Genome-Wide Identification and Salinity Response Analysis of the Germin-like Protein (GLP) Gene Family in Puccinellia tenuiflora
Abstract
1. Introduction
2. Results
2.1. Identification of PutGLPs in P. tenuiflora
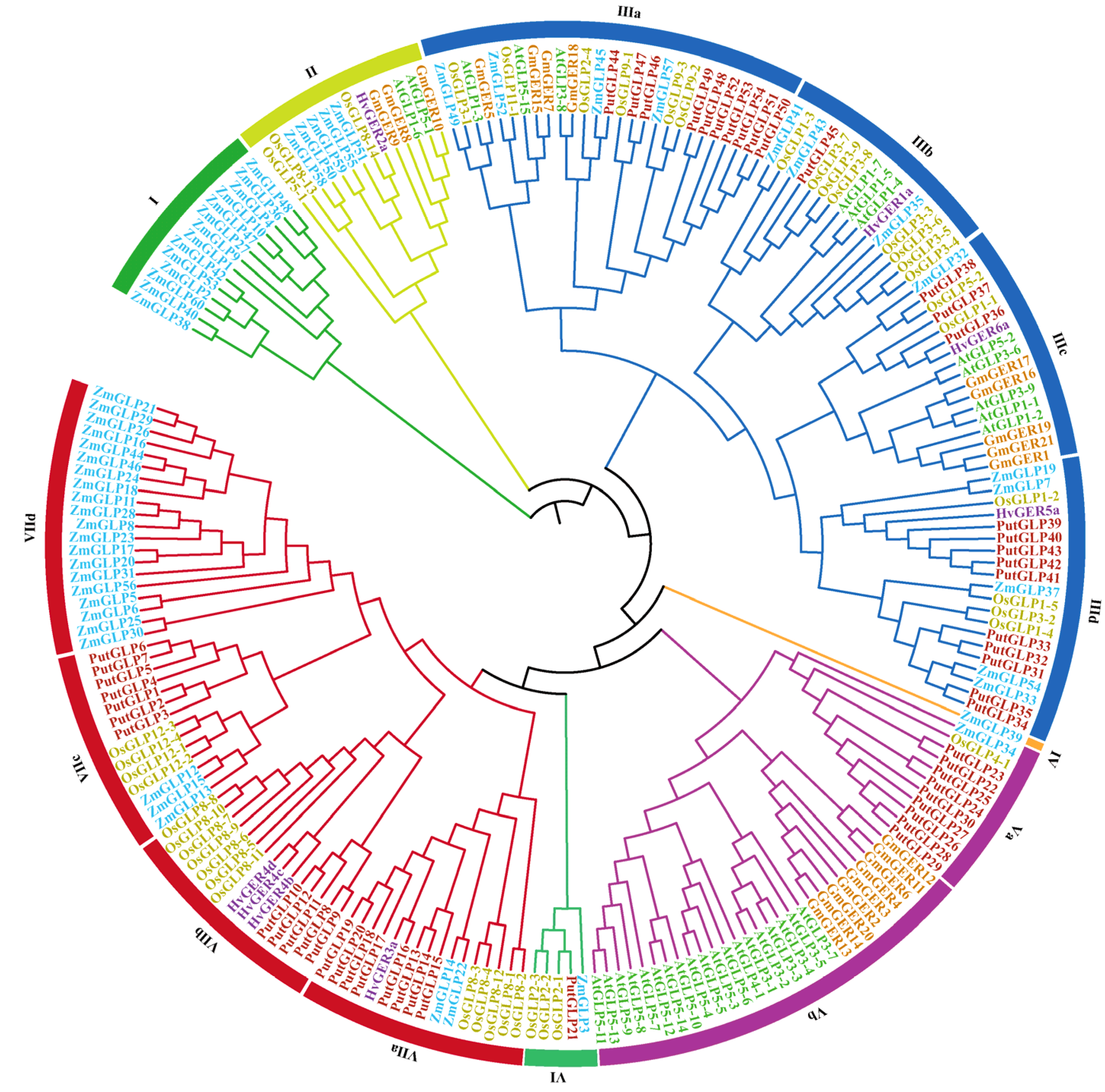
2.2. Phylogenetic and Evolutionary Analysis of the PutGLPs
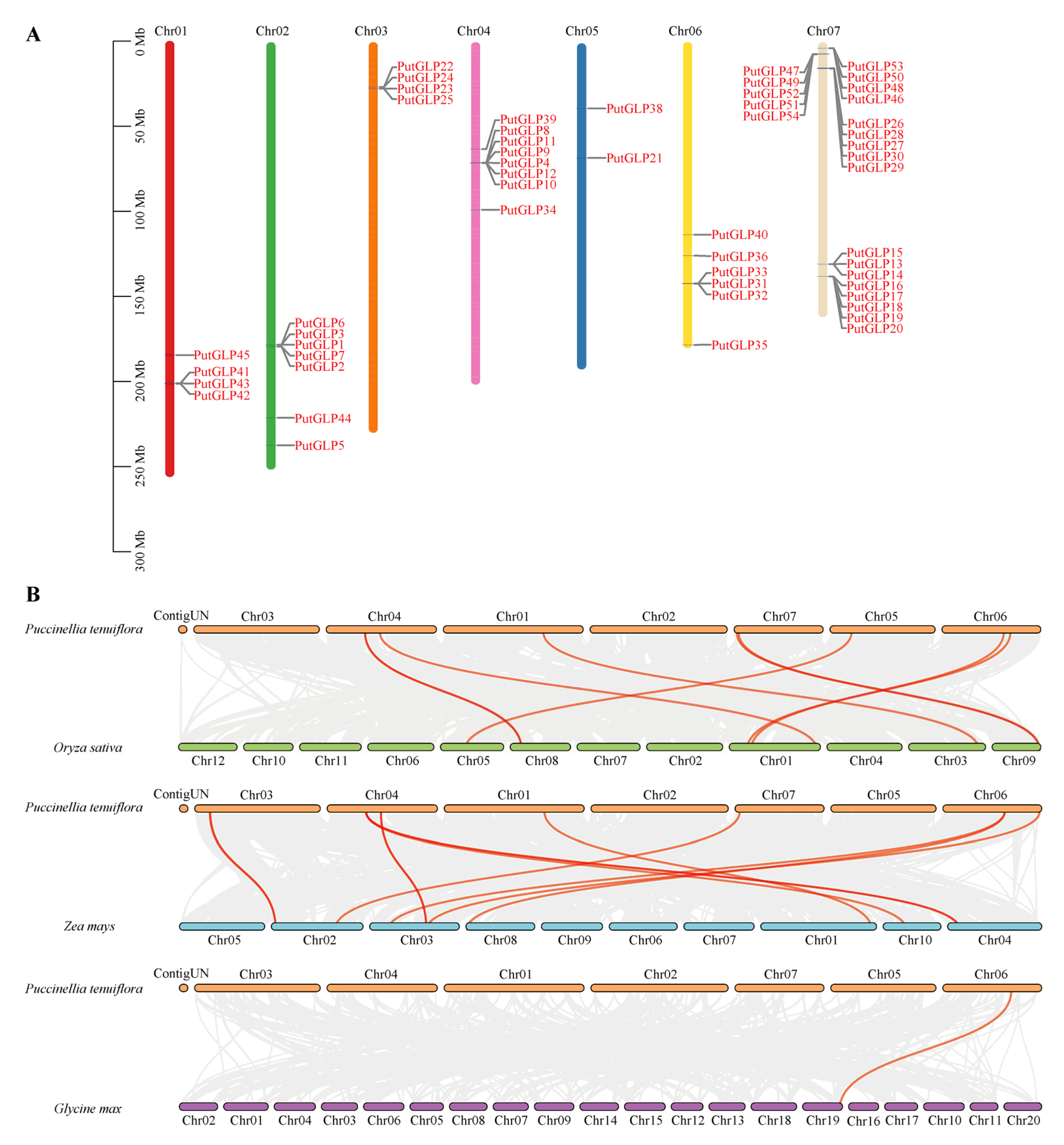
2.3. Chromosomal Distribution and Collinearity Analysis of the PutGLPs
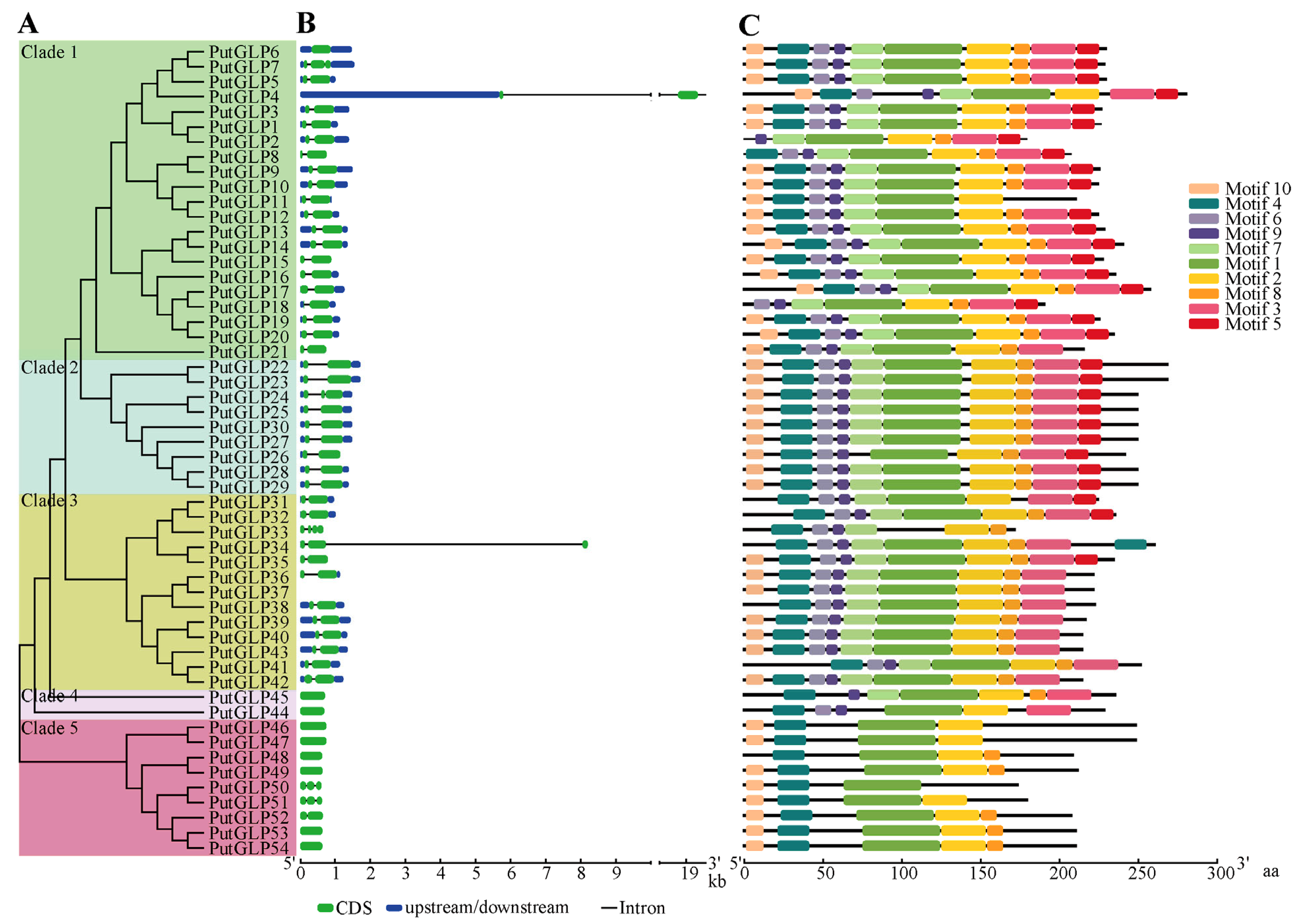
2.4. Gene Structure, Conserved Domain, and Motif Analysis of PutGLPs
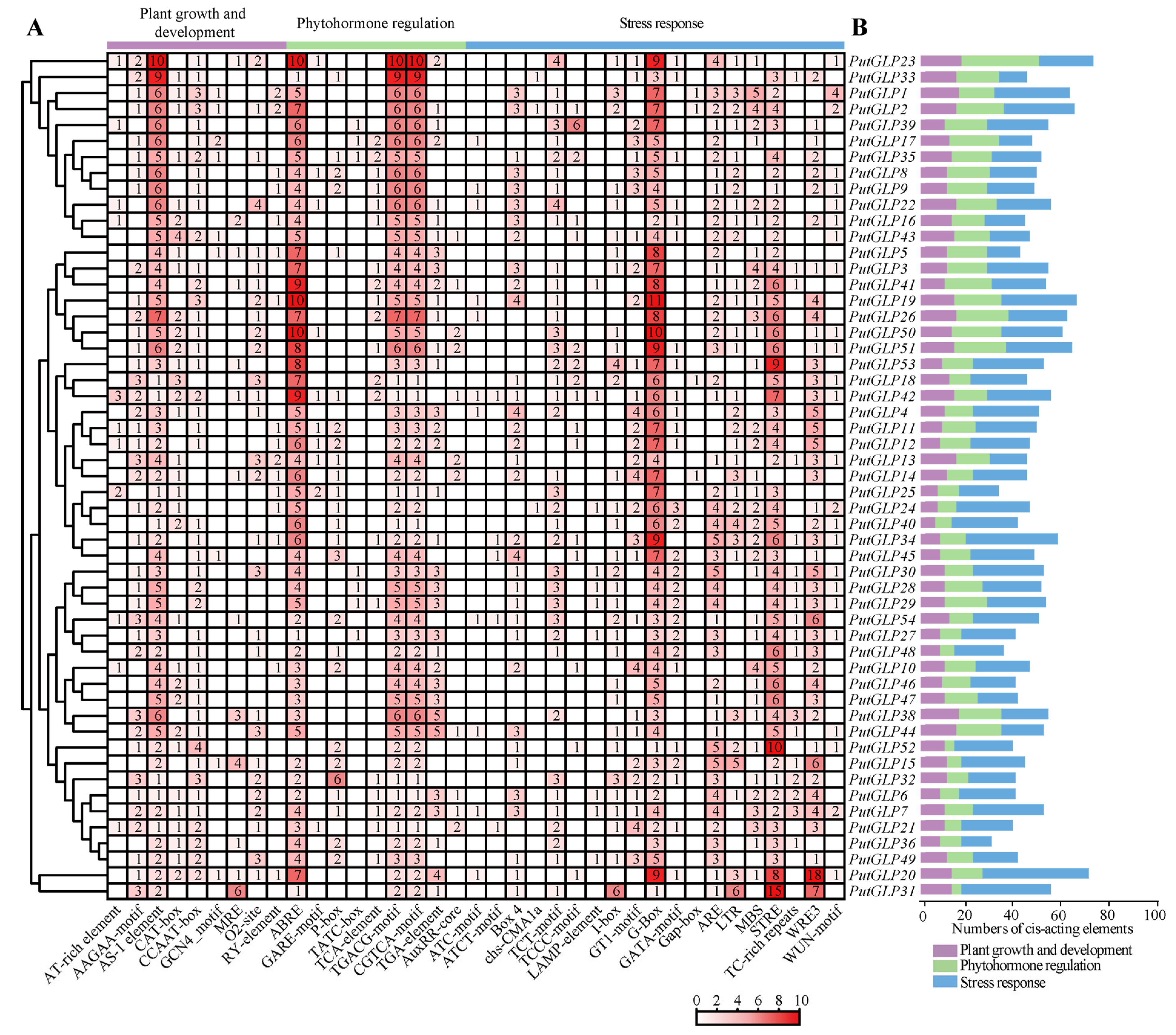
2.5. Analysis of Cis-Acting Elements of PutGLP Promoters
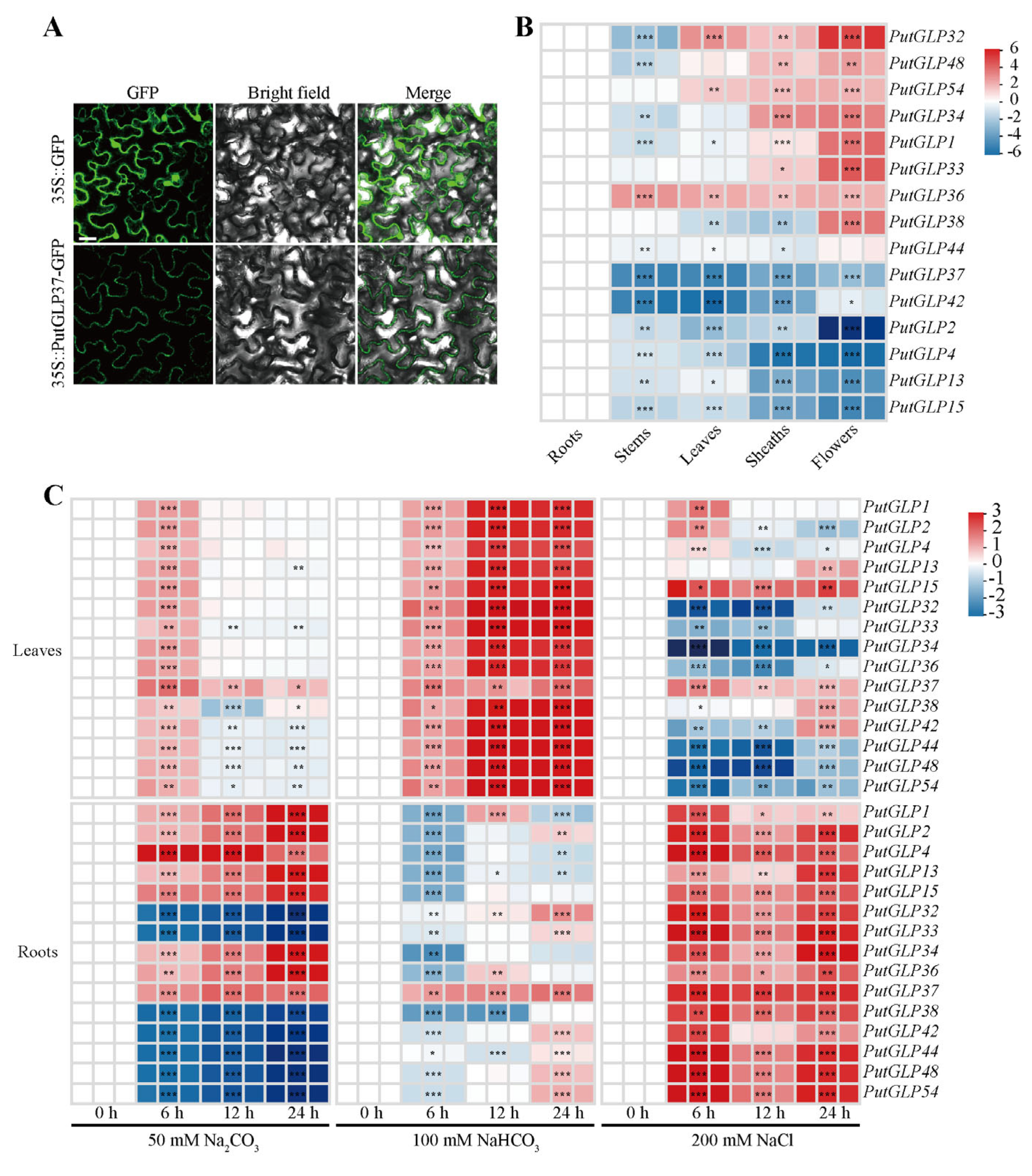
2.6. Subcellular Localization of PutGLP37 and Expression Profiles of PutGLPs in Various Organs
2.7. Expression Patterns of PutGLPs in Roots and Leaves Under Various Abiotic Stresses
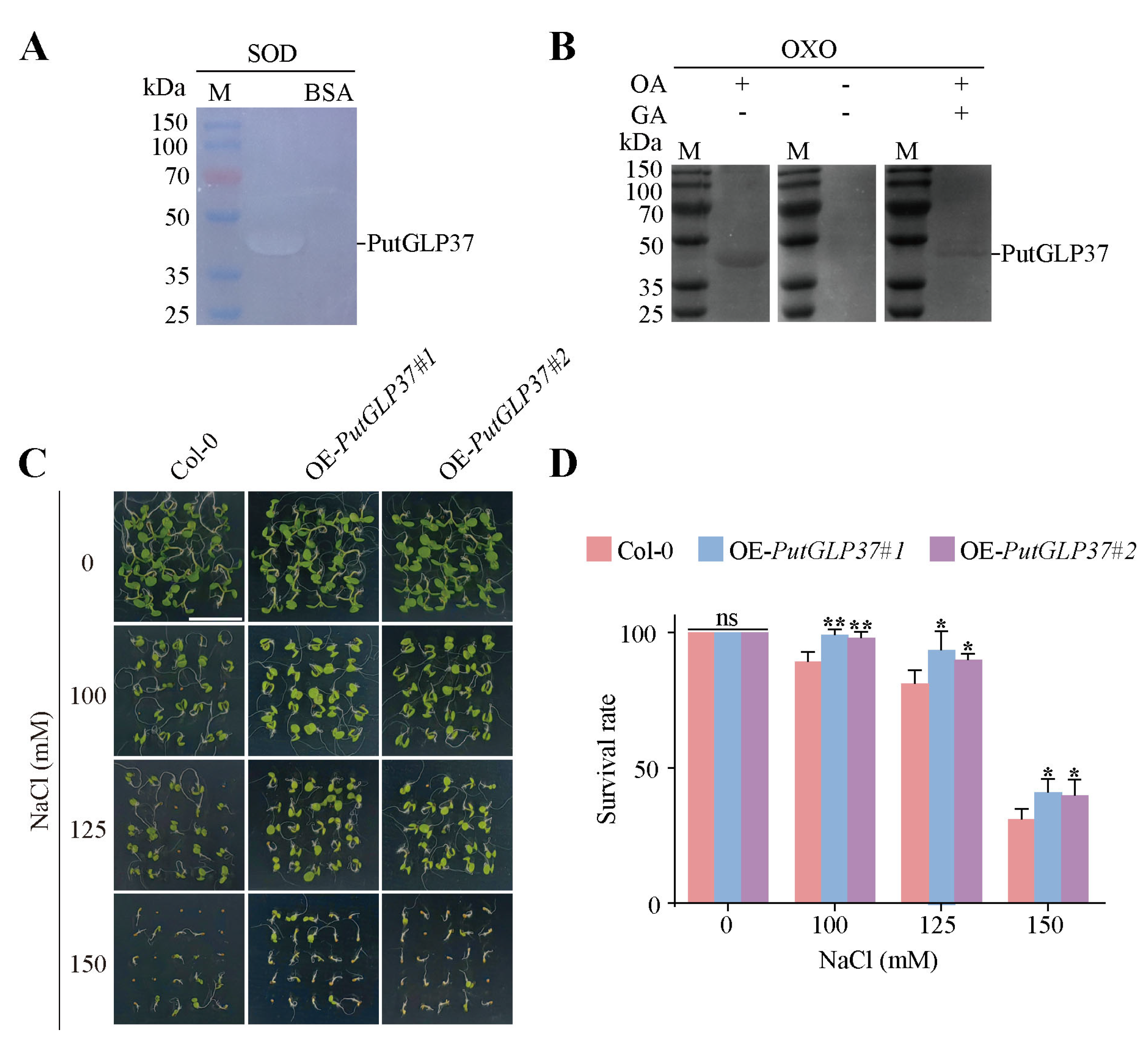
2.8. Tolerance Analysis by Overexpressing PutGLP37 in Yeast and E. coli
2.9. SOD and OXO Activity Assays of Recombinant PutGLP37 Protein
2.10. Salt-Responsive Phenotype of PutGLP37-Overexpressing Arabidopsis Plants
3. Discussion
3.1. Evolutionary Expansion and Stress-Adaptive Innovation of the PutGLP Family in P. tenuiflora
3.2. Organ-Specific and Stress-Inducible Expression Patterns of PutGLPs
3.3. Functional Significance of PutGLP37 in Salt Stress Adaptation
4. Materials and Methods
4.1. Sequence Search and Identification of PutGLPs
4.2. Phylogenetic and Classification Analysis of PutGLPs
4.3. Gene Structure and Conserved Motif Analysis of PutGLP Proteins
4.4. Analysis of Cis-Acting Elements in PutGLP Promoters
4.5. Chromosomal Distribution and Collinearity Analysis of PutGLPs
4.6. Plant Material and Abiotic Stress Treatment
4.7. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR) Analysis
4.8. Subcellular Localization Analysis
4.9. Stress Tolerance Assay of Yeast Transformants Expressing PutGLP37
4.10. Production and Purification of Recombinant PutGLP37 Protein
4.11. Salt Stress Tolerance Assay of E. coli Transformants Expressing PutGLP37
4.12. SOD and OXO Activity Assays of PutGLP37
4.13. Salinity Tolerance Analysis of PutGLP37-Overexpressing Transgenic Arabidopsis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breen, J.; Bellgard, M. Germin-like proteins (GLPs) in cereal genomes: Gene clustering and dynamic roles in plant defence. Funct. Integr. Genom. 2010, 10, 463–476. [Google Scholar] [CrossRef]
