RGB Imaging and Irrigation Management Reveal Water Stress Thresholds in Three Urban Shrubs in Northern China
Abstract
1. Introduction
2. Results
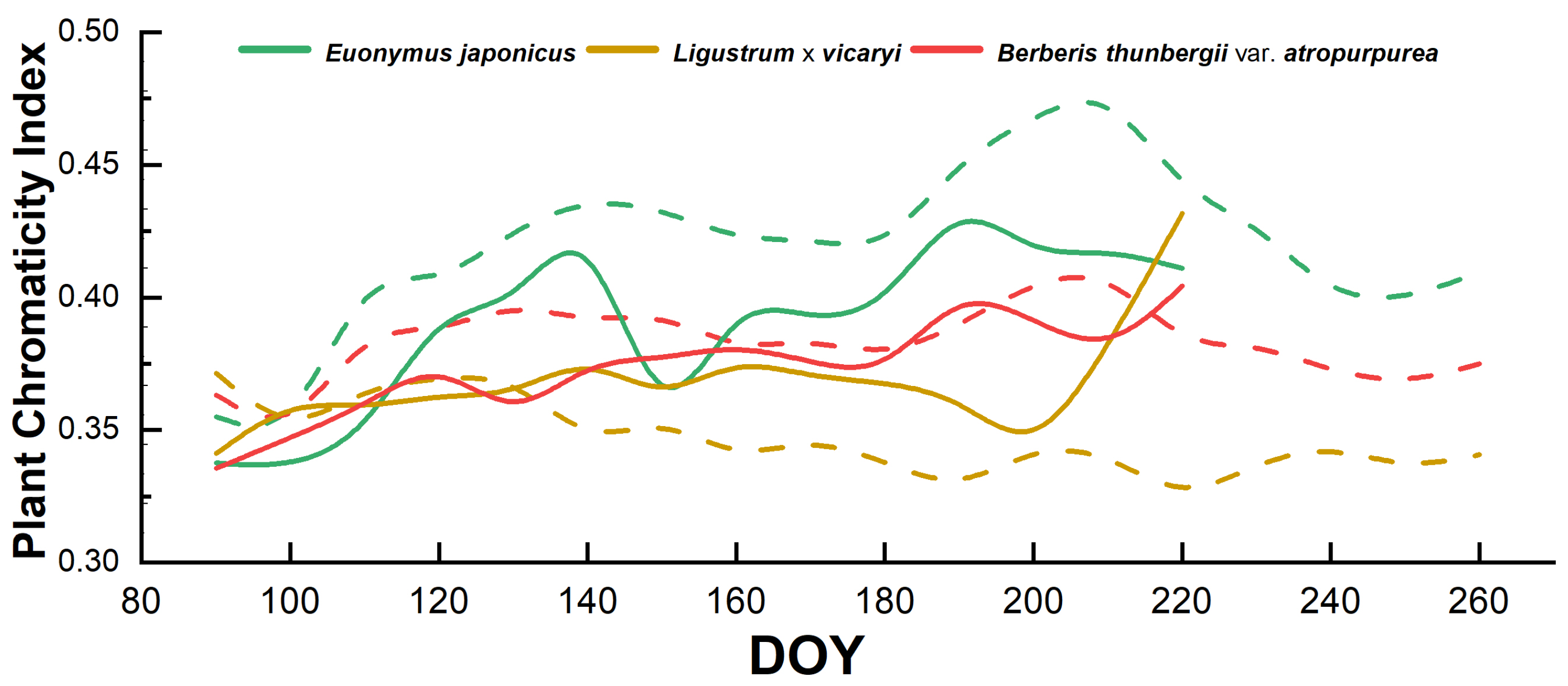
2.1. Color Indices of Plants Under Full Irrigation Condition
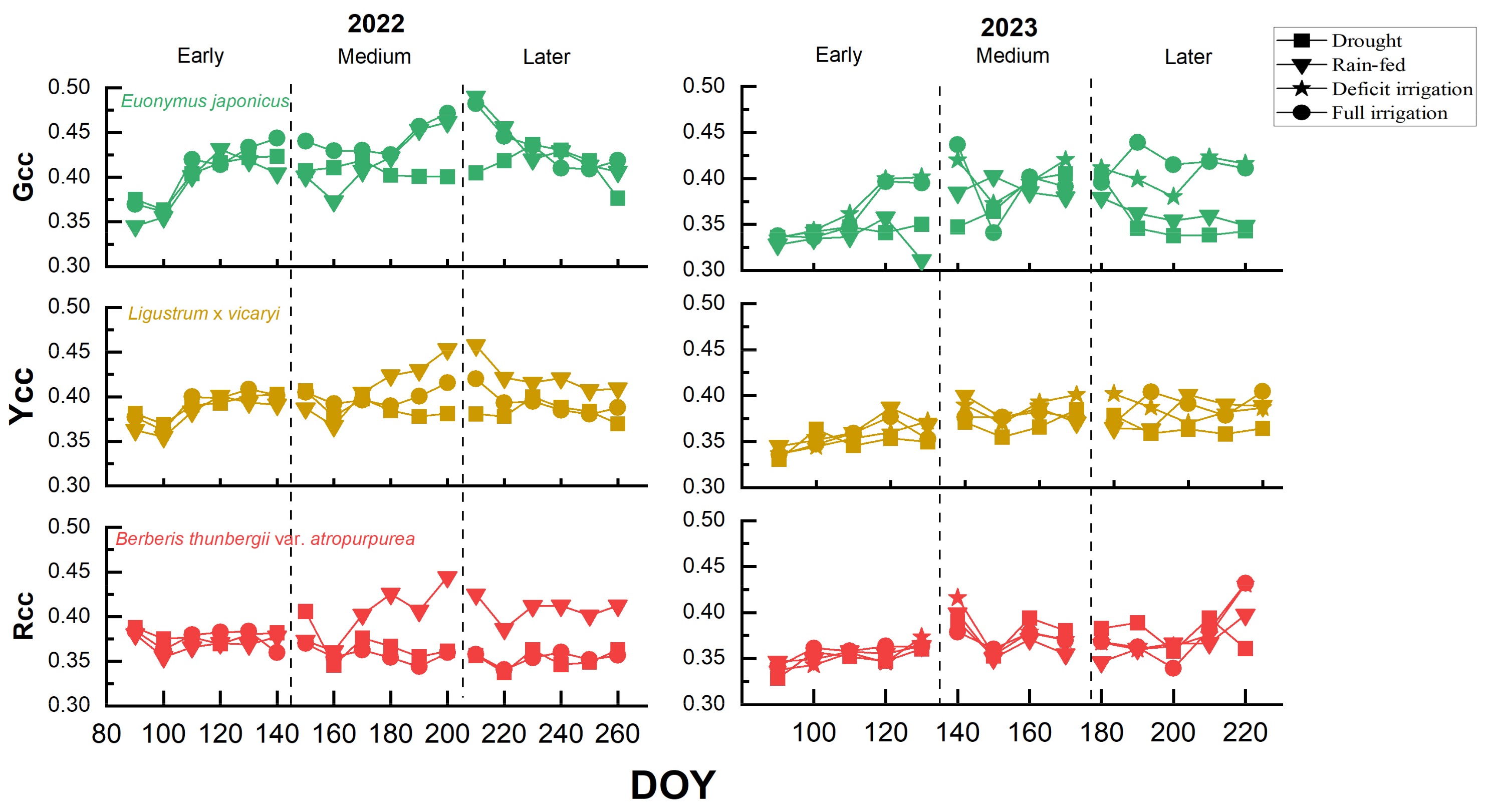
2.2. Changes in Plant Coloration and Response Under Different Treatments
2.3. Factors Influencing the Degree of Coloration in Plant Leaves
3. Discussion
3.1. Application of Shrub Color Index in Plant Drought-Tolerance Evaluation
3.2. Adaptation Strategies of Greening Shrubs to Water Stress
3.3. Climatic and Phenological Drivers of Seasonal Color Variation
3.4. Research Limitations and Outlook
4. Materials and Methods
4.1. Study Site
4.2. Experimental Materials
4.3. Experimental Design
4.4. Determination and Calculation of Leaf Color Parameters
4.5. Determination of Soil Water Content
4.6. Response Ratio
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. United Nations 2023 Water Conference Mid-Term Review of the Water Action Decade: Key Messages from the United Nations Regional Commissions; United Nations: Bangkok, Thailand, 2023. [Google Scholar]
- Escobedo, F.J.; Giannico, V.; Jim, C.Y.; Sanesi, G.; Lafortezza, R. Urban forests, ecosystem services, green infrastructure and nature-based solutions: Nexus or evolving metaphors? Urban For. Urban Green. 2019, 37, 3–12. [Google Scholar] [CrossRef]
- Neale, C.; Griffiths, A.; Chalmin-Pui, L.S.; Mendu, S.; Boukhechba, M.; Roe, J. Color aesthetics: A transatlantic comparison of psychological and physiological impacts of warm and cool colors in garden landscapes. Wellbeing Space Soc. 2021, 2, 100038. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Ugolini, F.; Tognetti, R.; Bussotti, F.; Raschi, A.; Ennos, A.R. Wood hydraulic and mechanical properties induced by low water availability on two ornamental species Photinia×fraseri var. Red Robin and Viburnum opulus L. Urban For. Urban Green. 2014, 13, 158–165. [Google Scholar] [CrossRef]
- Livesley, S.J.; Marchionni, V.; Cheung, P.K.; Daly, E.; Pataki, D.E. Water Smart Cities Increase Irrigation to Provide Cool Refuge in a Climate Crisis. Earth’s Future 2021, 9, e2020EF001806. [Google Scholar] [CrossRef]
- Li, X. Chlorophyll and Carotenoid Metabolism Varies with Growth Temperatures among Tea Genotypes with Different Leaf Colors in Camellia sinensis. Int. J. Mol. Sci. 2024, 25, 10772. [Google Scholar] [CrossRef]
