Elucidating Genotypic Variation in Quinoa via Multidimensional Agronomic, Physiological, and Biochemical Assessments
Abstract
1. Introduction
2. Results
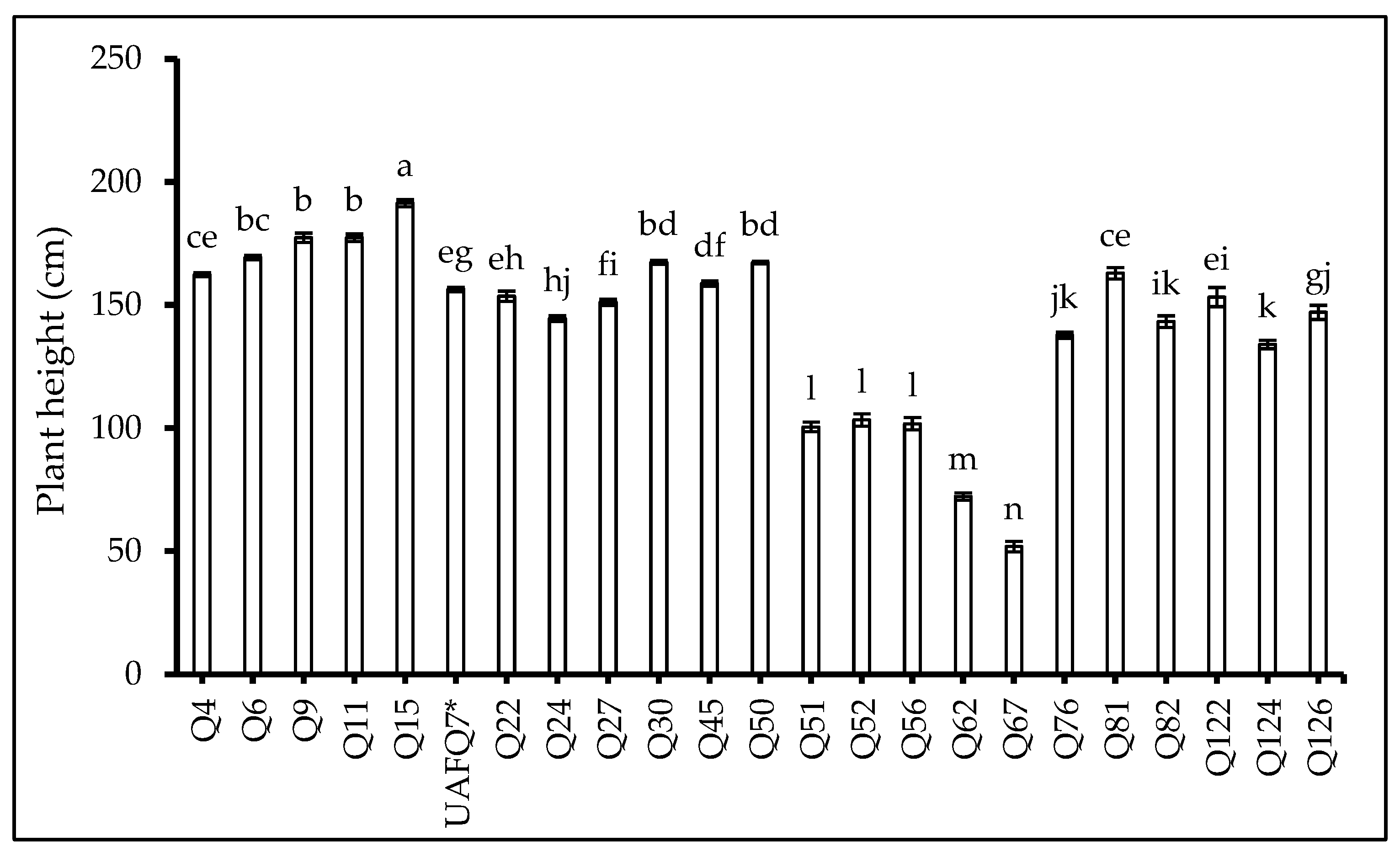
2.1. Phenological Development
2.2. Biochemical Parameters at Flowering
