh, B.-J.; Chen, H.-M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Donli, P.O.; Dauda, H. Evaluation of aqueous Moringa seed extract as a seed treatment biofungicide for groundnuts. Pest Manag. Sci. Former. Pestic. Sci. 2003, 59, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Ghasi, S.; Nwobodo, E.; Ofili, J. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed Wistar rats. J. Ethnopharmacol. 2000, 69, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Balaraman, R.; Amin, A.; Bafna, P.; Gulati, O. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- de Siqueira Patriota, L.L.; do Nascimento Santos, D.K.; da Silva Barros, B.R.; de Souza Aguiar, L.M.; Silva, Y.A.; Dos Santos, A.C.L.A.; Gama e Silva, M.; Barroso Coelho, L.C.B.; Paiva, P.M.G.; Pontual, E.V. Evaluation of the in vivo acute toxicity and in vitro hemolytic and immunomodulatory activities of the Moringa oleifera flower trypsin inhibitor (MoFTI). Protein Pept. Lett. 2021, 28, 665–674. [Google Scholar] [CrossRef]
- Anudeep, S.; Prasanna, V.K.; Adya, S.M.; Radha, C. Characterization of soluble dietary fiber from Moringa oleifera seeds and its immunomodulatory effects. Int. J. Biol. Macromol. 2016, 91, 656–662. [Google Scholar] [CrossRef]
- Coriolano, M.C.; de Santana Brito, J.; de Siqueira Patriota, L.L.; de Araujo Soares, A.K.; de Lorena, V.; Paiva, P.M.; Napoleao, T.H.; Coelho, L.C.; de Melo, C.M. Immunomodulatory effects of the water-soluble lectin from Moringa oleifera seeds (WSMoL) on human peripheral blood mononuclear cells (PBMC). Protein Pept. Lett. 2018, 25, 295–301. [Google Scholar] [CrossRef]
- Viera, G.H.F.; Mourão, J.A.; Ângelo, Â.M.; Costa, R.A.; Vieira, R.H.S.d.F. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev. Inst. Med. Trop. São Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Redha, A.; Perna, S.; Riva, A.; Petrangolini, G.; Peroni, G.; Nichetti, M.; Iannello, G.; Naso, M.; Faliva, M.; Rondanelli, M. Novel insights on anti-obesity potential of the miracle tree, Moringa oleifera: A systematic review. J. Funct. Foods 2021, 84, 104600. [Google Scholar] [CrossRef]
- Adarthaiya, S.; Sehgal, A. Moringa oleifera Lam. as a potential plant for alleviation of the metabolic syndrome—A narrative review based on in vivo and clinical studies. Phytother. Res. 2023, 38, 755–775. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Redondo-Useros, N.; Martínez-García, R.; Gómez-Martínez, S.; Díaz-Prieto, L.; Marcos, A. Potential of Moringa oleifera to Improve Glucose Control for the Prevention of Diabetes and Related Metabolic Alterations: A Systematic Review of Animal and Human Studies. Nutrients 2020, 12, 2050. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Dapar, M.; Jimam, N. Clinical antihypertensive efficacy and safety of Moringa oleifera Lam. (Moringaceae) leaf: A systematic review. J. Pharm. Bioresour. 2025, 22, 1–12. [Google Scholar] [CrossRef]
- Gambo, A.; Moodley, I.; Babashani, M.; Babalola, T.; Gqaleni, N. A double-blind, randomized controlled trial to examine the effect of Moringa oleifera leaf powder supplementation on the immune status and anthropometric parameters of adult HIV patients on antiretroviral therapy in a resource-limited setting. PLoS ONE 2021, 16, e0261935. [Google Scholar] [CrossRef]
- Fungtammasan, S.; Phupong, V. The effect of Moringa oleifera capsule in increasing breastmilk volume in early postpartum patients: A double-blind, randomized controlled trial. PLoS ONE 2021, 16, 100171. [Google Scholar] [CrossRef]
- Anumula, L.; Ramesh, S.; Chinni, S.; Punamalli, P.; Kolaparthi, V.S.K. Clinical Assessment of Moringa oleifera as a Natural Crosslinker for Enhanced Dentin Bond Durability: A Randomized Controlled Trial. Cureus 2023, 15, e46304. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ncube, B.; Madala, N.; Nyakudya, T.; Moyo, H.; Sibanda, M.; Ndhlala, A. Scribbling the Cat: A Case of the “Miracle” Plant, Moringa oleifera. Plants 2019, 8, 510. [Google Scholar] [CrossRef]
