The Impact of Colony Deployment Timing on Tetragonula carbonaria Crop Fidelity and Resource Use in Macadamia Orchards
Abstract
1. Introduction
2. Results
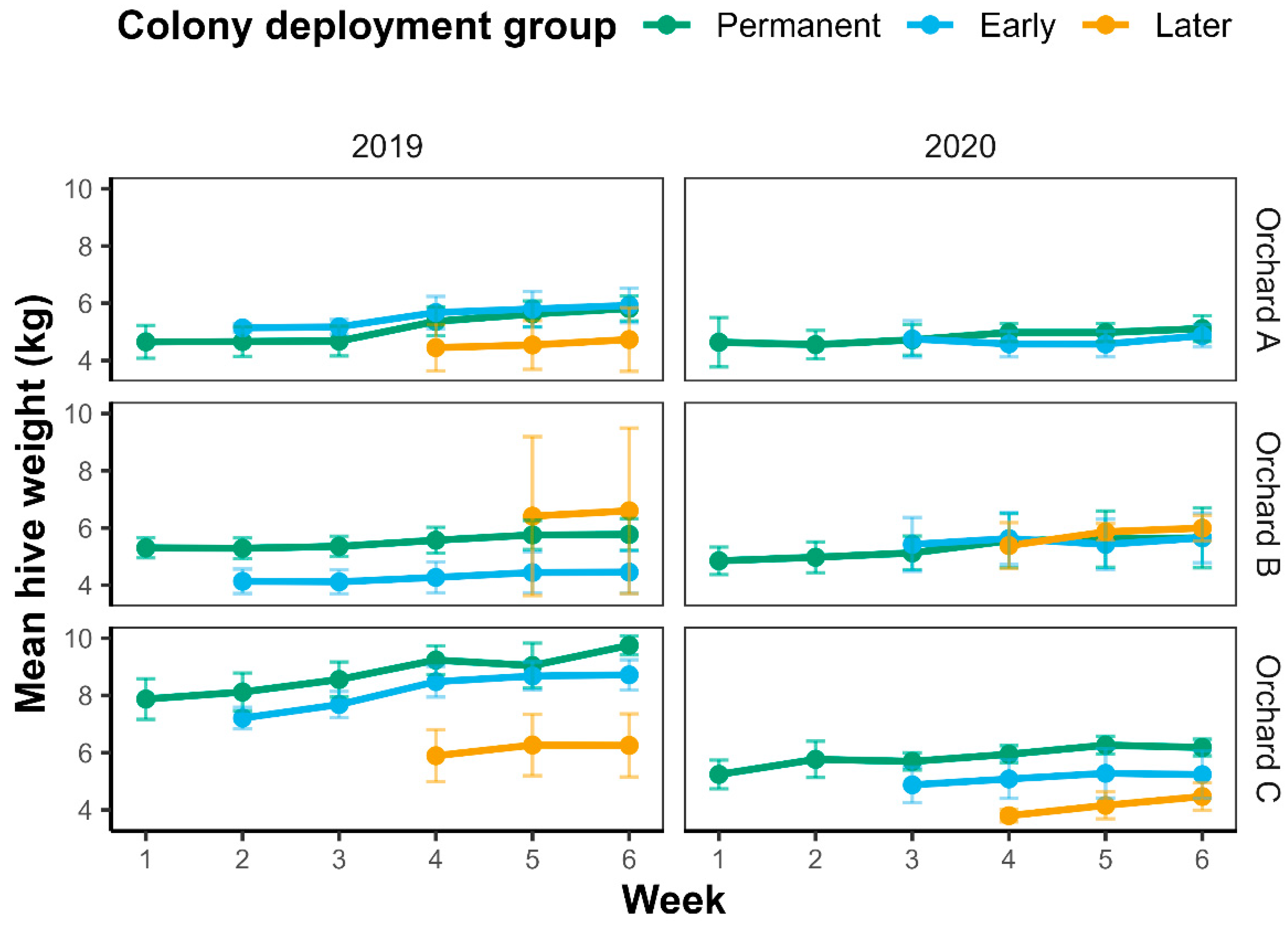
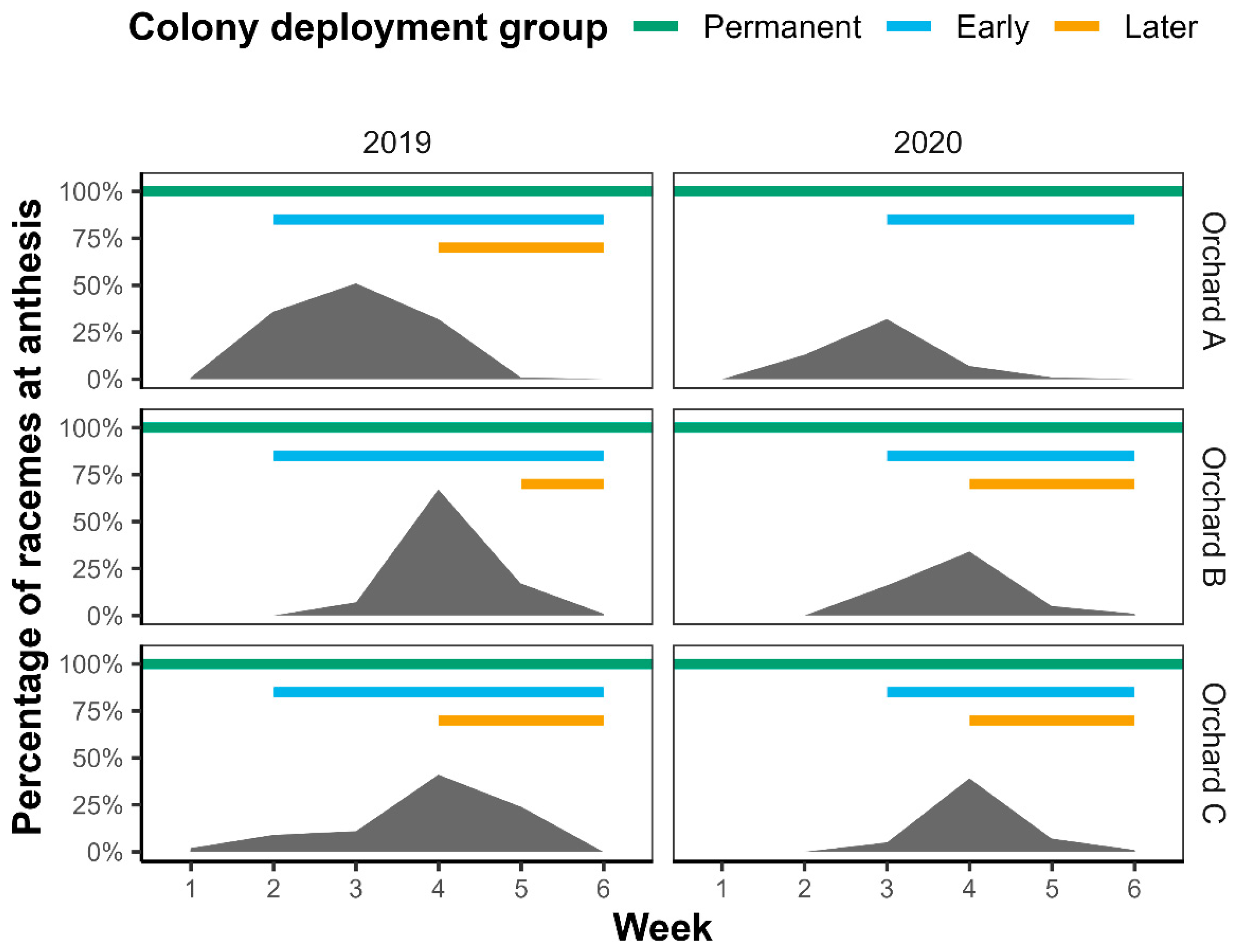
2.1. Macadamia Flowering Levels and Colony Introductions
2.2. Crop Fidelity
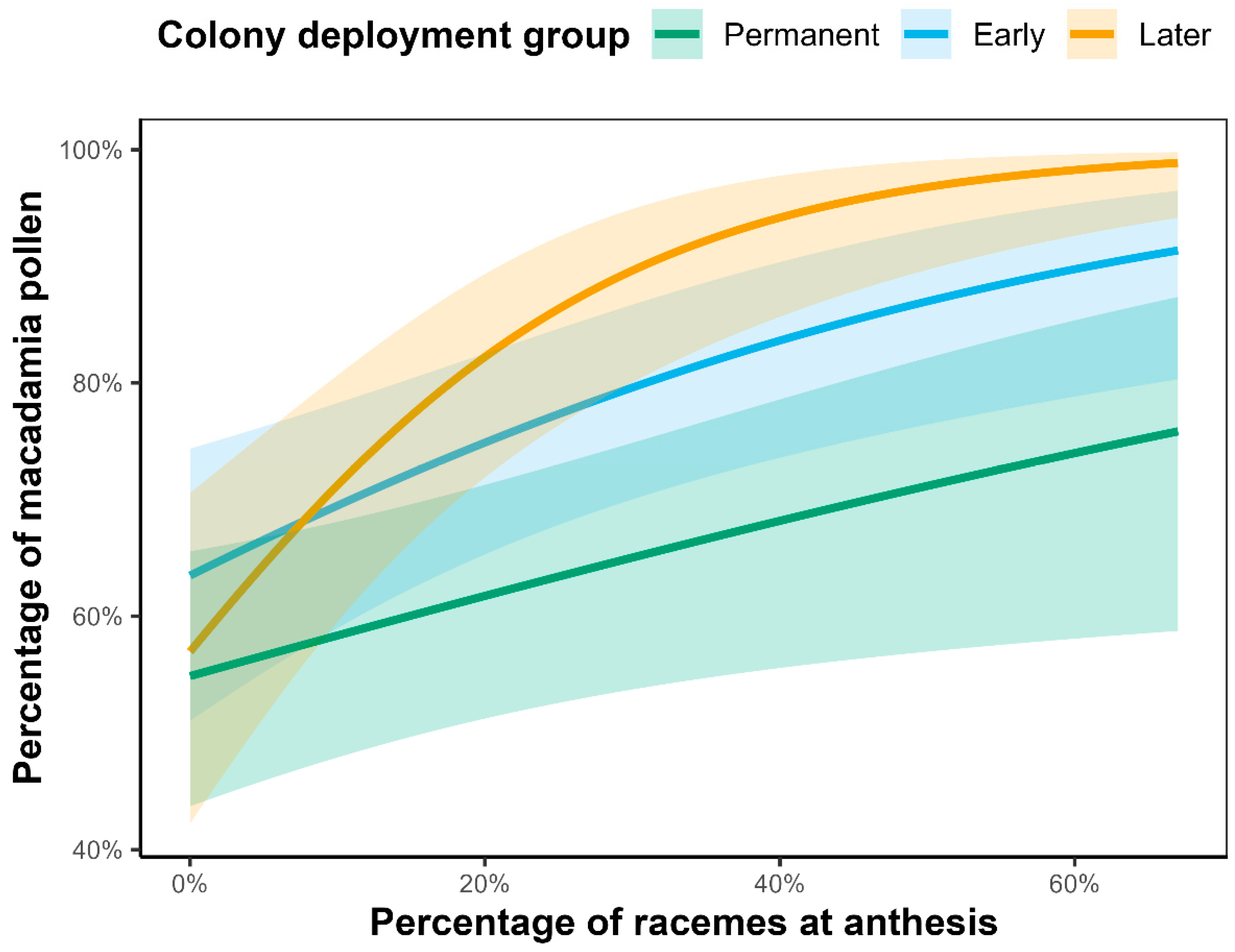
2.2.1. Factors Influencing Crop Fidelity
2.2.2. Weekly Differences in Crop Fidelity Between Colony Deployment Groups
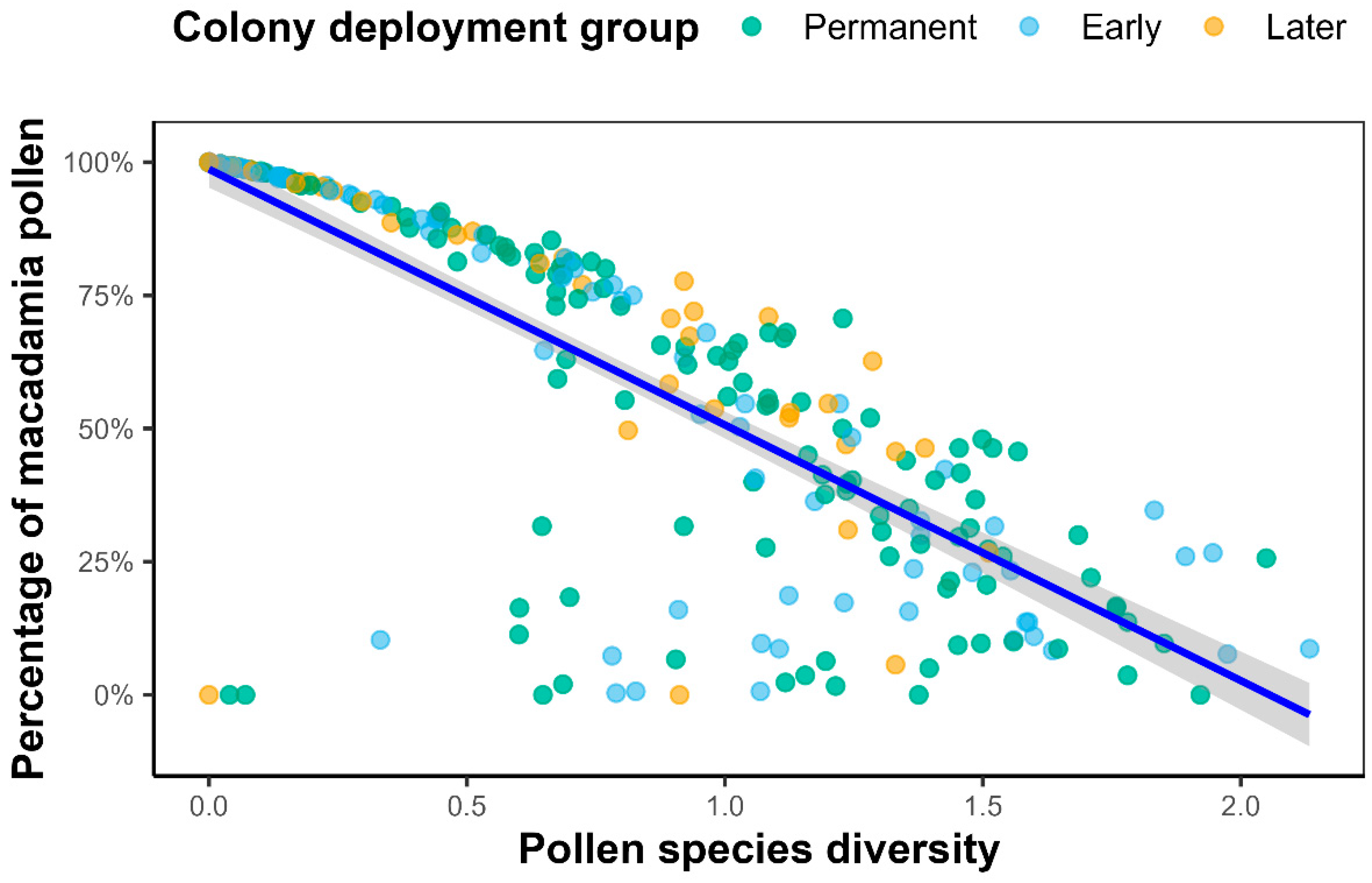
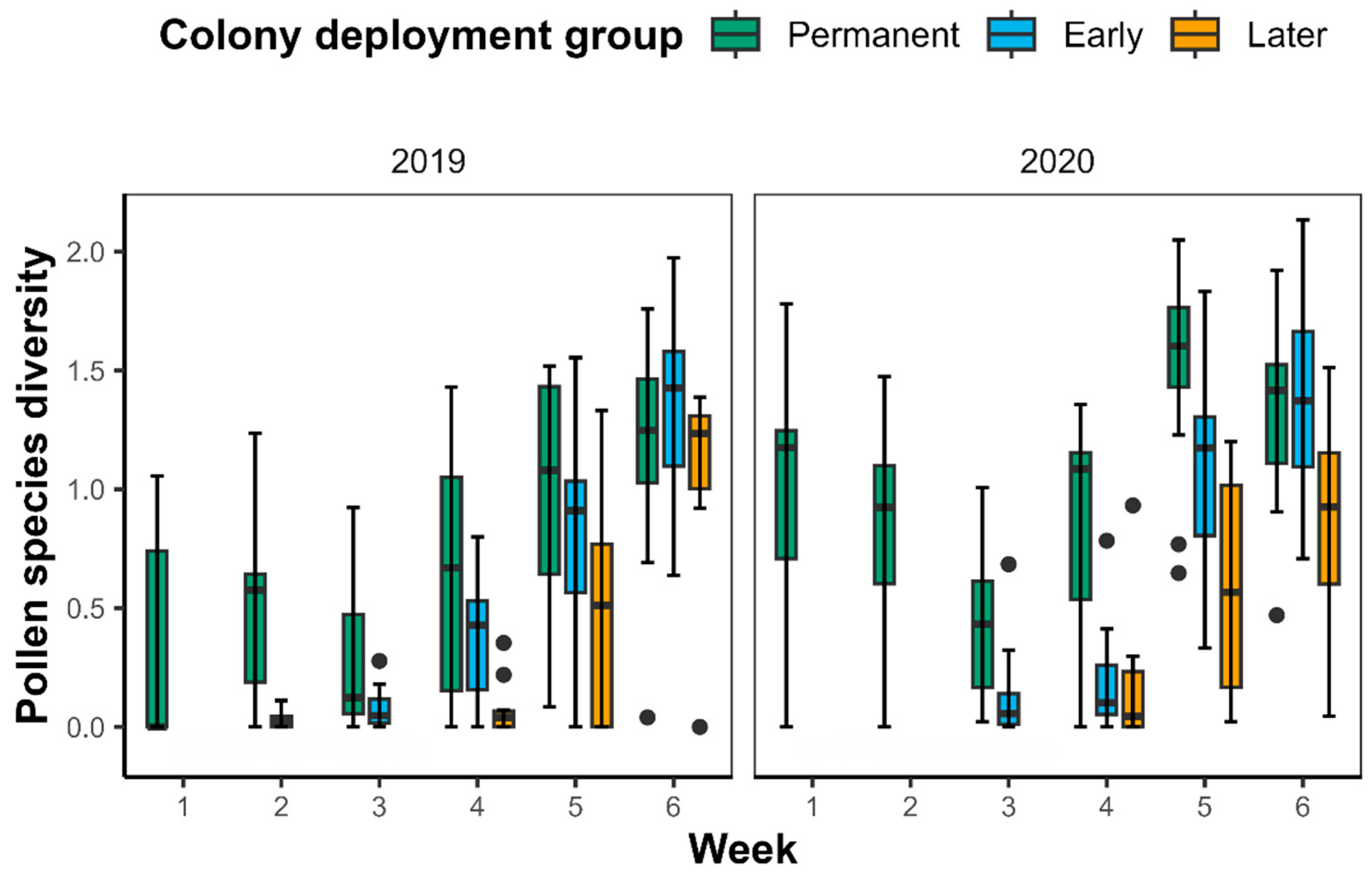
2.3. Pollen Diversity
3. Discussion
4. Materials and Methods
4.1. Experimental Set-Up
4.2. Monitoring Macadamia Flowering Levels
4.3. Pollen Sampling
4.4. Pollen Preparation and Counts
4.5. Recording Foraging Activity
4.6. Statistical Analyses
4.6.1. Factors Influencing Crop Fidelity
4.6.2. Weekly Comparisons of Crop Fidelity Between Colony Deployment Groups
4.6.3. Pollen Diversity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Week | Pairwise Comparison | Estimate | SE | Df * | z-Ratio | p |
|---|---|---|---|---|---|---|---|
| 2019 | 2 | Permanent–Early | −4.77 | 0.692 | Inf | −6.902 | <0.001 |
| 3 | Permanent–Early | −1.58 | 0.69 | Inf | −2.286 | 0.022 | |
| 4 | Permanent–Early | −0.901 | 0.760 | Inf | −1.186 | 0.416 | |
| Permanent–Later | −3.458 | 0.825 | Inf | −4.191 | <0.001 | ||
| Early–Later | −2.557 | 0.848 | Inf | −3.016 | 0.007 | ||
| 5 | Permanent–Early | −0.326 | 1.35 | Inf | −0.242 | 0.968 | |
| Permanent–Later | −2.259 | 1.37 | Inf | −1.644 | 0.227 | ||
| Early–Later | −1.933 | 1.38 | Inf | −1.401 | 0.340 | ||
| 6 | Permanent–Early | 0.393 | 0.773 | Inf | 0.509 | 0.867 | |
| Permanent–Later | −2.085 | 0.888 | Inf | −2.349 | 0.049 | ||
| Early–Later | −2.478 | 0.899 | Inf | −2.755 | 0.016 | ||
| 2020 | 3 | Permanent–Early | −3.84 | 1.08 | Inf | −3.557 | <0.001 |
| 4 | Permanent–Early | −2.69 | 0.788 | Inf | −3.415 | 0.002 | |
| Permanent–Later | −3.59 | 0.988 | Inf | −3.638 | <0.001 | ||
