Abstract
Finger millet (Eleusine coracana L.) is a nutrient-dense cereal with high flavonoid content, yet the mechanisms regulating its secondary metabolite biosynthesis remain underexplored. Various exogenous stimuli can readily activate the enzymatic pathways and gene expression associated with flavonoid biosynthesis in plants, which are regulated by developmental cues. Research has established that methyl jasmonate (MeJA) application enhances secondary metabolite production in plant systems. This investigation examined MeJA’s influence on flavonoid accumulation and physiological responses in finger millet sprouts to elucidate the molecular mechanisms underlying MeJA-mediated flavonoid accumulation. The findings revealed that MeJA treatment significantly suppressed sprout elongation while enhancing the biosynthesis of total flavonoids and phenolic compounds. MeJA treatment triggered oxidative stress responses, with hydrogen peroxide and superoxide anion concentrations increasing 1.84-fold and 1.70-fold compared to control levels at 4 days post-germination. Furthermore, the antioxidant defense mechanisms in finger millet were upregulated following treatment, resulting in significant enhancement of catalase and peroxidase enzymatic activities and corresponding transcript abundance. MeJA application augmented the activities of key phenylpropanoid pathway enzymes—phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H)—and upregulated their respective gene expression. At 4 days post-germination, EcPAL and EcC4H transcript levels were elevated 3.67-fold and 2.61-fold, respectively, compared to untreated controls. MeJA treatment significantly induced the expression of downstream structural genes and transcriptional regulators. This study provides a deeper understanding of the mechanism of flavonoid accumulation in foxtail millet induced by MeJA, and lays a foundation for exogenous conditions to promote flavonoid biosynthesis in plants.
1. Introduction
Flavonoids, which are secondary metabolites synthesized through the phenylpropanoid pathway in plants, are characterized by hydroxyl groups and conjugated double-bond systems in their molecular structures []. These structural features are known to confer exceptional free radical scavenging capacity, effectively mitigating cellular oxidative damage induced by reactive oxygen species (ROS) [,]. Substantial clinical and epidemiological evidence shows that regularly consuming adequate amounts of flavonoids can significantly improve human health through various protective mechanisms. Research indicates that moderate flavonoid intake protects cardiovascular health by reducing risks of vascular diseases, including atherosclerosis and hypertension, mainly by improving endothelial function and optimizing lipid balance []. These compounds also promote metabolic health by lowering the incidence of type 2 diabetes, an effect attributed to their ability to enhance insulin sensitivity and maintain glucose homeostasis []. Furthermore, flavonoids exhibit notable anticancer potential, particularly against hormone-related malignancies, including breast and prostate cancers, as they can modulate estrogen receptor activity and inhibit tumor cell proliferation [,]. These cumulative findings underscore the importance of incorporating dietary flavonoids as part of a balanced nutritional strategy for chronic disease prevention []. However, flavonoids cannot be endogenously synthesized by humans and must be acquired through dietary sources. Consequently, the development of flavonoid-enriched foods has gained increasing global attention.
Finger millet (Eleusine coracana L.), an annual cereal crop belonging to the Poaceae family, is native to Ethiopia in Africa and has been introduced to various regions in Asia []. In China, it is primarily cultivated in the southwestern regions []. Beyond its nutritional value as a staple crop, finger millet has gained increasing attention for its rich flavonoid content and associated health benefits. Epidemiological and clinical studies have documented its therapeutic potential, including anti-atherosclerotic [], anti-diabetic [], and anti-tumor activities []. Long-term consumption of finger millet has been reported to inhibit low-density lipoprotein oxidation, alleviate hypertension, hypercholesterolemia, and diabetes, and improve gastrointestinal function and vascular integrity [,]. To investigate the mechanisms underlying flavonoid biosynthesis, this study employed methyl jasmonate (MeJA) as an elicitor in finger millet sprouts. During plant growth stages, the application of phytohormones has been proven to effectively promote the accumulation of secondary metabolites such as flavonoids in sprouts [].
Research indicates that methyl jasmonate (MeJA), as a plant hormone, relies on the jasmonic acid (JA) metabolic pathway within plants for its endogenous synthesis []. Endogenous MeJA levels are regulated by multiple factors, including the expression of synthesis-related genes, induction by external signals, and regulation through epigenetic modifications []. MeJA performs various functions in plant physiological processes, particularly in stress resistance and defense metabolism regulation, where it can activate key enzyme genes in secondary metabolic pathways to promote the biosynthesis of defensive secondary metabolites in plants [,]. Recent studies have demonstrated that preharvest application of MeJA significantly influences secondary metabolite production in various berry crops. In raspberry plants, foliar MeJA treatment has been shown to induce a marked increase in flavonoid content, particularly quercetin and kaempferol derivatives, as reported by []. This phytohormonal elicitation effect extends to other Rubus species [], with experimental evidence indicating that MeJA treatment not only elevates flavonoid concentrations but also substantially enhances the antioxidant capacity in blackberry fruit []. The regulatory role of MeJA in phenylpropanoid pathway activation appears particularly pronounced in Vitis species. Research findings reveal that exogenous MeJA application stimulates anthocyanin biosynthesis in grape berries, resulting in significantly higher pigment accumulation in both the skin and flesh tissues []. Similarly, MeJA treatment has been observed to upregulate key anthocyanin synthesis genes, leading to enhanced coloration and improved phytochemical profiles in strawberry fruits []. Germination experiments in maize further confirmed that treatment with 0.15 μmol/L MeJA significantly elevated carotenoid biosynthesis gene expression, resulting in a 2.10-fold increase in free radical scavenging activity [].
