The Invasive Mechanism and Impact of Arundo donax, One of the World’s 100 Worst Invasive Alien Species
Abstract
1. Introduction
2. Literature Survey
3. Life History Traits of Arundo donax
3.1. Growth
3.2. Reproduction and Establishment
4. Adaptation of Arundo donax to Abiotic Stress
4.1. Cold Temperature
4.2. Drought
4.3. Flooding
4.4. Salinity
5. Adaptation of Arundo donax to Biotic Stresses
5.1. Defensive Response Against Herbivores and Pathogens
5.2. Defensive Response Against Competing Plant Species
6. Impacts of Arundo donax on Abiotic and Biotic Ecosystem Processes
6.1. Impacts on Abiotic Ecosystem Processes
6.2. Impacts on Biotic Ecosystem Processes
7. Conclusions
Funding
Conflicts of Interest
References
- Royal Botanic Garden Kew, Arundo donax L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:390837-1 (accessed on 11 June 2025).
- CABI Compendium Arundo donax (Giant Reed). Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.1940 (accessed on 11 June 2025).
- PROSEA—Plant Resources of South East Asia. Available online: https://prosea.prota4u.org/view.aspx?id=6525 (accessed on 11 June 2025).
- Pacific Island Ecosystems at Risk (PIER) Arundo donax. Available online: http://www.hear.org/pier/imagepages/singles/ardonp1.htm (accessed on 11 June 2025).
- Jiménez-Ruiz, J.; Hardion, L.; Del Monte, J.P.; Vila, B.; Santín-Montanyá, M.I. Monographs on invasive plants in Europe, no. 4: Arundo donax L. Bot. Lett. 2021, 168, 131–151. [Google Scholar] [CrossRef]
- Ngernsaengsaruay, C.; Puangsin, B.; Leksungnoen, N.; Khantayanuwong, S.; Chanton, P.; Thaepthup, T.; Wessapak, P.; Meeboonya, R.; Yimlamai, P.; Wanitpinyo, K.; et al. Morphology, taxonomy, culm internode and leaf anatomy, and palynology of the giant reed (Arundo donax L.), Poaceae, growing in Thailand. Plants 2023, 12, 1850. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Moran, P.J.; Jiménez, M.M.; Yang, C.; Canavan, K.; Paynter, Q.; Ota, N.; Kriticos, D.J. Biology of invasive plants 4. Arundo donax L. Invas. Plant Sci. Manag. 2023, 16, 81–109. [Google Scholar] [CrossRef]
- Pilu, R.; Cassani, E.; Landoni, M.; Badone, F.C.; Passera, A.; Cantaluppi, E.; Corno, L.; Adani, F. Genetic characterization of an Italian giant reed (Arundo donax L.) clone collection: Exploiting clonal selection. Euphytica 2013, 196, 169–181. [Google Scholar] [CrossRef]
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the invasive Arundo donax (Poaceae): A trans-Asian expedition in herbaria. Ann. Bot. 2014, 114, 455–462. [Google Scholar] [CrossRef]
- Danelli, T.; Laura, M.; Savona, M.; Landoni, M.; Adani, F.; Pilu, R. Genetic improvement of Arundo donax L.: Opportunities and challenges. Plants 2020, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Bucci, A.; Cassani, E.; Landoni, M.; Cantaluppi, E.; Pilu, R. Analysis of chromosome number and speculations on the origin of Arundo donax L. (giant reed). Cytol. Genet. 2013, 47, 237–241. [Google Scholar] [CrossRef]
- Hardion, L.; Verlaque, R.; Rosato, M.; Rossello, J.R.; Vila, B. Impact of polyploidy on fertility variation of Mediterranean Arundo donax L. (Poaceae). Curr. Rev. Biol. 2015, 338, 298–306. [Google Scholar]
- Touchell, D.H.; Ranney, T.G.; Panthee, D.R.; Gehl, R.J.; Krings, A. Genetic diversity, cytogenetics, and biomass yields among taxa of giant reeds (Arundo species). J. Am. Soc. Hortic. Sci. 2016, 141, 256–263. [Google Scholar] [CrossRef]
- Mariani, C.; Cabrini, R.; Danin, A.; Piffanelli, P.; Fricano, A.; Gomarasca, S.; Dicandilo, M.; Grassi, F.; Soave, C. Origin, diffusion and reproduction of the giant reed (Arundo donax L.): A promising weedy energy crop. Ann. Appl. Biol. 2010, 157, 191–202. [Google Scholar] [CrossRef]
- Balogh, E.; Herr, J.M., Jr.; Czakó, M.; Márton, L. Defective development of male and female gametophytes in Arundo donax L. (Poaceae). Biomass Bioenergy 2012, 45, 265–269. [Google Scholar] [CrossRef]
- Haddadchi, A.; Gross, C.L.; Fatemi, M. The expansion of sterile Arundo donax (Poaceae) in southeastern Australia is accompanied by genotypic variation. Aquat. Bot. 2013, 104, 153–161. [Google Scholar] [CrossRef]
- Jike, W.; Li, M.; Zadra, N.; Barbaro, E.; Sablok, G.; Bertorelle, G.; Rota-Stabelli, O.; Varotto, C. Phylogenomic proof of recurrent demipolyploidization and evolutionary stalling of the “Triploid Bridge” in Arundo (Poaceae). Int. J. Mol. Sci. 2020, 21, 5247. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Liu, F.; Han, X.; Wu, D.; Peng, H. Chromosome-scale genome assembly of the autoalloenneaploid Arundo donax. Grassl. Res. 2024, 3, 230–242. [Google Scholar] [CrossRef]
- Bell, G.P. Ecology and management of Arundo donax, and approaches to riparian habitat restoration in southern California. In Plant Invasion: Studies from North America and Europe; Brock, J.H., Wade, M., Pysek, P., Green, D., Eds.; Backhuys Publishers: Leiden, The Netherland, 1997; pp. 103–113. [Google Scholar]
- USDA-Field Release of the Arundo Wasp, Tetramesa romana (Hymenoptera: Eurytomidae), an Insect for Biological Control of Arundo donax (Poaceae). Available online: https://www.aphis.usda.gov/sites/default/files/Tetramesa-romana-ea.pdf (accessed on 11 June 2025).
