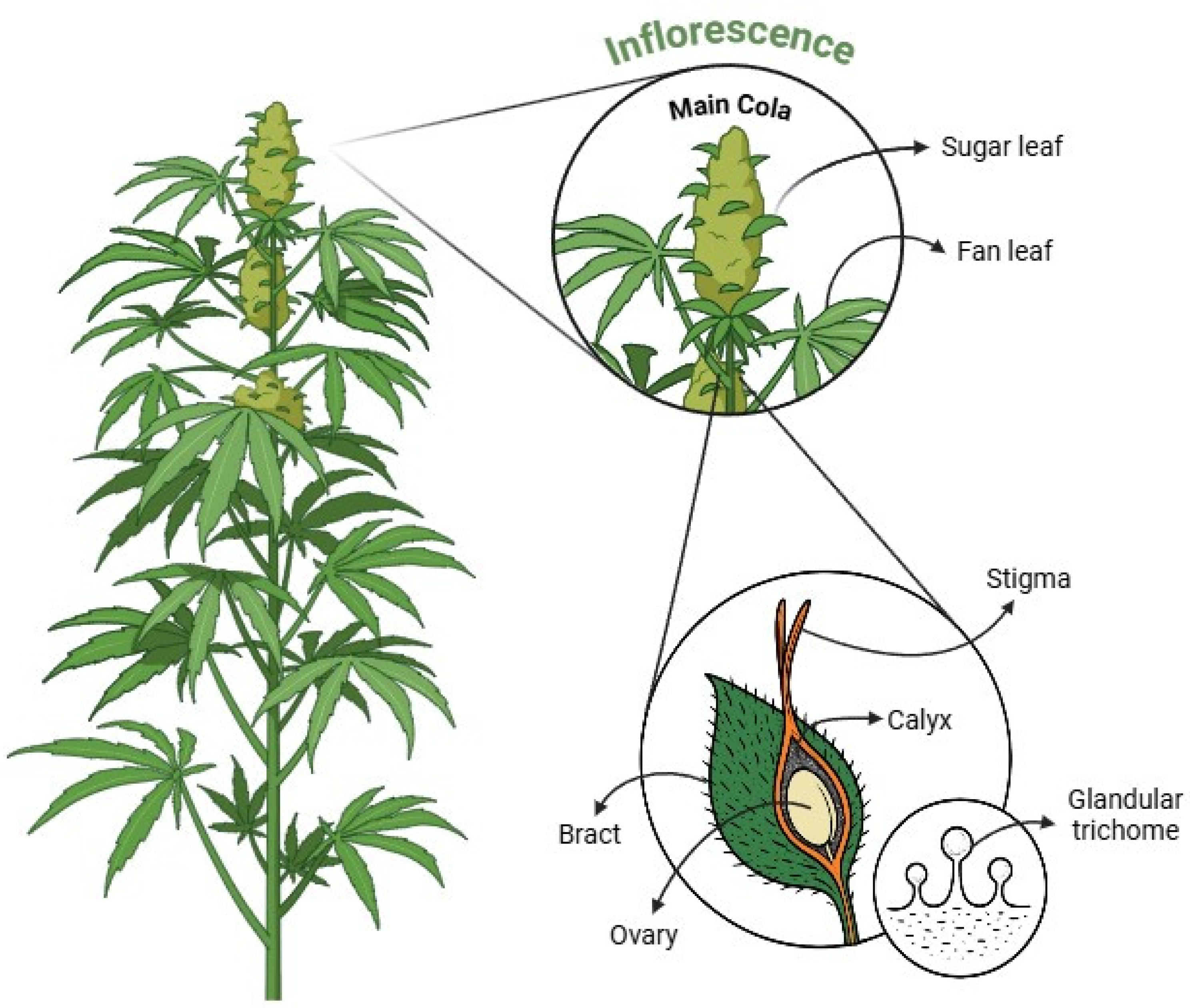
2. Morphological Targets for Trichome Quantification in Cannabis sativa
Accurate trichome quantification in
C. sativa is often challenging due to the uneven distribution of trichomes across different plant structures. Consequently, researchers and cultivators use various methods to determine where and how to count trichomes. Regardless of the chosen method, consistent sampling protocols and clearly defined reference areas are vital for reproducibility. In the following sections, we examine how trichome density varies across key cannabis structures, as depicted in
Figure 3, including the main cola, bracts, calyxes, and sugar leaves, and discuss the merits and limitations of focusing on each site for reliable trichome analysis.