- Villegas-Vázquez, E.; Gómez-Cansino, R.; Marcelino-Pérez, G.; Jiménez-López, D.; Quintas-Granados, L. Unveiling the Miracle Tree: Therapeutic Potential of Moringa oleifera in Chronic Disease Management and Beyond. Biomedicines 2025, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, H.; Hoque, A.; Kumari, L.; Sakure, K.; Baghel, M.; Giri, T. Moringa gum and its modified form as a potential green polymer used in biomedical field. Carbohydr. Polym. 2020, 249, 116893. [Google Scholar] [CrossRef]
- Bessalah, S.; Faraz, A.; Dbara, M.; Khorcheni, T.; Hammadi, M.; Ajose, D.; Saeed, S. Antibacterial, Anti-Biofilm, and Anti-Inflammatory Properties of Gelatin–Chitosan–Moringa-Biopolymer-Based Wound Dressings towards Staphylococcus aureus and Escherichia coli. Pharmaceuticals 2024, 17, 545. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B. Functional network copolymeric hydrogels derived from moringa gum: Physiochemical, drug delivery and biomedical properties. Int. J. Biol. Macromol. 2024, 275, 133352. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.; Dacrory, S.; Hesemann, P.; Bettache, N.; Ali, L.; Postel, L.; Akl, E.; El-Sakhawy, M. Wound Dressings Based on Sodium Alginate–Polyvinyl Alcohol–Moringa oleifera Extracts. Pharmaceutics 2023, 15, 1270. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, R.; Filip, R.; Lupăescu, A.-V.; Iavorschi, M.; Anchidin-Norocel, L.; Gutt, G. Innovative Materials with Possible Applications in the Wound Dressings Field: Alginate-Based Films with Moringa oleifera Extract. Gels 2023, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bal, T. Evaluation of a green synthesized biopolymer polymethyl methacrylate grafted Moringa gum amphiphilic graft copolymer (MOG-g-PMMA) with polymeric-surfactant like properties for biopharmaceutical applications. Polym. Bull. 2024, 81, 17017–17047. [Google Scholar] [CrossRef]
- Banik, S.; Biswas, S.; Karmakar, S. Extraction, purification, and activity of protease from the leaves of Moringa oleifera. F1000Research 2018, 7, 1151. [Google Scholar] [CrossRef]
- Garg, P.; Pundir, S.; Ali, A.; Panja, S.; Chellappan, D.K.; Dua, K.; Kulshrestha, S.; Negi, P. Exploring the potential of Moringa oleifera Lam in skin disorders and cosmetics: Nutritional analysis, phytochemistry, geographical distribution, ethnomedicinal uses, dermatological studies and cosmetic formulations. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 3635–3662. [Google Scholar]
- El-Sharkawy, R.M.; El-Hadary, A.E.; Essawy, H.S.; El-Sayed, A.S. Rutin of Moringa oleifera as a potential inhibitor to Agaricus bisporus tyrosinase as revealed from the molecular dynamics of inhibition. Sci. Rep. 2024, 14, 20131. [Google Scholar] [CrossRef]
- Abidin, Z.; Huang, H.-T.; Liao, Z.; Chen, B.; Wu, Y.-S.; Lin, Y.-J.; Nan, F. Moringa oleifera Leaves’ Extract Enhances Nonspecific Immune Responses, Resistance against Vibrio alginolyticus, and Growth in Whiteleg Shrimp (Penaeus vannamei). Animals 2021, 12, 42. [Google Scholar] [CrossRef]
- Shan, T.; Matar, M.A.; Makky, E.; Ali, E. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater treatment using Moringa oleifera Lam seeds: A review. J. Water Process Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Salem, S.A.; Abd El-Salam, A.M.; Abdel-Raheem, M.A. Moringa plant powders as repellent effect against the stored products insects. Plant Arch. 2020, 20, 939–945. [Google Scholar]
- Sanusi, L.; Ibrahim, N. Comparative efficacy of moringa, neem and lemon grass leaf powders in the control of bean beetle (Callosobruchus maculatus Fab.) infesting cowpea (Vigna unguiculata L. Walp). J. Agric. Environ. 2024, 20, 211–225. [Google Scholar] [CrossRef]
- Mohammed, A.L.; Iddriss, M. Effect of Moringa (Moringa oleifera) leaf powder, neem (Azadirachta indica) leaf powder, and camphor on weevil (Callosobruchus maculatus f.) In stored cowpea (Vigna unguiculata (l.) Walp) seeds. J. Appl. Life Sci. Environ. 2023, 55, 257–269. [Google Scholar] [CrossRef]
- Ria, E.R.; Hidayat, E.; Muliani, Y.; Komariah, A.; Abdullah, R.; Masnenah, E.; Kantikowati, E. Moringa Leaf Powder as Environmentally Friendly Repellent Agent for Controlling the Warehouse Insect Pest for Black Soybean Grain. J. Agrosci 2024, 1, 235–245. [Google Scholar] [CrossRef]
