Establishment of an Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation System for Functional Analysis in Passion Fruit
Abstract
1. Introduction
2. Results
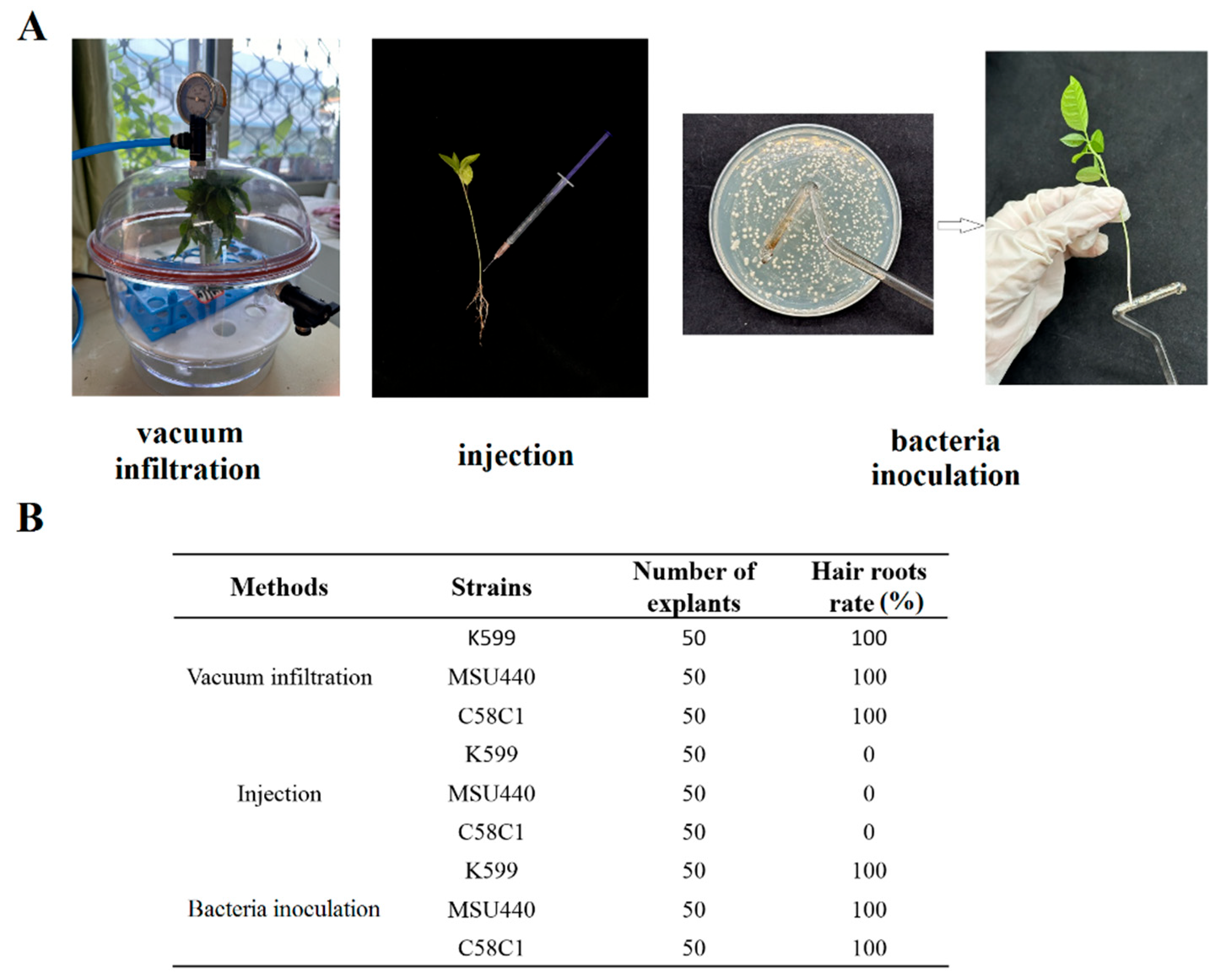
2.1. Hairy Root Induction of Passion Fruit Seedlings by A. rhizogenes
2.2. A. rhizogenes Strain K599 Was the Most Suitable Strain for Hairy Root Transformation in Passion Fruit
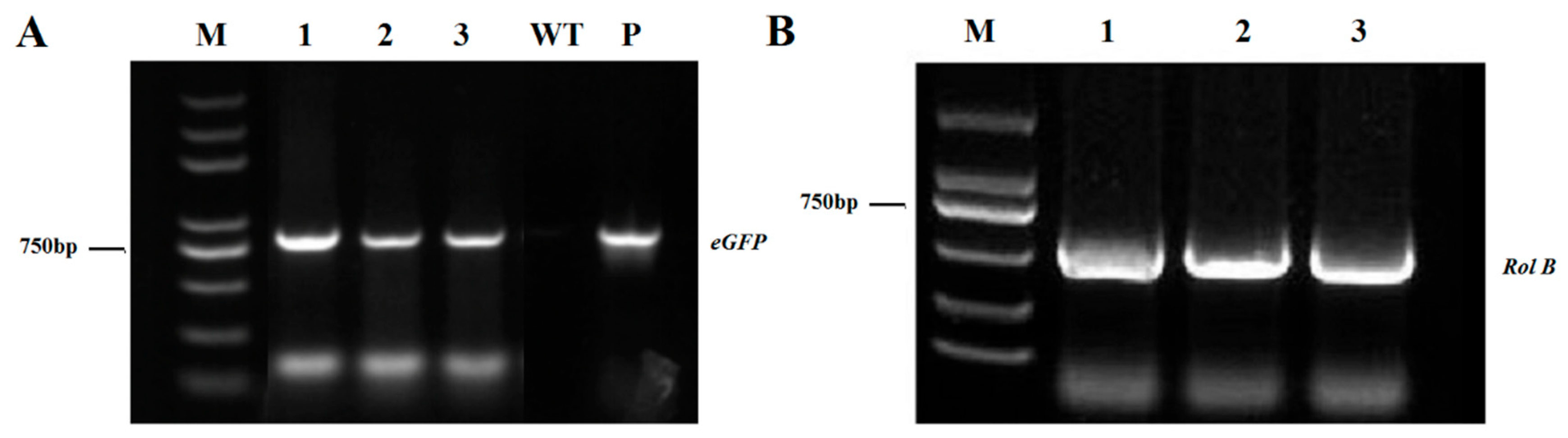
2.3. PCR Verification of eGFP-Positive Roots
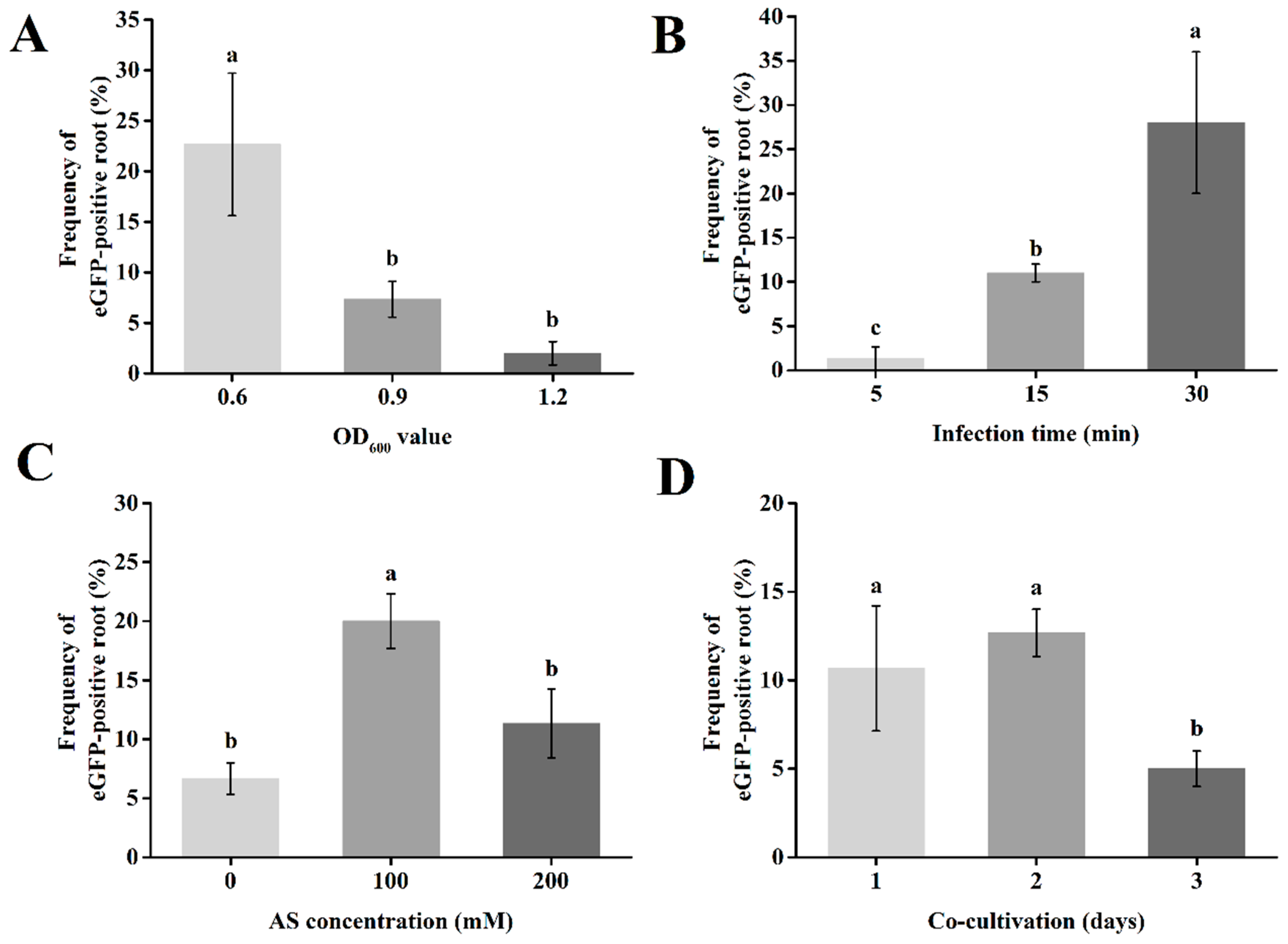
2.4. Optimization of the Hairy Root Transformation System in Passion Fruit
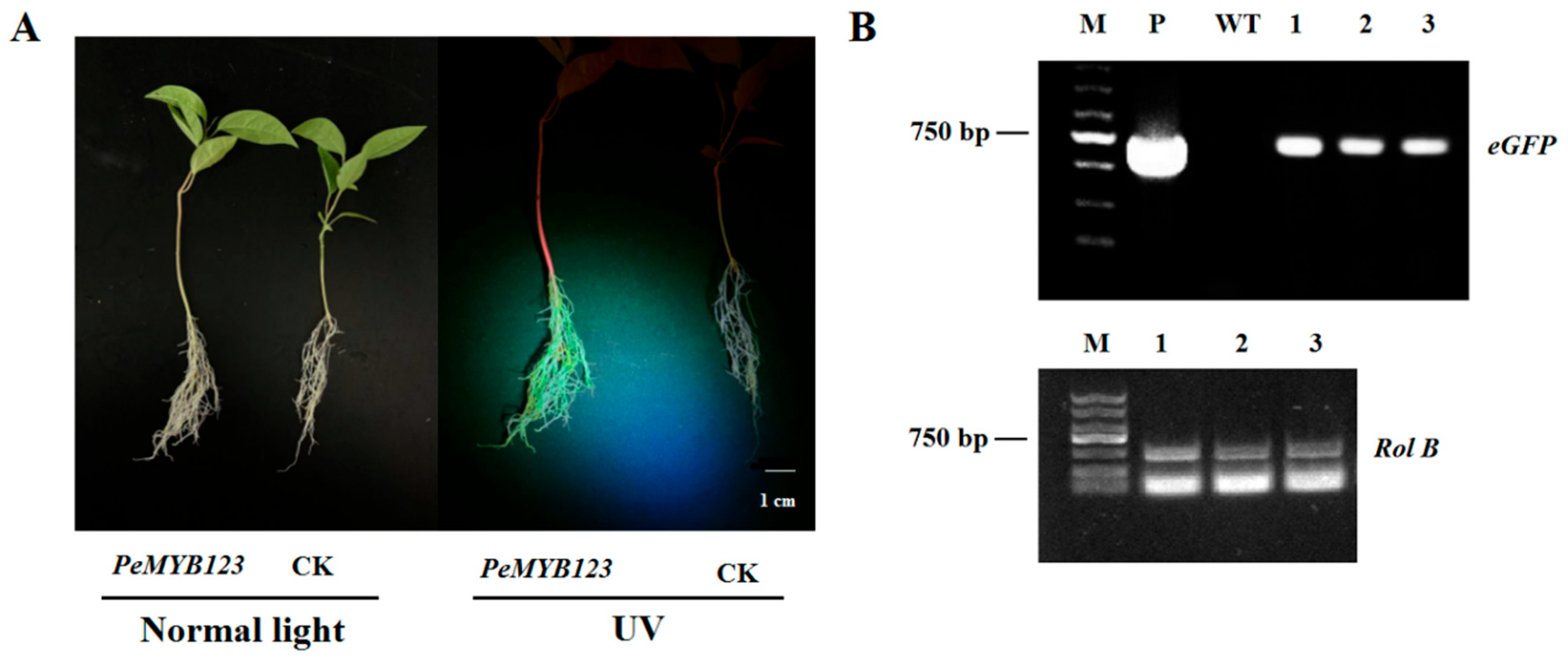
2.5. Gene Functional Analysis of PeMYB123 Using the Hairy Root Transgenic System
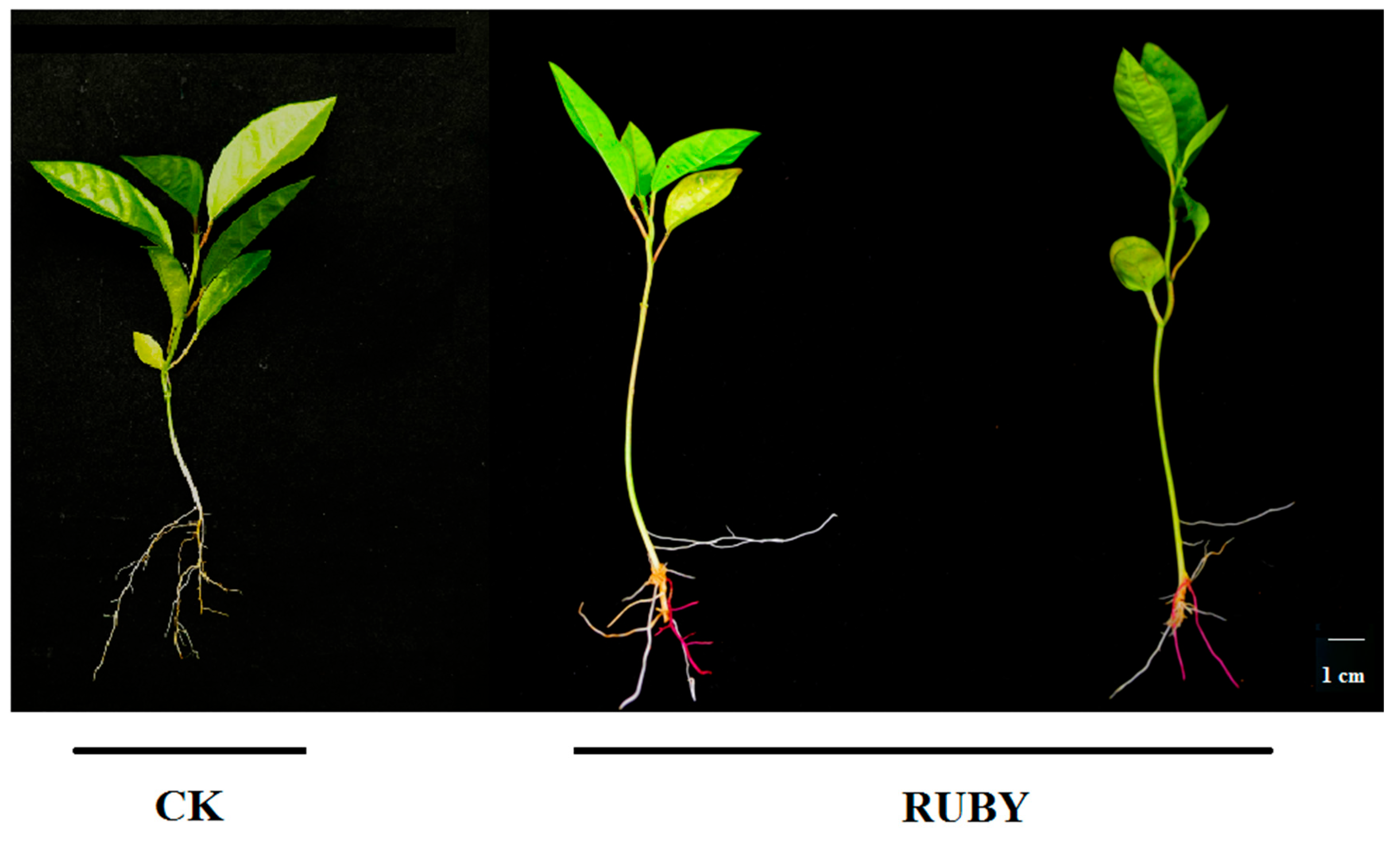
2.6. Application of RUBY in Hair Root Transformation System of Passion Fruit
3. Discussion
3.1. Establishment of A. rhizogenes-Mediated Hairy Root Transformation System in Passion Fruit
3.2. Factors Influencing the A. rhizogenes-Mediated Hairy Root Transformation Efficiency in Passion Fruit
3.3. Application of the A. rhizogenes-Mediated Hairy Root Transformation Efficiency in Passion Fruit
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. Plasmids and Agrobacterium Bacterial Strains
4.3. PeMYB123 Cloning
4.4. A. rhizogenes-Mediated Transformation
4.5. Determination of PA Content
4.6. Direct Observation of Transgenic Hairy Roots
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faleiro, F.G.; Junqueira, N.T.V.; Junghans, T.G.; Jesus, O.N.D.; Miranda, D.; Otoni, W.C. Advances in passion fruit (Passiflora spp.) propagation. Rev. Bras. Frutic. 2019, 41, e155. [Google Scholar] [CrossRef]
- Pereira, Z.C.; Cruz, J.M.D.A.; Correa, R.F.; Sanches, E.A.; Campelo, P.H.; Bezerra, J.A. Passion fruit (Passiflora spp.) pulp, A review on bioactive properties, health benefits and technological potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef]
- Shad, M.A.; Wu, S.; Rao, M.J.; Luo, X.; Huang, X.; Wu, Y.; Zhou, Y.; Wang, L.; Ma, C.; Hu, L. Evolution and functional dynamics of TCP transcription factor gene family in passion fruit (Passiflora edulis). Plants 2024, 13, 2568. [Google Scholar] [CrossRef]
