An Overview of Buckwheat—A Superfood with Applicability in Human Health and Food Packaging
Abstract
1. Introduction
1.1. Brief History and Buckwheat Varieties
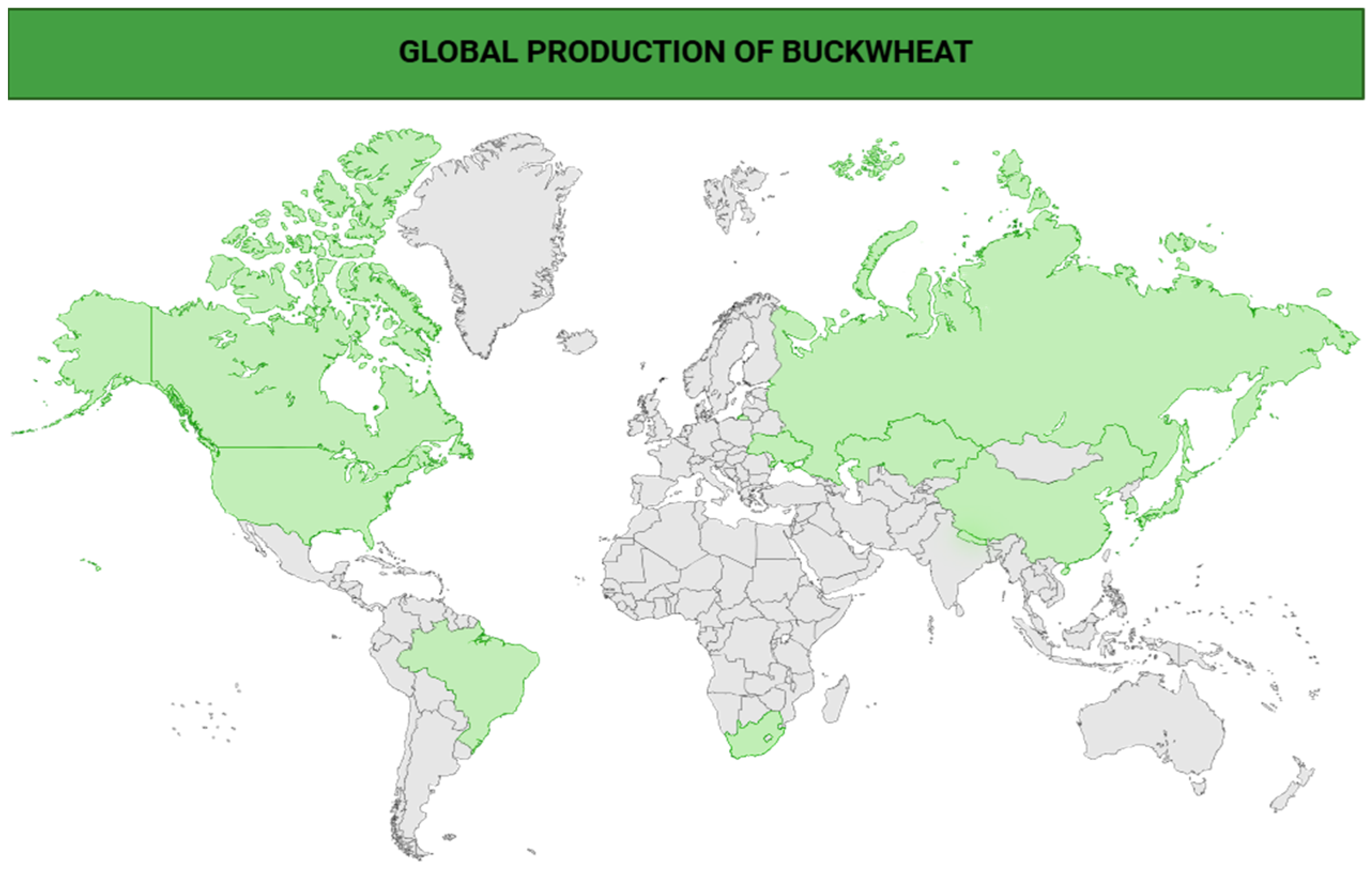
1.2. Cultivation and Production
1.3. Buckwheat Sustainability
2. Chemical Composition
2.1. Carbohydrates
2.2. Proteins
2.3. Dietary Fibers
2.4. Lipids
2.5. Vitamins and Minerals
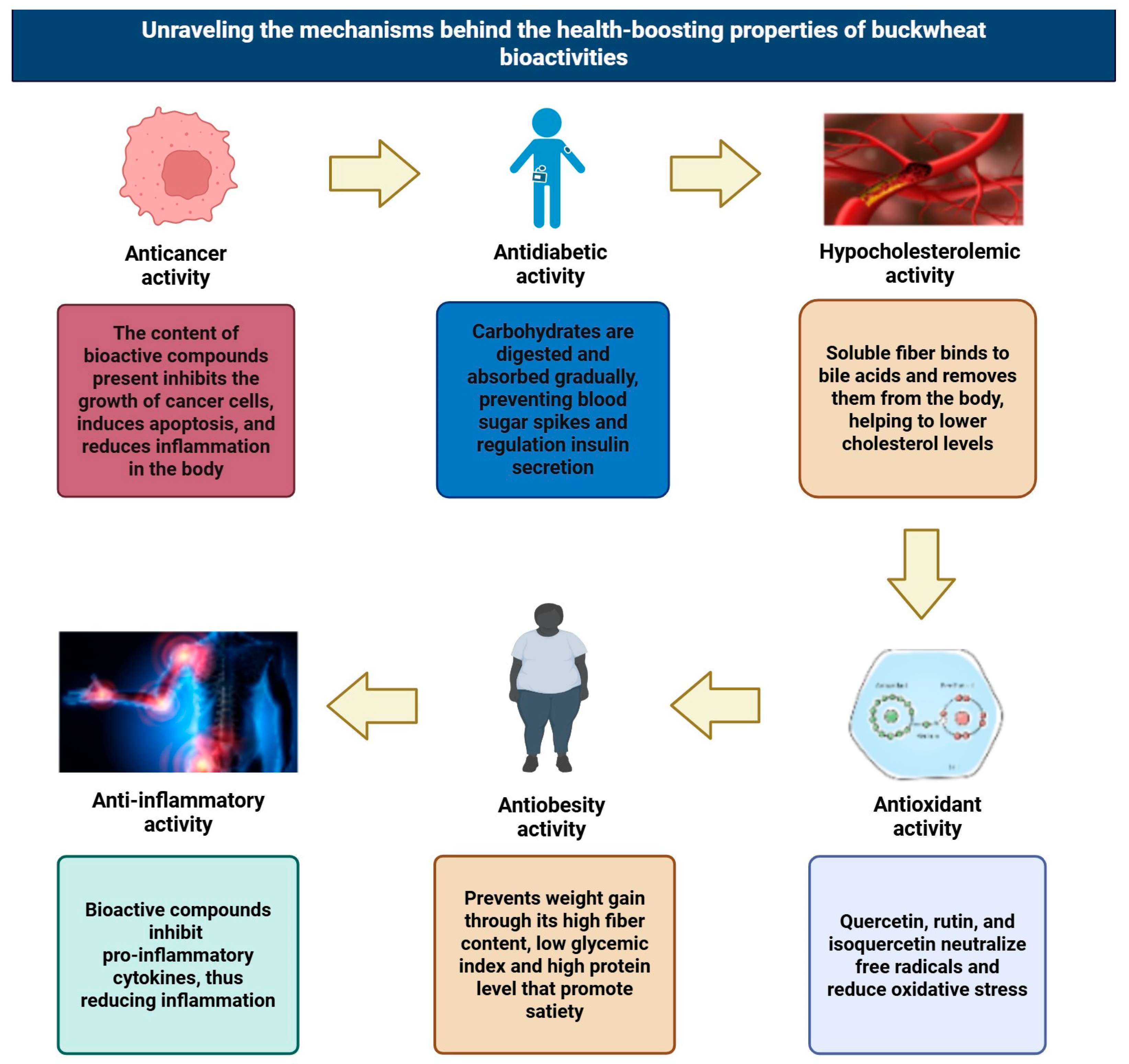
3. Bioactive Compounds, Health Effects, and Thermostability
| Category | Compounds | Detection Methods | Potential Health Benefits | Concentration | References |
|---|---|---|---|---|---|
| Components | |||||
| Flavonoids | Rutin | HPLC | Antioxidant Anti-inflammatory Cardiovascular and neuroprotective properties; antibacterial Prevention of human hepatic cancer | 1–2 mg/g in leaves, flower, bran | [4,91,92] |
| Quercetin | 0.02–0.30 mg/g in leaves, flower, seeds, flour | ||||
| Isoquercetin | 0.50–1.50 mg/g in leaves, flower, bran, flour | ||||
| Anthocyanins | Peonidin, petunidin 3-O-glucoside, cyanidin, cyanidin-3-O-glucoside, cyanidin-O-syringic acid, cyanidin-3-O-glucosyl-malonylglucoside | UPLC-ESI-MS/MS | Antioxidant Prevent colon, breast, and prostate cancer and cardiovascular and neurodegenerative diseases Diabetes prevention | 1–3 mg/g in leaves, flowers, shell, brain | [93] |
| Phenolic acids | Gallic acid | RP-UHPLC-ESI/MS | Cardioprotective Antioxidant effects Anti-inflammatory properties Cancer prevention Brain health and neuroprotection Blood sugar control Diabetes prevention | 0.10–0.40 mg/g in bran, seeds | [92,94] |
| Ferulic acid | 0.05–0.15 mg/g in bran and shell | ||||
| 4-Hydrobenzoic acid | 0.05–0.20 mg/g in bran | ||||
| Isovanilic acid | 0.02–0.1 mg/g in bran, whole grains | ||||
| Caffeic acid | 0.10–0.50 mg/g in leaves, flowers, and bran | ||||
| Chlorogenic acid | 0.10–0.50 mg/g in seeds | ||||
| Fagopyrins | Fagopyrin A-F | NMRS-MS | Reduce risk of cardiovascular disease Anticancer properties | 3–5 mg/g in leaves, flower, stem | [4,95] |
| Fagopyritol Steroids | Fagopyritol A1 and B1 | GC-MS NMRS | Reduce risk of cardiovascular disease Anticancer properties | 1.50–6.0 mg/g in bran, peeled buckwheat | [95] |
| β-sitosterol, β-sitosterol palmitate, daucosterol, ergosterol peroxide, stigmsat-4-en 3,6-dione, stigmast-5-en-3-ol | 0.60–1.0 mg/g in bran, whole seeds, peeled buckwheat | ||||
| Triterpenoids Phenylpropanoid glycosides | Glutinone, glutinol, olean-12-en-3-ol and urs-12-an-3-ol | HPLC-PDA/LTQ-FTICR-MS | Anti-inflammatory properties Reduce risk of cardiovascular diseases. | 0.20–0.70 mg/g in bran, whole seeds | [10,51] |
| Diboside A and tatarisides A-G | 0.50–1.50 mg/g in leaves, flowers, bran, whole seeds | ||||
| Proteins Carbohydrates | Amino acid compositions | HPLC IRS GC NMRS | Better muscle growth and repair Blood sugar control Digestive health Antitumor Hypolipidemic Antidiabetic | 14–18% in bran, whole seeds, peeled buckwheat | [93,96] |
| Polysaccharides and monosaccharides | 70–80% in whole seeds 72–75% in peeled buckwheat 60–65% in bran 10–20% in leaves and flowers | ||||
| Fatty acids | D-chiro-inositol | GLC GC | Improve heart health Improve brain function Skin health | 120–200 mg/g in bran, shell 90–150 mg/g in whole seeds 50–100 mg/g in peeled buckwheat | [10,34,96,97,98] |
| Fatty acid composition | 4–6% in bran | ||||
| Free fatty acid composition | 1.5–2.5% in bran | ||||
| Vitamins | Vitamin B1 | HPLC | Improve immune function Improve eye health Reduce inflammation Skin health | 0.0003–0.0060 mg/g in grains and bran | [97,98] |
| Vitamin B6 | |||||
| Vitamin C | 0.0003–0.0025 mg/g in leaves, flowers, bran | ||||
| Carotenoids | Lutein | ||||
| β-carotene |
4. Buckwheat Starch—A Complex-Based Film