| Early–Later | −0.90 | 1.010 | Inf | −0.893 | 0.645 | ||
| 5 | Permanent–Early | −0.921 | 0.614 | Inf | −1.051 | 0.29 | |
| Permanent–Later | −3.671 | 0.700 | Inf | −5.244 | <0.001 | ||
| Early–Later | −2.750 | 0.718 | Inf | −3.829 | <0.001 | ||
| 6 | Permanent–Early | −0.897 | 0.842 | Inf | −1.065 | 0.536 | |
| Permanent–Later | −1.845 | 0.978 | Inf | −1.886 | 0.143 | ||
| Early–Later | −0.948 | 0.969 | Inf | −0.979 | 0.59 |
References
- Aizen, M.A.; Aguiar, S.; Biesmeijer, J.C.; Garibaldi, L.A.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Morales, C.L.; Ngo, H. Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob. Change Biol. 2019, 25, 3516–3527. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Aizen, M.A.; Klein, A.M.; Cunningham, S.A.; Harder, L.D. Global growth and stability of agricultural yield decrease with pollinator dependence. Proc. Natl. Acad. Sci. USA 2011, 108, 5909–5914. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Osterman, J.; Aizen, M.A.; Biesmeijer, J.C.; Bosch, J.; Howlett, B.G.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Pauw, A.; et al. Global trends in the number and diversity of managed pollinator species. Agric. Ecosyst. Environ. 2021, 322, 107653. [Google Scholar] [CrossRef]
- Isaacs, R.; Williams, N.; Ellis, J.; Pitts-Singer, T.L.; Bommarco, R.; Vaughan, M. Integrated crop pollination: Combining strategies to ensure stable and sustainable yields of pollination-dependent crops. Basic Appl. Ecol. 2017, 22, 44–60. [Google Scholar] [CrossRef]
- Aleixo, K.P.; Menezes, C.; Imperatriz Fonseca, V.L.; da Silva, C.I. Seasonal availability of floral resources and ambient temperature shape stingless bee foraging behavior (Scaptotrigona aff. depilis). Apidologie 2017, 48, 117–127. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Born, M.; Lukács, S.; Sommeijer, M.J. The response of the stingless bee Melipona beecheii to experimental pollen stress, worker loss and different levels of information input. J. Apic. Res. 1999, 38, 33–41. [Google Scholar] [CrossRef]