This study examined how MeJA treatment affected the growth metabolism and flavonoid accumulation in finger millet sprouts. Furthermore, by examining the expression of genes encoding antioxidant and flavonoid biosynthesis pathway enzymes, the regulatory mechanisms behind finger millet’s reaction to MeJA treatment were clarified. For future studies on MeJA-mediated flavonoid production in finger millet sprouts, our results offer a theoretical basis.
2. Results
2.1. Effects of MeJA Treatment on the Total Phenolics and the Total Flavonoid Content of Finger Millet Sprouts
As shown in Figure 1A, MeJA treatment significantly increased phenolic compound production in finger millet sprouts (p < 0.05). MeJA-treated samples exhibited 1.11-, 1.15-, and 1.19-fold higher total phenolic concentrations compared to control samples at 2, 4, and 6 days after imbibition, respectively (Figure 1A). Total flavonoid levels increased steadily throughout germination, with MeJA-treated samples at 4 and 6 days after imbibition containing 1.14- and 1.28-fold higher concentrations than untreated controls (Figure 1B).
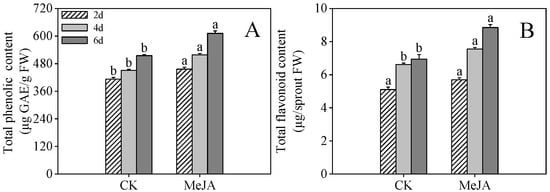
Figure 1.
Effects of MeJA treatment on total phenolic content (A) and total flavonoid content (B) in finger millet sprouts. Lowercase letters mark significant differences between treatments within the same germination period (one-way ANOVA with Tukey’s test, p < 0.05) 2d: two days after germination; 4d: four days after germination; 6d: six days after germination.
2.2. Effects of MeJA Treatment on the Growth and Development of Finger Millet Sprouts
Compared to the control, MeJA-treated finger millet sprouts exhibited retarded development at 4 and 6 days after germination (Figure 1A), although no significant difference in dry weight was observed (Figure 2C, p > 0.05). MeJA treatment caused severe growth inhibition, with sprout length significantly shorter than in the control group (Figure 2B, p < 0.05). While root development appeared sturdier in treated sprouts, shoot development was markedly delayed, particularly in sprouts germinated for over 2 days.
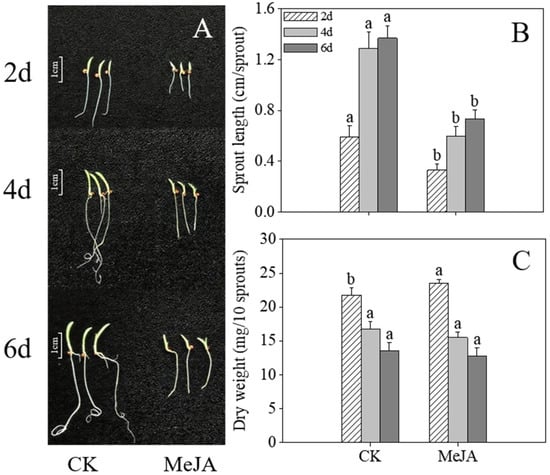
Figure 2.
Effects of MeJA treatment on morphology (A), sprout length (B), and dry weight (C) in finger millet sprouts. Lowercase letters mark significant differences between treatments within the same germination period (one-way ANOVA with Tukey’s test, p < 0.05). 2d: two days after germination; 4d: four days after germination; 6d: six days after germination.
2.3. Effects of MeJA Treatment on the Antioxidant Capacity of Finger Millet Sprouts
As depicted in Figure 3A, the O2−• concentrations in MeJA-treated finger millet sprouts increased progressively throughout the germination phase (p < 0.05, one-way ANOVA), attaining peak values 1.70-fold greater than control cohorts at 6 days post-imbibition. MeJA-treated specimens maintained significantly elevated H2O2 levels compared to controls at 2 and 4 days post-germination (1.69-fold and 1.84-fold, respectively, Figure 3B, p < 0.05). The observed accumulation of O2−• and H2O2 indicates ROS overproduction and consequent oxidative cellular impairment under MeJA treatment conditions.
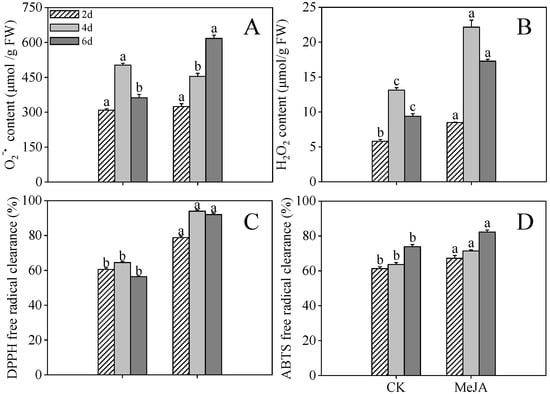
Figure 3.
Effects of MeJA treatment on O2−• (A), H2O2 (B), DPPH radical scavenging capacity (C), and ABTS free radical scavenging capacity (D) of finger millet sprouts. Lowercase letters mark significant differences between treatments within the same germination period (one-way ANOVA with Tukey’s test, p < 0.05). 2d: two days after germination; 4d: four days after germination; 6d: six days after germination.