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of Global Tartary Buckwheat Accessions Reveals Multiple Domestication Events and Key Loci Associated with Agronomic Traits. Genome Biol. 2021, 22, 23. [Google Scholar] [CrossRef]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present Status and Future Perspectives of Breeding for Buckwheat Quality. Breed. Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from Garden: Bioactive Compounds of Buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Joshi, D.C.; Zhang, K.; Wang, C.; Chandora, R.; Khurshid, M.; Li, J.; He, M.; Georgiev, M.I.; Zhou, M. Strategic Enhancement of Genetic Gain for Nutraceutical Development in Buckwheat: A Genomics-Driven Perspective. Biotechnol. Adv. 2020, 39, 107479. [Google Scholar] [CrossRef]
- Boukid, F.; Folloni, S.; Sforza, S.; Vittadini, E.; Prandi, B. Current Trends in Ancient Grains-Based Foodstuffs: Insights into Nutritional Aspects and Technological Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal Grains: Nutritional Value, Health Benefits and Current Applications for the Development of Gluten-Free Foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef] [PubMed]
- Graziano, S.; Agrimonti, C.; Marmiroli, N.; Gullì, M. Utilisation and Limitations of Pseudocereals (Quinoa, Amaranth, and Buckwheat) in Food Production: A Review. Trends Food Sci. Technol. 2022, 125, 154–165. [Google Scholar] [CrossRef]
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A Novel Source of Biologically Active Peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1537–1544. [Google Scholar] [CrossRef]
- Wieslander, G. Buckwheat in Human Health—A Medical Review/Ajda in Zdravje Ljudi—Pregledni Članek. Folia Biol. Geol. 2020, 61, 55–60. [Google Scholar] [CrossRef]
- Zhu, F. Chemical Composition and Health Effects of Tartary Buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Liu, C.; Shi, L.; Li, J.; Zeng, Y.; Guo, L.; Wang, S. Identification of Medicinal Compounds of Fagopyri Dibotryis Rhizome from Different Origins and Its Varieties Using UPLC-MS/MS-Based Metabolomics. Metabolites 2022, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.; Piskuła, M.; Zieliński, H. Recent Advances in Processing and Development of Buckwheat Derived Bakery and Non-Bakery Products—A Review. Pol. J. Food Nutr. Sci. 2015, 65, 9–20. [Google Scholar] [CrossRef]
- Chiş, M.S.; Păucean, A.; Man, S.M.; Vodnar, D.C.; Teleky, B.-E.; Pop, C.R.; Stan, L.; Borsai, O.; Kadar, C.B.; Urcan, A.C.; et al. Quinoa Sourdough Fermented with Lactobacillus plantarum ATCC 8014 Designed for Gluten-Free Muffins—A Powerful Tool to Enhance Bioactive Compounds. Appl. Sci. 2020, 10, 7140. [Google Scholar] [CrossRef]
- Hassan, H.F. Perceptions towards Gluten Free Products among Consumers: A Narrative Review. Appl. Food Res. 2024, 4, 100441. [Google Scholar] [CrossRef]
- Dalal, A. Nutritional, Functional, and Bioactive Characterization of Gluten-Free Noodles Using Germinated Buckwheat Flour. Cereal Res. Commun. 2025, 53, 123–125. [Google Scholar] [CrossRef]
- Sturza, A.; Păucean, A.; Chiș, M.S.; Mureșan, V.; Vodnar, D.C.; Man, S.M.; Urcan, A.C.; Rusu, I.E.; Fostoc, G.; Muste, S. Influence of Buckwheat and Buckwheat Sprouts Flours on the Nutritional and Textural Parameters of Wheat Buns. Appl. Sci. 2020, 10, 7969. [Google Scholar] [CrossRef]
- Thakur, D.; Kumar, Y.; Sharanagat, V.S.; Srivastava, T.; Saxena, D.C. Development of pH-Sensitive Films Based on Buckwheat Starch, Critic Acid and Rose Petal Extract for Active Food Packaging. Sustain. Chem. Pharm. 2023, 36, 101236. [Google Scholar] [CrossRef]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and Amaranth as Functional Foods: A Review. Food Rev. Int. 2023, 39, 2277–2296. [Google Scholar] [CrossRef]