- Maia-Silva, C.; Hrncir, M.; da Silva, C.I.; Imperatriz-Fonseca, V.L. Survival strategies of stingless bees (Melipona subnitida) in an unpredictable environment, the Brazilian tropical dry forest. Apidologie 2015, 46, 631–643. [Google Scholar] [CrossRef]
- Kaluza, B.F.; Wallace, H.M.; Heard, T.A.; Minden, V.; Klein, A.; Leonhardt, S.D. Social bees are fitter in more biodiverse environments. Sci. Rep. 2018, 8, 12353. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef]
- Heard, T.A. The Australian Native Bee Book: Keeping Stingless Bee Hives for Pets, Pollination and Sugarbag Honey; Sugarbag Bees: Brisbane, Australia, 2016. [Google Scholar]
- Requier, F.; Odoux, J.-F.; Tamic, T.; Moreau, N.; Henry, M.; Decourtye, A.; Bretagnolle, V. Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecol. Appl. 2015, 25, 881–890. [Google Scholar] [CrossRef]
- Bretagnolle, V.; Gaba, S. Weeds for bees? A review. Agron. Sustain. Dev. 2015, 35, 891–909. [Google Scholar] [CrossRef]
- Osterman, J.; Theodorou, P.; Radzevičiūtė, R.; Schnitker, P.; Paxton, R.J. Apple pollination is ensured by wild bees when honey bees are drawn away from orchards by a mass co-flowering crop, oilseed rape. Agric. Ecosyst. Environ. 2021, 315, 107383. [Google Scholar] [CrossRef]
- Moeller, F.E. Timing of placement of colonies of honey bees for pollination of cranberries. J. Econ. Entomol. 1973, 66, 370–372. [Google Scholar] [CrossRef]
- Al-Tikrity, W.; Benton, A.; Risius, M.; Clarke, W., Jr. The effect of length of stay of a honeybee colony in a crownvetch field on its foraging behaviour. J. Apic. Res. 1972, 11, 51–57. [Google Scholar] [CrossRef]
- Ish-Am, G.; Eisikowitch, D. Low attractiveness of avocado (Persea americana Mill.) flowers to honeybees (Apis mellifera L.) limits fruit set in Israel. J. Hortic. Sci. Biotechnol. 1998, 73, 195–204. [Google Scholar] [CrossRef]