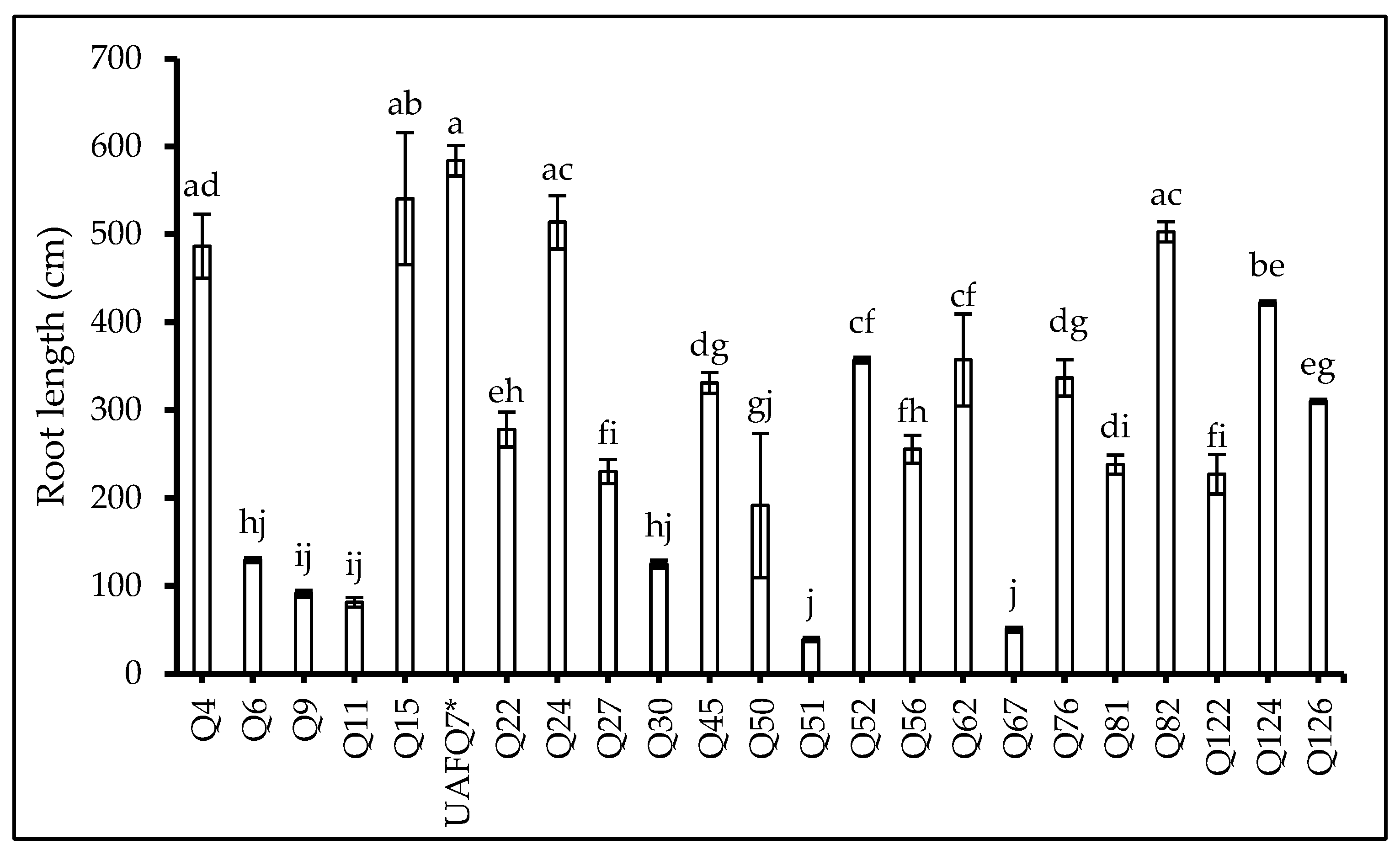
2.3. Root Scanning Attributes at Flowering
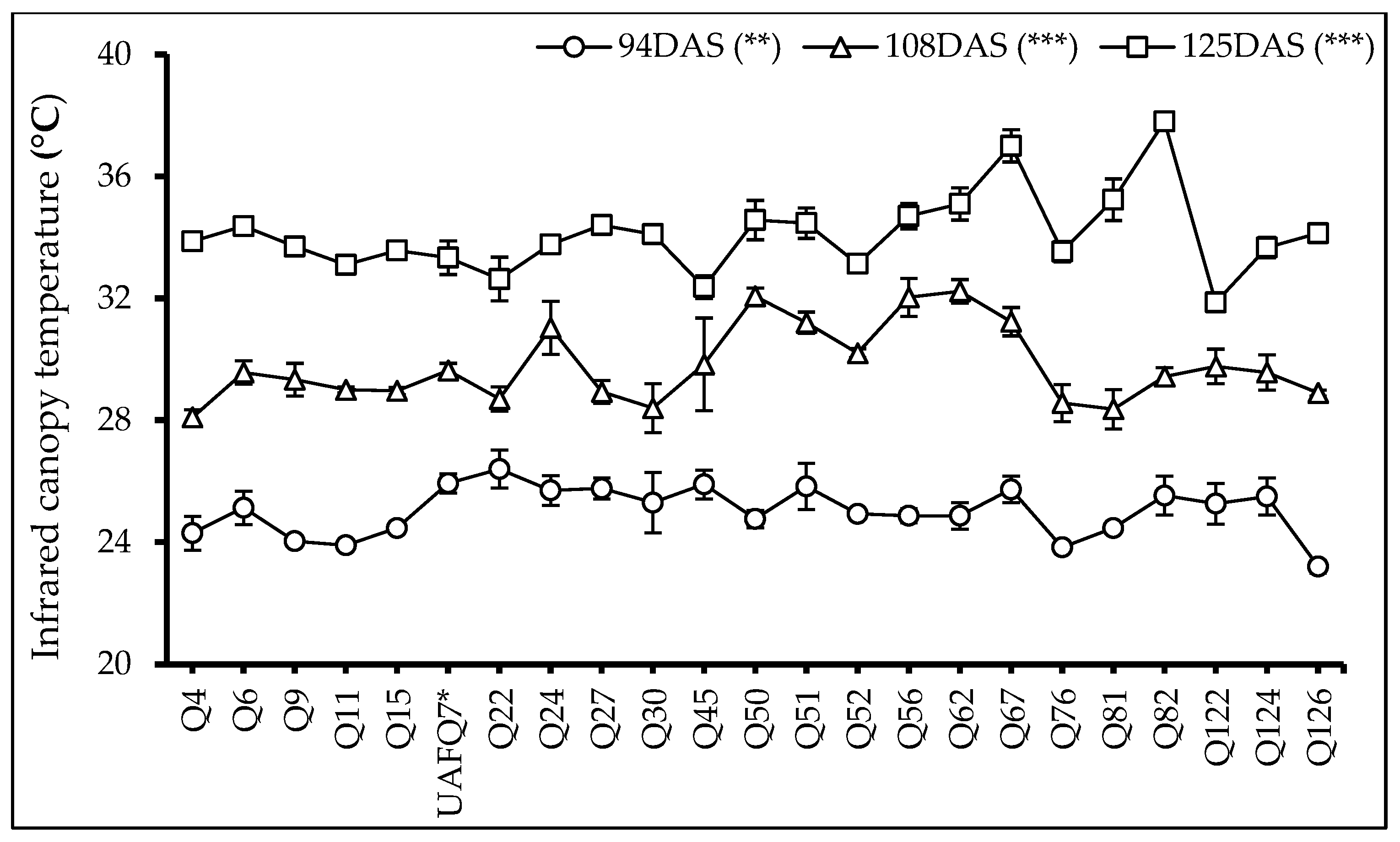
2.4. Infrared Canopy Temperature (ICT)
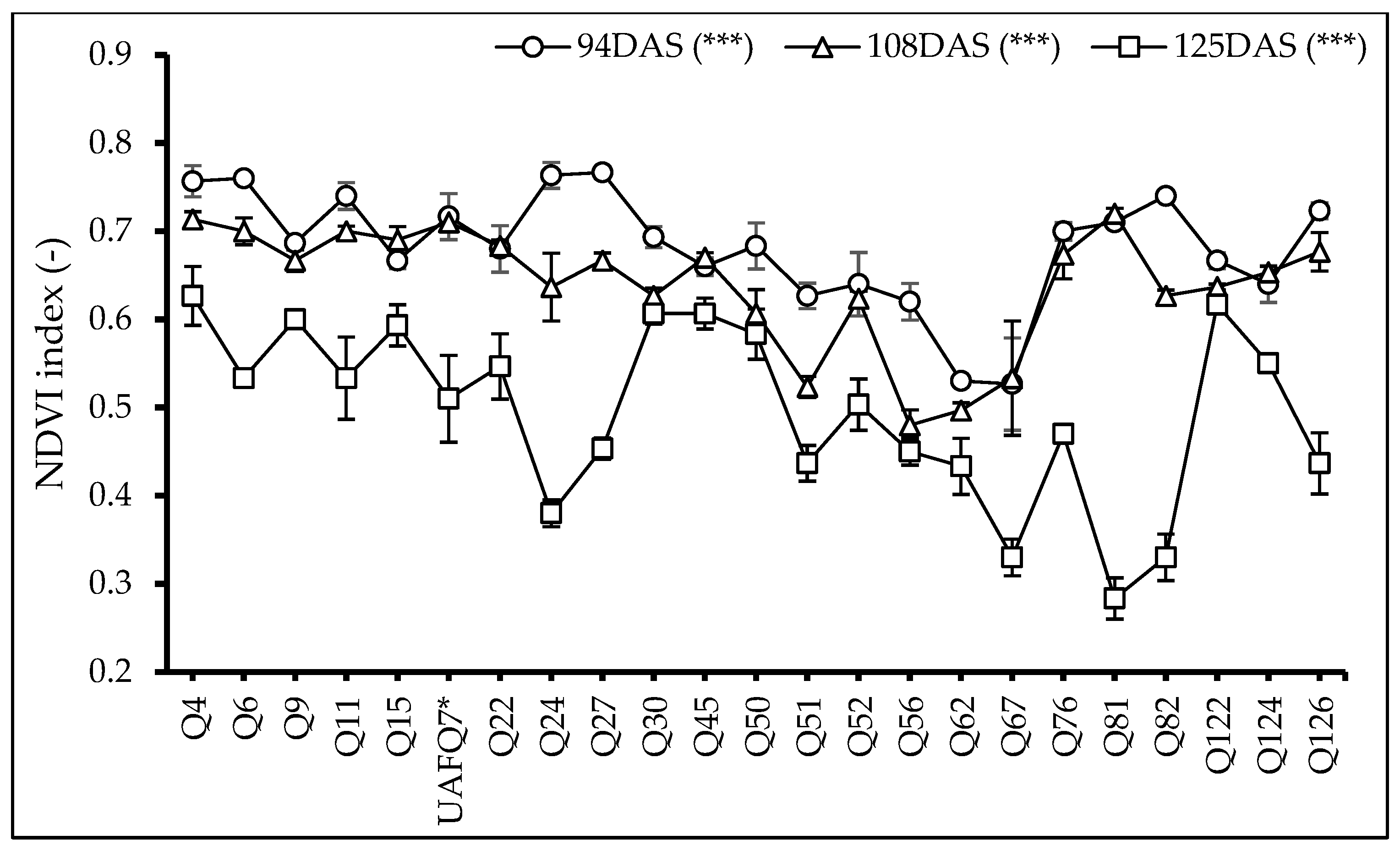
2.5. Normalized Difference Vegetation Index (NDVI)
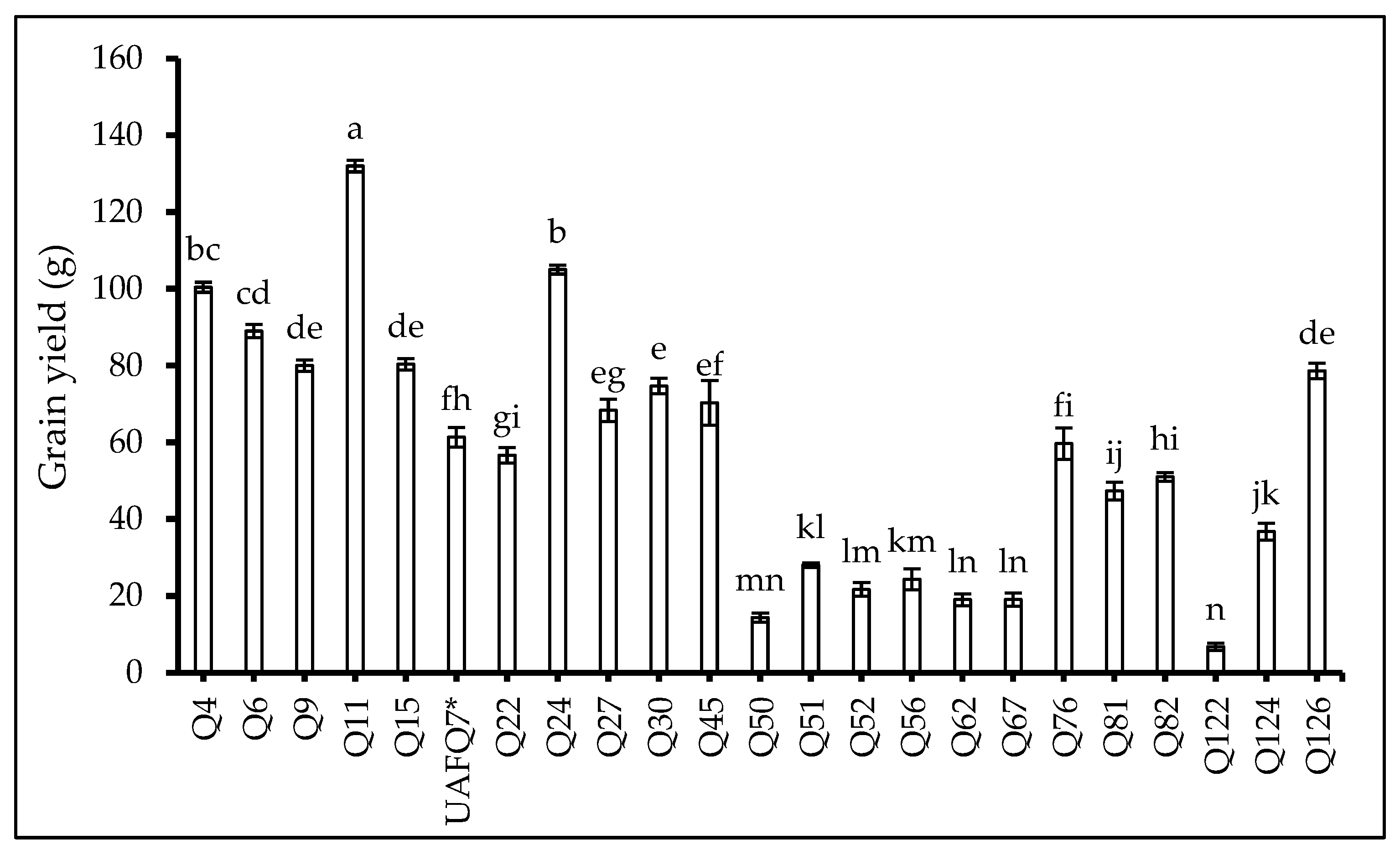