Additionally, DPPH scavenging activity reached maximum efficacy in 4-day post-imbibition specimens (Figure 3C), exhibiting 1.46-fold enhancement compared to controls, while ABTS scavenging potential peaked at 6 days post-imbibition (11.37% elevation relative to controls, Figure 3D). These findings substantiate that MeJA administration substantially augmented the antioxidant defense mechanisms in finger millet sprouts.
2.4. Effects of MeJA Treatment on Activity and Gene Expression Level of Antioxidant Enzyme in Finger Millet Sprouts
Figure 4A,C illustrate that the highest POD (253.38 U/g) and APX (226.87 U/g) activities were observed in 6-day post-germination sprouts, representing 1.61- and 3.20-fold increases over the control. CAT activity peaked at 436.70 U/g (Figure 4D, 1.28-fold of the control) in 4-day post-germination sprouts, while SOD activity reached 90.03 U/g (Figure 4B, 1.18-fold of the control) at 2 days post-germination. These findings indicate that finger millet sprouts alleviate stress damage by modulating POD, SOD, APX, and CAT activities.
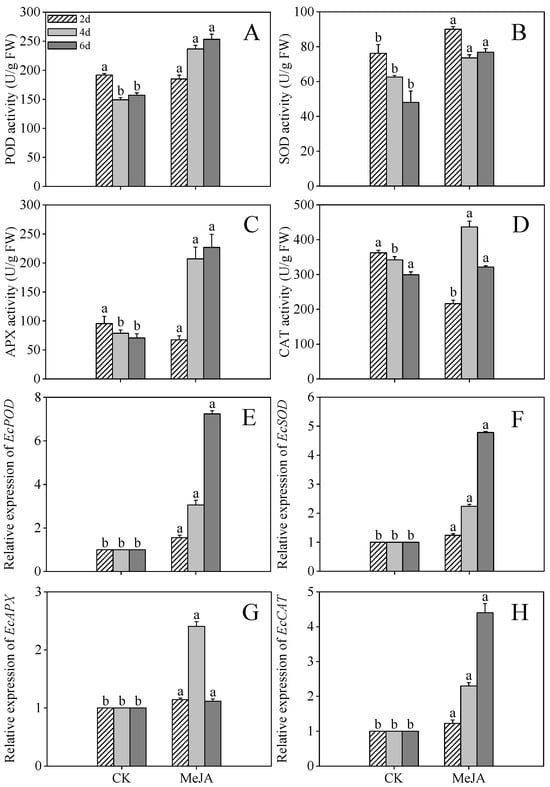
Figure 4.
Effects of MeJA treatment on peroxidase (POD) activity (A), superoxide dismutase (SOD) activity (B), ascorbate peroxidase (APX) activity (C), catalase (CAT) activity (D), and the gene expression levels of EcPOD (E), EcSOD (F), EcAPX (G), and EcCAT (H) of finger millet sprouts. Lowercase letters mark significant differences between treatments within the same germination period (one-way ANOVA with Tukey’s test, p < 0.05). 2d: two days after germination; 4d: four days after germination; 6d: six days after germination.
The relative expression levels of antioxidant enzyme genes (EcCAT, EcPOD, EcSOD, and EcAPX) under MeJA treatment are shown in Figure 4E–H. MeJA treatment significantly increased the expression of EcCAT, EcPOD, and EcSOD in 4- and 6-day post-germination sprouts compared to the control, while EcAPX expression in 4-day post-germination sprouts was elevated 2.25-fold compared to the control group (p < 0.05). These results suggest that oxidative stress induced by growth constraints triggers enhanced antioxidant enzyme activity as a compensatory mechanism.
2.5. Effects of MeJA Treatment on Activity and Gene Expression Level of Key Enzymes Associated with Flavonoid Biosynthesis in Finger Millet Sprouts
As illustrated in Figure 5A,C, by day 4 post-germination, PAL and C4H activities in MeJA-elicited sprouts reached maximal values of 130.17 U/g and 35.91 U/g, constituting 1.67-fold and 1.10-fold enhancements compared to untreated controls, respectively. However, 4CL activity exhibited no statistically significant variation between MeJA-treated and control sprouts at both 2 and 4 days following germination. 4CL activity in MeJA-treated sprouts decreased markedly by 54.19% compared to the control at 6 days post-germination. Data analysis revealed that the heightened flavonoid levels detected in MeJA-treated sprouts directly result from increased activity of essential biosynthetic enzymes within the phenolic pathway.
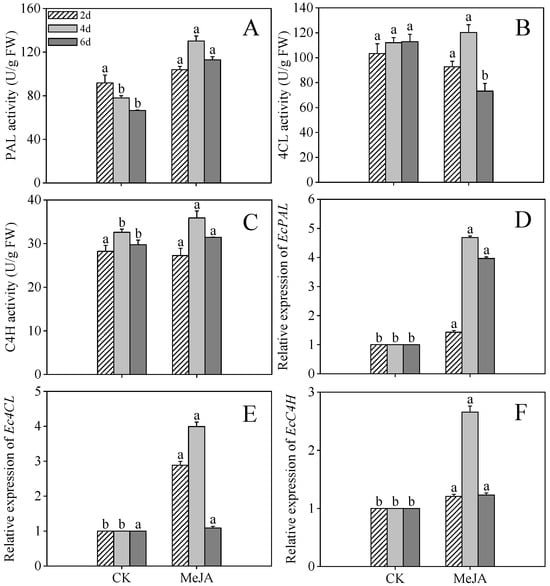
Figure 5.