- Fonseca, A.M.A.; Geraldi, M.V.; Junior, M.R.M.; Silvestre, A.J.D.; Rocha, S.M. Purple passion fruit (Passiflora edulis f. edulis), A comprehensive review on the nutritional value, phytochemical profile and associated health effects. Food Res. Int. 2022, 160, 111665. [Google Scholar] [CrossRef]
- Gadioli, I.L.; Correa, R.C.G.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Abreu, R.M.V.; Santos-Buelga, C.; Ferreira, I.C.F.R. A systematic review on phenolic compounds in Passiflora plants, exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Rudnicki, M.; Silveira, M.M.; Pereira, T.V.; Oliveira, M.R.; Moreira, J.C.F. Protective effects of Passiflora alata extract pretreatment on carbon tetrachloride induced oxidative damage in rats. Food Chem. Toxicol. 2007, 45, 656–661. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; Lu, J.; Sun, Y.; Cui, Y. Research progress of proanthocyanidins and anthocyanidins. Phytother. Res. 2023, 37, 2552–2577. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq, I.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins, A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Plaza, M.; Marina, M.L. High-performance thin-layer chromatography and direct analysis in real time-high resolution mass spectrometry of non-extractable polyphenols from tropical fruit peels. Food Res. Int. 2021, 147, 110455. [Google Scholar] [CrossRef]
- Pugliese, A.G.; Tomas-Barberan, F.A.; Truchado, P.; Genovese, M.I. Flavonoids, proanthocyanidins, vitamin C, and antioxidant activity of Theobroma grandiflorum (Cupuassu) pulp and seeds. J. Agric. Food Chem. 2013, 61, 2720–2728. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Wang, S.; Wei, L.; Cui, Y.Y.; Chen, Y.H. Proanthocyanidins, Components, Pharmacokinetics and Biomedical Properties. Am. J. Chin. Med. 2020, 48, 813–869. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef]
- Jiang, W.; Yin, Q.; Liu, J.; Su, X.; Han, X.; Li, Q.; Zhang, J.; Pang, Y. The APETALA2-MYBL2 module represses proanthocyanidin biosynthesis by affecting formation of the MBW complex in seeds of Arabidopsis thaliana. Plant Commun. 2024, 5, 100777. [Google Scholar] [CrossRef]