2.6. Yield-Related Traits at the End of the Experiment
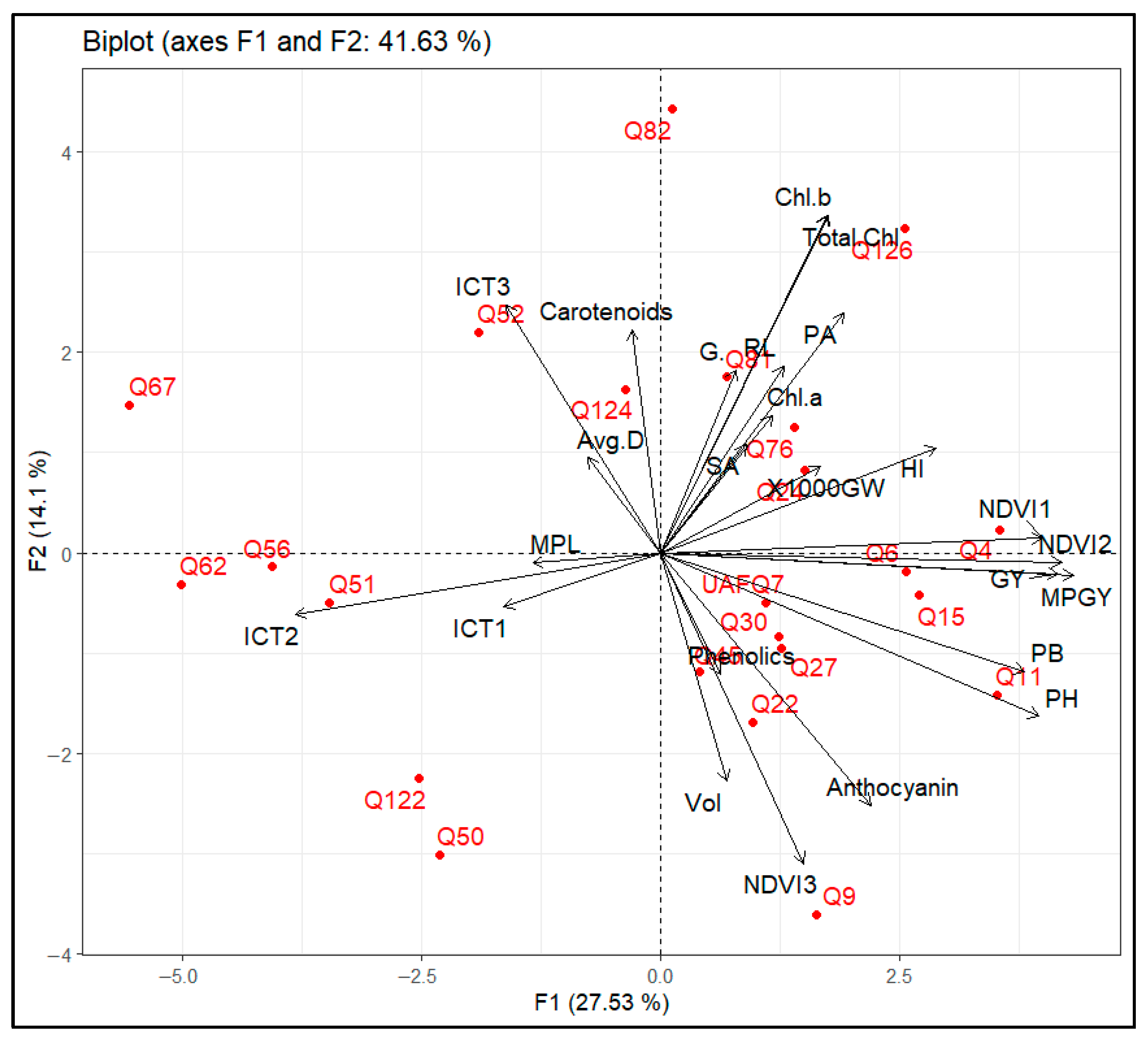
2.7. Principle Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Experimental Layout
4.2. Plant Measurments
4.2.1. Plant Phenology
4.2.2. Biochemical Attributes
4.2.3. Root Related Traits
4.2.4. Physiological Attributes
4.2.5. Plant Growth and Yield-Related Attributes
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Home-International Year of Quinoa 2013. 2013. Available online: http://www.fao.org/quinoa-2013/en (accessed on 10 July 2025).