- Ilyas, M.; Rasheed, A.; Mahmood, T. Functional characterization of germin and germin-like protein genes in various plant species using transgenic approaches. Biotechnol. Lett. 2016, 38, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Tahir Ul Qamar, M.; Fatima, K.; Rao, M.J.; Tang, Q.; Sadaqat, M.; Ding, B.; Chen, L.L.; Zhu, X.T. Comparative genomics profiling of Citrus species reveals the diversity and disease responsiveness of the GLP pangenes family. BMC Plant Biol. 2025, 25, 388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Wang, X.; Qiao, J.; Wang, H. Roles of germin-like protein family in response to seed germination and shoot branching in Brassica napus. Int. J. Mol. Sci. 2024, 25, 11518. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, D.; Khurana, P. Germins and germin like proteins: An overview. Indian J. Exp. Biol. 2001, 39, 191–200. [Google Scholar]
- Gangadhar, B.H.; Mishra, R.K.; Kappachery, S.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Thiruvengadam, M. Enhanced thermo-tolerance in transgenic potato (Solanum tuberosum L.) overexpressing hydrogen peroxide-producing germin-like protein (GLP). Genomics 2021, 113, 3224–3234. [Google Scholar] [CrossRef]
- To, H.T.M.; Pham, D.T.; Le Thi, V.A.; Nguyen, T.T.; Tran, T.A.; Ta, A.S.; Chu, H.H.; Do, P.T. The germin-like protein OsGER4 is involved in promoting crown root development under exogenous jasmonic acid treatment in rice. Plant J. 2022, 112, 860–874. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Xiao, N.; Zhang, G.; Wang, F.; Chen, X.; Fang, R. Rice GERMIN-LIKE PROTEIN 2-1 functions in seed dormancy under the control of abscisic acid and gibberellic acid signaling pathways. Plant Physiol. 2020, 183, 1157–1170. [Google Scholar] [CrossRef]
- Pei, Y.; Li, X.; Zhu, Y.; Ge, X.; Sun, Y.; Liu, N.; Jia, Y.; Li, F.; Hou, Y. GhABP19, a novel germin-like protein from Gossypium hirsutum, plays an important role in the regulation of resistance to Verticillium and Fusarium wilt pathogens. Front. Plant Sci. 2019, 10, 583. [Google Scholar] [CrossRef]
- Lane, B.G.; Dunwell, J.M.; Ray, J.A.; Schmitt, M.R.; Cuming, A.C. Germin, a protein marker of early plant development, is an oxalate oxidase. J. Biol. Chem. 1993, 268, 12239–12242. [Google Scholar] [CrossRef]
- Yamahara, T.; Shiono, T.; Suzuki, T.; Tanaka, K.; Takio, S.; Sato, K.; Yamazaki, S.; Satoh, T. Isolation of a germin-like protein with manganese superoxide dismutase activity from cells of a moss, Barbula unguiculata. J. Biol. Chem. 1999, 274, 33274–33278. [Google Scholar] [CrossRef]
