Phytochemical Analysis and Therapeutic Potential of Tuberaria lignosa (Sweet) Samp. Aqueous Extract in Skin Injuries
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characterization of Tuberaria lignosa (Sweet.) Samp. Aqueous Leaf Extract
2.1.1. Extraction Yield
2.1.2. Quantification of Phenolic Constituents Through Colorimetric Assays
2.1.3. UHPLC-HRMS/MS and HPLC-DAD Phenolic Profile Analysis
2.2. Evaluation of In Vitro Antioxidant Capacity of T. lignosa Aqueous Leaf Extract
2.2.1. DPPH• and ABTS•+ Free-Radical Scavenging Capacity
2.2.2. Reactive Oxygen and Nitrogen Species Scavenging Capacity
2.2.3. Transition Metal Chelating Capacity
2.2.4. Xanthine Oxidase Inhibition Capacity
2.3. Evaluation of Skin-Related Enzyme Inhibitory Activity of T. lignosa Aqueous Leaf Extract
2.4. Evaluation of T. lignosa Aqueous Leaf Extract on Cell Viability in NIH/3T3 Fibroblast and HaCaT Cells
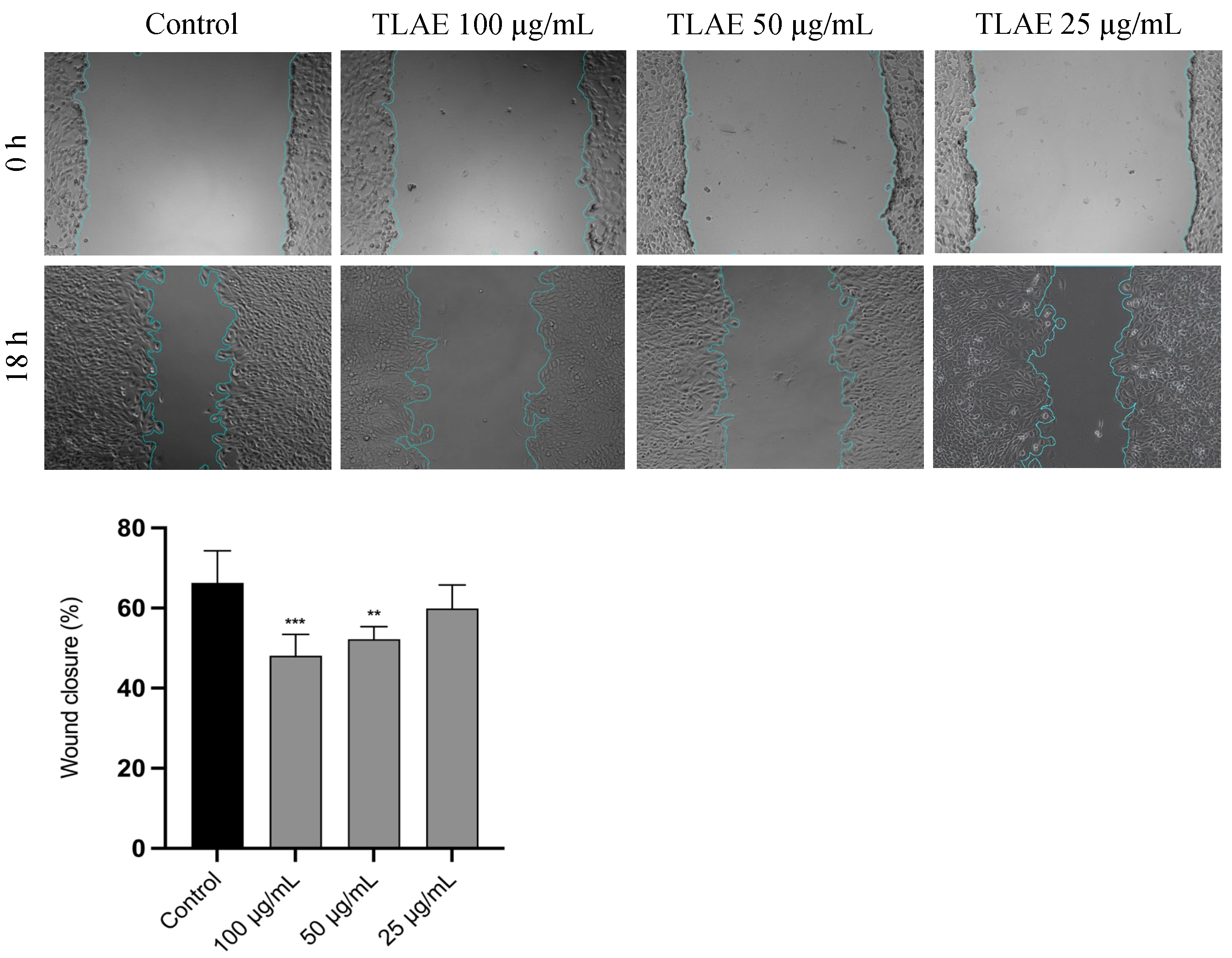
2.5. Evaluation of T. lignosa Aqueous Leaf Extract on Cell Migration in NIH/3T3 Fibroblast
2.6. Evaluation of the Antifungal Activity of T. lignosa Aqueous Leaf Extract
2.7. Evaluation of the Antibiofilm Activity Against Epidermophyton floccosum FF9 Biofilms of T. lignosa Aqueous Leaf Extract
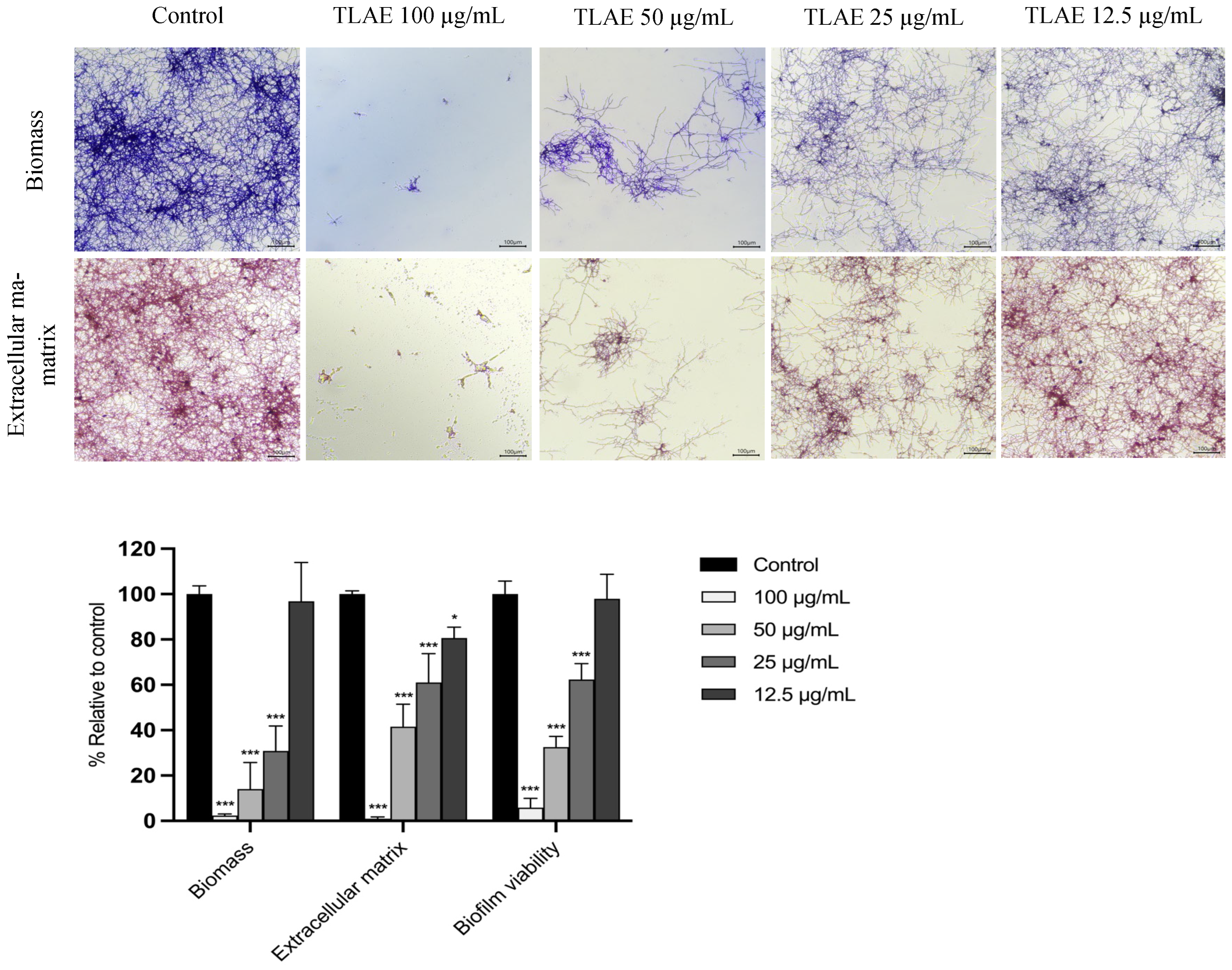
2.7.1. Effect on the Formation of E. floccosum Biofilms
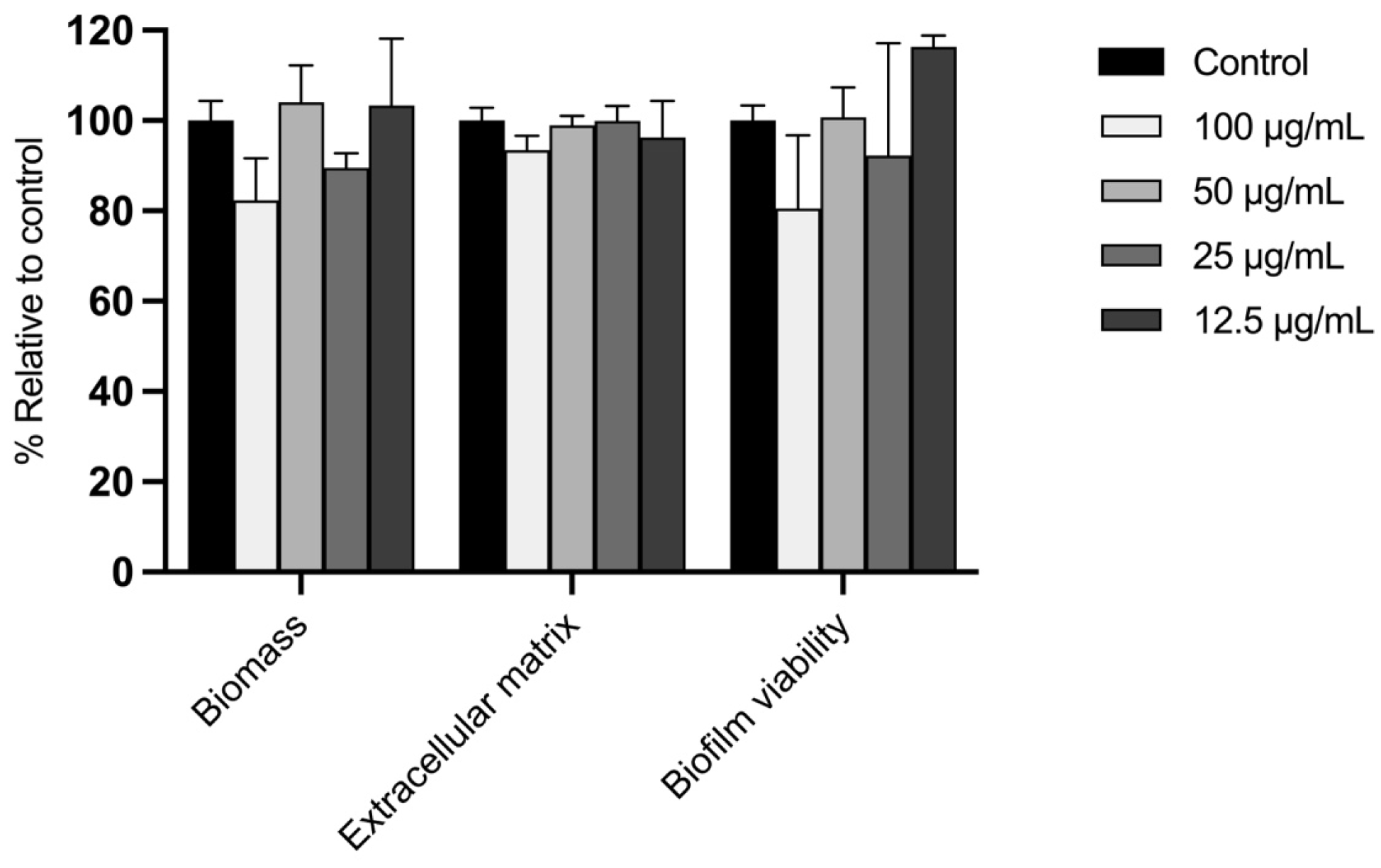
2.7.2. Effect on the Disruption of E. floccosum Mature Biofilms
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Equipment
4.2. Plant Material
4.3. Extraction Procedure
4.4. Phytochemical Characterization
4.4.1. Total Phenolic Content
4.4.2. Total Flavonoid Content
4.4.3. Total Hydroxycinnamic Acids Content
4.4.4. Total Proanthocyanidin Content
4.4.5. Total Tannin Content
4.4.6. UHPLC-HRMS/MS and HPLC-DAD Phenolic Profile Analysis
4.5. Evaluation of In Vitro Antioxidant Capacity
4.5.1. DPPH• Free-Radical Scavenging Assay
4.5.2. ABTS •+ Free-Radical Scavenging Assay
4.5.3. H2O2 Scavenging Assay
4.5.4. OH• Free-Radical Scavenging Assay
4.5.5. O2−• Free-Radical Scavenging Assay
4.5.6. •NO Free-Radical Scavenging Assay
4.5.7. Xanthine Oxidase Inhibition Assay
4.5.8. Fe2+ Chelation Assay
4.6. Evaluation of Skin-Related Enzyme Inhibitory Activity
4.6.1. Tyrosinase Inhibitory Activity
4.6.2. Elastase Inhibitory Activity
4.6.3. Collagenase Inhibitory Activity
4.6.4. Hyaluronidase Inhibitory Activity
4.7. Cell Culture
4.8. Cell Viability
4.9. Cell Migration
4.10. Antifungal Activity
4.11. Antibiofilm Activity Against Epidermophyton floccosum FF9 Biofilms
4.11.1. Effect on Biofilm Formation
4.11.2. Effect Towards Mature Biofilms
4.11.3. Biofilm Mass Quantification
4.11.4. Biofilm Extracellular Matrix Quantification
4.11.5. Biofilm Metabolic Activity Evaluation
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLAE | Tuberaria lignosa (Sweet) Samp. leaf aqueous extract |
| ECM | Extracellular matrix |
| GAE | Gallic acid equivalents |
| RE | Rutin equivalents |
| CAE | Caffeic acid equivalents |
| CE | Catechin equivalents |
| TRP | Tannin-related phenolics |
| UHPLC-HRMS/MS | Ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry |
| HPLC-DAD | High-performance liquid chromatography with diode array detection |
| IC50 | Inhibitory concentration 50 |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| EDTA | Ethylenediaminetetraacetic acid |
| XO | Xanthine oxidase |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide |
| XTT | 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide |
| MIC | Minimum inhibitory concentration |
| MLC | Minimum lethal concentration |
References
- Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; Lopez, J.A.M.; Barrera Lopez, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.L.; Prescott, T.A.K.; Mas-Claret, E.; Steadman, R.; Moseley, R.; Sloan, A.J. Evidence for Natural Products as Alternative Wound-Healing Therapies. Biomolecules 2023, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Lordani, T.V.A.; de Lara, C.E.; Ferreira, F.B.P.; de Souza Terron Monich, M.; Mesquita da Silva, C.; Felicetti Lordani, C.R.; Giacomini Bueno, F.; Vieira Teixeira, J.J.; Lonardoni, M.V.C. Therapeutic Effects of Medicinal Plants on Cutaneous Wound Healing in Humans: A Systematic Review. Mediat. Inflamm. 2018, 2018, 7354250. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound dressings as growth factor delivery platforms for chronic wound healing. Expert Opin. Drug Deliv. 2021, 18, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Mazumder, A. A critical overview of challenging roles of medicinal plants in improvement of wound healing technology. Daru 2024, 32, 379–419. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Atkin, L.; Dissemond, J.; Hurlow, J.; Tan, Y.K.; Apelqvist, J.; James, G.; Salles, N.; Wu, J.; Tachi, M.; et al. Defying hard-to-heal wounds with an early antibiofilm intervention strategy: ‘Wound hygiene’. J. Wound Care 2019, 28, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. mBio 2016, 7, e01058-16. [Google Scholar] [CrossRef] [PubMed]
- Short, B.; Bakri, A.; Baz, A.; Williams, C.; Brown, J.; Ramage, G. There Is More to Wounds than Bacteria: Fungal Biofilms in Chronic Wounds. Curr. Clin. Microbiol. Rep. 2023, 10, 9–16. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q. Current research on fungi in chronic wounds. Front. Mol. Biosci. 2022, 9, 1057766. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, C.; Zhang, Y.; Li, R. Invasive dermatophyte infection: A systematic review. Mycoses 2021, 64, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Barac, A.; Stjepanovic, M.; Krajisnik, S.; Stevanovic, G.; Paglietti, B.; Milosevic, B. Dermatophytes: Update on Clinical Epidemiology and Treatment. Mycopathologia 2024, 189, 101. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Tsuboi, R.; Iozumi, K.; Ishizaki, S.; Ushigami, T.; Ogawa, Y.; Kaneko, T.; Kawai, M.; Kitami, Y.; Kusuhara, M.; et al. Guidelines for the management of dermatomycosis (2019). J. Dermatol. 2020, 47, 1343–1373. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1-50. [Google Scholar] [CrossRef] [PubMed]
- Verdu-Soriano, J.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Caballero-Villarraso, J.; Moreno-Moreno, P.; Casado-Diaz, A.; Berenguer-Perez, M.; Guler-Caamano, I.; Laosa-Zafra, O.; et al. Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial. J. Clin. Med. 2022, 11, 1260. [Google Scholar] [CrossRef] [PubMed]
- Castroviejo, S.C. Flora Ibérica. Plantas Vasculares de la Península Ibérica e Islas Baleares; Real Jardín Botánico, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2005; Volume III. [Google Scholar]
- Gonzalez-Tejero, M.R.; Casares-Porcel, M.; Sanchez-Rojas, C.P.; Ramiro-Gutierrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M. Plantas y Sabiduría Popular del Parque Natural de Montesinho. un Estudio Etnobotánico en Portugal; Biblioteca de Ciencias n° 35; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2010. [Google Scholar]
- Martín-Aragón, S.; Benedí, J.; Villar, A. Studies on the anti-inflammatory and antiulcerogenic activities of Tuberaria lignosa extract in experimental animals. Int. J. Pharmacogn. 1994, 32, 27–32. [Google Scholar] [CrossRef]
- Bedoya, L.M.; Abad, M.J.; Sanchez-Palomino, S.; Alcami, J.; Bermejo, P. Ellagitannins from Tuberaria lignosa as entry inhibitors of HIV. Phytomedicine 2010, 17, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Barros, L.; Duenas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: Effects of drying and oral preparation methods. Food Chem. 2012, 135, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Lopes-Rodrigues, V.; Xavier, C.P.; Lima, M.J.; Lima, R.T.; Ferreira, I.C.; Vasconcelos, M.H. An Aqueous Extract of Tuberaria lignosa Inhibits Cell Growth, Alters the Cell Cycle Profile, and Induces Apoptosis of NCI-H460 Tumor Cells. Molecules 2016, 21, 595. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Castro, L.A.; Alvarez, M.I. Nail dermatophytoma in HIV-infected patients in Cali, Colombia. J. Mycol. Med. 2021, 31, 101172. [Google Scholar] [CrossRef] [PubMed]