- Eseabasi, R.; Ime, O.U. Effect of Moringa oleifera Leaf Powder and Seed Oil on Insect Pests of Stored Maize and Cowpea. Int. J. Life Sci. Agric. Res. 2024, 3, 267–273. [Google Scholar] [CrossRef]
- Santos, N.; Santos, P.É.M.D.; Da Silva Lira, T.L.; Da Silva Santos, A.R.; De Oliveira Silva, J.N.; Santos, A.N.S.D.; De Amorim, M.M.R.; Barros, M.R.; Coelho, L.; Paiva, P.; et al. Insecticidal Activity of Lectin Preparations from Moringa oleifera Lam. (Moringaceae) Seeds Against Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Plants 2025, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Damilola, A.M.; Temitope, M.F.O. Assessment of Moringa oleifera as Bio-Pesticide against Podagrica spp on the growth and yield of Okra (Abelmoschus esculentus L. Moench). J. Hortic. 2020, 7, 1–11. [Google Scholar]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Rumbos, C.; Athanassiou, C.; Lalas, S. Enhancing the Nutritional Profile of Tenebrio molitor Using the Leaves of Moringa oleifera. Foods 2023, 12, 2612. [Google Scholar] [CrossRef]
- Mirhashemi, M.S.; Mohseni, S.; Hasanzadeh, M.; Pishvaee, M. Moringa oleifera biomass-to-biodiesel supply chain design: An opportunity to combat desertification in Iran. J. Clean. Prod. 2018, 203, 313–327. [Google Scholar] [CrossRef]
- Al-Khalifah, N.; Shanavaskhan, A. Moringa oleifera Lam., a promising crop species for arid conditions of Saudi Arabia and Moringa peregrina (Forssk.) Fiori, a native wild species for crop improvement. I Int. Symp. Moringa 2017, 1158, 159–170. [Google Scholar] [CrossRef]
- Vaknin, Y.; Mishal, A. The potential of the tropical “miracle tree” Moringa oleifera and its desert relative Moringa peregrina as edible seed-oil and protein crops under Mediterranean conditions. Sci. Hortic. 2017, 225, 431–437. [Google Scholar] [CrossRef]
- Bayomy, H.; Alamri, E.; Alharbi, B.; Almasoudi, S.; Ozaybi, N.; Mohammed, G.; Genaidy, E.; Atteya, A. Oil Yield and Bioactive Compounds of Moringa oleifera Trees Grown Under Saline Conditions. Plants 2025, 14, 509. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A. Seed characteristics, oil content and fatty acid composition of moringa (Moringa oleifera Lam.) seeds from three arid land locations in Ecuador. Ind. Crops Prod. 2019, 140, 111575. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef]
- Mashamaite, C.; Mothapo, P.; Albien, A.; Pieterse, P.; Phiri, E. A SUSPECT under the National Environmental Management Biodiversity Act (NEM:BA) Moringa oleifera’s ecological and social costs and benefits. S. Afr. J. Bot. 2020, 129, 249–254. [Google Scholar] [CrossRef]
- Ayoub, M.; Hussain, S.; Khan, A.; Zahid, M.; Wahid, J.A.; Zhifang, L.; Rehman, R.A. A Predictive Machine Learning and Deep Learning Approach on Agriculture Datasets for New Moringa oleifera Varieties Prediction. Pak. J. Eng. Technol. 2022, 5, 68–77. [Google Scholar] [CrossRef]
- Ndayakunze, A.; Steyn, J.M.; Du Plooy, C.; Araya, N.A. Measurement and modelling of Moringa transpiration for improved irrigation management. Agric. Water Manag. 2024, 305, 109127. [Google Scholar] [CrossRef]
| Moringaceae Species | Total of Phenolic Compounds | Total of Flavonoids | Antioxidant Activity | Ref. |
|---|---|---|---|---|
| M. peregrina | 200.12 mg of gallic acid equivalents (GAE)/100 g dry weight (DW) | 7 mg quercetin equivalents (QE)/100 g DW | 1066.39 mg ascorbic acid equivalents (AAE)/100 g DW | [7] |
| M. stenopetala | 243.00 mg GAE/100 g DW | 3.05 mg QE/100 g DW | 1226.75 mg AAE/100 g DW | [7] |
| M. oleifera | 241.05 mg GAE/100 g DW | 6.06 mg QE/100 g DW | 745.64 mg AAE/100 g DW | [7] |
| M. concanensis | 152.81 mg GAE/g ± 3.30 (methanolic leaves) | 123.22 mg QE/g ± 6.5 (methanolic leaves) | Strong DPPH/ABTS activity via multiple assays; IC50 hydroxyl scavenging ≈ 45.3 µg/mL | [4,9] |
| M. ovalifolia | Not provided | Not provided | Contains antioxidants (e.g., quercetin, kaempferol, myricetin) with confirmed DPPH and ferric reducing activity | [4] |