- Sun, B.; Li, W.; Ma, Y.; Yu, T.; Huang, W.; Ding, J.; Yu, H.; Jiang, L.; Zhang, J.; Lv, S.; et al. OsGLP3-7 positively regulates rice immune response by activating hydrogen peroxide, jasmonic acid, and phytoalexin metabolic pathways. Mol. Plant Pathol. 2023, 24, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.D.T.; Freitas, D.C.; Cruz, W.T.; Porfírio, C.; Silva, M.Z.R.; Oliveira, J.S.; Carvalho, C.P.S.; Ramos, M.V. Identification and characterization of two germin-like proteins with oxalate oxidase activity from Calotropis procera latex. Int. J. Biol. Macromol. 2017, 105, 1051–1061. [Google Scholar] [CrossRef]
- Sakamoto, A.; Nishimura, T.; Miyaki, Y.I.; Watanabe, S.; Takagi, H.; Izumi, S.; Shimada, H. In vitro and in vivo evidence for oxalate oxidase activity of a germin-like protein from azalea. Biochem. Bioph. Res. 2015, 458, 536–542. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, W.T.; Bezerra, E.H.S.; Grangeiro, T.B.; Lopes, J.L.S.; Silva, M.Z.R.; Ramos, M.V.; Rocha, B.A.M.; Oliveira, J.S.; Freitas, D.C.; Freitas, C.D.T. Structural and enzymatic characterization of Peruvianin-I, the first germin-like protein with proteolytic activity. Int. J. Biol. Macromol. 2019, 126, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Han, Y.P.; Gao, J.G.; Wang, X.J.; Li, W.B. Identification and analysis of the germin-like gene family in soybean. BMC Genom. 2010, 11, 620. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.C.; Do, P.T.; To, H.T.M. Effect of gibberellin on crown root development in the mutant of the rice plasmodesmal germin-like protein OsGER4. Funct. Integr. Genom. 2024, 24, 59. [Google Scholar] [CrossRef]
- Ham, B.K.; Li, G.; Kang, B.H.; Zeng, F.; Lucas, W.J. Overexpression of Arabidopsis plasmodesmata germin-like proteins disrupts root growth and development. Plant Cell 2012, 24, 3630–3648. [Google Scholar] [CrossRef]
- Giarola, V.; Chen, P.; Dulitz, S.J.; König, M.; Manduzio, S.; Bartels, D. The dehydration- and ABA-inducible germin-like protein CpGLP1 from Craterostigma plantagineum has SOD activity and may contribute to cell wall integrity during desiccation. Planta 2020, 252, 84. [Google Scholar] [CrossRef]
- Beracochea, V.C.; Almasia, N.I.; Peluffo, L.; Nahirñak, V.; Hopp, E.H.; Paniego, N.; Heinz, R.A.; Vazquez-Rovere, C.; Lia, V.V. Sunflower germin-like protein HaGLP1 promotes ROS accumulation and enhances protection against fungal pathogens in transgenic Arabidopsis thaliana. Plant Cell Rep. 2015, 34, 1717–1733. [Google Scholar] [CrossRef]