- Nokdhes, Y.N.; Leeyaphan, C.; Jirawattanadon, P.; Pongkittilar, B.; Sereeaphinan, C.; Bunyaratavej, S. Prevalence and characteristics of Epidermophyton floccosum skin infections: A 12-year retrospective study. Mycoses 2024, 67, e13702. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Dong, M.; Niu, Y. Lead toxicity and potential therapeutic effect of plant-derived polyphenols. Phytomedicine 2023, 114, 154789. [Google Scholar] [CrossRef] [PubMed]
- Kerbab, K.; Sansone, F.; Zaiter, L.; Esposito, T.; Celano, R.; Franceschelli, S.; Pecoraro, M.; Benayache, F.; Rastrelli, L.; Picerno, P.; et al. Halimium halimifolium: From the Chemical and Functional Characterization to a Nutraceutical Ingredient Design. Planta Med. 2019, 85, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Starzec, A.; Wlodarczyk, M.; Kunachowicz, D.; Drys, A.; Kepinska, M.; Fecka, I. Polyphenol Profile of Cistus x incanus L. and Its Relevance to Antioxidant Effect and alpha-Glucosidase Inhibition. Antioxidants 2023, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus sp.: Phytochemical and Antimicrobial Activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Moraga, A.; Argandona, J.; Mota, B.; Perez, J.; Verde, A.; Fajardo, J.; Gomez-Navarro, J.; Castillo-Lopez, R.; Ahrazem, O.; Gomez-Gomez, L. Screening for polyphenols, antioxidant and antimicrobial activitiesof extracts from eleven Helianthemum taxa (Cistaceae) used in folk medicine in south-eastern Spain. J. Ethnopharmacol. 2013, 148, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (Cistaceae) Compared to Six Further, Partly Sympatric Cistus Species. Plants 2021, 10, 615. [Google Scholar] [CrossRef]
- Gallego, M.J.; Aparicio, A. Karyological study in the genusTuberaria sect.Scorpioides (Cistaceae): Taxonomic and evolutionary inferences. Plant Syst. Evol. 1993, 184, 11–25. [Google Scholar] [CrossRef]
- Osório, M.L.; Osório, J.; Romano, A. Photosynthesis, energy partitioning, and metabolic adjustments of the endangered Cistaceae species Tuberaria major under high temperature and drought. Photosynthetica 2013, 51, 75–84. [Google Scholar] [CrossRef]
- Shabir, I.; Dar, A.H.; Dash, K.K.; Manzoor, S.; Srivastava, S.; Pandey, V.K.; Shams, R.; Bashir, I.; Khan, S.A.; Mukarram, S.A.; et al. Bioactive potential of punicalagin: A comprehensive review. Appl. Food Res. 2024, 4, 100572. [Google Scholar] [CrossRef]
- Lin, J.; Ran, H.; Feng, Q.; Shen, Q.; Zhou, S.; Sun, Y.; Hou, D. Unveiling the differences between vitexin and isovitexin: From the perspective of sources, green advanced extraction technologies, biological activities, and safety. Food Chem. 2025, 485, 144600. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; Lopez-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants: The basics—What they are and how to evaluate them. Adv. Pharmacol. 1997, 38, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Attaguile, G.; Russo, A.; Campisi, A.; Savoca, F.; Acquaviva, R.; Ragusa, N.; Vanella, A. Antioxidant activity and protective effect on DNA cleavage of extracts from Cistus incanus L. and Cistus monspeliensis L. Cell Biol. Toxicol. 2000, 16, 83–90. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.C.; McEnaney, R.M.; Shukla, A.J.; Hong, G.; Kelley, E.E.; Tarpey, M.M.; Gladwin, M.; Zuckerbraun, B.S.; Tzeng, E. Xanthine Oxidoreductase Function Contributes to Normal Wound Healing. Mol. Med. 2015, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Mahal, H.S.; Kapoor, S.; Aradhya, S.M. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J. Agric. Food Chem. 2007, 55, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Hormesis: Wound healing and keratinocytes. Pharmacol. Res. 2022, 183, 106393. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Hormesis: Wound healing and fibroblasts. Pharmacol. Res. 2022, 184, 106449. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, S.; Mandim, F.; Taofiq, O.; Pires, T.; Finimundy, T.C.; Prieto, M.A.; Barros, L. Valorization of Punica granatum L. Leaves Extracts as a Source of Bioactive Molecules. Pharmaceuticals 2023, 16, 342. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, K.; Zeng, S.; Liu, W.; Cui, T.; Chen, Z.; Lin, L.; Chen, D.; Ouyang, H. Punicalagin Inhibited Inflammation and Migration of Fibroblast-Like Synoviocytes Through NF-kappaB Pathway in the Experimental Study of Rheumatoid Arthritis. J. Inflamm. Res. 2021, 14, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Celiksoy, V.; Moses, R.L.; Sloan, A.J.; Moseley, R.; Heard, C.M. Evaluation of the In Vitro Oral Wound Healing Effects of Pomegranate (Punica granatum) Rind Extract and Punicalagin, in Combination with Zn (II). Biomolecules 2020, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Illescas-Montes, R.; Rueda-Fernandez, M.; Gonzalez-Acedo, A.; Melguizo-Rodriguez, L.; Garcia-Recio, E.; Ramos-Torrecillas, J.; Garcia-Martinez, O. Effect of Punicalagin and Ellagic Acid on Human Fibroblasts In Vitro: A Preliminary Evaluation of Their Therapeutic Potential. Nutrients 2023, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Al-Naqeb, G.; Zorzi, G.; Oldani, A.; Azzalin, A.; Avesani, L.; Guzzo, F.; Pascale, A.; De Giuseppe, R.; Cena, H. Phytochemical Profile and In Vitro Cytotoxic, Genotoxic, and Antigenotoxic Evaluation of Cistus monspeliensis L. Leaf Extract. Int. J. Mol. Sci. 2024, 25, 13707. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Martinez, F.J.; Rodriguez, J.C.; Borras-Rocher, F.; Barrajon-Catalan, E.; Micol, V. The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their polyphenolic composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Miled, K.; Boubaker, S.; Bellasfar, Z.; Abedrabba, M.; Iwaski, H.; Oku, H.; Matsui, T.; Limam, F.; Hamdi, M. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine 2011, 18, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Caprifico, A.E.; Calabrese, G.; Tornese, R.; Montefusco, A.; Placi, R.; Semeraro, T.; Durante, M.; De Caroli, M.; Lenucci, M.S. Pomegranate Extracts as Dual Regulators of Angiogenesis: A Systematic Review of Preclinical Evidence in Cancer and Chronic Wound Healing. Mol. Nutr. Food Res. 2025, 69, e70060. [Google Scholar] [CrossRef] [PubMed]
- Che Zain, M.S.; Lee, S.Y.; Sarian, M.N.; Fakurazi, S.; Shaari, K. In Vitro Wound Healing Potential of Flavonoid C-Glycosides from Oil Palm (Elaeis guineensis Jacq.) Leaves on 3T3 Fibroblast Cells. Antioxidants 2020, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodriguez, S.; Folgueras, A.R.; Lopez-Otin, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Gallego, J.S.; Pedroza-Escobar, D.; Caicedo-Ortega, A.R.; Berumen-Murra, M.T.; Novelo-Aguirre, A.L.; de Sotelo-Leon, R.D.; Delgadillo-Guzman, D. Human neutrophil elastase inhibitors: Classification, biological-synthetic sources and their relevance in related diseases. Fundam. Clin. Pharmacol. 2024, 38, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [PubMed]
- Gawel-Beben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M. Characterization of Cistus x incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Emir, A.; Nilofar, N.; Emir, C.; Coban, G.; Yildiztugay, E.; Zengin, G. Helianthemum oelandicum subsp. incanum and Fumana thymifolia: Characterization of LC-ESI-QTOF-MS profiles and their biological activities based on plant parts and extraction solvents. Kuwait J. Sci. 2025, 52, 100378. [Google Scholar] [CrossRef]