Effects of MeJA treatment on phenylalanine ammonia-lyase (PAL) activity (A), 4-coumarate-CoA ligase (4CL) activity (B), cinnamate 4-hydroxylase (C4H) activity (C), and the gene expression levels of EcPAL (D), Ec4CL (E), and EcC4H (F) of finger millet sprouts. Lowercase letters mark significant differences between treatments within the same germination period (one-way ANOVA with Tukey’s test, p < 0.05). 2d: two days after germination; 4d: four days after germination; 6d: six days after germination.
MeJA treatment significantly upregulated the relative expression of EcPAL, Ec4CL, and EcC4H in 4-day post-germination sprouts (Figure 5D–F, p < 0.05), increasing 3.67-, 3.98-, and 2.61-fold compared to the control, respectively. The concordance between enzyme activity and gene expression highlights the critical role of transcriptional regulation in phenolic compound accumulation.
2.6. Effects of MeJA Treatment on Gene Expression in the Middle and Downstream Pathways of Flavonoid Metabolism in Finger Millet Sprouts
Compared to the control, MeJA-treated 4-day post-germination sprouts exhibited 0.79-, 4.43-, 0.68-, and 3.22-fold higher expression of EcCHI, EcCHS, EcCHR, and EcIFS, respectively. Elevated expression of these genes persisted in 6-day post-germination sprouts under MeJA treatment. In contrast, EcCHS expression initially increased but subsequently declined (Figure 6B).
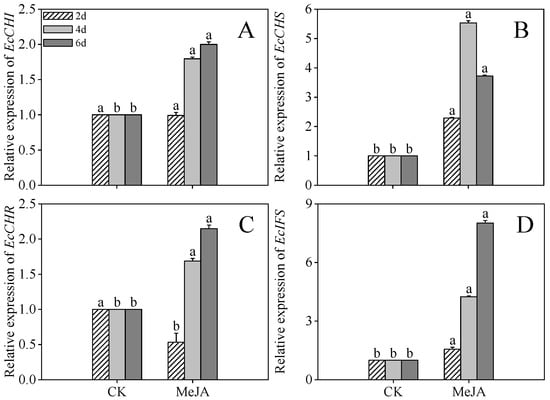
Figure 6.
The effects of MeJA treatment on EcCHI (A), EcCHS (B), EcCHR (C), and EcIFS (D) relative expression of finger millet sprouts. Lowercase letters mark significant differences between treatments within the same germination period. CHI: chalcone isomerase; CHS: chalcone synthase; CHR: chalcone reductase; IFS: isoflavone synthase (one-way ANOVA with Tukey’s test, p < 0.05). 2d: two days after germination; 4d: four days after germination; 6d: six days after germination.
2.7. Effects of MeJA Treatment on Stress-Related Gene Expression in Finger Millet Sprouts
Figure 7A revealed that MeJA treatment significantly enhanced EcMYB expression in 4- and 6-day post-germination sprouts (6.27- and 4.69-fold compared to the control, respectively, p < 0.05). Similarly, EcNAC expression in 6-day post-germination sprouts was 3.91-fold higher than the control (Figure 7B, p < 0.05). These findings suggest that the transcription factors EcMYB and EcNAC play pivotal roles in mitigating stress during the mid-to-late germination stages.
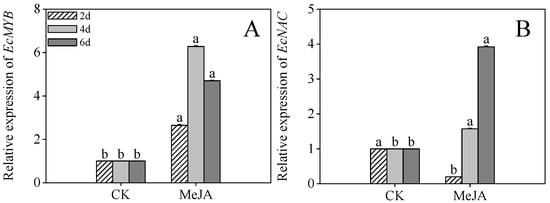
Figure 7.
The effects of MeJA treatment on EcMYB (A) and EcNAC (B) relative expression of finger millet sprouts. Lowercase letters mark significant differences between treatments within the same germination period (one-way ANOVA with Tukey’s test, p < 0.05). 2d: two days after germination; 4d: four days after germination; 6d: six days after germination.
3. Discussion
During germination, environmental stress induces ROS generation, potentially reducing the nutritional value of food. Plants counter this stress through antioxidant defense systems, including phenolic and flavonoid compounds synthesized via the phenylpropanoid metabolic cascade in non-enzymatic pathways. Plant hormones such as MeJA effectively activate these biosynthetic pathways. This research demonstrates that MeJA treatment significantly increased H2O2 (1.84-fold) and O2−• (1.70-fold) concentrations on the fourth day post-germination in finger millet sprouts, while simultaneously promoting flavonoid/phenolic compound accumulation. Notably, H2O2 peaked 48 h post-treatment, preceding the maximum transcription levels of phenylpropanoid metabolism-related genes (such as EcPAL, upregulated 3.67-fold at 72 h), suggesting ROS may function as signaling molecules for flavonoid biosynthesis. Additionally, MeJA upregulated the activity and gene expression of POD and SOD during days 4–6 of finger millet germination, as well as CAT and APX on day 4, exhibiting a coordinated antioxidant enzyme system activation pattern. This integrated response confirms MeJA’s central role in regulating plant oxidative stress defense networks, consistent with findings from germinating peanut studies [,].
Morphologically, MeJA treatment significantly reduced sprout elongation at 4 and 6 days post-germination, likely attributable to its function as an endogenous signaling molecule that activates plant defense mechanisms, including enhanced stress tolerance, regulated growth, secondary metabolite synthesis via signal transduction, and improved self-repair capacity []. Analogous to our observations, previous research demonstrated that MeJA treatment inhibited peanut sprout growth and induced resveratrol accumulation []. Under abiotic stress conditions, plants frequently accumulate soluble sugars for osmotic adjustment, thereby adapting to environmental perturbations [].