- Mayer, D.F. Sequential introduction of honey bee colonies for pear pollination. Acta Hortic. 1994, 367, 267–269. [Google Scholar] [CrossRef]
- Sapir, G.; Goldway, M.; Shafir, S.; Stern, R. Multiple introduction of honeybee colonies increases cross-pollination, fruit-set and yield of ‘Black Diamond’ Japanese plum (Prunus salicina Lindl.). J. Hortic. Sci. Biotechnol. 2007, 82, 590–596. [Google Scholar] [CrossRef]
- Stern, R.A.; Goldway, M.; Zisovich, A.H.; Shafir, S.; Dag, A. Sequential introduction of honeybee colonies increases cross-pollination, fruit-set and yield of ‘Spadona’ pear (Pyrus communis L.). J. Hortic. Sci. Biotechnol. 2004, 79, 652–658. [Google Scholar] [CrossRef]
- Free, J.; Free, N.W.; Jay, S.C. The effect on foraging behavior of moving honey bee colonies to crops before or after flowering has begun. J. Econ. Entomol. 1960, 53, 564–566. [Google Scholar] [CrossRef]
- Bartareau, T. Foraging behaviour of Trigona carbonaria (Hymenoptera: Apidae) at multiple-choice feeding stations. Aust. J. Zool. 1996, 44, 143–153. [Google Scholar] [CrossRef]
- White, D.; Cribb, B.W.; Heard, T.A. Flower constancy of the stingless bee Trigona carbonaria Smith (Hymenoptera: Apidae: Meliponini). Aust. J. Entomol. 2001, 40, 61–64. [Google Scholar] [CrossRef]
- Elliott, B.; Wilson, R.; Shapcott, A.; Keller, A.; Newis, R.; Cannizzaro, C.; Burwell, C.; Smith, T.; Leonhardt, S.D.; Kämper, W.; et al. Pollen diets and niche overlap of honey bees and native bees in protected areas. Basic Appl. Ecol. 2021, 50, 169–180. [Google Scholar] [CrossRef]
- Nieh, J.C. Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 2004, 35, 159–182. [Google Scholar] [CrossRef]
- Grüter, C. Stingless Bees: An overview. In Stingless Bees; Springer: Cham, Switzerland, 2020; pp. 17–24. [Google Scholar]
- Goodwin, M. Honey bee biology and behaviour. In Pollination of crops in Australia and New Zealand; RIRDC: Canberra, Australia, 2012; pp. 21–32. [Google Scholar]