- Taherkhani, A.; Moradkhani, S.; Orangi, A.; Jalalvand, A.; Khamverdi, Z. Molecular docking study of flavonoid compounds for possible matrix metalloproteinase-13 inhibition. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chai, W.M.; Ma, Z.Y.; Deng, W.L.; Wei, Q.M.; Song, S.; Zou, Z.R.; Peng, Y.Y. Antityrosinase mechanism of ellagic acid in vitro and its effect on mouse melanoma cells. J. Food Biochem. 2019, 43, e12996. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Hao, P.C.; Peng, L.Y.; Peng, M.J.; Li, W.Y.; Zhang, S.Y.; Zhao, Q.S. Ellagitannins from Pomegranate Flower with Whitening and Anti-Skin Photoaging Effect. Chem. Biodivers. 2025, 22, e202403292. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Mushroom tyrosinase inhibitors from mung bean (Vigna radiatae L.) extracts. Int. J. Food Sci. Nutr. 2012, 63, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.H.; Mosher, M.L.; Jarquin, P.; Smith, P.; Chironis, A. Effects of short-duration treatment of cartilage with punicalagin and genipin and the implications for treatment of osteoarthritis. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentao, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.S.; Carollo, C.A.; de Magalhães, J.C.; Palumbo, J.M.C.; Boaretto, A.G.; Nunes e Sá, I.C.; Ferraz, A.C.; Lima, W.G.; de Siqueira, J.M.; Ferreira, J.M.S. Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. S. Afr. J. Bot. 2018, 114, 181–187. [Google Scholar] [CrossRef]
- Jin, Y.S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef] [PubMed]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.; Endo, E.H.; Dias Filho, B.P. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Lin, Y.; Wang, C.; Niu, B.; Xu, Y.; Zhao, G.; Zhao, J. Chemical Profile, Antimicrobial and Antioxidant Activity Assessment of the Crude Extract and Its Main Flavonoids from Tartary Buckwheat Sprouts. Molecules 2022, 27, 374. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, M.E.; Ashraf, M.; Ahmed, A.; Usman, A.; Hamdoon, A.A.; Elawad, M.A.; Almalki, M.G.; Mosa, O.F.; Niyazov, L.N.; Ayaz, M. Polyphenols and their nanoformulations as potential antibiofilm agents against multidrug-resistant pathogens. Future Microbiol. 2024, 19, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, X.; Shen, Z.; Cheng, W.; Zeng, Z.; Chen, Y.; Tang, C.; Jiang, T. The effect of isoflavaspidic acid PB extracted from Dryopteris fragrans (L.) Schott on planktonic and biofilm growth of dermatophytes and the possible mechanism of antibiofilm. J. Ethnopharmacol. 2019, 241, 111956. [Google Scholar] [CrossRef] [PubMed]
- Junta de Andalucía. Estación Meteorológica de Aroche—Datos de la estación. Available online: https://www.juntadeandalucia.es/agriculturaypesca/ifapa/riaweb/web/estacion/21/6 (accessed on 21 September 2024).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bobo-Garcia, G.; Davidov-Pardo, G.; Arroqui, C.; Virseda, P.; Marin-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Lamaison, J.L.; Carnat, A. The amount of main flavonoids in flowers and leaves of Crataegus monogyna Jacq. and Crataegus laevigata (Poiret) DC. (Rosaceae). Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Arnow, L.E. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine-tyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar] [CrossRef]
- Lopez-Orenes, A.; Bueso, M.C.; Conesa, H.M.; Calderon, A.A.; Ferrer, M.A. Seasonal changes in antioxidative/oxidative profile of mining and non-mining populations of Syrian beancaper as determined by soil conditions. Sci. Total Env. 2017, 575, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Semmarath, W.; Srisawad, K.; Arjsri, P.; Umsumarng, S.; Yodkeeree, S.; Jamjod, S.; Prom, U.T.C.; Dejkriengkraikul, P. Protective Effects of Proanthocyanidin-Rich Fraction from Red Rice Germ and Bran on Lung Cell Inflammation via Inhibition of NF-kappaB/NLRP3 Inflammasome Pathway. Nutrients 2023, 15, 3793. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method. In Quantification of Tannins in Tree and Shrub Foliage; Springer: Berlin/Heidelberg, Germany, 2003; pp. 49–51. [Google Scholar]