- Békés, F.; Schoenlechner, R.; Tömösközi, S. Ancient Wheats and Pseudocereals for Possible Use in Cereal-Grain Dietary Intolerances. In Cereal Grains; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–389. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional Constituents of Pseudo Cereals and Their Potential Use in Food Systems: A Review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Hunt, H.V.; Shang, X.; Jones, M.K. Buckwheat: A Crop from Outside the Major Chinese Domestication Centres? A Review of the Archaeobotanical, Palynological and Genetic Evidence. Veg. Hist. Archaeobotany 2017, 27, 493–506. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Deng, X.; Ruan, C.; Kreft, I.; Tang, Y.; Wu, Y. Overview of Buckwheat Resources in the World. In Buckwheat Germplasm in the World; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Reddy, M.V.K.; Dubey, P.K.; Mishra, A.A.; Ahada Sabeel, V. Potential Processing Techniques for Safe Utilisation of Pseudo Cereals in the Food System. J. Food Compos. Anal. 2024, 135, 106609. [Google Scholar] [CrossRef]
- Ohsako, T.; Li, C. Classification and Systematics of the Fagopyrum Species. Breed. Sci. 2020, 70, 93–100. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Fabjan, N. Nutritional Comparison of Tartary Buckwheat with Common Buckwheat and Minor Cereals. Acta Agric. Slov. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Vollmannová, A.; Kreft, S.; Luthar, Z. Phytochemistry, Bioactivities of Metabolites, and Traditional Uses of Fagopyrum tataricum. Molecules 2022, 27, 7101. [Google Scholar] [CrossRef]
- Wahlsten, A. Phenolic Compounds in Fagopyrum Sp. Grains (Buckwheat); Profile, Bioactivity and Effect of Processing. Mol. Vetenskaper 2019, 3, 8–34. [Google Scholar]
- Kreft, I.; Vollmannová, A.; Lidiková, J.; Musilová, J.; Germ, M.; Golob, A.; Vombergar, B.; Kocjan Ačko, D.; Luthar, Z. Molecular Shield for Protection of Buckwheat Plants from UV-B Radiation. Molecules 2022, 27, 5577. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Gerhardt, K.; Knicky, M.; Wendin, K. Buckwheat: An Underutilized Crop with Attractive Sensory Qualities and Health Benefits. Crit. Rev. Food Sci. Nutr. 2024, 64, 12303–12318. [Google Scholar] [CrossRef]
- FAO STAT. Crops and Livestock Products. 2025. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 March 2025).
- Jin, X.; He, C.; Guo, Z.; Li, Y.; Li, Y.; Gao, J.; Wang, M.; Han, L. The Hypoglycemic Activity of Buckwheat and the Underlying Mechanisms: A Mechanistic Review. Food Biosci. 2024, 62, 105046. [Google Scholar] [CrossRef]
- Swamy, K.R.M. Origin, domestication, taxonomy, botanical description, genetics and cytogenetics, genetic diversity and breeding of Buckwheat (Fagopyrum esculentum Moench). Int. J. Curr. Res. 2024, 16, 27028–27051. [Google Scholar] [CrossRef]
- Small, E. 54. Buckwheat—The World’s Most Biodiversity-Friendly Crop? Biodiversity 2017, 18, 108–123. [Google Scholar] [CrossRef]
- Sinkovič, L.; Deželak, M.; Kopinč, R.; Meglič, V. Macro/Microelements, Nutrients and Bioactive Components in Common and Tartary Buckwheat (Fagopyrum Spp.) Grain and Stone-Milling Fractions. LWT 2022, 161, 113422. [Google Scholar] [CrossRef]
- Tao, J.; Leng, J.; Lei, X.; Wan, C.; Li, D.; Wu, Y.; Yang, Q.; Wang, P.; Feng, B.; Gao, J. Effects of Selenium (Se) Uptake on Plant Growth and Yield in Common Buckwheat (Fagopyrum esculentum Moench). Field Crop. Res. 2023, 302, 109070. [Google Scholar] [CrossRef]
- Dębski, H.; Wiczkowski, W.; Szawara-Nowak, D.; Bączek, N.; Chrzanowski, G.; Horbowicz, M. Effects of glyphosate and fluazifop-P-butyl on flavonoids content and growth of common buckwheat (Fagopyrum esculentum Moench). Fresenius Environ. Bull. 2018, 27, 91–97. [Google Scholar]