- Xiao, T.; ShangGuan, X.; Wang, Y.; Tian, Z.; Peng, K.; Shen, Z.; Hu, Z.; Xia, Y. The germin-like protein OsGLP8-7 is involved in lignin synthesis for acclimation to copper toxicity in rice. J. Plant Physiol. 2024, 303, 154335. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Zhang, Y.; Qiu, J.; Li, Y.; Zheng, B.; Hu, F.; Dai, S.; Huang, X. A high-quality genome sequence of alkaligrass provides insights into halophyte stress tolerance. Sci. China Life Sci. 2020, 63, 1269–1282. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.; Wang, Z.; Wang, T. Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J. Integr. Plant Biol. 2013, 55, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Wang, P.; Bao, Z.; Ma, Q.; Duan, L.J.; Bao, A.K.; Zhang, J.L.; Wang, S.M. SOS1, HKT1;5, and NHX1 synergistically modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora. Front. Plant Sci. 2017, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fu, Z.; Zhang, S.; Zhang, X.; Xue, X.; Chen, Y.; Zhang, Z.; Lai, Z.; Lin, Y. Genome-wide analysis of the GLP gene family and overexpression of GLP1-5-1 to promote lignin accumulation during early somatic embryo development in Dimocarpus longan. BMC Genom. 2023, 24, 138. [Google Scholar] [CrossRef]
- Yang, Q.; Sharif, Y.; Zhuang, Y.; Chen, H.; Zhang, C.; Fu, H.; Wang, S.; Cai, T.; Chen, K.; Raza, A.; et al. Genome-wide identification of germin-like proteins in peanut (Arachis hypogea L.) and expression analysis under different abiotic stresses. Front. Plant Sci. 2022, 13, 1044144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, Y.; Yuan, L.; Zhang, Y.; Liu, J.; Zhou, F.; Wang, Q.; Hu, X. Genome-wide identification, characterization, and expression analysis related to low-temperature stress of the CmGLP gene family in Cucumis melo L. Int. J. Mol. Sci. 2022, 23, 8190. [Google Scholar] [CrossRef]
- Jin, Y.; Fan, L.; Zhang, Y.; Hu, W.; Han, X.; Yan, Q.; Yang, J.; Li, F.; Yang, Z. Functional divergence of GLP genes between G. barbadense and G. hirsutum in response to Verticillium dahliae infection. Genomics 2022, 114, 110470. [Google Scholar] [CrossRef]
- Mao, L.; Ge, L.; Ye, X.; Xu, L.; Si, W.; Ding, T. ZmGLP1, a Germin-like protein from maize, plays an important role in the regulation of pathogen resistance. Int. J. Mol. Sci. 2022, 23, 14316. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Chen, C.; Shen, Z. Genome-wide characterization and expression analysis of the germin-like protein family in rice and Arabidopsis. Int. J. Mol. Sci. 2016, 17, 1622. [Google Scholar] [CrossRef]