| M. drouhardii | Minor phenolic content reported | Minor flavonoid content reported | Not provided | [4] |
| M. hildebrandtii | Not provided | Not provided | A non-quantitative phytochemical profile has been reported, including alkaloids, tannins, flavonoids, saponins, steroids, and phenolic compounds. | [10] |
| M. longituba | Not provided | Not provided | Not provided | |
| M. ruspoliana | Not provided | Not provided | Not provided | |
| M. pygmaea | Not provided | Not provided | Not provided | |
| M. borziana | Not provided | Not provided | Not provided | |
| M. arborea | Not provided | Not provided | Not provided | |
| M. rivae | Not provided | Not provided | Not provided |
| Color Clusters | Description | Keywords | Focus |
|---|---|---|---|
| Red Cluster | Health and Biomedical Applications | antioxidant, oxidative stress, apoptosis, cancer, inflammation, flavonoids, quercetin | Pharmacological and therapeutic effects of M. oleifera, particularly in managing oxidative stress, inflammation, and diseases like cancer and Alzheimer’s. |
| Green Cluster | Environmental and Water Treatment Applications | adsorption, coagulation, removal, biosorption, flocculation, heavy metals, optimization | Use of M. oleifera as a natural coagulant or biosorbent for water purification and environmental cleanup. |
| Blue Cluster | Animal Nutrition and Feed | growth-performance, digestibility, fermentation, supplementation, metabolism, sheep, goats | Application of M. oleifera in livestock nutrition, enhancing animal growth, digestion, and health. |
| Purple Cluster | Agricultural and Plant-Based Research | germination, photosynthesis, biomass, biosynthesis, yield, phytohormones, quality | M. oleifera in plant growth, productivity, and sustainable agriculture. |
| Yellow Cluster | Oil Extraction and Biofuel | seed oil, biodiesel, transesterification, extraction, stability | Industrial and biochemical extraction of oil from M. oleifera seeds for use in biodiesel and bio-based industries. |
| Strengths (S) | Weaknesses (W) | Opportunities (O) | Threats (T) |
|---|---|---|---|
| Rich in bioactive compounds, such as proteins, flavonoids, phenolics, and enzymes, making it suitable for various industrial applications. | Advanced extraction techniques, like supercritical CO2 and MAE, are expensive and currently not suitable for large-scale manufacturing. | Growing demand for eco-friendly and carbon-neutral energy sources like biofuels and bioplastics. | Environmental trade-offs of large-scale monoculture plantations, such as biodiversity loss and soil depletion. |
| Seeds with high oil content (40% w/w) are suitable for biodiesel production, and lignocellulosic biomass can be used for bioethanol. | Traditional techniques, such as Soxhlet extraction, require a lot of solvents, take a long time, and pose environmental concerns. | Development of high-yield cultivars like MOMAX3 and bioengineered enzymes to optimize bioprocesses. | High production costs and technological barriers hinder the transition to industrial-scale operations. |
| Suitable for arid climates and poor soils; ideal for areas affected by climate change. | There is limited quantitative data available for non-oleifera Moringa species, and long-term toxicology studies are insufficient. | Growth in the functional food and nutraceutical sector driven by Moringa’s health benefits. | Regulatory challenges for new food ingredients, particularly in affluent nations. |
| Applications are diverse, encompassing biofuels, nutraceuticals, biopolymers, and water purification systems. | Variation in yield and quality is caused by environmental and farming factors. | Utilized water purification membranes and biopolymer-based packaging to promote environmental sustainability. | Socioeconomic challenges include a lack of awareness, limited access to planting materials, and the marginalization of smallholders. |