2.1. Main Cola and Its Substructures for Trichome Analysis
As illustrated in
Figure 4, the main cola, or apical bud, stands out as the plant’s primary flowering site and is frequently cited as having one of the highest trichome densities [
13]. This dense coverage is often attributed to favorable light exposure at the canopy’s apex, and with preferential nutrient allocation during flowering. However, trichome distribution within the main cola is not uniform, reflecting micro-environmental variations (e.g., light penetration, airflow) and internal plant dynamics [
9]. To address this variability, researchers commonly recommend standardized sampling protocols, such as selecting a consistent 1–2 cm section of the upper cola [
10]. This approach helps capture a comparable physiological zone across samples, reducing intra- and inter-plant variability.
Importantly, even when focusing on the main cola, it is necessary to define which specific substructures—such as upper bracts, calyxes, or sugar leaf tips—are being analyzed. Providing the definition enhances accuracy in trichome quantification and facilitates cross-study comparisons. By combining well-defined sampling sites with digital imaging tools, researchers can further reduce observer bias and obtain more reliable data.
2.2. Bracts
Bracts in cannabis are small, leaf-like structures that surround and protect the reproductive organs of the plant (
Figure 5). They are often described as the most reliable site for trichome analysis due to their dense and homogeneous resin coverage. Unlike other floral structures, bracts display a relatively consistent distribution of glandular trichomes, making them particularly suitable for microscopic imaging and cannabinoid quantification [
12]. In addition, bracts are larger and more exposed than inner floral parts; they provide an accessible and efficient target for cannabinoid research. This accessibility streamlines the quantification of cannabinoids and terpenes, which in turn facilitates both breeding research and commercial quality control efforts [
5].
The protective role of bracts aligns with their high density of glandular trichomes. The secondary metabolites produced in these trichomes not only deter herbivores and pathogens but also form the basis for the plant’s medicinal and recreational value [
10]. As a result, focusing on bracts offers researchers and cultivators insight into one of the primary sites of cannabinoid and terpene biosynthesis. This dual role makes bracts a particularly appealing target for those aiming to optimize resin output or investigate the genetic and physiological mechanisms of trichome development.
By combining consistent bract sampling with rigorous imaging protocols, studies can achieve high reproducibility of trichome density measurements, an essential requirement for both breeding programs and product standardization. For these reasons, bracts represent one of the most reliable benchmarks for comparing genotypic variations and environmental influences on trichome production in C. sativa.
2.3. Calyxes
Although the term
bract is sometimes used interchangeably with
bractlet or
calyx, botanical classifications distinguish bracts as distinct structures that envelop the developing flowers and seeds [
14]. Calyx’s primary function is to protect the plant’s reproductive components, and it generally lacks the glandular trichomes abundant on the surrounding bracts. The term
calyx in
C. sativa remains a point of contention, as some sources equate it with the bract. In contrast, others view it as a distinct inner structure that encases the ovule [
14,
15], reflecting overlapping morphology and inconsistent usage in the literature. Nevertheless, the generally accepted definition positions the calyx as an inward, protective layer situated within the bracts, playing a critical role in the development of the reproductive organs.
Despite logistical challenges, calyxes are important due to their role in enclosing the ovules. However, since calyxes potentially lack the glandular trichomes responsible for resin production, their direct contribution to cannabinoid and terpene biosynthesis is limited compared to bracts. Therefore, any methodologies referring to trichome analysis on calyxes likely involve bracts due to the morphological confusion and the absence of glandular trichomes on the true calyx. It is also important to note that, to date, there are no standardized studies on sampling and quantification protocols specifically for calyxes, distinct from those used for bracts, which contributes to variability in reported data.
2.4. Sugar Leaves
Sugar leaves, as shown in
Figure 6, are the small, “frosty” leaves that surround cannabis buds and represent another key site for trichome density analysis. These leaves can contribute significantly to overall trichome yield [
15], with higher densities observed closer to their tips, an attribute that makes them particularly valuable for cannabinoid extraction. In addition to their role as trichome-bearing structures, sugar leaves retain some photosynthetic capacity, which may support local energy and nutrient supply for developing buds and resin production [
11]. Although their photosynthetic contribution is modest compared to larger fan leaves, the combination of limited photosynthesis and high trichome content in sugar leaves makes them an intriguing focus for both morphological and biochemical analyses in cannabis research.