- Akram, M.Z.; Libutti, A.; Rivelli, A.R. Evaluation of vegetative development of quinoa under water stress by applying different organic amendments. Agronomy 2023, 13, 1412. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Farooq, H.U.; Hashim, M.; Rey, E.; Curti, R.; Morris, A.; Maughan, P.J.; Jellen, E.N.; Jarvis, D.E.; Bertero, D. Wild relatives to improve heat tolerance of cultivated quinoa (Chenopodium quinoa Willd.): Pollen viability and seed number. J. Exp. Bot. 2025, eraf235. [Google Scholar] [CrossRef] [PubMed]
- Dehghanian, Z.; Ahmadabadi, M.; Asgari Lajayer, B.; Gougerdchi, V.; Hamedpour-Darabi, M.; Bagheri, N.; Sharma, R.; Vetukuri, R.R.; Astatkie, T.; Dell, B. Quinoa: A promising crop for resolving the bottleneck of cultivation in soils affected by multiple environmental abiotic stresses. Plants 2024, 13, 2117. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Shahzad, M.; Akram, M.Z.; Quratulain, M.S.; Nazeer, S. Assessment of growth and physiological traits of Chenopodium quinoa lines under different salt levels. Sarhad J. Agric. 2024, 40, 315–324. [Google Scholar] [CrossRef]
- Iqbal, S.; Baloch, H.; Hafeez, M.B.; Zahra, N.; Fatima, E.M.; Raza, A.; Raza, S.; Saddiq, M.S. Salt tolerance in quinoa genotypes: Ion-specific adaptations and growth performance under hydroponic conditions. Agrociencia Urug. 2025, 29, e1538. [Google Scholar] [CrossRef]
- Akram, M.Z.; Libutti, A.; Andreasen, C.; Liu, F.; Rivelli, A.R. Woody biochar differently influences plant water status and growth of five Quinoa (Chenopodium Quinoa Willd.) varieties under water stress. J. Soil Sci. Plant Nut. 2025, 25, 5140–5156. [Google Scholar] [CrossRef]
- Akram, M.Z.; Rivelli, A.R.; Libutti, A.; Liu, F.; Andreasen, C. Mitigation of drought stress for Quinoa (Chenopodium quinoa Willd.) varieties using woodchip biochar-amended soil. Plants 2024, 13, 2279. [Google Scholar] [CrossRef] [PubMed]
- Rivelli, A.R.; Akram, M.Z.; Libutti, A. Woody biochar rate and water shortage impact on early growth stages of Chenopodium quinoa Willd. Agronomy 2023, 14, 53. [Google Scholar] [CrossRef]
- Akram, M.Z.; Libutti, A.; Rivelli, A.R. Enhancing vegetative growth of quinoa and soil properties under water shortage through targeted organic amendments. Biol. Life Sci. For. 2023, 30, 4. [Google Scholar] [CrossRef]
- Matías, J.; Rodríguez, M.J.; Carrillo-Vico, A.; Casals, J.; Fondevilla, S.; Haros, C.M.; Pedroche, J.; Aparicio, N.; Fernández-García, N.; Aguiló-Aguayo, I. From ‘farm to fork’: Exploring the potential of nutrient-rich and stress-resilient emergent crops for sustainable and healthy food in the Mediterranean region in the face of climate change challenges. Plants 2024, 13, 1914. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, N.; Kumar, A. Enhancing resource use efficiency in crops through plant functional traits. In Plant Functional Traits for Improving Productivity; Kumar, N., Singh, H., Eds.; Springer: Singapore, 2024; pp. 97–117. [Google Scholar] [CrossRef]
- Santamarina, C.; Mathieu, L.; Bitocchi, E.; Pieri, A.; Bellucci, E.; Di Vittori, V.; Susek, K.; Scossa, F.; Nanni, L.; Papa, R. Agroecological genomics and participatory science: Optimizing crop mixtures for agricultural diversification. Trends Plant Sci. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.B.; Iqbal, S.; Li, Y.; Saddiq, M.S.; Basra, S.M.; Zhang, H.; Zahra, N.; Akram, M.Z.; Bertero, D.; Curti, R.N. Assessment of phenotypic diversity in the USDA collection of quinoa links genotypic adaptation to germplasm origin. Plants 2022, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Quinoa (Chenopodium quinoa Willd): A Potential Crop for Future Food, Healthy Security, Livelihood Generation and Poverty Eradication; Scientific Publishers: Jodhpur, India, 2019. [Google Scholar]
- Akram, M.Z.; Basra, S.M.A.; Hafeez, M.B.; Khan, S.; Nazeer, S.; Iqbal, S.; Saddiq, M.S.; Zahra, N. Adaptability and yield potential of new quinoa lines under agro-ecological conditions of Faisalabad-Pakistan. Asian J. Agric. Biol. 2021, 2, 202005301. [Google Scholar] [CrossRef]
- Coţovanu, I.; Mironeasa, C.; Mironeasa, S. Nutritionally improved wheat bread supplemented with quinoa flour of large, medium and small particle sizes at typical doses. Plants 2023, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, E.; Roura, R.; Rizzolo, D.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.; Redouane, C.A. Phenotyping the Combined Effect of Heat and Water Stress on Quinoa. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Environment & Policy; Springer: Singapore, 2020; Volume 58, pp. 163–183. [Google Scholar] [CrossRef]