- An, X.H.; Tian, Y.; Chen, K.Q.; Liu, X.J.; Liu, D.D.; Xie, X.B.; Cheng, C.G.; Cong, P.H.; Hao, Y.J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, W.; Guan, X.; Wang, F.; Fan, Z.; Gao, S.; Yao, Y. VvMYB14 participates in melatonin-induced proanthocyanidin biosynthesis by upregulating expression of VvMYBPA1 and VvMYBPA2 in grape seeds. Hortic. Res. 2023, 10, uhac274. [Google Scholar] [CrossRef]
- Zhou, H.; Lin-Wang, K.; Liao, L.; Gu, C.; Lu, Z.; Allan, A.C.; Han, Y. Peach MYB7 activates transcription of the proanthocyanidin pathway gene encoding leucoanthocyanidin reductase, but not anthocyanidin reductase. Front. Plant Sci. 2015, 6, 908. [Google Scholar] [CrossRef]
- Su, W.; Xu, M.; Radani, Y.; Yang, L. Technological Development and Application of Plant Genetic Transformation. Int. J. Mol. Sci. 2023, 24, 10646. [Google Scholar] [CrossRef]
- Wang, P.; Si, H.; Li, C.; Xu, Z.; Guo, H.; Jin, S.; Cheng, H. Plant genetic transformation, achievements, current status and future prospects. Plant Biotechnol. J. 2025, 23, 2034–2058. [Google Scholar] [CrossRef]
- Rahman, S.U.; Khan, M.O.; Ullah, R.; Ahmad, F.; Raza, G. Agrobacterium-Mediated Transformation for the Development of Transgenic Crops; Present and Future Prospects. Mol. Biotechnol. 2024, 66, 1836–1852. [Google Scholar] [CrossRef]
- Tiwari, M.; Mishra, A.K.; Chakrabarty, D. Agrobacterium-mediated gene transfer, recent advancements and layered immunity in plants. Planta 2022, 256, 37. [Google Scholar] [CrossRef]
- Schröpfer, S.; Lempe, J.; Emeriewen, O.F.; Flachowsky, H. Recent Developments and Strategies for the Application of Agrobacterium-Mediated Transformation of Apple Malus × domestica Borkh. Front. Plant Sci. 2022, 13, 928292. [Google Scholar] [CrossRef]
- Conti, G.; Xoconostle-Cázares, B.; Marcelino-Pérez, G.; Hopp, H.E.; Reyes, C.A. Citrus Genetic Transformation, An Overview of the Current Strategies and Insights on the New Emerging Technologies. Front. Plant Sci. 2021, 12, 768197. [Google Scholar] [CrossRef]