Sugar leaves exhibit a less uniform distribution of trichomes than bracts, with elevated densities being found at the margins and tips. This variability requires meticulous sample techniques, often focusing on areas rich in trichomes, to ensure precise density assessments. Whilst sugar leaves lack the uniformity of bracts, their inclusion in trichome analysis offers valuable supplementary data, especially for evaluating whole-plant cannabinoid concentration and understanding spatial variability in trichome production across different leaf areas and developmental stages.
4. Discussion
Establishing reliable methods for trichome density analysis remains central to Cannabis sativa L. research, given that secondary metabolites, primarily cannabinoids and terpenes, accumulate in these glandular structures.
Whilst the present review focuses on identifying reliable sampling targets for trichome quantification, it also highlights the methodological gaps that hinder standardization. Current approaches are predominantly manual or semi-automated, relying on dissecting microscopy and software for counting trichomes in defined areas. Although advanced tools, such as scanning electron microscopy, confocal imaging, and machine learning-based detection, offer high-resolution and high-throughput alternatives, they remain underutilized and largely unvalidated in cannabis research. Comparative studies employing multiple quantification techniques across cultivars and growth conditions are still lacking, limiting confidence in cross-study reproducibility. Therefore, despite progress in imaging and computational tools, the field urgently requires consensus on validated, reproducible methods tailored to cannabis trichome analysis.
Among the plant tissues evaluated, bracts consistently demonstrate the highest reproducibility due to their relatively uniform trichome distribution and significant cannabinoid content [
10]. This consistency also translates into lower plant-to-plant variance, a critical attribute for industrial-scale applications, where maintaining batch consistency directly impacts market value and therapeutic efficacy [
11].
Still, sugar leaves and calyxes offer valuable perspectives depending on experimental objectives. Sugar leaves, although less uniform in trichome dispersion, can illuminate extraction efficiency since their edges and tips often host abundant glandular heads [
15]. Calyxes, despite posing sampling challenges due to their smaller size and proximity to reproductive tissues, represent strategic focal points for investigating cannabinoid biosynthesis pathways [
14]. In contrast, although visually prominent, the main cola can exhibit substantial variability in trichome coverage; hence, standardization for this region typically involves precisely defined substructures (e.g., uppermost bracts) rather than the entire apical bud [
13].
Despite trichomes’ pivotal role, higher density alone does not guarantee greater cannabinoid potency [
12]. Recent studies underscore that while trichome density is a valuable morphological marker, it does not consistently predict cannabinoid content across genotypes. Multiple studies, including recent metabolomic and transcriptomic work, confirm that biosynthetic enzyme activity, genotypic expression profiles, and resin storage capacity are equally, if not more, influential in determining the final amounts of cannabinoids [
8,
10]. A cultivar with moderate trichome density but upregulated synthase genes may surpass a denser but genetically less active cultivar in total cannabinoid contents. Consequently, relying solely on trichome counting could obscure the true biochemical potential of a given plant genotype. Integrating morphological data (trichome density, distribution, shape, and size) with chemical assays (cannabinoid and terpene identification and quantification) thus provides a more robust assessment of cannabis potency and value.
From a breeding standpoint, uniform and precise trichome density metrics enable phenotypic selection for cultivars that exhibit stable, high-resin traits under diverse environmental conditions [
9]. Furthermore, environmental factors such as plant density and architecture significantly influence cannabinoid uniformity. Studies indicate that higher planting densities can reduce cannabinoid uniformity across different parts of the plant, although they may increase overall yield per area. Architectural modifications, such as defoliation or pruning, have been shown to mitigate some of these effects, thereby enhancing chemical standardization [
16].
Moreover, anatomical and morphological differences among cannabis trichomes have been linked to variations in metabolite profiles. Microcapillary sampling of individual glandular trichomes revealed that stalked trichomes on floral tissues predominantly accumulate monoterpenes, with monoterpene-to-sesquiterpene ratios exceeding 12:1. In contrast, sessile trichomes on vegetative leaves exhibited lower monoterpene content and higher sesquiterpene proportions. Interestingly, despite these differences in terpene profiles, cannabinoid compositions between stalked and sessile trichomes were found to be similar. These findings underscore the importance of considering trichome type and sampling location when analyzing secondary metabolite profiles, as morphological variations can significantly influence terpene composition, whilst cannabinoid content may remain relatively consistent across trichome types. The study by Livingston et al. (2020) [
11] examines the dynamic changes in cannabis trichome morphology and their relationship with metabolite content throughout the plant’s maturation, offering insights into the underlying mechanisms that drive metabolite synthesis.