- Nickerson, M.N.; Moore, L.P.; U’Ren, J.M. The impact of polyphenolic compounds on the in vitro growth of oak-associated foliar endophytic and saprotrophic fungi. Fungal Ecol. 2023, 62, 101226. [Google Scholar] [CrossRef]
- Rea, J.; Garcia-Gimenez, M.D.; Santiago, M.; De la Puerta, R.; Fernandez-Arche, M.A. Hydroxycinnamic acid derivatives isolated from hempseed and their effects on central nervous system enzymes. Int. J. Food Sci. Nutr. 2021, 72, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Rea Martinez, J.; Montserrat-de la Paz, S.; De la Puerta, R.; Garcia-Gimenez, M.D.; Fernandez-Arche, M.A. Characterization of bioactive compounds in defatted hempseed (Cannabis sativa L.) by UHPLC-HRMS/MS and anti-inflammatory activity in primary human monocytes. Food Funct. 2020, 11, 4057–4066. [Google Scholar] [CrossRef] [PubMed]
- Uhrovcik, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Cuendet, M.; Potterat, O.; Hostettmann, K. Flavonoids and phenylpropanoid derivatives from Campanula barbata. Phytochemistry 2001, 56, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garcia, R.; Sanchez-Hidalgo, M.; Montoya, T.; Alcarranza, M.; Ortega-Vidal, J.; Altarejos, J.; Alarcon-de-la-Lastra, C. Effects of Oleacein, a New Epinutraceutical Bioproduct from Extra Virgin Olive Oil, in LPS-Activated Murine Immune Cells. Pharmaceuticals 2022, 15, 1338. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef] [PubMed]
- García-Prado, M.E. Aportación al Estudio Farmacológico de Uncaria tomentosa (Willd) DC. Ph.D. Thesis, University of Seville, Seville, Spain, 2005. [Google Scholar]
- Guo, T.; Wei, L.; Sun, J.; Hou, C.-l.; Fan, L. Antioxidant activities of extract and fractions from Tuber indicum Cooke & Massee. Food Chem. 2011, 127, 1634–1640. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Murcia, A.; Butler, J.; Halliwell, B. Evaluation of the antioxidant and prooxidant actions of gallic acid and its derivatives. J. Agric. Food Chem. 2002, 41, 1880–1885. [Google Scholar] [CrossRef]
- Mingarro, D.M.; Plaza, A.; Galan, A.; Vicente, J.A.; Martinez, M.P.; Acero, N. The effect of five Taraxacum species on in vitro and in vivo antioxidant and antiproliferative activity. Food Funct. 2015, 6, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.). Front. Life Sci. 2014, 7, 224–228. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci. 1999, 65, PL241–PL246. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.T.; Tawata, S. Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef] [PubMed]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.J. Protective Effect of Sulfated Polysaccharides from Celluclast-Assisted Extract of Hizikia fusiforme Against Ultraviolet B-Induced Skin Damage by Regulating NF-kappaB, AP-1, and MAPKs Signaling Pathways In Vitro in Human Dermal Fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabud, A.; Deodhar, M. Anti-hyaluronidase, Anti-elastase Activity of Garcinia indica. Int. J. Bot. 2010, 6, 299–303. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Salgueiro, L.; Cocco, E.; Ghiani, V.; Falconieri, D.; Maccioni, D.; Maxia, A. Agroprospecting of Biowastes: Globe Artichoke (Cynara scolymus L. Cultivar Tema, Asteraceae) as Potential Source of Bioactive Compounds. Molecules 2024, 29, 3960. [Google Scholar] [CrossRef] [PubMed]
- Avila-Roman, J.; Gomez-Villegas, P.; de Carvalho, C.; Vigara, J.; Motilva, V.; Leon, R.; Talero, E. Up-Regulation of the Nrf2/HO-1 Antioxidant Pathway in Macrophages by an Extract from a New Halophilic Archaea Isolated in Odiel Saltworks. Antioxidants 2023, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbelaez, P.; Cruz, J.C.; Munoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2008.