- Ren, C.; Lin, Y.; Liang, Z. CRISPR/Cas genome editing in grapevine, recent advances, challenges and future prospects. Fruit. Res. 2022, 2, 7. [Google Scholar] [CrossRef]
- Manders, G.; Otoni, W.C.; Vaz, F.B.D.; Blackhall, N.W.; Power, J.B.; Davey, M.R. Transformation of passionfruit (Passiflora edulis fv flavicarpa Degener.) using Agrobacterium tumefaciens. Plant Cell Rep. 1994, 13, 697–702. [Google Scholar] [CrossRef]
- Huang, D.; Wu, B.; Chen, G.; Xing, W.; Xu, Y.; Ma, F.; Li, H.; Hu, W.; Huang, H.; Yang, L.; et al. Genome-wide analysis of the passion fruit invertase gene family reveals involvement of PeCWINV5 in hexose accumulation. BMC Plant Biol. 2024, 24, 836. [Google Scholar] [CrossRef]
- Monteiro-Hara, A.C.; Jadao, A.S.; Mendes, B.M.J.; Rezende, J.A.; Trevisan, F.; Mello, A.P.O.; Vieira, M.L.C.; Meletti, L.M.M.; De Piedade, S.M. Genetic transformation of passionflower and evaluation of R1 and R2 generations for resistance to Cowpea aphid borne mosaic virus. Plant Dis. 2011, 95, 1021–1025. [Google Scholar] [CrossRef]
- Trevisan, F.; Mendes, B.M.J.; Maciel, S.C.; Vieira, M.L.C.; Meletti, L.; Rezende, J.A.M. Resistance to Passion fruit woodiness virus in transgenic passionflower expressing the virus coat protein gene. Plant Dis. 2006, 90, 1026–1030. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Méndez-Hernández, H.A.; Quintana-Escobar, A.O. The History of Agrobacterium Rhizogenes, From Pathogen to a Multitasking Platform for Biotechnology. Methods Mol. Biol. 2024, 2827, 51–69. [Google Scholar]
- Shi, M.; Liao, P.; Nile, S.H.; Georgiev, M.I.; Kai, G. Biotechnological Exploration of Transformed Root Culture for Value-Added Products. Trends Biotechnol. 2021, 39, 137–149. [Google Scholar] [CrossRef]
- Gantait, S.; Mukherjee, E. Hairy root culture technology, applications, constraints and prospect. Appl. Microbiol. Biotechnol. 2021, 105, 35–53. [Google Scholar] [CrossRef]
- Liu, L.; Qu, J.; Wang, C.; Liu, M.; Zhang, C.; Zhang, X.; Guo, C.; Wu, C.; Yang, G.; Huang, J.; et al. An efficient genetic transformation system mediated by Rhizobium rhizogenes in fruit trees based on the transgenic hairy root to shoot conversion. Plant Biotechnol. J. 2024, 22, 2093–2103. [Google Scholar] [CrossRef]