- Karlik, E. Potential stress tolerance roles of barley germins and GLPs. Dev. Genes Evol. 2021, 231, 109–118. [Google Scholar] [CrossRef]
- Nakata, M.; Watanabe, Y.; Sakurai, Y.; Hashimoto, Y.; Matsuzaki, M.; Takahashi, Y.; Satoh, T. Germin-like protein gene family of a moss, Physcomitrella patens, phylogenetically falls into two characteristic new clades. Plant Mol. Biol. 2004, 56, 381–395. [Google Scholar] [CrossRef]
- Abdullah, M.; Cheng, X.; Cao, Y.; Su, X.; Manzoor, M.A.; Gao, J.; Cai, Y.; Lin, Y. Zinc finger-homeodomain transcriptional factors (ZHDs) in upland cotton (Gossypium hirsutum): Genome-wide identification and expression analysis in fiber development. Front. Genet. 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; An, J.M.; Shin, Y.C.; Kim, K.J.; Lee, B.J.; Paek, K.H. Molecular characterization of pepper germin-like protein as the novel PR-16 family of pathogenesis-related proteins isolated during the resistance response to viral and bacterial infection. Planta 2004, 219, 797–806. [Google Scholar] [CrossRef]
- Woo, E.J.; Dunwell, J.M.; Goodenough, P.W.; Marvier, A.C.; Pickersgill, R.W. Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat. Struct. Biol. 2000, 7, 1036–1040. [Google Scholar] [CrossRef]
- Manosalva, P.M.; Davidson, R.M.; Liu, B.; Zhu, X.; Hulbert, S.H.; Leung, H.; Leach, J.E. A germin-like protein gene family functions as a complex quantitative trait locus conferring broad-spectrum disease resistance in rice. Plant Physiol. 2009, 149, 286–296. [Google Scholar] [CrossRef]
- Barman, A.R.; Banerjee, J. Versatility of germin-like proteins in their sequences, expressions, and functions. Funct. Integr. Genom. 2015, 15, 533–548. [Google Scholar] [CrossRef]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef]
- Gao, S.; Gao, J.; Zhu, X.; Song, Y.; Li, Z.; Ren, G.; Zhou, X.; Kuai, B. ABF2, ABF3, and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in Arabidopsis. Mol. Plant 2016, 9, 1272–1285. [Google Scholar] [CrossRef]
- Hong, S.Y.; Roze, L.V.; Wee, J.; Linz, J.E. Evidence that a transcription factor regulatory network coordinates oxidative stress response and secondary metabolism in aspergilli. Microbiol. Open 2013, 2, 144–160. [Google Scholar] [CrossRef]