- García-Parra, M.; Zurita-Silva, A.; Stechauner-Rohringer, R.; Roa-Acosta, D.; Jacobsen, S.-E. Quinoa (Chenopodium quinoa Willd.) and its relationship with agroclimatic characteristics: A Colombian perspective. Chil. J. Agric. Res. 2020, 80, 290–302. [Google Scholar] [CrossRef]
- Ren, A.; Jiang, Z.; Dai, J.; Sun, M.; Anwar, S.; Tang, P.; Wang, R.; Ding, P.; Li, L.; Wu, X. Phenotypic characterization and yield screening of quinoa germplasms in diverse low-altitude regions: A preliminary study. Agronomy 2024, 14, 1354. [Google Scholar] [CrossRef]
- Vasconcelos, E.S.d.; Echer, M.d.M.; Kliemann, M.A.; Lang, M.J. Selection and recommend of quinoa (Chenopodim quinoa) genotypes based on the yield genotypic adaptability and stability. Rev. Ceres 2019, 66, 117–123. [Google Scholar] [CrossRef]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.-L. Thermal imaging for plant stress detection and phenotyping. Remote Sens. 2020, 13, 68. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fan, J.; Yang, C.; Xu, F.; Zhang, X. Novel vegetation indices for estimating photosynthetic and non-photosynthetic fractional vegetation cover from Sentinel data. Int. J. Appl. Earth Obs. Geoinf. 2022, 109, 102793. [Google Scholar] [CrossRef]
- Mbebi, A.J.; Mercado, F.; Hobby, D.; Tong, H.; Nikoloski, Z. Advances in multi-trait genomic prediction approaches: Classification, comparative analysis, and perspectives. Brief. Bioinform. 2025, 26, bbaf211. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.S.; Shiri, M.; Mostafavi, K.; Mohammadi, A.; Miri, S.M. Genetic analysis and association detection of agronomic traits in maize genotypes. Sci. Rep. 2025, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Sembada, A.A.; Faizal, A.; Sulistyawati, E. Photosynthesis efficiency as key factor in decision-making for forest design and redesign: A systematic literature review. Ecol. Front. 2024, 44, 1128–1139. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The scope for adaptation of quinoa in northern latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Cui, H.; Yao, Q.; Xing, B.; Zhou, B.; Shah, S.S.; Qin, P. The performance of agronomic and quality traits of Quinoa under different altitudes in northwest of China. Agronomy 2024, 14, 1194. [Google Scholar] [CrossRef]
- Hou, M.; Tian, F.; Zhang, T.; Huang, M. Evaluation of canopy temperature depression, transpiration, and canopy greenness in relation to yield of soybean at reproductive stage based on remote sensing imagery. Agric. Water Manag. 2019, 222, 182–192. [Google Scholar] [CrossRef]
- Contreras, R.A.; Pizarro, M.; Batista-Gonzalez, A. Exploring plant metabolites for functional food applications: A review of bioactive compounds and their potential use in the food industry. Preprints 2025, 2025040688. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-W.; Chen, M.; Tian, F.; Yao, R.; Qin, N.-N.; Wu, W.-H.; Turner, N.C.; Li, F.-M.; Du, Y.-L. Root morphology, exudate patterns, and mycorrhizal symbiosis are determinants to improve phosphorus acquisition in alfalfa. J. Exp. Bot. 2025, eraf107. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U.; Viola, E.; Keswani, C.; Minkina, T. Impact of the sustainable agricultural practices for governing soil health from the perspective of a rising agri-based circular bioeconomy. Appl. Soil Ecol. 2024, 194, 105199. [Google Scholar] [CrossRef]
- Mahmood, U.; Li, X.; Fan, Y.; Chang, W.; Niu, Y.; Li, J.; Qu, C.; Lu, K. Multi-omics revolution to promote plant breeding efficiency. Front. Plant Sci. 2022, 13, 1062952. [Google Scholar] [CrossRef] [PubMed]
- Gharib, M.; Qabil, N.; Salem, A.; Ali, M.; Awaad, H.; Mansour, E. Characterization of wheat landraces and commercial cultivars based on morpho-phenological and agronomic traits. Cereal Res. Commun. 2021, 49, 149–159. [Google Scholar] [CrossRef]
- Vitale, P.; Laidò, G.; Dono, G.; Pecorella, I.; Ramasubramanian, V.; Lorenz, A.; De Vita, P.; Pecchioni, N. Univariate and multivariate genomic prediction for agronomic traits in durum wheat under two field conditions. PLoS ONE 2024, 19, e0310886. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Karunathilake, E.M.; Tuan, T.T.; Chung, Y.S. Genomics, phenomics, and machine learning in transforming plant research: Advancements and challenges. Hortic. Plant J. 2025, 11, 486–503. [Google Scholar] [CrossRef]
- Thiam, E.; Allaoui, A.; Benlhabib, O. Quinoa productivity and stability evaluation through varietal and environmental interaction. Plants 2021, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Zuniga, V.; Brito, V.; Fuentes, F.; Steinfort, U. Phenological growth stages of quinoa (Chenopodium quinoa) based on the BBCH scale. Ann. Appl. Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons: New York, NY, USA, 2001; Volume 6, pp. I1. 1.1–I1. 1.8. [Google Scholar]