The flavonoid biosynthetic pathway initiates via the phenylpropanoid pathway, with PAL, C4H, and 4CL functioning as key enzymatic regulators. To elucidate the mechanism underlying flavonoid enrichment in finger millet sprouts, we assessed these enzyme activities. MeJA treatment significantly enhanced PAL activity at 4 and 6 days post-germination and C4H activity at 4 days post-germination compared to controls, indicating that MeJA augments flavonoid synthesis by enhancing key enzymatic activities. Similar strategies for enhancing secondary metabolism have been documented, such as abscisic acid-induced PAL activation promoting phenolic acid accumulation in germinating wheat [].
To further investigate the regulatory mechanisms, we analyzed gene expression profiles in the flavonoid biosynthetic pathway. The expression patterns of EcPAL and EcC4H in the phenylpropanoid pathway correlated with their corresponding enzyme activities under MeJA treatment, confirming that MeJA promotes flavonoid accumulation through transcriptional upregulation. Similar molecular mechanisms were observed in peanuts, where [] MeJA treatment elevated PAL, C4H, and 4CL activities and gene expression in cotyledonous and non-cotyledonous tissues to enhance resveratrol biosynthesis [].
In our study, chalcone isomerase (CHI), chalcone synthase (CHS), and chalcone reductase (CHR)—key enzymes in downstream flavonoid branch pathways—facilitated flavonoid conversion []. Isoflavone synthase (IFS), which specifically determines flavonoid production, was also investigated []. MeJA treatment significantly upregulated the relative expression of EcCHI, EcCHS, EcCHR, and EcIFS compared to controls. While acid-treated germinating soybeans exhibited similar upregulation of EcCHI, EcCHS, and EcIFS, discrepancies in EcCHR expression may result from treatment-specific or species-dependent variations.
MYB and NAC transcription factors are key regulators of stress adaptation and secondary metabolism [,]. Our data reveal that MeJA significantly upregulates EcMYB and EcNAC expression in finger millet sprouts, aligning with the reported roles of MYB factors in jasmonate-mediated flavonoid biosynthesis and NAC induction by MeJA in other species [,]. Future work will define the specific functions of EcMYB/EcNAC in stress mitigation and metabolic regulation during elicitor treatments.
MeJA-induced flavonoid enrichment demonstrates significant application value. From a nutritional perspective, millet sprouts with high flavonoid content can serve as preventive dietary supplements for cardiovascular diseases, holding particular significance in regions with limited food diversity [,]. From a commercial standpoint, millet sprouts show tremendous potential as high value-added functional food ingredients: market research indicates that grain products rich in bioactive compounds can command a premium price [], while their germination-active characteristics simplify large-scale production processes. Compared to transgenic technology, this biofortification strategy circumvents regulatory barriers associated with genetically modified organisms, accelerating the product commercialization process.
4. Materials and Methods
4.1. Plant Materials and Treatments
Homogeneous, fully developed finger millet sprouts were selected and subjected to rigorous washing using distilled water for complete removal of surface particulates. Subsequent sterilization involved immersion in 1% (v/v) sodium hypochlorite solution for 10 min, with thorough rinsing in deionized water until pH neutrality was attained. Post-sterilization, samples underwent imbibition at 31 °C for 6 h before transfer to growth chambers. Germination proceeded under dark conditions at 31 °C for 6 days. The experimental design comprised two treatments: control (CK) receiving aqueous sprays and MeJA-treated samples administered 150 μM methyl jasmonate foliar applications. This concentration was optimized through preliminary trials. Nutrient solutions were replenished at 12 h intervals throughout cultivation. Germinated specimens were collected at 2-, 4-, and 6-day intervals, rapidly frozen in liquid nitrogen, and preserved at −80 °C for subsequent biochemical assays.
4.2. Instrumentation
The main instruments used in this study are listed in Table 1.

Table 1.
Instruments used in this study.
4.3. Determination of Sprout Length and Dry Weight
For each experimental condition, a randomized subset of thirty sprouts was isolated. Morphometric assessment of sprout elongation and gravimetric analysis of desiccated biomass were conducted in accordance with the methodological framework established in Ma et al.’s research [].
4.4. Determination of Total Flavonoid and Total Phenolic Content
Total flavonoid extraction employed freshly harvested sprouts using 80% (v/v) ethanolic solution as the extraction solvent. This homogenate underwent ultrasonic processing (25 °C, 25 min) prior to high-speed centrifugation (8000× g, 10 min). The resulting supernatant was diluted 25-fold before spectrophotometric quantification at 265 nm, with genistein-based calibration enabling flavonoid quantification []. For phenolic analysis, sprout homogenates prepared in 50% methanol were centrifuged to obtain soluble fractions. These extracts were reacted with Folin–Ciocalteu reagent and sodium carbonate solution, incubated under light-protected conditions (25 °C, 2 h), and quantified spectrophotometrically at 765 nm against a gallic acid standard curve [].
4.5. Determination of H2O2 and O2−• Content, DPPH, and ABTS
H2O2 concentration was determined using the xylenol orange oxidation assay according to the protocol described by Zhao et al. []. Sprout specimens were homogenized in acidic extraction buffer (2.5 M H2SO4) containing 25 mM FeSO4 and 25 mM (NH4)2SO4. The homogenate was supplemented with 125 μM xylenol orange and 100 mM sorbitol. Following centrifugation (9000× g, 5 min), 100 μL of the supernatant was combined with 1 mL xylenol orange reagent. After 30 min incubation, the absorbance of the Fe3+ complex was measured spectrophotometrically at 560 nm.