- M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Alves-Silva, J.M.; Maccioni, D.; Cocco, E.; Goncalves, M.J.; Porcedda, S.; Piras, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil. Plants 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Satti, N.K.; Dutt, P.; Prasad, R.; Khan, I.A. Hydroxychavicol: A phytochemical targeting cutaneous fungal infections. Sci. Rep. 2016, 6, 37867. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.; Zuzarte, M.; Cavaleiro, C.; Salgueiro, L. Antibiofilm Effect of Lavandula multifida Essential Oil: A New Approach for Chronic Infections. Pharmaceutics 2023, 15, 2142. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, D.; Aguiar, L.; Araujo, G.D.S.; Lopes, R.G.P.; Sales, J.A.; Pereira-Neto, W.A.; Pinheiro, A.Q.; Paixao, G.C.; Cordeiro, R.A.; Sidrim, J.J.C.; et al. In vitro and ex vivo biofilms of dermatophytes: A new panorama for the study of antifungal drugs. Biofouling 2020, 36, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Sardi, J.C.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Goncalves, M.J.; Zuzarte, M.; Alves-Silva, J.M.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Unveiling the Antifungal Potential of Two Iberian Thyme Essential Oils: Effect on C. albicans Germ Tube and Preformed Biofilms. Front. Pharmacol. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed]


| Assay | Content |
|---|---|
| Total Phenolic Content (mg GAE/g dry extract) | 425.33 ± 9.02 |
| Total Flavonoid Content (mg RE/g dry extract) | 181.21 ± 6.04 |
| Total Hydroxycinnamic Acid Content (mg CAE/g dry extract) | 28.88 ± 3.08 |
| Total Proanthocyanidin Content (mg CE/g dry extract) | 38.64 ± 4.01 |
| Total Tannin Content (TRP % w/w) | 71.96 ± 2.25 |
| RT (min) | Molecular Formula | Expected m/z | Measured m/z | Error (ppm) | MS/MS Fragments | Attribution | Content µg/g Dry Extract 1 |
|---|---|---|---|---|---|---|---|
| 5.52 | C21H20O10 | 431.09837 | 431.098465 | 0.220285 | 77.03976; 117.0347; 283.06152; 311.05661; 341.06689 | Isovitexin | 375.44 ± 7.49 |
| 5.32 | C21H20O10 | 431.09837 | 431.09831 | 0.220285 | 77.03976; 117.0347; 283.06152; 311.05661; 341.06689 | Vitexin | 216.12 ± 2.54 |
| 5.66 | C21H20O12 | 463.0882 | 463.08832 | 0.25453 | 227.03548; 243.02995; 255.03014; 271.02521; 300.0278 | Hyperoside | 37.73 ± 1.67 |
| 5.99 | C27H30O14 | 577.15628 | 577.15613 | −0.26348 | 117.03459; 183.04515; 255.0299; 285.04046; 430.09054 | Kaempferol-3,7-O-α-di- rhamnopyranoside | 27.30 ± 1.65 |
| 6.82 | C15H12O5 | 271.0612 | 271.061235 | 0.123665 | 65.00333; 83.01394; 107.01404; 119.0503; 151.00375 | Naringenin | N/A |
| 4.03 | C9H8O4 | 179.03498 | 179.03497 | −0.0595975 | 107.05025; 134.03757; 135.04532; 179.03532 | Caffeic acid | N/A |
| 5.02 | C15H12O7 | 303.05103 | 303.050975 | −0.16626 | 57.03462; 125.02451; 151.00342; 175.04033; 285.04071 | Taxifolin | N/A |
| 0.52 | C7H12O6 | 191.05611 | 191.056085 | −0.137765 | 85.02961; 93.03466; 109.02957; 127.04015; 173.0459 | Quinic acid | N/A |
| 1.11 | C7H6O5 | 169.01425 | 169.0141875 | −0.3735825 | 69.03467; 79.01904; 81.03464; 97.02959; 125.02452 | Gallic acid | N/A |
| 1.83 | C15H14O7 | 305.06668 | 305.06662 | −0.1970925 | 109.02959; 125.02451; 137.02457; 167.03516; 219.06656 | (-) Gallocatechin | N/A |
| 2.20 | C7H6O4 | 153.01933 | 153.01931 | −0.13072 | 65.00337; 81.03465; 91.01901; 108.02183; 109.0296 | Protocatechuic acid | N/A |
| 3.13 | C48H28O30 | 1083.05926 | 1083.06042 | 1.07104 | 300.99921; 600.99017; 781.05371 | Punicalagin | N/A |
| 3.31 | C30H26O12 | 577.13515 | 577.13495 | −0.34874 | 109.02943; 125.02448; 161.02473; 289.07266; 407.07812 | Procyanidin B1 | N/A |
| 3.59 | C15H14O6 | 289.07176 | 289.0718625 | 0.3503175 | 109.02957; 123.04529; 137.02441; 203.0719; 245.0822 | Catechin | N/A |
| 4.50 | C8H8O3 | 151.04007 | 151.040025 | −0.280595 | 92.02686; 108.02182; 136.01671; 151.0403 | Vanillin | N/A |
| 4.82 | C9H8O3 | 163.04007 | 163.040055 | −0.0727675 | 65.03967; 91.05544; 93.03464; 104.02702; 119.05034 | p-Coumaric acid | N/A |