- Martin-Guay, M.-O.; Paquette, A.; Dupras, J.; Rivest, D. The New Green Revolution: Sustainable Intensification of Agriculture by Intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Farooq, S.; Rehman, R.U.; Pirzadah, T.B.; Malik, B.; Dar, F.A.; Tahir, I. Cultivation, Agronomic Practices, and Growth Performance of Buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Elsevier: Amsterdam, The Netherlands, 2016; pp. 299–319. [Google Scholar] [CrossRef]
- Hore, D.; Rathi, R.S. Collection, Cultivation and Characterization of Buckwheat in Northeastern Region of India. Fagopyrum 2002, 19, 11–15. [Google Scholar]
- Björkman, T. Buckwheat Production: Planting. In Agronomy Fact Sheet Series; College of Agriculture and Life Sciences: College Station, TX, USA, 2010; pp. 1–2. [Google Scholar]
- Lee HanBum, L.H.; Lee KiCheol, L.K.; Kim SunLim, K.S.; Chang KwangJin, C.K.; Shin YoungBhum, S.Y.; Yoon KyoungMin, Y.K.; Kim NamSoo, K.N.; Park CheolHo, P.C. Productivity of the Whole Buckwheat Plant and Its Rutin Content under Different Quality of Light. In Proceedings of the 8th International Symposium on Buckwheat 2001, Chunchon, Republic of Korea, 30 August–2 September 2001. No. 84–89. [Google Scholar]
- Ratan, P.; Kothiyal, P. Fagopyrum esculentum Moench (Common Buckwheat) Edible Plant of Himalayas: A Review. Asian J. Pharm. Life Sci. 2011, 1, 426–442. [Google Scholar]
- Koskey, G.; Leoni, F.; Carlesi, S.; Avio, L.; Bàrberi, P. Exploiting Plant Functional Diversity in Durum Wheat–Lentil Relay Intercropping to Stabilize Crop Yields under Contrasting Climatic Conditions. Agronomy 2022, 12, 210. [Google Scholar] [CrossRef]
- Niro, S.; D’Agostino, A.; Fratianni, A.; Cinquanta, L.; Panfili, G. Gluten-Free Alternative Grains: Nutritional Evaluation and Bioactive Compounds. Foods 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, L.; Tallarico, R.; Mariotti, M.; Romagnoli, S.; Baglio, A.P.; Donnarumma, P.; Benedettelli, S. Agronomic and Nutritional Characteristics of Three Buckwheat Cultivars Under Organic Farming in Three Environments of the Garfagnana Mountain District. Ital. J. Agron. 2016, 11, 729. [Google Scholar] [CrossRef]
- Zargar, S.M.; Hami, A.; Manzoor, M.; Mir, R.A.; Mahajan, R.; Bhat, K.A.; Gani, U.; Sofi, N.R.; Sofi, P.A.; Masi, A. Buckwheat OMICS: Present Status and Future Prospects. Crit. Rev. Biotechnol. 2024, 44, 717–734. [Google Scholar] [CrossRef]
- Vieites-Álvarez, Y.; Reigosa, M.J.; Sánchez-Moreiras, A.M. A Decade of Advances in the Study of Buckwheat for Organic Farming and Agroecology (2013–2023). Front. Plant Sci. 2024, 15, 1354672. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jia, Z.; Hou, Y.; Ma, X.; Li, L.; Jin, X.; An, L. Roles of DNA Methylation in Cold Priming in Tartary Buckwheat. Front. Plant Sci. 2020, 11, 608540. [Google Scholar] [CrossRef]
- Sobhani, M.R.; Rahmikhdoev, G.; Mazaheri, D.; Majidian, M. Influence of Different Sowing Date and Planting Pattern and N Rate on Buckwheat Yield and Its Quality. Aust. J. Crop Sci. 2014, 8, 1402–1414. [Google Scholar]
- Sofi, S.A.; Ahmed, N.; Farooq, A.; Rafiq, S.; Zargar, S.M.; Kamran, F.; Dar, T.A.; Mir, S.A.; Dar, B.N.; Mousavi Khaneghah, A. Nutritional and Bioactive Characteristics of Buckwheat, and Its Potential for Developing Gluten-free Products: An Updated Overview. Food Sci. Nutr. 2023, 11, 2256–2276. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Li, H.-Q.; Hu, C.-L.; Jiang, Y.-P.; Qin, L.-P.; Zheng, C.-J. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. Int. J. Mol. Sci. 2016, 17, 589. [Google Scholar] [CrossRef]
- Kumar, H.; Guleria, S.; Kimta, N.; Dhalaria, R.; Nepovimova, E.; Dhanjal, D.S.; Alomar, S.Y.; Kuca, K. Amaranth and Buckwheat Grains: Nutritional Profile, Development of Functional Foods, Their Pre-Clinical Cum Clinical Aspects and Enrichment in Feed. Curr. Res. Food Sci. 2024, 9, 100836. [Google Scholar] [CrossRef]
- D’Amico, S.; Schoenlechner, R.; Tömösköszi, S.; Langó, B. Proteins and Amino Acids of Kernels. In Pseudocereals; Haros, C.M., Schonlechner, R., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 94–118. [Google Scholar] [CrossRef]