| Potential use as a natural preservative and food fortifier to fight against malnutrition. | Incorporating sensory issues like taste and color changes into food requires extra processing. | Potential for circular bioeconomy initiatives utilizing agricultural residues, such as pod husks for bioethanol production. | Yield may still be influenced by climate variability, even with drought tolerance. |
| Strengths (S) | Weaknesses (W) | Opportunities (O) | Threats (T) |
|---|---|---|---|
| MO’s broad-spectrum bioactivity and compatibility with sustainable practices establish it as a crucial agent for green biotechnology and the bioeconomy. Its multifunctionality—from soil enhancer to nanoparticle precursor—makes it especially relevant for integrated systems (e.g., agriculture and water treatment). | Most applications are still in the experimental stage, especially nanoparticle synthesis and bioprocess scaling. Cost-effectiveness and standardization remain major obstacles. Changes in food sensory properties and environmental trade-offs (such as large-scale monocultures) are not fully addressed. | Advancing bioprocess optimization, integrating MO into circular economy models, and improving knowledge transfer to smallholders could maximize impact. | Regulatory frameworks for nanoparticle use, potential allergenicity or toxicity in food systems, and scalability of in vitro propagation are essential issues requiring further research. |
| Study Title | NCT Number | Locations | Study Status | Sex | Age | Phases | Study Type | Conditions | Summary |
|---|---|---|---|---|---|---|---|---|---|
| Effect of Moringa oleifera mouthwash | NCT05191069 | Islamabad Capital Territory, Pakistan | Unknown * | All | Child, adult | NA | Interventional | Orthodontic appliance complication | This study evaluates the effectiveness of MO mouthwash in enhancing oral hygiene during orthodontic treatment. It examines its role in preventing gingivitis, periodontitis, plaque formation, enamel demineralization, tooth discoloration, and reducing the bacterial load in plaque. |
| Moringa oleifera on bone density | NCT03026660 | Boone, North Carolina, United States | Completed | Female | Adult, older adult | NA | Interventional | Osteoporosis, osteopenia, postmenopausal osteoporosis | This study aims to evaluate the effects of daily 1000 mg MO supplementation over 12 weeks on bone structure and function in postmenopausal women. |
| Moringa oleifera–antiretroviral pharmacokinetic drug interaction | NCT01410058 | Harare, Zimbabwe | Completed | All | Adult, older adult | - | Observational | HIV | The use of MO Lam leaf powder at its traditional dosage did not significantly affect the steady-state pharmacokinetics of nevirapine. |
| Moringa supplementation for improved milk output | NCT05333939 | Lexington, Kentucky, United States | Completed | All | Child, adult, older adult | NA | Interventional | Breastfeeding | This study gathers data on whether daily 4 g MO supplementation for four weeks enhances breast milk quantity, quality, and infant health versus placebo. Moringa is expected to boost milk output and the proportion of the infant’s intake from the mother. |
| Effect of Moringa oleifera infusion on health | NCT04314258 | Moka, Mauritius | Unknown * | All | Adult, older adult | NA | Interventional | Metabolic syndrome | This study explores the effects of MO leaf tea on health markers in hyperglycemic individuals (fasting blood glucose ≥ 5.5 mmol/L). Objectives include assessing impacts on blood glucose, lipid profiles, and antioxidant levels, comparing healthy and hyperglycemic individuals. |
| Effect of Moringa oleifera leaves on glycemic control of women with type 2 diabetes | NCT06517602 | Tindouf, Algeria | Completed | Female | Adult, older adult | NA | Interventional | Type 2 diabetes | This clinical trial assessed if daily MO leaf powder supplementation, alongside oral hypoglycemic therapy, improved glycemic control in Sahrawi women with type 2 diabetes. Researchers measured changes in glycosylated hemoglobin, fasting blood glucose, and clinical, metabolic, and body composition parameters at the study’s start and end. |