- Smith, J.P.; Heard, T.A.; Beekman, M.; Gloag, R. Flight range of the Australian stingless bee Tetragonula carbonaria (Hymenoptera: Apidae). Aust. Entomol. 2017, 56, 50–53. [Google Scholar] [CrossRef]
- Evans, L.J.; Jesson, L.; Read, S.F.J.; Jochym, M.; Cutting, B.T.; Gayrard, T.; Jammes, M.A.S.; Roumier, R.; Howlett, B.G. Key factors influencing forager distribution across macadamia orchards differ among species of managed bees. Basic. Appl. Ecol. 2021, 53, 74–85. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef]
- Woyke, J.; Wilde, J.; Wilde, M. Flight activity reaction to temperature changes in Apis dorsata, Apis laboriosa and Apis mellifera. J. Apic. Sci. 2003, 47, 73–80. [Google Scholar]
- Heard, T.A.; Hendrikz, J.K. Factors influencing flight activity of colonies of the stingless bee Trigona carbonaria (Hymenoptera, Apidae). Aust. J. Zool. 1993, 41, 343–353. [Google Scholar] [CrossRef]
- Halcroft, M.T.; Spooner-Hart, R.; Haigh, A.M.; Heard, T.A.; Dollin, A. The Australian stingless bee industry: A follow-up survey, one decade on. J. Apic. Res. 2013, 52, 1–7. [Google Scholar] [CrossRef]
- Trueman, S.J. The reproductive biology of macadamia. Sci. Hortic. 2013, 150, 354–359. [Google Scholar] [CrossRef]
- Trueman, S.J.; Kämper, W.; Nichols, J.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; Hosseini Bai, S.; Wallace, H.M. Pollen limitation and xenia effects in a cultivated mass-flowering tree, Macadamia integrifolia (Proteaceae). Ann. Bot. 2022, 129, 135–146. [Google Scholar] [CrossRef]
- Wallace, H.M.; Vithanage, V.; Exley, E. The effect of supplementary pollination on nut set of Macadamia (Proteaceae). Ann. Bot. 1996, 78, 765–773. [Google Scholar] [CrossRef]
- Howlett, B.G.; Read, S.F.; Alavi, M.; Cutting, B.T.; Nelson, W.R.; Goodwin, R.M.; Cross, S.; Thorp, T.G.; Pattemore, D.E. Cross-pollination enhances macadamia yields, even with branch-level resource limitation. Hortscience 2019, 54, 609–615. [Google Scholar] [CrossRef]