- Ilyas, M.; Akhtar, W.; Rehman, S.; Naqvi, S.M.S.; Mahmood, T. Functional characterization of the rice root germin-like protein gene-1 (OsRGLP1) promoter in Nicotiana tabacum. 3 Biotech 2019, 9, 130. [Google Scholar] [CrossRef]
- Suo, J.; Zhang, H.; Zhao, Q.; Zhang, N.; Zhang, Y.; Li, Y.; Song, B.; Yu, J.; Cao, J.; Wang, T.; et al. Na2CO3-responsive photosynthetic and ROS scavenging mechanisms in chloroplasts of alkaligrass revealed by phosphoproteomics. Genom. Proteom. Bioinf 2020, 18, 271–288. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Ji, J.; Zhao, W.; Guo, W.; Li, J.; Bai, Y.; Wang, D.; Yan, Z.; Guo, C. Flavonol synthase gene MsFLS13 regulates saline-alkali stress tolerance in alfalfa. Crop J. 2023, 11, 1218–1229. [Google Scholar] [CrossRef]
- Hurkman, W.J.; Tao, H.P.; Tanaka, C.K. Germin-like polypeptides increase in barley roots during salt stress. Plant Physiol. 1991, 97, 366–374. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Zhu, F.; Li, H.; Li, L.; Yang, Q.; Chi, X.; Yu, S.; Liang, X. Characterization of peanut germin-like proteins, AhGLPs in plant development and defense. PLoS ONE 2013, 8, e61722. [Google Scholar] [CrossRef]
- Anum, J.; O’Shea, C.; Skriver, K.; Saeed, M.; Hyder, M.; Farrakh, S.; Yasmin, T. The promoters of OsGLP genes exhibited differentially methylated sites under drought and salt stress in rice cultivars. Euphytica 2023, 219, 42. [Google Scholar] [CrossRef]
- Li, Y.; Takano, T.; Liu, S. Discovery and characterization of two novel salt-tolerance genes in Puccinellia tenuiflora. Int. J. Mol. Sci. 2014, 15, 16469–16483. [Google Scholar] [CrossRef]
- Du, H.; Liang, C. Assembly of chromosome-scale contigs by efficiently resolving repetitive sequences with long reads. Nat. Commun. 2019, 10, 5360. [Google Scholar] [CrossRef]
- Zerpa-Catanho, D.; Zhang, X.; Song, J.; Hernandez, A.G.; Ming, R. Ultra-long DNA molecule isolation from plant nuclei for ultra-long read genome sequencing. STAR Protoc. 2021, 2, 100343. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Yan, S.; Zhang, S.; Zhao, J.; Wang, W.; Yang, T.; Wang, X.; Mao, X.; Dong, J.; et al. The germin-like protein OsGLP2-1 enhances resistance to fungal blast and bacterial blight in rice. Plant Mol. Biol. 2016, 92, 411–423. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, W.; Zayed, O.; Liu, X.; Tang, K.; Nie, W.; Li, Y.; Xie, S.; Li, Y.; Long, T.; et al. The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl. Sci. Rev. 2021, 8, nwaa149. [Google Scholar] [CrossRef]