- Yan, H.; Ma, D.; Yi, P.; Sun, G.; Chen, X.; Yi, Y.; Huang, X. Highly efficient Agrobacterium rhizogenes-mediated transformation for functional analysis in woodland strawberry. Plant Methods 2023, 19, 99. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, D.; Fu, J.; Zhang, Z.; Qin, Y.; Hu, G.; Zhao, J. Agrobacterium rhizogenes-mediated hairy root transformation as an efficient system for gene function analysis in Litchi chinensis. Plant Methods 2021, 17, 103. [Google Scholar] [CrossRef]
- Fang, T.; Zheng, Y.; Ma, Q.; Ren, R.; Xian, H.; Zeng, L. Integrated Transcriptomic and Metabolomic Analysis Revealed Regulatory Mechanisms on Flavonoids Biosynthesis in the Skin of Passion Fruit (Passiflora spp.). J. Agric. Food Chem. 2025, 73, 967–978. [Google Scholar] [CrossRef]
- Grimplet, J. Genomic and Bioinformatic Resources for Perennial Fruit Species. Curr. Genom. 2022, 23, 217–233. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; He, R.; Chen, Q.; He, D.; Chen, X.; Li, Y.; Ren, R.; Yu, W.; Zeng, L. A 224-bp Indel in the Promoter of PeMYB114 Accounts for Anthocyanin Accumulation of Skin in Passion Fruit (Passiflora spp.). J. Agric. Food Chem. 2024, 72, 10138–10148. [Google Scholar] [CrossRef]
- Ren, R.; Chen, Y.; Yu, X.; Peng, X.; Zeng, L.; Fang, T. Identification and characterization of SWEET gene family in passion fruit reveals the involvement of PeSWEET3 in soluble sugar accumulation. Plant Physiol. Biochem. 2025, 224, 109943. [Google Scholar] [CrossRef]
- Yin, M.; Jiang, Y.H.; Wen, Y.J.; Shi, F.C.; Huang, H.; Yan, Q.; Liu, H.L. Establishment of an efficient Agrobacterium rhizogenes-mediated hairy root transformation method for subtropical fruit trees. Hortic. Plant J. 2025, 11, 1699–1702. [Google Scholar] [CrossRef]