- Sonawane, S.; Shams, R.; Dash, K.K.; Patil, V.; Pandey, V.K.; Dar, A.H. Nutritional Profile, Bioactive Properties and Potential Health Benefits of Buckwheat: A Review. eFood 2024, 5, e171. [Google Scholar] [CrossRef]
- Jha, R.; Zhang, K.; He, Y.; Mendler-Drienyovszki, N.; Magyar-Tábori, K.; Quinet, M.; Germ, M.; Kreft, I.; Meglič, V.; Ikeda, K.; et al. Global Nutritional Challenges and Opportunities: Buckwheat, a Potential Bridge between Nutrient Deficiency and Food Security. Trends Food Sci. Technol. 2024, 145, 104365. [Google Scholar] [CrossRef]
- Liu, F.; He, C.; Wang, L.; Wang, M. Effect of Milling Method on the Chemical Composition and Antioxidant Capacity of Tartary Buckwheat Flour. Int. J. Food Sci. Technol. 2018, 53, 2457–2464. [Google Scholar] [CrossRef]
- Yu, D.; Chen, J.; Ma, J.; Sun, H.; Yuan, Y.; Ju, Q.; Teng, Y.; Yang, M.; Li, W.; Fujita, K.; et al. Effects of Different Milling Methods on Physicochemical Properties of Common Buckwheat Flour. LWT 2018, 92, 220–226. [Google Scholar] [CrossRef]
- Skřivan, P.; Chrpová, D.; Klitschová, B.; Švec, I.; Sluková, M. Buckwheat Flour (Fagopyrum esculentum Moench)—A Contemporary View on the Problems of Its Production for Human Nutrition. Foods 2023, 12, 3055. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic Chemical Composition and Bioactive Compounds Content in Selected Cultivars of Buckwheat Whole Seeds, Dehulled Seeds and Hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Bobkov, S. Biochemical and Technological Properties of Buckwheat Grains. In Molecular Breeding and Nutritional Aspects of Buckwheat; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–440. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional Composition and Flavonoids Content of Flour from Different Buckwheat Cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Taylor, J.; Campanella, O.H.; Hamaker, B.R. Functionality of the Storage Proteins in Gluten-Free Cereals and Pseudocereals in Dough Systems. J. Cereal Sci. 2016, 67, 22–34. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Zhou, M.-L.; Tang, Y.; Li, F.-L.; Tang, Y.-X.; Shao, J.-R.; Xue, W.-T.; Wu, Y.-M. Bioactive Compounds in Functional Buckwheat Food. Food Res. Int. 2012, 49, 389–395. [Google Scholar] [CrossRef]
- Wefers, D.; Bunzel, M. Characterization of Dietary Fiber Polysaccharides from Dehulled Common Buckwheat (Fagopyrum esculentum) Seeds. Cereal Chem. 2015, 92, 598–603. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Zhou, Z.; Wang, L.; Cao, R. Effects of Different Treatments on Composition, Physicochemical and Biological Properties of Soluble Dietary Fiber in Buckwheat Bran. Food Biosci. 2023, 53, 102517. [Google Scholar] [CrossRef]
- Koç, S.T.; Coşkun, F. Buckwheat: Nutritional Value, Health Effects and Applications in Foods. Turk. J. Agric.-Food Sci. Technol. 2025, 13, 1665–1674. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Yan, J.; Zhou, M.; Woo, S.-H.; Weng, W.; Cheng, J.; Zhang, K. Tartary Buckwheat: An Under-Utilized Edible and Medicinal Herb for Food and Nutritional Security. Food Rev. Int. 2022, 38, 440–454. [Google Scholar] [CrossRef]
- Krumina-Zemture, G.; Beitane, I. Fatty acid composition în Buckwheat (Fagopyrum esculentum M.) flours and their extruded products. In Proceedings of International Scientific Conference “RURAL DEVELOPMENT 2017”; Aleksandras Stulginskis University: Kauno r. sav., Lithuania, 2018. [Google Scholar] [CrossRef]
- Sinkovič, L.; Kokalj, D.; Vidrih, R.; Meglič, V. Milling Fractions Fatty Acid Composition of Common (Fagopyrum esculentum Moench) and Tartary (Fagopyrum Tataricum (L.) Gaertn) Buckwheat. J. Stored Prod. Res. 2020, 85, 101551. [Google Scholar] [CrossRef]