| Remineralization efficacy of Moringa oleifera varnish vs MI varnish in initial carious lesions over 6-month follow up: a randomized controlled clinical trial | NCT06905379 | Cairo, Egypt | Not yet recruiting | All | Adult | NA | Interventional | White spot lesions [initial caries] on smooth surface of tooth | This clinical trial assessed the remineralization efficacy of MO varnish versus MI Varnish (CPP-ACP) on incipient carious lesions. Participants aged 25–35 with at least one active white spot lesion (WSL) and good oral hygiene provided informed consent |
| Effects of Moringa oleifera on hsCRP and Hgba1c level of patients in Hospital ng Maynila medical center diabetic clinic | NCT02308683 | Location not provided | Completed | All | Adult, older adult | Phase 1 | Interventional | Diabetes | This cohort study investigates the effects of MO leaf supplementation on inflammation and glycemic control in patients with type 2 diabetes. This study focuses on high-sensitivity C-reactive protein (hsCRP) as a key inflammatory marker, along with HbA1c. clinical outcomes. |
| Effect of Moringa leaf extract on disease activity in rheumatoid arthritis patients | NCT05665985 | Surakarta, Central Java, Indonesia | Completed | Female | Adult | Phase 1, phase 2 | Interventional | Rheumatoid arthritis | This study evaluated the effects of MO extract on rheumatoid arthritis activity. Patients received MO in a 30-day treatment regimen to assess changes in disease activity during the intervention. |
| Effect of aerobic training and Moringa oleifera on dyslipidemia and cardiac endurance | NCT04164771 | Location not provided | Unknown * | Male | Adult | NA | Interventional | Dyslipidemias | Moringa leaves are highly effective against various diseases, particularly diabetes, blood pressure issues, dyslipidemia, and cancer. |
| Effect of Moringa oleifera on metformin plasma level in type 2 diabetes mellitus patients | NCT03189407 | Location not provided | Completed | All | Adult, older adult | NA | Interventional | Type 2 diabetes mellitus | This study evaluated the effects of a seven-day, twice-daily hot water infusion of dried MO leaves on the plasma concentrations of Metformin in type 2 diabetes patients already on Metformin for at least three years months. |
| Moringa oleifera (drumstick leaves) for improving hemoglobin, vitamin A status and underweight among adolescent girls in rural Bangladesh: a quasi-experimental study | NCT04156321 | Dhaka, Bangladesh | Unknown * | Female | Child | Phase 3 | Interventional | Assess the impact of Moringa leaves on serum hemoglobin and vitamin A level among the adolescent girls | NA |
| Anticariogenic effect of Moringa oleifera mouthwash compared to chlorhexidine mouthwash | NCT04575948 | Location not provided | Not yet recruiting | All | Adult | Phase 2, phase 3 | Interventional | Plaque, dental, antimicrobial, mouthwash, cytotoxicity | Part I: This in vitro study aims to formulate a non-toxic mouthwash from MO leaf extract, which has antimicrobial activity, for use in Part II. Additionally, the mouthwash’s stability and efficacy will be evaluated. Part II: This randomized controlled trial assesses the antibacterial, antiplaque, and anticariogenic effects of MO mouthwash versus chlorhexidine mouthwash. |
| Effects of Moringa oleifera leaves on glycemia, lipemia, and inflammatory profile in prediabetic patients | NCT04734132 | Madrid, Spain | Completed | All | Adult, older adult | NA | Interventional | Prediabetes | This proposal studies the efficacy of MO in controlling glycaemia in prediabetic subjects. A 3-month dietary intervention with MO dry leaf capsules will be compared to a placebo. |
| Nutritional impact of Moringa oleifera leaf supplementation in mothers and children | NCT04587271 | Kisumu, Kenya | Completed | All | Child, adult, older adult | NA | Interventional | Malnutrition, wasting, and growth failure | The primary outcomes were infant growth and maternal milk production, while secondary outcomes included maternal and infant vitamin A and iron status and changes in their intestinal health. |