Adding to this, Ferri et al. (2024) [
17] demonstrated that even individual glandular trichomes isolated from the same cannabis flower can display detectable variations in their phytocannabinoid profiles. In their study, single trichomes from two
Cannabis sativa L. varieties—a medicinal strain (FM2) and a CBD-rich hemp cultivar—were analyzed using an untargeted metabolomics approach. Around 70 phytocannabinoids, including carboxylated and decarboxylated forms of CBD, THC, and CBG, were identified. Although the profiles of isolated trichomes and whole inflorescences were broadly similar, absolute cannabinoid levels varied among trichomes, while the CBD-to-THC ratio remained consistent, reflecting genetic chemotypes. These results highlight that chemical variability exists even within a single anatomical region, suggesting that differences across broader plant structures, such as leaves, bracts, and flowers, could be even more significant. Despite the overall consistency observed between isolated trichomes and whole flower extracts, subtle differences reinforce the hypothesis that cannabinoid biosynthesis is influenced not only by genetics but also by tissue-specific factors. Therefore, taking into account more than one floral structure mentioned in this review could increase trichome density reliability when correlating it with total cannabinoid concentration.
Despite ongoing advancements in microscopic imaging and software-assisted quantification, standardizing sampling protocols still faces significant hurdles. Variability introduced by genotype, developmental stage, and environmental factors often leads to inconsistent data across research labs and commercial operations [
5,
9]. Moreover, cultivars can manifest unique developmental timelines for trichome initiation, and environmental stressors (e.g., UV-B light, nutrient shifts) can differentially modulate secondary metabolite biosynthesis and accumulation [
8]. Such variability underscores the need for harmonized guidelines, including standardized sampling areas (e.g., 1 mm
2 or 2 mm
2 sectors), consistent plant growth stages (e.g., late flowering or post-harvest), and documented environmental parameters (e.g., light intensity and humidity ranges).
Microscopic imaging (optical, confocal, electron) combined with macro-photographic methods improves the resolution and accuracy of trichome assessments. Platforms like ImageJ or custom machine learning algorithms help reduce observer bias, permitting faster and more reproducible data collection [
5]. However, uniform calibration of imaging equipment and consistent magnification levels are crucial for cross-study comparability [
10]. Tanney et al. (2021) [
5] demonstrated that portable Raman spectroscopy can non-destructively and rapidly profile cannabinoids and terpenes in trichomes without extensive sample preparation, offering a powerful tool for real-time, in situ analysis. In parallel, machine learning approaches have been utilized to automate trichome identification and quantification from microscopic images, as demonstrated by Ebersbach et al. (2018) [
18] in Arabidopsis, thereby improving throughput and reducing observer bias—methods that could be adapted for cannabis research. Comparative studies employing multiple quantification techniques—such as manual counting, ImageJ analysis, and AI-based methods—across various cannabis cultivars under controlled conditions are warranted to validate the reliability and reproducibility of these approaches.
As regulatory frameworks evolve worldwide, robust methods for trichome evaluation will be increasingly important not only for academic research but also for quality control in commercial settings. Validated trichome density assays may become integral to legal compliance, product labeling, and ensuring batch-to-batch consistency, particularly in medicinal markets where patients rely on reproducible dosage [
11]. Also, it creates a metric for a trait that is desirable to consumers. The development and adoption of standardized best practices will be essential for advancing
Cannabis sativa as a scientifically validated crop.
5. Conclusions
Establishing standardized methods for trichome density assessment remains a critical priority for advancing cannabis research.
This review highlights that, among different plant tissues, bracts stand out as the most reliable sampling site due to their consistent glandular coverage and high cannabinoid concentrations—key attributes for reproducible and robust quantification. Nevertheless, morphological evaluations alone are insufficient to fully capture the potency and chemical variability of cannabis. Moreover, using several floral structures or tissues could increase the relevance and accuracy of the whole-plant cannabinoid total concentration. Future methodologies must integrate trichome density, distribution, shape, and size metrics with biochemical profiling, thereby reflecting not only anatomical structure but also tissue-specific metabolic potential. Advanced imaging technologies, automated quantification tools, and harmonized sampling protocols will be crucial for enhancing reproducibility across both research and industrial applications. As the cannabis industry matures and regulatory frameworks evolve, validated trichome analysis methods will play an increasingly central role in quality control, cultivar selection, and therapeutic product development. Building a foundation of validated, multidimensional assessment practices, including morphological, chemical, and computational tools, will be key to unlocking the full scientific and commercial potential of Cannabis sativa.