- Xu, C.; Liu, H.; Williams, A.P.; Yin, Y.; Wu, X. Trends toward an earlier peak of the growing season in Northern Hemisphere mid-latitudes. Glob. Change Biol. 2016, 22, 2852–2860. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, Y.; Qu, H. Physiological and psychological responses to tended plant communities with varying color characteristics. J. For. Res. 2024, 35, 32. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase–mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of Anthocyanins in Plant Tolerance to Drought and Salt Stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Vollmann, J.; Walter, H.; Sato, T.; Schweiger, P. Digital image analysis and chlorophyll metering for phenotyping the effects of nodulation in soybean. Comput. Electron. Agric. 2011, 75, 190–195. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, N.; Kaiser, E.; Li, G.; An, D.; Sun, Q.; Chen, W.; Liu, W.; Luo, W. Integrating chlorophyll fluorescence parameters into a crop model improves growth prediction under severe drought. Agric. For. Meteorol. 2021, 303, 108367. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. Detection of nutrition deficiencies in plants using proximal images and machine learning: A review. Comput. Electron. Agric. 2019, 162, 482–492. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Lu, X.; Zhao, X.; Yang, L.; Sun, Z.; Bai, Y. Hyperspectral retrieval of leaf physiological traits and their links to ecosystem productivity in grassland monocultures. Ecol. Indic. 2021, 122, 107267. [Google Scholar] [CrossRef]
- Sirisathitkul, Y.; Natthinee, D.; Wannasan, N.; Sirisathitkul, C. Accuracy and precision of smartphone colorimetry: A comparative analysis in RGB, HSV, and CIELAB color spaces for archaeological research. STAR Sci. Technol. Archaeol. Res. 2025, 11, e2444168. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gratani, L.; Varone, L. Leaf key traits of Erica arborea L., Erica multiflora L. and Rosmarinus officinalis L. co-occurring in the Mediterranean maquis. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 58–69. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gamon, J.A. Remote sensing of plant functional types. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.-L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef]