- Hou, Y.; Wong, D.C.J.; Sun, X.; Li, Q.; Zhou, H.; Meng, L.; Liao, X.; Liang, Z.; Aryal, R.; Wang, Q.; et al. VvbHLH036, a basic helix-loop-helix transcription factor regulates the cold tolerance of grapevine. Plant Physiol. 2024, 196, 2871–2889. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Luo, R.F.; Wu, Y.X.; Zhang, T.T.; Yusuf, A.; Wang, N.; Li, M.; Duan, S. Establishment and application of high-pressure propagation breeding (HPPB)-mediated genetic transformation system in citrus rootstocks. Plant Biotechnol. J. 2025, 23, 2790–2792. [Google Scholar] [CrossRef]
- Otoni, W.C.; Soares, J.R.; Souza, C.S.; Silva, L.A.S.; Dias, L.L.L.; Robledo, K.J.M.; Paim-Pinto, D.L.; Koehler, A.D.; Sodrzeieski, P.A.; Fernandes, A.M.; et al. Advances in Tissue Culture and Transformation Studies in Non-model Species, Passiflora spp. (Passifloraceae). Methods Mol. Biol. 2024, 2827, 207–222. [Google Scholar]
- Rizwan, H.M.; Yang, Q.; Yousef, A.F.; Zhang, X.; Sharif, Y.; Kaijie, J.; Shi, M.; Li, H.; Munir, N.; Yang, X.; et al. Establishment of a Novel and Efficient Agrobacterium-Mediated in Planta Transformation System for Passion Fruit (Passiflora edulis). Plants 2021, 10, 2459. [Google Scholar] [CrossRef]
- Ajdanian, L.; Niazian, M.; Torkamaneh, D. Optimizing ex vitro one-step RUBY-equipped hairy root transformation in drug- and hemp-type Cannabis. Plant Biotechnol. J. 2024, 22, 1957–1959. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, Y.; Tan, L.; Li, Q.; Li, W.; Kafle, S.; Xu, M.; Kiselev, K.V.; Meng, L.; Xin, H. VaEIN3.1-VaERF057-VaFBA1 Module Positively Regulates Cold Tolerance by Accumulating Soluble Sugar in Grapevine. Plant Cell Environ. 2025, 48, 5429–5447. [Google Scholar] [CrossRef]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019, 37, 107405. [Google Scholar] [CrossRef]
- Meng, D.; Yang, Q.; Dong, B.; Song, Z.; Niu, L.; Wang, L.; Cao, H.; Li, H.; Fu, Y. Development of an efficient root transgenic system for pigeon pea and its application to other important economically plants. Plant Biotechnol. J. 2019, 17, 1804–1813. [Google Scholar] [CrossRef]
- Xie, X.T.; Huang, Q.Y.; Wen, G.C.; Yuan, H.W.; He, Y.; Yan, D.L.; Huang, J.Q.; Wang, X.F.; Zheng, B.S. Construction of Agrobacterium rhizogenes-mediated transformation system of Carya illinoinensis without dependence on tissue culture. J. Fruit Sci. 2022, 39, 131–140. [Google Scholar]