- Golijan, J.; Milinčić, D.D.; Petronijević, R.; Pešić, M.B.; Barać, M.B.; Sečanski, M.; Lekić, S.; Kostić, A.Ž. The Fatty Acid and Triacylglycerol Profiles of Conventionally and Organically Produced Grains of Maize, Spelt and Buckwheat. J. Cereal Sci. 2019, 90, 102845. [Google Scholar] [CrossRef]
- Klepacka, J.; Najda, A.; Klimek, K. Effect of Buckwheat Groats Processing on the Content and Bioaccessibility of Selected Minerals. Foods 2020, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, R. A Review of Nutritional and Nutraceutical Components of Buckwheat. Eur. J. Plant Sci. Biotechnol. 2009, 3, 10–19. [Google Scholar]
- Pongrac, P.; Vogel-Mikuš, K.; Potisek, M.; Kovačec, E.; Budič, B.; Kump, P.; Regvar, M.; Kreft, I. Mineral and Trace Element Composition and Importance for Nutritional Value of Buckwheat Grain, Groats, and Sprouts. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Woo, S.H., Wieslander, G., Kreft, I., Chrungoo, N., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 261–271. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Martínez-Monzó, J.; García-Segovia, P.; Becze, A.; Török, A.I.; Cadar, O.; Coldea, T.E.; Igual, M. In Vitro Digestibility of Minerals and B Group Vitamins from Different Brewers’ Spent Grains. Nutrients 2022, 14, 3512. [Google Scholar] [CrossRef]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Olejnik, A.; Rychlik, J.; Kreft, I.; Drożdżyńska, A.; Walkowiak, J. The Cytotoxic Effect of Artificially Digested Buckwheat Products on HT-29 Colon Cancer Cells. J. Cereal Sci. 2018, 83, 68–73. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Mao, Y.; Liu, L.; Li, C.; Wu, H.; Zhao, H.; Wu, Q. Tartary Buckwheat Rutin: Accumulation, Metabolic Pathways, Regulation Mechanisms, and Biofortification Strategies. Plant Physiol. Biochem. 2024, 208, 108503. [Google Scholar] [CrossRef]
- Molinari, R.; Costantini, L.; Timperio, A.M.; Lelli, V.; Bonafaccia, F.; Bonafaccia, G.; Merendino, N. Tartary Buckwheat Malt as Ingredient of Gluten-Free Cookies. J. Cereal Sci. 2018, 80, 37–43. [Google Scholar] [CrossRef]
- Soosai, D.; Ramalingam, R.; Perumal, E.; Veeramani, K.; Pancras, C.; Almutairi, M.H.; Savarimuthu, L.A.R.; Veeramuthu, D.; Antony, S. Anticancer Effects of Rutin from Fagopyrum tataricum (Tartary Buckwheat) against Osteosarcoma Cell Line. Mol. Biol. Rep. 2024, 51, 312. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Li, Y.; Lu, K.; Yin, R.; Ming, J. Phenolics Extracted from Tartary (Fagopyrum tartaricum L. Gaerth) Buckwheat Bran Exhibit Antioxidant Activity, and an Antiproliferative Effect on Human Breast Cancer MDA-MB-231 Cells through the P38/MAP Kinase Pathway. Food Funct. 2017, 8, 177–188. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Chen, Z.-D.; Zhou, Y.-M.; Shi, R.-H.; Li, Z.-J. The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination. Molecules 2019, 24, 3092. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive Compounds, Health Benefits, and Industrial Applications of Tartary Buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2023, 63, 657–673. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat Phenolic Metabolites in Health and Disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Valido, E.; Stoyanov, J.; Gorreja, F.; Stojic, S.; Niehot, C.; Kiefte-de Jong, J.; Llanaj, E.; Muka, T.; Glisic, M. Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health. Nutrients 2022, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mao, Y.; Tang, Y.; Zhao, J.; Wang, A.; Li, C.; Wu, H.; Wu, Q.; Zhao, H. Rutin Distribution in Tartary Buckwheat: Identifying Prime Dietary Sources through Comparative Analysis of Post-Processing Treatments. Food Chem. 2025, 464, 141641. [Google Scholar] [CrossRef] [PubMed]