- Eckhardt, M.; Haider, M.; Dorn, S.; Müller, A. Pollen mixing in pollen generalist solitary bees: A possible strategy to complement or mitigate unfavourable pollen properties? J. Anim. Ecol. 2014, 83, 588–597. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Wilson, R.S.; Keller, A.; Shapcott, A.; Leonhardt, S.D.; Sickel, W.; Hardwick, J.L.; Heard, T.A.; Kaluza, B.F.; Wallace, H.M. Many small rather than few large sources identified in long-term bee pollen diets in agroecosystems. Agric. Ecosyst. Environ. 2021, 310, 107296. [Google Scholar] [CrossRef]
- Leonhardt, S.; Heard, T.; Wallace, H. Differences in the resource intake of two sympatric Australian stingless bee species. Apidologie 2014, 45, 514–527. [Google Scholar] [CrossRef]
- Kaluza, B.F.; Wallace, H.; Keller, A.; Heard, T.A.; Jeffers, B.; Drescher, N.; Blüthgen, N.; Leonhardt, S.D. Generalist social bees maximize diversity intake in plant species-rich and resource-abundant environments. Ecosphere 2017, 8, e01758. [Google Scholar] [CrossRef]
- Urata, U. Pollination Requirements of Macadamia; Hawaii Agricultural Experiment Station: Honolulu, HI, USA, 1954; pp. 1–40. [Google Scholar]
- Heard, T.A.; Exley, E.M. Diversity, abundance, and distribution of insect visitors to macadamia flowers. Environ. Entomol. 1994, 23, 91–100. [Google Scholar] [CrossRef]
- Howlett, B.G.; Goodwin, M.; Read, S.; Evans, L.; Cutting, B.; Cross, S.; Pattemore, D. Optimising Pollination of Macadamia and Avocado in Australia; Horticulture Innovation Australia Limited: Sydney, NSW, Australia, 2017. [Google Scholar]
- Cook, S.M.; Awmack, C.S.; Murray, D.A.; Williams, I.H. Are honey bees’ foraging preferences affected by pollen amino acid composition? Ecol. Entomol. 2003, 28, 622–627. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Spaethe, J.; Leonhardt, S.D. Hungry for quality—Individual bumblebees forage flexibly to collect high-quality pollen. Behav. Ecol. Sociobiol. 2016, 70, 1209–1217. [Google Scholar] [CrossRef]