| Effects of Allium sativum and Moringa oleifera extract on dental enamel | NCT05744752 | Karachi, Sindh, Pakistan | Unknown * | Male | Child | NA | Interventional | Lead poisoning | The objective is to compare the protective effects of Allium sativum (AS) and MO on dental enamel defects from lead and to determine their benefits in remineralizing dental enamel. |
| Effect of Moringa oleifera leaf on hemoglobin levels in anemia | NCT05737862 | Bandung, West Java, Indonesia | Completed | Female | Child, adult | Phase 3 | Interventional | Anemia of pregnancy | This study aimed to compare hemoglobin levels in pregnant women between the treatment group, which received Moringa leaf capsules and iron tablets, and the control group, which received only iron tablets. |
| Evaluation of Artemisia annua and Moringa | NCT03366922 | Mbarara, South Western, Uganda | Completed | All | Adult, older adult | NA | Interventional | HIV infections | Determine the effect of A. annua L. and MO leaf powder on CD4 cell count and immunological indices in HIV patients receiving highly active antiretroviral therapy. |
| Anti-plaque and anti-gingivitis effects of Moringa plant extract and fluoride toothpastes | NCT05390099 | Giza, Egypt | Unknown * | All | Child | NA | Interventional | Oral disease | This study assesses and compares the anti-plaque and anti-gingivitis effects of Moringa plant extract and fluoride toothpastes in Egyptian children. |
| Effect of Moringa oleifera capsule in increasing breast milk volume in early postpartum patients | NCT04487613 | Bangkok, Thailand | Completed | Female | Adult, older adult | Phase 4 | Interventional | Postpartum women | This study aims to assess how MO leaf capsules influence breast milk production. |
| Effects of Moringa oleifera leaf powders on hematological profiles in pregnant women with iron deficiency anemia | NCT06875947 | Cianjur, West Java, Indonesia | Not yet recruiting | Female | Adult | Phase 4 | Interventional | Iron deficiency anemia of pregnancy, pregnancy complications, inflammation, Moringa oleifera, cytokines (IL-1, IL-6), hepcidin | This study investigates micronized Moringa leaf powders as a natural supplement to enhance hemoglobin levels in pregnant women with iron deficiency anemia. Participants will undergo regular blood tests to assess hemoglobin levels, iron status markers (hepcidin, TIBC), and inflammatory cytokines (IL-1, IL-6). This study also evaluates the safety of Moringa supplements, focusing on liver and kidney functions. |
| Impact of dried Moringa oleifera leaves in enhancing hemoglobin status | NCT03514472 | Location not provided | Completed | Female | Child, adult | NA | Interventional | Anemia, iron deficiency | This research project targets nutritional deficiencies, particularly iron deficiency anemia in reproductive-aged females from underprivileged groups. Anemia can result in stillbirths, preterm deliveries, and low birth weight, potentially leading to cognitive disabilities, emphasizing the need for priority treatment. |
| Effect of Moringa leaf capsules on glycemic control of type 2 diabetic patients | NCT06125873 | Islamabad, Federal, Pakistan | Enrolling by invitation | All | Adult, older adult | Phase 2 | Interventional | Diabetes mellitus type 2 | A clinical trial will involve 50 patients randomly divided into two groups to compare glycemic control in Type 2 diabetes mellitus using MO capsules. |
| Evaluation of Moringa oleifera leaf extract versus sodium hypochlorite in pulpectomy of nonvital primary molars | NCT06948526 | El-Manial, Giza, Egypt | Not yet recruiting | All | Child | NA | Interventional | Nonvital primary molars | This trial compares the success of MO leaf extract and sodium hypochlorite as intracanal irrigants in pulpectomy of nonvital primary molars in children aged 3 to 7. It evaluates clinical parameters (pain, swelling, mobility) and radiographic healing (periapical changes, root resorption) over 12 months. |