- Vogrinčič, M.; Timoracka, M.; Melichacova, S.; Vollmannova, A.; Kreft, I. Degradation of Rutin and Polyphenols during the Preparation of Tartary Buckwheat Bread. J. Agric. Food Chem. 2010, 58, 4883–4887. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Olech, M. The Impact of Processing Parameters on the Content of Phenolic Compounds in New Gluten-Free Precooked Buckwheat Pasta. Molecules 2019, 24, 1262. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Castillo-Martínez, W.E.; Simpalo-Lopez, W.D.; Verona-Ruiz, A.; Lavado-Cruz, A.; Martínez-Villaluenga, C.; Peñas, E.; Frias, J.; Schmiele, M. Performance of Thermoplastic Extrusion, Germination, Fermentation, and Hydrolysis Techniques on Phenolic Compounds in Cereals and Pseudocereals. Foods 2022, 11, 1957. [Google Scholar] [CrossRef]
- Yalcin, S. Quality Characteristics, Mineral Contents and Phenolic Compounds of Gluten Free Buckwheat Noodles. J. Food Sci. Technol. 2021, 58, 2661–2669. [Google Scholar] [CrossRef]
- Ge, R.H.; Wang, H. Nutrient Components and Bioactive Compounds in Tartary Buckwheat Bran and Flour as Affected by Thermal Processing. Int. J. Food Prop. 2020, 23, 127–137. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, D.; Bose, A.; Mandal, S.C. Natural Flavonoids and Its Pharmaceutical Importance. Pharma Rev. 2018, 94, 61–63. [Google Scholar]
- Li, J.; Yang, P.; Yang, Q.; Gong, X.; Ma, H.; Dang, K.; Chen, G.; Gao, X.; Feng, B. Analysis of Flavonoid Metabolites in Buckwheat Leaves Using UPLC-ESI-MS/MS. Molecules 2019, 24, 1310. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Qiu, J.; Li, Z. The Analysis of Fagopyritols from Tartary Buckwheat and Their Anti-Diabetic Effects in KK-Ay Type 2 Diabetic Mice and HepG2 Cells. J. Funct. Foods 2018, 50, 137–146. [Google Scholar] [CrossRef]
- Tien, N.N.T.; Trinh, L.N.D.; Inoue, N.; Morita, N.; Hung, P.V. Nutritional Composition, Bioactive Compounds, and Diabetic Enzyme Inhibition Capacity of Three Varieties of Buckwheat in Japan. Cereal Chem. 2018, 95, 615–624. [Google Scholar] [CrossRef]
- Tuan, P.A.; Thwe, A.A.; Kim, J.K.; Kim, Y.B.; Lee, S.; Park, S.U. Molecular Characterisation and the Light–Dark Regulation of Carotenoid Biosynthesis in Sprouts of Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Food Chem. 2013, 141, 3803–3812. [Google Scholar] [CrossRef]
- Ji, X.; Han, L.; Liu, F.; Yin, S.; Peng, Q.; Wang, M. A Mini-Review of Isolation, Chemical Properties and Bioactivities of Polysaccharides from Buckwheat (Fagopyrum mill). Int. J. Biol. Macromol. 2019, 127, 204–209. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Henning, F.G.; Demiate, I.M.; Salem, R.D.S.; De Almeida, V.S.; Lacerda, L.G. Effect of Organic Acids as Additives on Buckwheat Starch Films Produced by Casting. Starch-Stärke 2025, 77, 2400030. [Google Scholar] [CrossRef]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M.; Nhubu, T. Integrated and Consolidated Review of Plastic Waste Management and Bio-Based Biodegradable Plastics: Challenges and Opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Tafa, K.D.; Satheesh, N.; Abera, W. Mechanical Properties of Tef Starch Based Edible Films: Development and Process Optimization. Heliyon 2023, 9, e13160. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and Intelligent Starch-Based Films: A Review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Koca, E.; Kahraman, K.; Oskaybaş-Emlek, B.; Özbey, A.; Aydemir, L.Y. Development of Buckwheat Starch-Capric Acid Complex-Based Film: Process Optimization and Film Characterization. Starch Stärke 2025, 77, e70001. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Major Factors Affecting the Characteristics of Starch Based Biopolymer Films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Oskaybaş-Emlek, B.; Özbey, A.; Aydemir, L.Y.; Kahraman, K. Production of Buckwheat Starch-Myristic Acid Complexes and Effect of Reaction Conditions on the Physicochemical Properties, X-Ray Pattern and FT-IR Spectra. Int. J. Biol. Macromol. 