| 5.78 | C7H6O3 | 137.02442 | 137.02435 | −0.516605 | 65.03975; 93.03467; 137.02455 | Salicylic acid | N/A |
| 5.61 | C15H12O6 | 287.05611 | 287.056165 | 0.200665 | 65.00336; 83.01392; 125.02451; 177.05606; 259.06152 | Aromadendrin | N/A |
| 5.67 | C14H6O8 | 300.99899 | 300.99887 | −0.39585 | 117.03472; 145.02966; 173.0246; 201.01962; 283.99649 | Ellagic acid | N/A |
| 6.32 | C15H10O7 | 301.03538 | 301.0352275 | −0.5151725 | 65.00338; 83.01395; 149.02454; 151.00392 | Morin | N/A |
| 6.57 | C15H20O4 | 263.12888 | 263.1288325 | −0.19019 | 122.03747; 153.09236; 203.10808; 204.11569; 219.13937 | Abscisic acid | N/A |
| 7.23 | C15H10O6 | 285.04046 | 285.04047 | 0.0221325 | 65.00337; 93.03457; 117.03468; 159.04544; 187.04065 | Kaempferol | N/A |
| Sample | Free Radicals | Reactive Oxygen and Nitrogen Species (ROS; RNS) | Enzymes | Transition Metals | ||||
|---|---|---|---|---|---|---|---|---|
| △DPPH• | ▽ABTS •+ | H2O2 | OH• | O2−• | •NO | Xanthine Oxidase | Fe2+ | |
| TLAE | 9.65 ± 0.49 c | 4.97 ± 0.47 ns | 9.43 ± 0.44 ns | 313.51 ± 24.82 b | 8.81 ± 0.71 c | 35.22 ± 1.33 c | 27.6 ± 1.59 c | 10.54 ± 0.35 c |
| Allopurinol | - | - | - | - | - | - | 0.33 ± 0.01 | - |
| Ascorbic acid | - | - | - | 250.36 ± 0.920 | - | - | - | - |
| EDTA-Na2 | - | - | - | - | - | - | - | 7.83 ± 0.30 |
| Caffeic acid | - | - | - | - | - | 7.02 ± 0.22 | - | - |
| Trolox | 6.25 ± 0.07 | 5.37 ± 0.15 | 9.93 ± 0.45 | - | - | - | - | - |
| Gallic acid | - | - | - | - | 4.70 ± 0.73 | - | - | - |
| Sample | Skin-Related Enzymes | |||
|---|---|---|---|---|
| % Inhibition at 200 µg/mL | IC50 (µg/mL) | |||
| Collagenase | Elastase | Hyaluronidase | Tyrosinase | |
| TLAE | 58.49 ± 4.08 ns | 219.02 ± 11.31 b | >675 | 67.48 ± 2.78 c |
| EGCG | 62.52 ± 1.86 | - | - | - |
| Kojic acid | - | - | - | 22.38 ± 1.01 |
| Quercetin | - | 100.26 ± 8.66 | - | - |
| Tannic acid | - | - | 171.96 ± 11.28 | - |
| Strains | TLAE | Fluconazole | ||
|---|---|---|---|---|
| MIC | MLC | MIC | MLC | |
| Epidermophyton floccosum FF9 | 100 | 100 | 16 | 16 |
| Microsporum canis FF1 | 100 | 100 | 128 | 128 |
| Microsporum gypseum CECT 2908 | 400 | 400 | 128 | >128 |
| Trichophyton mentagrophytes FF7 | 50 | 50 | 32 | 32–64 |
| Trichophyton mentagrophytes var interdigitale CECT 2958 | 100 | 100–200 | 128 | >128 |
| Trichophyton rubrum CECT 2794 | 100–200 | 200 | 16 | 64 |
| Trichophyton verrucosum CECT 2992 | >1000 | - | >128 | - |
| Candida krusei H9 | 1000 | >1000 | 64 | 64–128 |
| Candida albicans ATCC 10231 | >1000 | - | 1 | >128 |
| Candida guilliermondii MAT23 | >1000 | - | 4 | >128 |
| Candida parapsilosis ATCC 90018 | >1000 | - | 1 | 2 |
| Candida tropicalis ATCC 13803 | >1000 | - | 4 | >128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Vázquez, M.; Guerrero, A.Q.; Zuzarte, M.; Salgueiro, L.; Alves-Silva, J.; González-Rodríguez, M.L.; Puerta, R.D.l. Phytochemical Analysis and Therapeutic Potential of Tuberaria lignosa (Sweet) Samp. Aqueous Extract in Skin Injuries. Plants 2025, 14, 2299. https://doi.org/10.3390/plants14152299
González-Vázquez M, Guerrero AQ, Zuzarte M, Salgueiro L, Alves-Silva J, González-Rodríguez ML, Puerta RDl. Phytochemical Analysis and Therapeutic Potential of Tuberaria lignosa (Sweet) Samp. Aqueous Extract in Skin Injuries. Plants. 2025; 14(15):2299. https://doi.org/10.3390/plants14152299
Chicago/Turabian StyleGonzález-Vázquez, Manuel, Ana Quílez Guerrero, Mónica Zuzarte, Lígia Salgueiro, Jorge Alves-Silva, María Luisa González-Rodríguez, and Rocío De la Puerta. 2025. "Phytochemical Analysis and Therapeutic Potential of Tuberaria lignosa (Sweet) Samp. Aqueous Extract in Skin Injuries" Plants 14, no. 15: 2299. https://doi.org/10.3390/plants14152299
APA StyleGonzález-Vázquez, M., Guerrero, A. Q., Zuzarte, M., Salgueiro, L., Alves-Silva, J., González-Rodríguez, M. L., & Puerta, R. D. l. (2025). Phytochemical Analysis and Therapeutic Potential of Tuberaria lignosa (Sweet) Samp. Aqueous Extract in Skin Injuries. Plants, 14(15), 2299. https://doi.org/10.3390/plants14152299