| Antifungal potential of Moringa oleifera against otomycosis | NCT04768829 | Minya, Egypt | Completed | All | Adult | Early phase 1 | Interventional | Otomycosis | One group of patients with otomycosis received Nystatin ear drops, while the other received Moringa ear drops. An otolaryngologist performs an endoscopic examination, and their swabs will be analyzed using ELISA assays. |
| A study to explore the effect of Moringa oleifera (E-HS-01) on flow mediated dilatation and hemodynamics | NCT05002881 | Mumbai, Maharashtra, India | Unknown * | Male | Adult | NA | Interventional | Endothelial function | This study evaluates how MO affects vascular endothelial function, investigating its vasodilation potential by analyzing flow-mediated dilation (FMD) and blood flow velocity (BFV) in healthy males. |
| Effect of Moringa oleifera leaf extract on postoperative pain and bacterial reduction in mandibular premolars | NCT05348824 | Location not provided | Unknown * | All | Adult | Phase 2, phase 3 | Interventional | Necrotic pulp | This study clinically compares post-operative pain intensity and bacterial reduction with MO leaf extract solution versus 2.5% NaOCl in asymptomatic necrotic mandibular premolars treated in a single visit. |
| The cardiovascular and renal effects of Moringa oleifera extracts and Stevia rebaudiana Bertoni in patients with type 2 diabetes mellitus | NCT04254029 | Yaoundé, Cameroon | Completed | All | Adult, older adult | Phase 4 | Interventional | Benefits of capsules of M. oleifera and Stevia rebaudiana Bertoni in patients with type 2 diabetes mellitus before and after 45 days of add-on therapy | This study aimed to evaluate MO and stevia’s cardiovascular and renal benefits in type 2 diabetes patients over 8 weeks. |
| Antidiabetic potential of Moringa and Dom extract | NCT05898750 | Minya, Egypt | Completed | All | Adult | Early phase 1 | Interventional | Diabetes | The antidiabetic properties of Hyphaene thebaica fruits and MO leaves will be studied in type 2 diabetic patients consuming tea from both for six weeks. Their fasting blood glucose levels will be monitored daily, alongside other biomarkers, such as insulin concentration, lipid profile, liver enzymes, c-peptide, and glycated hemoglobin. |
| Moringa; delivering nutrition and economic value to the people of Malawi | NCT04092517 | Aberdeen, United Kingdom | Completed | All | Adult, older adult | NA | Interventional | Malnourishment | This study compares Moringa as a substitute in supplementary foods to evaluate nutrient bioavailability, bioactivity, and the plant’s activities. It assesses Moringa’s potential as an economically viable crop to support a resilient food supply chain in Malawi, ensuring access to essential nutrients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas-Vazquez, E.Y.; Padilla-Mendoza, J.R.; Carrillo-Pérez, M.S.; Gómez-Cansino, R.; Altamirano-Garcia, L.; Cruz Muñoz, R.; Diaz-Badillo, A.; López-Reyes, I.; Quintas-Granados, L.I. The “Colors” of Moringa: Biotechnological Approaches. Plants 2025, 14, 2338. https://doi.org/10.3390/plants14152338
Villegas-Vazquez EY, Padilla-Mendoza JR, Carrillo-Pérez MS, Gómez-Cansino R, Altamirano-Garcia L, Cruz Muñoz R, Diaz-Badillo A, López-Reyes I, Quintas-Granados LI. The “Colors” of Moringa: Biotechnological Approaches. Plants. 2025; 14(15):2338. https://doi.org/10.3390/plants14152338
Chicago/Turabian StyleVillegas-Vazquez, Edgar Yebran, Juan Ramón Padilla-Mendoza, Mayra Susana Carrillo-Pérez, Rocío Gómez-Cansino, Liliana Altamirano-Garcia, Rocío Cruz Muñoz, Alvaro Diaz-Badillo, Israel López-Reyes, and Laura Itzel Quintas-Granados. 2025. "The “Colors” of Moringa: Biotechnological Approaches" Plants 14, no. 15: 2338. https://doi.org/10.3390/plants14152338
APA StyleVillegas-Vazquez, E. Y., Padilla-Mendoza, J. R., Carrillo-Pérez, M. S., Gómez-Cansino, R., Altamirano-Garcia, L., Cruz Muñoz, R., Diaz-Badillo, A., López-Reyes, I., & Quintas-Granados, L. I. (2025). The “Colors” of Moringa: Biotechnological Approaches. Plants, 14(15), 2338. https://doi.org/10.3390/plants14152338