- Bhagat, P.; Verma, S.K.; Singh, A.K.; Aseri, G.K.; Khare, N. Evaluation of influence of different strains of Agrobacterium rhizogenes on efficiency of hairy root induction in Rauwolfia serpentina. Indian J. Genet. Pl. Br. 2019, 79, 760–764. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Li, C.; Ji, J.; Liu, T.; Duan, K. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene functional and gene editing analysis in soybean. Plant Methods 2021, 17, 73. [Google Scholar] [CrossRef]
- Yu, W.L.; Yang, L.; Xiang, Y.Y.; Li, R.D.; Zhou, X.Q.; Gan, L.C.; Xiang, X.Y.; Zhang, Y.Y.; Yuan, L.; Luo, Y.Q.; et al. Development of a rapid and efficient system for CR genes identification based on hairy root transformation in Brassicaceae. Hortic. Plant J. 2024, 10, 1049–1060. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Prasad, B.; Gururaj, H.; Ravishankar, G. Agrobacterium rhizogenes mediated genetic transformation resulting in hairy root formation is enhanced by ultrasonication and acetosyringone treatment. Electron. J. Biotechnol. 2006, 9, 349–357. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Luo, D.; Jia, J. Regeneration of plants from callus cultures of roots induced by Agrobacterium rhizogenes on Alhagi pseudoalhagi. Cell Res. 2001, 11, 279–284. [Google Scholar] [CrossRef]
- Niazian, M.; Belzile, F.; Curtin, S.; de Ronne, M.; Torkamaneh, D. Optimization of in vitro and ex vitro Agrobacterium rhizogenes-mediated hairy root transformation of soybean for visual screening of transformants using RUBY. Front. Plant Sci. 2023, 14, 1207762. [Google Scholar] [CrossRef]
- Yi, X.; Sun, X.; Tian, R.; Li, K.; Ni, M.; Zhang, Z.; Liu, J.; Wang, Q.; Ma, Q.; Li, J.; et al. Genome-wide characterization of the aquaporin gene family in radish and functional analysis of RsPIP2-6 involved in salt stress. Front. Plant Sci. 2022, 13, 86072. [Google Scholar] [CrossRef]
- Plasencia, A.; Soler, M.; Dupas, A.; Ladouce, N.; Silva-Martins, G.; Meilan, R.; Marron, N.; Jouanin, L. Eucalyptus hairy roots, a fast, efficient and versatile tool to explore function and expression of genes involved in wood formation. Plant Biotechnol. J. 2016, 14, 1381–1393. [Google Scholar] [CrossRef]