- Lees, D.; Francis, F. Quantitative methods for anthocyanins: 6. Flavonols and anthocyanins in cranberries. J. Food Sci. 1971, 36, 1056–1060. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://cir.nii.ac.jp/crid/1370298755636824325 (accessed on 10 July 2025).
| Genotypes | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q4 | 5 | 6 | 14 | 24 | 44 | 57 | 70 | 81 | 110 | 125 | 138 |
| Q6 | 6 | 8 | 13 | 21 | 46 | 53 | 64 | 78 | 106 | 124 | 146 |
| Q9 | 6 | 7 | 14 | 24 | 46 | 55 | 66 | 80 | 115 | 132 | 143 |
| Q11 | 7 | 6 | 13 | 24 | 47 | 55 | 68 | 80 | 105 | 124 | 147 |
| Q15 | 5 | 6 | 14 | 23 | 44 | 53 | 65 | 79 | 113 | 128 | 145 |
| UAFQ7* | 5 | 6 | 14 | 23 | 51 | 58 | 68 | 78 | 110 | 126 | 145 |
| Q22 | 5 | 7 | 15 | 23 | 48 | 59 | 70 | 82 | 108 | 122 | 145 |
| Q24 | 5 | 6 | 14 | 24 | 48 | 58 | 70 | 82 | 102 | 115 | 128 |
| Q27 | 6 | 7 | 14 | 21 | 45 | 56 | 68 | 79 | 104 | 126 | 146 |
| Q30 | 6 | 7 | 15 | 25 | 46 | 59 | 68 | 79 | 115 | 129 | 144 |
| Q45 | 5 | 6 | 14 | 24 | 57 | 65 | 76 | 87 | 110 | 125 | 145 |
| Q50 | 6 | 6 | 13 | 24 | 55 | 61 | 68 | 78 | 107 | 142 | 170 |
| Q51 | 7 | 7 | 15 | 24 | 46 | 58 | 68 | 79 | 111 | 128 | 145 |
| Q52 | 6 | 7 | 13 | 23 | 44 | 58 | 69 | 82 | 106 | 125 | 143 |
| Q56 | 6 | 4 | 14 | 24 | 45 | 57 | 67 | 77 | 108 | 122 | 142 |
| Q62 | 6 | 7 | 14 | 22 | 44 | 57 | 70 | 79 | 112 | 130 | 145 |
| Q67 | 6 | 4 | 14 | 24 | 45 | 55 | 68 | 81 | 108 | 128 | 146 |
| Q76 | 6 | 7 | 14 | 24 | 55 | 68 | 79 | 89 | 115 | 130 | 147 |
| Q81 | 6 | 6 | 14 | 25 | 57 | 66 | 77 | 87 | 105 | 127 | 144 |
| Q82 | 6 | 7 | 14 | 24 | 48 | 64 | 73 | 85 | 106 | 129 | 128 |
| Q122 | 7 | 6 | 15 | 25 | 51 | 62 | 75 | 86 | 104 | 135 | 149 |
| Q124 | 6 | 7 | 15 | 22 | 54 | 57 | 69 | 81 | 117 | 132 | 145 |
| Q126 | 6 | 7 | 15 | 22 | 55 | 65 | 77 | 84 | 115 | 135 | 146 |
| Genotypes | Chl a (mg/g FW) | Chl b (mg/g FW) | Total Chl (mg/g FW) | Carotenoids (mg/g FW) | Phenolics (µg/g FW) | Anthocyanin (mg CGE 100 g−1 DW) |
|---|---|---|---|---|---|---|
| Q4 | 0.045 ab | 0.243 ac | 0.288 ac | 3.354 ab | 0.701 ac | 0.584 gi |
| Q6 | 0.034 cg | 0.447 ac | 0.482 ac | 2.884 ab | 0.586 bc | 1.144 ac |
| Q9 | 0.031 dh | 0.150 bc | 0.181 bc | 2.727 b | 0.825 a | 1.287 a |
| Q11 | 0.030 eh | 0.266 ac | 0.296 ac | 3.336 ab | 0.655 ac | 1.130 ad |
| Q15 | 0.036 af | 0.218 ac | 0.255 ac | 3.701 ab | 0.673 ac | 1.215 a |
| UAFQ7* | 0.026 gh | 0.165 bc | 0.191 bc | 3.688 ab | 0.684 ac | 1.149 ac |
| Q22 | 0.036 af | 0.221 ac | 0.257 ac | 3.092 ab | 0.659 ac | 1.212 a |
| Q24 | 0.027 gh | 0.202 ac | 0.228 ac | 3.526 ab | 0.646 ac | 1.180 ab |
| Q27 | 0.032 dg | 0.219 ac | 0.251 ac | 3.153 ab | 0.809 ab | 1.085 ae |
| Q30 | 0.045 a | 0.321 ac | 0.366 ac | 3.330 ab | 0.683 ac | 1.063 af |
| Q45 | 0.039 ae | 0.276 ac | 0.315 ac | 2.541 b | 0.753 ac | 0.799 di |
| Q50 | 0.034 cg | 0.159 bc | 0.193 bc | 2.991 ab | 0.799 ac | 1.204 a |
| Q51 | 0.034 cg | 0.354 ac | 0.388 ac | 2.831 b | 0.712 ac | 0.788 ei |
| Q52 | 0.043 ac | 0.379 ac | 0.422 ac | 4.915 a | 0.748 ac | 0.517 hi |
| Q56 | 0.034 cg | 0.051 c | 0.085 c | 3.388 ab | 0.564 c | 0.671 gi |
| Q62 | 0.022 h | 0.056 c | 0.078 c | 3.267 ab | 0.711 ac | 0.807 di |
| Q67 | 0.034 cg | 0.219 ac | 0.253 ac | 3.955 ab | 0.599 ac | 0.699 gi |
| Q76 | 0.037 af | 0.564 ab | 0.600 ab | 2.771 b | 0.694 ac | 0.745 fi |
| Q81 | 0.038 ae | 0.388 ac | 0.425 ac | 3.937 ab | 0.717 ac | 0.841 ch |
| Q82 | 0.039 ad | 0.576 ab | 0.615 ab | 2.648 b | 0.713 ac | 0.500 i |
| Q122 | 0.028 fh | 0.081 c | 0.109 c | 3.183 ab | 0.644 ac | 0.741 fi |
| Q124 | 0.036 bf | 0.525 ac | 0.561 ac | 3.873 ab | 0.657 ac | 0.854 bh |
| Q126 | 0.033 dg | 0.669 a | 0.702 a | 4.508 ab | 0.687 ac | 1.124 ae |
| Level of significance | ||||||
| *** | *** | *** | ** | ** | *** | |
| Genotypes | SA (cm2) | PA (cm2) | Vol (cm3) | Avg. D (mm) |
|---|---|---|---|---|
| Q4 | 94.2 a | 32.1 ab | 1.453 | 0.550 d |
| Q6 | 36.3 gh | 25.1 ag | 1.717 | 0.823 cd |
| Q9 | 53.5 ef | 14.1 eg | 1.691 | 0.934 bd |
| Q11 | 34.2 gh | 19.1 bg | 1.670 | 1.782 a |
| Q15 | 95.5 a | 33.3 a | 1.940 | 1.837 a |
| UAFQ7* | 84.5 ac | 33.9 a | 1.547 | 1.837 a |
| Q22 | 59.8 df | 23.9 ag | 1.790 | 1.620 ab |
| Q24 | 93.8 a | 32.7 a | 1.530 | 1.647 a |
| Q27 | 61.3 de | 23.5 ag | 1.610 | 0.857 cd |
| Q30 | 44.7 fg | 17.0 cg | 1.813 | 1.670 a |
| Q45 | 27.0 h | 26.5 af | 1.640 | 1.773 a |
| Q50 | 54.4 ef | 12.7 g | 1.833 | 1.723 a |
| Q51 | 33.2 gh | 12.1 g | 1.533 | 0.071 a |
| Q52 | 60.0 df | 31.3 ab | 1.443 | 1.573 ab |
| Q56 | 68.6 ce | 21.8 ag | 1.797 | 1.947 a |
| Q62 | 75.7 bd | 24.7 ag | 1.560 | 1.807 a |