- Virtue, J.G.; Reynolds, T.; Malone, J.; Preston, C.; Williams, C. Managing the weed risk of cultivated Arundo donax L. New Frontiers in New Zealand: Together We Can Beat the Weeds. In Proceedings of the Seventeenth Australasian Weeds Conference, Christchurch, New Zealand, 26–30 September 2010; pp. 176–179. [Google Scholar]
- Bacher, W.; Sauerbeck, G.; Mix-Wagner, G.; El-Bassam, N. Giant Reed (Arundo donax L.) Network: Improvement Productivity and Biomass Quality; Final Report FAIR-CT-96-2028. Available online: https://www.openagrar.de/receive/timport_mods_00001974 (accessed on 11 June 2025).
- Antal, G. Giant reed (Arundo donax L.) from ornamental plant to dedicated bioenergy species: Review of economic prospects of biomass production and utilization. Int. J. Hortic. Sci. 2018, 24, 39–46. [Google Scholar] [CrossRef]
- Jambor, A.; Török, A. The economics of Arundo donax—A systematic literature review. Sustainability 2019, 11, 4225. [Google Scholar] [CrossRef]
- Ortega, Z.; Bolaji, I.; Suárez, L.; Cunningham, E. A review of the use of giant reed (Arundo donax L.) in the biorefineries context. Rev. Chem. Eng. 2024, 40, 305–328. [Google Scholar] [CrossRef]
- Canavan, K.; Paterson, I.D.; Hill, M.P. Exploring the origin and genetic diversity of the giant reed, Arundo donax in South Africa. Invas. Plant Sci. Manag. 2017, 10, 53–60. [Google Scholar] [CrossRef]
- EDDMapS, State and County Distribution Map for Giant Reed. Early Detection & Distribution Mapping System. Available online: https://www.eddmaps.org/distribution/viewmap.cfm?sub=3009 (accessed on 11 June 2025).
- Instituto Méxicano de Tecnologia del Agua, Invasive Species with High Impact on Biodiversity, Priorities in Mexico. Available online: https://www.invasive.org/gist/products/library/mex-especies-invadoras.pdf (accessed on 11 June 2025).
- Yang, C.; Everitt, J.H.; Goolsby, J.A. Mapping giant reed (Arundo donax) infestations along the Texas–Mexico portion of the Rio Grande with aerial photography. Invas. Plant Sci. Manag. 2011, 4, 402–410. [Google Scholar] [CrossRef]
- Yang, C.; Goolsby, J.A.; Everitt, J.H. Mapping giant reed with Quick-Bird imagery in the Mexican portion of the Rio Grande Basin. J. Appl. Remote Sens. 2009, 3, 033530. [Google Scholar] [CrossRef]
- Giessow, J.; Casanova, J.; MacArthur, R.; Leclerc, R.; Fleming, G. Arundo Donax Distribution and Impact Report; 06-374-559-0, Cal-IPC, 2011; California Invasive Plant Council (Cal-IPC): Berkeley, CA, USA, 2011; Available online: https://www.cal-ipc.org/wp-content/uploads/2017/11/Arundo_Distribution_Impact_Report_Cal-IPC_March-2011_small.pdf (accessed on 11 June 2025).
- Arundo donax Workshop Proceedings, California Exotic Pest Plant Council (CalEPPC), Pismo Beach, CA, USA, 19 November 1993; Jackson, N.E., Frandsen, P., Douthit, P., Eds. Available online: https://www.cal-ipc.org/docs/symposia/archive/pdf/Arundo_Proceedings_1993.pdf (accessed on 11 June 2025).
- IUCN, 100 of the World’s Worst Invasive Alien Species. Available online: https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf (accessed on 11 June 2025).