- Kai, G.; Xu, H.; Zhou, C.; Liao, P.; Xiao, J.; Luo, X.; You, L.; Zhang, L. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 2011, 13, 319–327. [Google Scholar] [CrossRef]
- Hao, X.; Pu, Z.; Cao, G.; You, D.; Zhou, Y.; Deng, C.; Shi, M.; Nile, S.H.; Wang, Y.; Zhou, W.; et al. Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Ma, H.; Liu, N.; Sun, X.; Zhu, M.; Mao, T.; Huang, S.; Meng, X.; Li, H.; Wang, M.; Liang, H. Establishment of an efficient transformation system and its application in regulatory mechanism analysis of biological macromolecules in tea plants. Int. J. Biol. Macromol. 2023, 244, 125372. [Google Scholar] [CrossRef]
- Ramasamy, M.; Dominguez, M.M.; Irigoyen, S.; Padilla, C.S.; Mandadi, K.K. Rhizobium rhizogenes-mediated hairy root induction and plant regeneration for bioengineering citrus. Plant Biotechnol. J. 2023, 21, 1728–1730. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Zheng, Y.; Wang, T.; Xiong, P.; Cui, K.; Zeng, L.; Fang, T. Establishment of an Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation System for Functional Analysis in Passion Fruit. Plants 2025, 14, 2312. https://doi.org/10.3390/plants14152312
Pan J, Zheng Y, Wang T, Xiong P, Cui K, Zeng L, Fang T. Establishment of an Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation System for Functional Analysis in Passion Fruit. Plants. 2025; 14(15):2312. https://doi.org/10.3390/plants14152312
Chicago/Turabian StylePan, Jiayi, Yiping Zheng, Tiancai Wang, Pengpeng Xiong, Kaibo Cui, Lihui Zeng, and Ting Fang. 2025. "Establishment of an Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation System for Functional Analysis in Passion Fruit" Plants 14, no. 15: 2312. https://doi.org/10.3390/plants14152312
APA StylePan, J., Zheng, Y., Wang, T., Xiong, P., Cui, K., Zeng, L., & Fang, T. (2025). Establishment of an Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation System for Functional Analysis in Passion Fruit. Plants, 14(15), 2312. https://doi.org/10.3390/plants14152312