O2−• levels were assessed by monitoring hydroxylamine-mediated nitrite formation. Fresh sprout tissues were homogenized in 65 mM phosphate buffer (pH 7.8) and centrifuged at 8000× g for 10 min. A 1 mL aliquot of the supernatant was mixed with 0.9 mL phosphate buffer and 0.1 mL hydroxylammonium chloride (10 mM). Following a 20 min incubation at 25 °C, the mixture was treated with sulfanilic acid (17 mM) and α-naphthylamine (7 mM) solutions. Spectrophotometric analysis at 530 nm enabled quantification of O2−• generation rates via calibration against a sodium nitrite standard curve.
DPPH radical scavenging activity was evaluated according to Rumpf et al. [] by monitoring absorbance reduction at 515 nm with ascorbic acid serving as the positive control. ABTS radical scavenging capacity was determined by spectrophotometrically measuring absorbance at 734 nm.
4.6. Determination of Flavonoid Biosynthetic Enzyme Activities
A 0.1 M Tris-HCl buffer (pH 8.9) was utilized as the homogenization medium for sprout tissue samples. After mechanical disruption, samples underwent centrifugation at 12,000× g for 25 min at 4 °C to separate the pellet. The enzyme-rich supernatant was promptly collected to preserve enzymatic integrity for subsequent activity assays. PAL, C4H, and 4CL activities were quantified according to the protocol described by Ma et al. [], with one unit of enzyme activity defined as an absorbance increase of 0.01 per min at OD290 nm for PAL, OD340 nm for C4H, and OD333 nm for 4CL.
4.7. Determination of Antioxidant Enzyme Activities
The sprout samples were homogenized in an ice-cold 50 mM sodium phosphate buffer (pH 7.0) containing 1% polyvinylpyrrolidone (v/v). Following homogenization, the suspension was centrifuged at 12,000× g for 15 min at 4 °C, and the supernatant was subsequently collected for enzymatic assays. SOD activity was measured in units (U), with one unit defined as the amount of enzyme required to inhibit the rate of epinephrine autoxidation by 50% under specified assay conditions []. For APX, activity was defined as one unit per min for every 0.01 absorbance shift at 290 nm. POD and CAT activities were measured in units per gram of protein (U/g), with a single unit corresponding to a 0.01 absorbance change per min at OD470 nm (POD) or OD240 nm (CAT) [].
4.8. RNA Extraction and Quantitative Real-Time PCR Analysis
Total RNA was extracted from cotyledon and non-cotyledon tissues utilizing a Plant RNA Isolation Kit (R6827-01, OMEGA, Norcross, GA, USA). Complementary DNA synthesis was conducted with PrimeScriptTM RT Master Mix (RR036A, Takara Bio, Kusatsu, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was performed using SYBR® Premix Ex TaqTM (RR420A, Takara, Japan) on an ABI 7500 system (Applied Biosystems, Foster City, CA, USA), with triplicate biological samples. Primer sequences are listed in Table 2. Gene expression was quantified using the comparative Ct (2−ΔΔCt) method [].

Table 2.
Primer sequences used for qRT-PCR analysis of relevant genes in finger millet. Reference gene: actin.
4.9. Statistical Analysis
Experimental results are presented as means with standard deviations (±SD), calculated from three independent experiments. Statistical analysis between treatment groups was conducted using one-way ANOVA followed by Tukey’s HSD post hoc test, with p < 0.05 considered statistically significant. All statistical analyses were performed using SPSS Statistics software (Version 22.0; IBM Corp., Armonk, NY, USA).
5. Conclusions
MeJA application enhanced flavonoid and phenolic compound accumulation while simultaneously triggering ROS oxidative stress, as demonstrated by increased H2O2 and O2−• concentrations at 4 days post-germination. MeJA treatment upregulated enzymatic activities of key biosynthetic enzymes and elevated expression levels of associated genes while concurrently augmenting antioxidant enzyme activities. This investigation establishes that MeJA treatment effectively stimulates flavonoid biosynthesis in finger millet sprouts through modulation of the phenylpropanoid metabolic pathway and antioxidant defense mechanisms.