- Trinkl, M.; Kaluza, B.F.; Wallace, H.; Heard, T.A.; Keller, A.; Leonhardt, S.D. Floral species richness correlates with changes in the nutritional quality of larval diets in a stingless bee. Insects 2020, 11, 125. [Google Scholar] [CrossRef]
- Aleixo, K.P.; Faria, L.B.d.; Garófalo, C.A.; Imperatriz Fonseca, V.L.; Silva, C.I.d. Pollen collected and foraging activities of Frieseomelitta varia (Lepeletier) (Hymenoptera: Apidae) in an urban landscape. Sociobiology 2013, 60, 266–276. [Google Scholar] [CrossRef]
- Eltz, T.; Brühl, C.A.; van der Kaars, S.; Chey, V.K.; Linsenmair, K.E. Pollen foraging and resource partitioning of stingless bees in relation to flowering dynamics in a Southeast Asian tropical rainforest. Insectes Soc. 2001, 48, 273–279. [Google Scholar] [CrossRef]
- Biesmeijer, J.; Slaa, J. Information flow and organization of stingless bee foraging. Apidologie 2004, 35, 143–157. [Google Scholar] [CrossRef]
- Heard, T.A. Behaviour and pollinator efficiency of stingless bees and honey bees on macadamia flowers. J. Apic. Res. 1994, 33, 191–198. [Google Scholar] [CrossRef]
- Goodwin, M. Crops. In Pollination of Crops in Australia and New Zealand; RIRDC: Canberra, Australia, 2012; pp. 67–99. [Google Scholar]
- Boyton, S.J.; Hardner, C.M. Phenology of flowering and nut production in macadamia. Acta Hortic. 2002, 575, 381–387. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Dag, A.; Danka, R.; Freitas, B.; Garibaldi, L.; Goodwin, R.; Hormaza, J. Standard methods for pollination research with Apis mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Gilpin, A.-M.; O’Brien, C.; Kobel, C.; Brettell, L.E.; Cook, J.M.; Power, S.A. Co-flowering plants support diverse pollinator populations and facilitate pollinator visitation to sweet cherry crops. Basic Appl. Ecol. 2022, 63, 36–48. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Veldtman, R.; Shenkute, A.G.; Tesfay, G.B.; Pirk, C.W.W.; Donaldson, J.S.; Nicolson, S.W. Natural and within-farmland biodiversity enhances crop productivity. Ecol. Lett. 2011, 14, 251–259. [Google Scholar] [CrossRef]