- Garrity, S.R.; Bohrer, G.; Maurer, K.D.; Mueller, K.L.; Vogel, C.S.; Curtis, P.S. A comparison of multiple phenology data sources for estimating seasonal transitions in deciduous forest carbon exchange. Agric. For. Meteorol. 2011, 151, 1741–1752. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Dao, P.D.; He, Y.; Proctor, C. Plant drought impact detection using ultra-high spatial resolution hyperspectral images and machine learning. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102364. [Google Scholar] [CrossRef]
- Singh, A.K.; Ganapathysubramanian, B.; Sarkar, S.; Singh, A. Deep Learning for Plant Stress Phenotyping: Trends and Future Perspectives. Trends Plant Sci. 2018, 23, 883–898. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Horike, H.; Kinoshita, T.; Kume, A.; Hanba, Y.T. Responses of leaf photosynthetic traits, water use efficiency, and water relations in five urban shrub tree species under drought stress and recovery. Trees 2023, 37, 53–67. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Liu, F.; Yang, W.; Wang, Z.; Xu, Z.; Liu, H.; Zhang, M.; Liu, Y.; An, S.; Sun, S. Plant size effects on the relationships among specific leaf area, leaf nutrient content, and photosynthetic capacity in tropical woody species. Acta Oecologica 2010, 36, 149–159. [Google Scholar] [CrossRef]
- Nolan, R.H.; Foster, B.; Griebel, A.; Choat, B.; Medlyn, B.E.; Yebra, M.; Younes, N.; Boer, M.M. Drought-related leaf functional traits control spatial and temporal dynamics of live fuel moisture content. Agric. For. Meteorol. 2022, 319, 108941. [Google Scholar] [CrossRef]
- Zhang, X.; Zang, R.; Li, C. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Sci. 2004, 166, 791–797. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Comparative field study of Quercus ilex and Phillyrea latifolia: Photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003, 50, 137–148. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H. Climate controls on vegetation phenological patterns in northern mid- and high latitudes inferred from MODIS data. Glob. Change Biol. 2004, 10, 1133–1145. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Lee, D.W.; Gould, K.S. Anthocyanins in leaves and other vegetative organs: An introduction. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2002; Volume 37, pp. 1–16. [Google Scholar]
- Gould, K.S. Nature′s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. BioMed Res. Int. 2004, 2004, 415423. [Google Scholar] [CrossRef]