| Q67 | 24.7 h | 16.3 dg | 1.360 | 1.817 a |
| Q76 | 66.6 de | 29.6 ad | 2.163 | 1.517 ac |
| Q81 | 44.2 fg | 27.5 ae | 1.773 | 1.757 a |
| Q82 | 95.2 a | 30.1 ac | 1.067 | 1.753 a |
| Q122 | 33.8 gh | 13.1 fg | 1.527 | 1.747 a |
| Q124 | 85.8 ab | 27.9 ad | 1.773 | 1.893 a |
| Q126 | 28.6 gh | 20.8 ag | 1.187 | 1.717 a |
| Level of significance | ||||
| *** | *** | ns | *** | |
| Genotypes | PB (g) | MPL (cm) | MPGY (g) | 1000 GW (g) | HI (-) | GP (%) |
|---|---|---|---|---|---|---|
| Q4 | 174.7 bd | 31.7 bd | 71.8 ab | 0.567 ab | 0.576 ac | 72.0 j |
| Q6 | 172.9 be | 34.0 bd | 67.9 b | 0.467 ab | 0.515 ad | 98.0 ab |
| Q9 | 195.9 ab | 26.0 d | 54.5 c | 0.400 b | 0.408 cf | 84.0 hi |
| Q11 | 216.3 a | 34.0 bd | 80.8 a | 0.533 ab | 0.610 ab | 94.0 cd |
| Q15 | 193.8 ac | 29.0 cd | 52.9 cd | 0.467 ab | 0.415 cf | 100.0 a |
| UAFQ7* | 158.3 df | 35.3 bd | 49.6 ce | 0.467 ab | 0.388 dg | 86.0 gh |
| Q22 | 177.4 bd | 31.3 bd | 46.5 cg | 0.467 ab | 0.319 eg | 90.0 ef |
| Q24 | 156.4 dg | 26.3 d | 39.9 ei | 0.500 ab | 0.672 a | 100.0 a |
| Q27 | 135.7 ei | 27.0 d | 43.7 dh | 0.500 ab | 0.504 ad | 88.0 fg |
| Q30 | 168.4 df | 34.7 bd | 39.2 fi | 0.500 ab | 0.444 be | 96.0 bc |
| Q45 | 153.7 dh | 35.3 bd | 31.9 ik | 0.367 b | 0.458 be | 96.0 bc |
| Q50 | 141.1 dh | 39.7 ab | 7.2 m | 0.433 ab | 0.102 h | 84.0 hi |
| Q51 | 98.9 ij | 46.3 a | 23.8 kl | 0.400 b | 0.283 eg | 98.0 ab |
| Q52 | 98.9 ij | 38.3 ac | 13.8 lm | 0.467 ab | 0.220 gh | 92.0 de |
| Q56 | 100.4 ij | 35.3 bd | 11.3 m | 0.533 ab | 0.242 fh | 94.0 cd |
| Q62 | 73.4 jk | 32.0 bd | 5.4 m | 0.500 ab | 0.260 fh | 88.0 fg |
| Q67 | 36.7 kl | 28.0 d | 7.7 m | 0.333 b | 0.523 ad | 82.0 i |
| Q76 | 104.7 jk | 35.7 bd | 39.9 ei | 0.400 b | 0.571 ac | 92.0 de |
| Q81 | 130.6 fi | 28.0 d | 34.8 hj | 0.367 b | 0.363 dg | 98.0 ab |
| Q82 | 140.1 dh | 31.7 bd | 37.4 gi | 0.433 ab | 0.364 dg | 100.0 a |
| Q122 | 19.4 l | 28.3 bd | 4.6 m | 0.467 ab | 0.383 dg | 82.0 i |
| Q124 | 116.1 hi | 38.3 ac | 26.2 jk | 0.467 ab | 0.316 eg | 94.0 cd |
| Q126 | 120.1 gi | 29.0 cd | 47.9 cf | 0.733 a | 0.655 a | 96.0 bc |
| Level of significance | ||||||
| *** | *** | *** | * | *** | *** | |
| Genotypes Local Coding | Origin |
|---|---|
| Q4 | United States, New Mexico |
| Q6 | United States, New Mexico |
| Q9 | United States, New Mexico |
| Q11 | United States, New Mexico |
| Q15 | United States, New Mexico |
| UAFQ7* | United States, New Mexico (Approved quinoa variety in Pakistan) |
| Q22 | United States, New Mexico |
| Q24 | United States, New Mexico |
| Q27 | Bolivia |
| Q30 | United States, New Mexico |
| Q45 | Chile |
| Q50 | Bolivia, La Paz |
| Q51 | Bolivia, La Paz |
| Q52 | Bolivia, La Paz |
| Q56 | Peru |
| Q62 | Peru |
| Q67 | Peru |
| Q76 | Chile, Los Lagos |
| Q81 | Chile, Los Lagos |
| Q82 | Bolivia, Oruro |
| Q122 | Bolivia, La Paz |
| Q124 | Bolivia, La Paz |
| Q126 | United States, Colorado |
| Soil Properties | Value |
|---|---|
| pH | 6.8 to 7.3 |
| Electric conductivity | 3.62–3.72 dS/m |
| Organic matter | 0.88–0.91% |
| Total Nitrogen | 0.07–0.08% |
| Exchangeable Potassium | 175–177 ppm |
| Available Phosphorous | 4.44–4.60 ppm |
| Months | Temperature | Relative Humidity | Total Rainfall | Sunshine Radiation | Wind Speed | ||
|---|---|---|---|---|---|---|---|
| Max. | Min. | Mean | |||||
| °C | °C | °C | % | mm | h | km/h | |
| November | 24.1 | 11.8 | 18 | 84.6 | 1.5 | 3.7 | 1.9 |
| December | 22 | 6.7 | 14.4 | 69.3 | 4.2 | 6 | 2.4 |
| January | 21.5 | 5.5 | 13.5 | 75.9 | 0 | 6.4 | 3.5 |
| February | 24 | 9.5 | 16.7 | 73.3 | 9.5 | 6.5 | 3.8 |
| March | 32.2 | 16.4 | 23.8 | 61.4 | 12.5 | 8.6 | 5.2 |
| April | 36.8 | 20.8 | 28.8 | 47.3 | 7.9 | 9.1 | 3.1 |
| May | 40.3 | 23.7 | 32 | 29.8 | 21.6 | 8.6 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazeer, S.; Akram, M.Z. Elucidating Genotypic Variation in Quinoa via Multidimensional Agronomic, Physiological, and Biochemical Assessments. Plants 2025, 14, 2332. https://doi.org/10.3390/plants14152332
Nazeer S, Akram MZ. Elucidating Genotypic Variation in Quinoa via Multidimensional Agronomic, Physiological, and Biochemical Assessments. Plants. 2025; 14(15):2332. https://doi.org/10.3390/plants14152332
Chicago/Turabian StyleNazeer, Samreen, and Muhammad Zubair Akram. 2025. "Elucidating Genotypic Variation in Quinoa via Multidimensional Agronomic, Physiological, and Biochemical Assessments" Plants 14, no. 15: 2332. https://doi.org/10.3390/plants14152332
APA StyleNazeer, S., & Akram, M. Z. (2025). Elucidating Genotypic Variation in Quinoa via Multidimensional Agronomic, Physiological, and Biochemical Assessments. Plants, 14(15), 2332. https://doi.org/10.3390/plants14152332