- Heard, T.A. Propogation of hives of Trigona carbonaria Smith (Hymenoptera: Apidae). Aust. J. Entomol. 1988, 27, 303–304. [Google Scholar] [CrossRef]
- Jones, G.D. Pollen analyses for pollination research, acetolysis. J. Pollinat. Ecol. 2014, 13, 203–217. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 22 January 2025).
- Harti, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 22 January 2025).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kind, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 22 January 2025).

| Pairwise Comparison | Estimate | SE | Df * | z-Ratio | p |
|---|---|---|---|---|---|
| Permanent–Early | −0.541 | 0.189 | Inf | −2.863 | 0.012 |
| Permanent–Late | −0.779 | 0.237 | Inf | −3.291 | 0.003 |
| Early–Late | −0.238 | 0.252 | Inf | −0.947 | 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allison, C.E.; Makinson, J.C.; Spooner-Hart, R.N.; Cook, J.M. The Impact of Colony Deployment Timing on Tetragonula carbonaria Crop Fidelity and Resource Use in Macadamia Orchards. Plants 2025, 14, 2313. https://doi.org/10.3390/plants14152313
Allison CE, Makinson JC, Spooner-Hart RN, Cook JM. The Impact of Colony Deployment Timing on Tetragonula carbonaria Crop Fidelity and Resource Use in Macadamia Orchards. Plants. 2025; 14(15):2313. https://doi.org/10.3390/plants14152313
Chicago/Turabian StyleAllison, Claire E., James C. Makinson, Robert N. Spooner-Hart, and James M. Cook. 2025. "The Impact of Colony Deployment Timing on Tetragonula carbonaria Crop Fidelity and Resource Use in Macadamia Orchards" Plants 14, no. 15: 2313. https://doi.org/10.3390/plants14152313
APA StyleAllison, C. E., Makinson, J. C., Spooner-Hart, R. N., & Cook, J. M. (2025). The Impact of Colony Deployment Timing on Tetragonula carbonaria Crop Fidelity and Resource Use in Macadamia Orchards. Plants, 14(15), 2313. https://doi.org/10.3390/plants14152313