- Yahdjian, L.; Sala, O.E. A rainout shelter design for intercepting different amounts of rainfall. Oecologia 2002, 133, 95–101. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Wang, W.; Hu, G.; Wu, X.; Song, Z. Effects of manipulated precipitation on aboveground net primary productivity of grassland fields: Controlled rainfall experiments in Inner Mongolia, China. Land Degrad. Dev. 2021, 32, 1981–1992. [Google Scholar] [CrossRef]
- Li, J.; Lu, S.; Li, S.; Li, B.; Hou, L.; Zhao, N.; Xu, X. Seasonal Photosynthetic and Water Relation Responses of Three Cool Temperate Garden Shrubs to Drought Stress. Agronomy 2024, 14, 1772. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Forkel, M.; Wingate, L.; Tomelleri, E.; Morra di Cella, U.; Richardson, A.D. Phenopix: A R package for image-based vegetation phenology. Agric. For. Meteorol. 2016, 220, 141–150. [Google Scholar] [CrossRef]
- Gillespie, A.R.; Kahle, A.B.; Walker, R.E. Color enhancement of highly correlated images. II. Channel ratio and “chromaticity” transformation techniques. Remote Sens. Environ. 1987, 22, 343–365. [Google Scholar] [CrossRef]




| Source | df | MS | F-Value | p-Value | Partial η2 |
|---|---|---|---|---|---|
| Species | 2 | 0.001 | 1.566 | 0.210 | 0.008 |
| Season | 2 | 0.045 | 53.859 | <0.001 | 0.207 |
| Treatment | 3 | 0.014 | 16.890 | <0.001 | 0.109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Xu, X.; Huang, W.; Li, J.; Li, S.; Zhao, N.; Li, B.; Xu, C.; Lu, S. RGB Imaging and Irrigation Management Reveal Water Stress Thresholds in Three Urban Shrubs in Northern China. Plants 2025, 14, 2253. https://doi.org/10.3390/plants14152253
Niu Y, Xu X, Huang W, Li J, Li S, Zhao N, Li B, Xu C, Lu S. RGB Imaging and Irrigation Management Reveal Water Stress Thresholds in Three Urban Shrubs in Northern China. Plants. 2025; 14(15):2253. https://doi.org/10.3390/plants14152253
Chicago/Turabian StyleNiu, Yuan, Xiaotian Xu, Wenxu Huang, Jiaying Li, Shaoning Li, Na Zhao, Bin Li, Chengyang Xu, and Shaowei Lu. 2025. "RGB Imaging and Irrigation Management Reveal Water Stress Thresholds in Three Urban Shrubs in Northern China" Plants 14, no. 15: 2253. https://doi.org/10.3390/plants14152253
APA StyleNiu, Y., Xu, X., Huang, W., Li, J., Li, S., Zhao, N., Li, B., Xu, C., & Lu, S. (2025). RGB Imaging and Irrigation Management Reveal Water Stress Thresholds in Three Urban Shrubs in Northern China. Plants, 14(15), 2253. https://doi.org/10.3390/plants14152253