- León-Galván, F.; de Jesús Joaquín-Ramos, A.; Torres-Pacheco, I.; Barba de la Rosa, A.P.; Guevara-Olvera, L.; González-Chavira, M.M.; Ocampo-Velazquez, R.V.; Rico-García, E.; Guevara-González, R.G. A germin-like protein gene (CchGLP) of Capsicum chinense Jacq. is induced during incompatible interactions and displays Mn-superoxide dismutase activity. Int. J. Mol. Sci. 2011, 12, 7301–7313. [Google Scholar] [CrossRef]
- Rietz, S.; Bernsdorff, F.E.; Cai, D. Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. J. Exp. Bot. 2012, 63, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bidney, D.L.; Yalpani, N.; Duvick, J.P.; Crasta, O.; Folkerts, O.; Lu, G. Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol. 2003, 133, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Maiti, M.K. Functional role of rice germin-like protein1 in regulation of plant height and disease resistance. Biochem. Bioph. Res. Co. 2010, 394, 178–183. [Google Scholar] [CrossRef]
- He, Z.D.; Tao, M.L.; Leung, D.W.M.; Yan, X.Y.; Chen, L.; Peng, X.X.; Liu, E.E. The rice germin-like protein OsGLP1 participates in acclimation to UV-B radiation. Plant Physiol. 2021, 186, 1254–1268. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, D.T.; Nguyen, N.H.; Do, P.T.; To, H.T.M. The Germin-like protein gene OsGER4 is involved in heat stress response in rice root development. Funct. Integr. Genom. 2023, 23, 271. [Google Scholar] [CrossRef]
- Vidović, M.; Battisti, I.; Pantelić, A.; Morina, F.; Arrigoni, G.; Masi, A.; Jovanović, S.V. Desiccation tolerance in Ramonda serbica Panc.: An integrative transcriptomic, proteomic, metabolite and photosynthetic study. Plants 2022, 11, 1199. [Google Scholar] [CrossRef]
- Ma, L.; Qin, D.B.; Sun, L.; Zhang, K.; Yu, X.; Dang, A.K.; Hou, S.; Zhao, X.; Yang, Y.; Wang, Y.; et al. SALT OVERLY SENSITIVE2 and AMMONIUM TRANSPORTER1;1 contribute to plant salt tolerance by maintaining ammonium uptake. Plant Cell 2025, 37, koaf034. [Google Scholar] [CrossRef]
- Gámez-Arjona, F.; Park, H.J.; García, E.; Aman, R.; Villalta, I.; Raddatz, N.; Carranco, R.; Ali, A.; Ali, Z.; Zareen, S.; et al. Inverse regulation of SOS1 and HKT1 protein localization and stability by SOS3/CBL4 in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2024, 121, e2320657121. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, Y.; Ma, M.; Li, L.; Wei, Y.; Fan, L.; Xie, Z.; Qi, K.; Wu, J.; Tao, S.; et al. PbNAC3 coordinates AsA generation and ABA biosynthesis to improve salt tolerance in pear. Plant J. 2025, 122, e70171. [Google Scholar] [CrossRef]