Author Contributions
Writing—original draft, Z.Y. (Zhangqin Ye); Writing—review and editing, Z.Y. (Zhangqin Ye) and J.Z. (Jing Zhang); Formal analysis, J.Z. (Jing Zhang); Validation, X.T.; Project administration, Z.Y. (Zhengfei Yang) and J.Z. (Jiangyu Zhu); Supervision, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Majeed, I.; Nisa, M.U.; Rahim, M.A.; Ramadan, M.F.; Al-Asmari, F.; Alissa, M.; Zongo, E. Role of Seed Therapy on Estrous and Non-estrous Cycle in Healthy Female Rats. Food Sci. Nutr. 2025, 13, e4692. [Google Scholar] [CrossRef] [PubMed]
- Canivenc-Lavier, M.C.; Bennetau-Pelissero, C. Phytoestrogens and Health Effects. Nutrients 2023, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Omidian, M.; Kord-Varkaneh, H. Effect of Soy Protein Containing Isoflavones on Endothelial and Vascular Function in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Menopause 2020, 27, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Appleby, P.N.; Boeing, H.; Gil, L.; Kyrø, C.; Ricceri, F.; Murphy, N.; Trichopoulou, A.; Tsilidis, K.K.; Khaw, K.-T.; et al. Circulating Isoflavone and Lignan Concentrations and Prostate Cancer Risk: A Meta-Analysis of Individual Participant Data from Seven Prospective Studies Including 2828 Cases and 5593 Controls. Int. J. Cancer 2018, 143, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Hillman, G.G.; Singh-Gupta, V. Soy Isoflavones Sensitize Cancer Cells to Radiotherapy. Free Radic. Biol. Med. 2011, 51, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhang, Y.J.; Zhu, G.Y.; Shi, X.C.; Chen, X.; Herrera-Balandrano, D.D.; Liu, F.Q.; Laborda, P. Occurrence of Isoflavones in Soybean Sprouts and Strategies to Enhance Their Content: A Review. J. Food Sci. 2022, 87, 1961–1982. [Google Scholar] [CrossRef] [PubMed]
- Nickhil, C.; Singh, R.; Deka, S.C.; Nisha, R. Exploring Finger Millet Storage: An in-Depth Review of Challenges, Innovations, and Sustainable Practices. Cereal Res. Commun. 2025, 53, 57–79. [Google Scholar] [CrossRef]
- Udeh, H.O.; Duodu, K.G.; Jideani, A.I.O. Finger Millet Bioactive Compounds, Bioaccessibility, and Potential Health Effects—A Review. Czech J. Food Sci. 2017, 35, 7–17. [Google Scholar] [CrossRef]
- Gaikwad, V.; Kaur, J.; Rasane, P.; Kaur, S.; Singh, J.; Kumar, A.; Kumar, A.; Sharma, N.; Mehta, C.M.; Patel, A.S. Nutritional Significance of Finger Millet and Its Potential for Using in Functional Products. Foods Raw Mater. 2023, 12, 110–123. [Google Scholar] [CrossRef]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Finger Millet (Ragi, Eleusine coracana L.): A Review of Its Nutritional Properties, Processing, and Plausible Health Benefits. Adv. Food Nutr. Res. 2013, 69, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wan, Z.; Perry, L.; Lu, H.; Wang, Q.; Zhao, C.; Li, J.; Xie, F.; Yu, J.; Cui, T.; et al. Early Millet Use in Northern China. Proc. Natl. Acad. Sci. USA 2012, 109, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Shingare, S.P.; Thorat, B.N. Effect of Drying Temperature and Pretreatment on Protein Content and Color Changes during Fluidized Bed Drying of Finger Millets (Ragi, Eleusine coracana) Sprouts. Dry. Technol. 2013, 31, 507–518. [Google Scholar] [CrossRef]
- George, N.; Mildred, N.; Wanzala, E.; Munga, J.; Oduori, C.; Kinyuru, J.; Hudson, N. Nutritional Composition and Anti-Nutrient Levels in Raw and Processed Varieties of Finger Millet Promoted for Nutritional Security. Food Nutr. Sci. 2023, 14, 1183–1205. [Google Scholar] [CrossRef]
- Yan, H.; Chen, H.; Xia, M.; Liao, Q.; Zhao, J.; Peng, L.; Zou, L.; Zhao, G. The Impacts of Plant Hormones on the Growth and Quality of Sprouts. Food Bioprocess Technol. 2024, 17, 2913–2942. [Google Scholar] [CrossRef]
- Cao, J.; Li, M.; Chen, J.; Liu, P.; Li, Z. Effects of MeJA on Arabidopsis Metabolome under Endogenous JA Deficiency. Sci. Rep. 2016, 6, 37674. [Google Scholar] [CrossRef] [PubMed]
- Kortbeek, R.W.J.; Van Der Gragt, M.; Bleeker, P.M. Endogenous Plant Metabolites against Insects. Eur. J. Plant Pathol. 2019, 154, 67–90. [Google Scholar] [CrossRef]
- Pilaisangsuree, V.; Somboon, T.; Tonglairoum, P.; Keawracha, P.; Wongsa, T.; Kongbangkerd, A.; Limmongkon, A. Enhancement of Stilbene Compounds and Anti-Inflammatory Activity of Methyl Jasmonate and Cyclodextrin Elicited Peanut Hairy Root Culture. Plant Cell Tissue Organ Cult. 2018, 132, 165–179. [Google Scholar] [CrossRef]
- Shang, C.; Liu, X.; Chen, G.; Zheng, H.; Khan, A.; Li, G.; Hu, X. SlWRKY80-Mediated Jasmonic Acid Pathway Positively Regulates Tomato Resistance to Saline–Alkali Stress by Enhancing Spermidine Content and Stabilizing Na+/K+ Homeostasis. Hortic. Res. 2024, 11, uhae028. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; del Castillo, M.L.R. Influence of Preharvest and Postharvest Methyl Jasmonate Treatments on Flavonoid Content and Metabolomic Enzymes in Red Raspberry. Postharvest Biol. Technol. 2014, 97, 77–82. [Google Scholar] [CrossRef]