2022, 207, 978–989. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, P.; Yang, F.; Zhao, T.; Wang, J.; Li, S.; Chen, Z.; Jin, J.; Gong, J. Unraveling In-Kernel Starch Multiscale Structure and Nutritional Profiles of Thermal Remodeling Whole Grain Black Tartary Buckwheat. Int. J. Biol. Macromol. 2025, 316, 144730. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, M.; Zhou, Z.; Xiao, S.; Lin, P.; Wang, Y.; Fu, Y.; Wang, X. Effect of Ultrasonic Treatment on the Physicochemical Properties of Buckwheat Starch: Based on the Ultrasonic Power and Moisture Content. Ultrason. Sonochem. 2025, 116, 107333. [Google Scholar] [CrossRef]
- Sarker, A.; Rennie, T.; Shaheb, M.R.; Matak, K.; Jaczynski, J. Optimization of the Properties of Underutilized Buckwheat Starch Films through Different Modification Approaches. Food Packag. Shelf Life 2025, 49, 101513. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Wang, Y.; Xiong, G.; Mei, X.; Wu, W.; Ding, A.; Li, X.; Qiao, Y.; Liao, L. Effects of Fatty Acid Chain Length on Properties of Potato Starch–Fatty Acid Complexes under Partially Gelatinization. Int. J. Food Prop. 2018, 21, 2121–2134. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.B. Antimicrobial Activity of Buckwheat Starch Films Containing Zinc Oxide Nanoparticles against Listeria Monocytogenes on Mushrooms. Int. J. of Food Sci. Tech. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Bansal, H.; Sharma, A.; Singh, S.; Mehta, S.K. Fabrication and Application of Buckwheat Starch-Based Sustained-Release Composite Films Infused with Curry Leaf Essential Oil/β-Cyclodextrin Micro-Capsules for Preservation of Green Grapes. Food Hydrocoll. 2025, 159, 110666. [Google Scholar] [CrossRef]
- An, T.; Liu, W.; Zhang, Q.; Pei, L.; Liu, W.; Duan, Y.; Zhang, Y. An active packaging film based on esterified starch with Tartary buckwheat bran extract and chitosan and its application for mutton preservation. J. Food Process. Preserv. 2024, 45, e1600. [Google Scholar] [CrossRef]
- Chumsri, P.; Panpipat, W.; Cheong, L.-Z.; Chaijan, M. Formation of Intermediate Amylose Rice Starch–Lipid Complex Assisted by Ultrasonication. Foods 2022, 11, 2430. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Singh, S.; Amonsou, E.O. A Review on Structural, Digestibility and Physicochemical Properties of Legume Starch-Lipid Complexes. Food Chem. 2021, 349, 129165. [Google Scholar] [CrossRef]
| Parameters | Tartary Buckwheat | Common Buckwheat |
|---|---|---|
| Ash (%) | 1.8–2.70 | 1.53–2.50 |
| Proteins (%) | 5.7–16.4 | 8.51–18.57 |
| Lipids (%) | 3–4.7 | 1.5–4 |
| Total dietary fibers (%) | 6.1–8.4 | 8.4–10.0 |
| Starch (%) | 60.0–70.2 | 59–69 |
| Carbohydrates (%) | 55.4–57.40 | 54.50–73.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lițoiu, A.A.; Păucean, A.; Lung, C.; Zmuncilă, A.; Chiș, M.S. An Overview of Buckwheat—A Superfood with Applicability in Human Health and Food Packaging. Plants 2025, 14, 2200. https://doi.org/10.3390/plants14142200
Lițoiu AA, Păucean A, Lung C, Zmuncilă A, Chiș MS. An Overview of Buckwheat—A Superfood with Applicability in Human Health and Food Packaging. Plants. 2025; 14(14):2200. https://doi.org/10.3390/plants14142200
Chicago/Turabian StyleLițoiu, Alexandra Andreea, Adriana Păucean, Claudiu Lung, Alexandru Zmuncilă, and Maria Simona Chiș. 2025. "An Overview of Buckwheat—A Superfood with Applicability in Human Health and Food Packaging" Plants 14, no. 14: 2200. https://doi.org/10.3390/plants14142200
APA StyleLițoiu, A. A., Păucean, A., Lung, C., Zmuncilă, A., & Chiș, M. S. (2025). An Overview of Buckwheat—A Superfood with Applicability in Human Health and Food Packaging. Plants, 14(14), 2200. https://doi.org/10.3390/plants14142200