- Zha, D.; He, Y.; Song, J. Regulatory role of ABA-responsive element binding factors in plant abiotic stress response. Physiol. Plant. 2025, 177, e70233. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. Expansins in salt and drought stress adaptation: From genome-wide identification to functional characterisation in crops. Plants 2025, 14, 1327. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Gigli-Bisceglia, N.; van Zelm, E.; Kokkinopoulou, P.; Julkowska, M.M.; Besten, M.; Nguyen, T.P.; Li, H.; Lamers, J.; de Zeeuw, T.; et al. Arabinosylation of cell wall extensin is required for the directional response to salinity in roots. Plant Cell 2024, 36, 3328–3343. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–w296. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Zhao, Q.; Zhang, Z.; Chen, S.; Cao, J.; Liu, G.; Wei, X.; Wang, T.; Yang, C.; Dai, S. Cytological and proteomic analyses of Osmunda cinnamomea germinating spores reveal characteristics of fern spore germination and rhizoid tip growth. Mol. Cell. Proteom. 2015, 14, 2510–2534. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, J.; Collinge, D.B.; Thordal-Christensen, H. Ethanol increases sensitivity of oxalate oxidase assays and facilitates direct activity staining in SDS gels. Plant Mol. Biol. Rep. 1996, 14, 266–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, Z.; Li, B.; Zheng, H.; Wu, Z.; Li, Y.; Sun, M.; Dai, S. Genome-Wide Identification and Salinity Response Analysis of the Germin-like Protein (GLP) Gene Family in Puccinellia tenuiflora. Plants 2025, 14, 2259. https://doi.org/10.3390/plants14152259
Li Y, Zhao Z, Li B, Zheng H, Wu Z, Li Y, Sun M, Dai S. Genome-Wide Identification and Salinity Response Analysis of the Germin-like Protein (GLP) Gene Family in Puccinellia tenuiflora. Plants. 2025; 14(15):2259. https://doi.org/10.3390/plants14152259
Chicago/Turabian StyleLi, Yueyue, Zhe Zhao, Bo Li, Hongxia Zheng, Zhen Wu, Ying Li, Meihong Sun, and Shaojun Dai. 2025. "Genome-Wide Identification and Salinity Response Analysis of the Germin-like Protein (GLP) Gene Family in Puccinellia tenuiflora" Plants 14, no. 15: 2259. https://doi.org/10.3390/plants14152259
APA StyleLi, Y., Zhao, Z., Li, B., Zheng, H., Wu, Z., Li, Y., Sun, M., & Dai, S. (2025). Genome-Wide Identification and Salinity Response Analysis of the Germin-like Protein (GLP) Gene Family in Puccinellia tenuiflora. Plants, 14(15), 2259. https://doi.org/10.3390/plants14152259