- Hama, J.R.; Hooshmand, K.; Laursen, B.B.; Vestergård, M.; Fomsgaard, I.S. Clover Root Uptake of Cereal Benzoxazinoids (BXs) Caused Accumulation of BXs and BX Transformation Products Concurrently with Substantial Increments in Clover Flavonoids and Abscisic Acid. J. Agric. Food Chem. 2022, 70, 14633–14640. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, P.; Cao, S.; Shang, H.; Yang, Z.; Zheng, Y. Methyl Jasmonate Reduces Decay and Enhances Antioxidant Capacity in Chinese Bayberries. J. Agric. Food Chem. 2009, 57, 5809–5815. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.; Liang, N.; Pan, Q.; Wang, J.; Reeves, M.J.; Duan, C. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.d.L.; Blanch, G.P.; Flores, G.; del Castillo, M.L.R. Impact of Postharvest Methyl Jasmonate Treatment on the Volatile Composition and Flavonol Content of Strawberries. J. Sci. Food Agric. 2010, 90, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; He, W.; Li, D.; Bao, Y.; Riaz, A.; Xiao, Y.; Song, J.; Liu, C. Effect of Methyl Jasmonate on Carotenoids Biosynthesis in Germinated Maize Kernels. Food Chem. 2020, 307, 125525. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xue, J.; Hu, J.; Yang, Z.; Fang, W. Exogenous Methyl Jasmonate Combined with Ca2+ Promote Resveratrol Biosynthesis and Stabilize Sprout Growth for the Production of Resveratrol-Rich Peanut Sprouts. Plant Physiol. Biochem. 2023, 203, 107988. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of Methyl Jasmonate on Plant Growth and Leaf Properties. J. Plant Nutr. Soil Sci. 2018, 181, 409–418. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The Sugar-Signaling Hub: Overview of Regulators and Interaction with the Hormonal and Metabolic Network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, C.; Huang, J.; Xu, D.; Zhang, C.; Chen, J. Effect of Exogenous Abscisic Acid and Salicylic Acid on Germination and Physiological Characteristics of Wheat Seed. Chin. J. Appl. Environ. Biol. 2014, 20, 139–143. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, J.; Yang, Z.; Fang, W.; Yang, J. Effects of Methyl Jasmonate and NaCl Treatments on the Resveratrol Accumulation and Defensive Responses in Germinated Peanut (Arachis hypogaea L.). Plant Physiol. Biochem. 2023, 194, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, Y.; Wang, Q.; Wang, P.; Han, Y.; Gu, Z.; Yang, R. NaCl Treatment on Physio-Biochemical Metabolism and Phenolics Accumulation in Barley Seedlings. Food Chem. 2020, 331, 127282. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gonzalez, J.J.; Guttikonda, S.K.; Tran, L.S.; Aldrich, D.L.; Zhong, R.; Yu, O.; Nguyen, H.T.; Sleper, D.A. Differential Expression of Isoflavone Biosynthetic Genes in Soybean during Water Deficits. Plant Cell Physiol. 2010, 51, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Yu, O.; Lau, S.M.; O’Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and Expression of Isoflavone Synthase, the Key Enzyme for Biosynthesis of Isoflavones in Legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liu, B.; Geng, X.; Ding, X.; Yan, N.; Sun, X.; Wang, W.; Sun, X.; Zheng, C. Biological Function and Stress Response Mechanism of MYB Transcription Factor Family Genes. J. Plant Growth Regul. 2023, 42, 83–95. [Google Scholar] [CrossRef]
- Han, K.; Zhao, Y.; Sun, Y.; Li, Y. NACs, Generalist in Plant Life. Plant Biotechnol. J. 2023, 21, 2433–2457. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhu, L.; Xu, L.; Guo, W.; Zhang, X. GhATAF1, a NAC Transcription Factor, Confers Abiotic and Biotic Stress Responses by Regulating Phytohormonal Signaling Networks. Plant Cell Rep. 2016, 35, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Wang, J.; Zou, G.; Wang, L.; Li, X. Two Responses to MeJA Induction of R2R3-MYB Transcription Factors Regulate Flavonoid Accumulation in Glycyrrhiza Uralensis Fisch. PLoS ONE 2020, 15, e0236565. [Google Scholar] [CrossRef] [PubMed]
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An Overview and Update on the Epidemiology of Flavonoid Intake and Cardiovascular Disease Risk. Food Funct. 2020, 11, 6777–6806. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose–Response Meta-analysis. Mol. Nutr. Food Res. 2021, 65, 2001019. [Google Scholar] [CrossRef] [PubMed]
- Bińkowska, W.; Szpicer, A.; Stelmasiak, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Utilization of Microencapsulated Polyphenols to Enhance the Bioactive Compound Content in Whole Grain Bread: Recipe Optimization. Appl. Sci. 2024, 14, 10156. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Gu, Z.; Sun, M.; Yang, R. Effects of Germination on Physio-Biochemical Metabolism and Phenolic Acids of Soybean Seeds. J. Food Compost. Anal. 2022, 112, 104717. [Google Scholar] [CrossRef]
- Wang, G.; Wu, L.; Zhang, H.; Wu, W.; Zhang, M.; Li, X.; Wu, H. Regulation of the Phenylpropanoid Pathway: A Mechanism of Selenium Tolerance in Peanut (Arachis hypogaea L.) Seedlings. J. Agric. Food Chem. 2016, 64, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile Maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, C.; Yang, M.; Yin, D. ZnCl2 Treatment Improves Nutrient Quality and Zn Accumulation in Peanut Seeds and Sprouts. Sci. Rep. 2020, 10, 2364. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, P.; Yang, R.; Zhou, T.; Gu, Z. UV-B Mediates Isoflavone Accumulation and Oxidative-Antioxidant System Responses in Germinating Soybean. Food Chem. 2019, 275, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, P.; Gu, Z.; Ma, M.; Yang, R. Spermidine Improves Antioxidant Activity and Energy Metabolism in Mung Bean Sprouts. Food Chem. 2020, 309, 125759. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Dawidziak, M. Influence of Stress Conditions on the Quality of Obtained Sprouts—Modification of Their Chemical Composition. Qual. Assur. Saf. Crops Foods 2021, 13, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).