- Howe, C.E. A review of the removal of Arundo donax from a riparian area within San Timoteo Canyon. Electronic Theses, Projects, and Dissertations. 2014, 106. Available online: https://scholarworks.lib.csusb.edu/etd/106 (accessed on 11 June 2025).
- Briggs, M.K.; Poulos, H.M.; Renfrow, J.; Ochoa-Espinoza, J.; Larson, D.; Manning, P.; Sirotnak, J.; Crawford, K. Choked out: Battling invasive giant cane along the Rio Grande/Bravo borderlands. River Res. Appl. 2021, 37, 1471–1479. [Google Scholar] [CrossRef]
- Bruno, D.; Zapata, V.; Guareschi, S.; Picazo, F.; Dettori, E.; Carbonell, J.A.; Millán, A.; Velasco, J.; Robledano, F. Short-term responses of aquatic and terrestrial biodiversity to riparian restoration measures designed to control the invasive Arundo donax L. Water 2021, 11, 2551. [Google Scholar] [CrossRef]
- Coffey, J.E.; Pomara, L.Y.; Mackey, H.L.; Wood, E.M. Removing invasive giant reed reshapes desert riparian butterfly and bird communities. J. Wildlife Manag. 2023, 87, e22380. [Google Scholar] [CrossRef]
- Holmes, P.M.; Richardson, D.M.; Esler, K.J.; Witkowski, E.T.F.; Fourie, S. A decision-making framework for restoring riparian zones degraded by invasive alien plants in South Africa. S. Afr. J. Sci. 2005, 101, 553–564. [Google Scholar]
- Lawson, D.M.; Giessow, J.A.; Giessow, J.H. The Santa Margarita River Arundo donax control project: Development of methods and plant community response. In Planning for Biodiversity: Bringing Research and Management Together; Gen. Tech. Rep. PSW-GTR-195; Pacific Southwest Research Station, Forest Service, US Department of Agriculture: Albany, CA, USA, 2005; pp. 229–244. Available online: https://www.fs.usda.gov/psw/publications/documents/psw_gtr195/psw_gtr195_2_14_Lawson.pdf (accessed on 11 June 2025).
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Warren, R.J.; Matt Candeias, M.; Labatore, A.; Olejniczak, M.; Yang, L. Multiple mechanisms in woodland plant species invasion. J. Plant Ecol. 2019, 12, 201–209. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The impact and invasive mechanisms of Pueraria montana var. lobata, one of the world’s worst alien species. Plants 2023, 12, 3066. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. The invasive mechanisms of the noxious alien plant species Bidens pilosa. Plants 2024, 13, 356. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Invasive characteristics of Robinia pseudoacacia and its impacts on the species diversity. Diversity 2024, 16, 773. [Google Scholar] [CrossRef]
- Clements, D.R.; Kato-Noguchi, H. Defensive mechanisms of Mikania micrantha likely enhance its invasiveness as one of the world’s worst alien species. Plants 2025, 14, 269. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Defense molecules of the invasive plant species Ageratum conyzoides. Molecules 2024, 29, 4673. [Google Scholar] [CrossRef] [PubMed]
- Coffman, G.C.; Ambrose, R.F.; Rundel, P.W. Wildfire promotes dominance of invasive giant reed (Arundo donax) in riparian ecosystems. Biol. Invasions 2010, 12, 2723–2734. [Google Scholar] [CrossRef]
- Dudley, T.L. Arundo donax. In Invasive Plants of California Wildlands; Bossard, C.C., Randal, J.M., Hosovsky, M.C., Eds.; University of California Press: Berkeley, CA, USA, 2000; pp. 53–58. [Google Scholar]
- Erickson, J.E.; Soikaew, A.; Sollenberger, L.E.; Bennet, J.M. Water use and water-use efficiency of three perennial bioenergy grass crops in Florida. Agriculture 2012, 2, 325–338. [Google Scholar] [CrossRef]
- Sánchez, E.; Lino, G.; Arias, C.; Serrat, X.; Nogués, S. Photosynthesis, resource acquisition and growth responses of two biomass crops subjected to water stress. J. Plant Sci. 2018, 6, 68–86. [Google Scholar]
- Haworth, M.; Catola, S.; Marino, G.; Brunetti, C.; Michelozzi, M.; Riggi, E.; Avola, G.; Cosentino, S.L.; Loreto, F.; Centritto, M. Moderate drought stress induces increased foliar dimethylsulphoniopropionate (DMSP) concentration and isoprene emission in two contrasting ecotypes of Arundo donax. Front. Plant Sci. 2017, 8, 1016. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patanè, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Mohr, H.; Schopfer, P. Plant Physiology; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–629. [Google Scholar]
- Rossa, B.; Rüffers, A.V.; Naidoo, G.; von Willert, D.J. Arundo donax L. (Poaceae)—A C3 species with unusually high photosynthetic capacity. Plant Biol. 1998, 111, 216–221. [Google Scholar] [CrossRef]
- Webster, R.J.; Driever, S.M.; Kromdijk, J.; McGrath, J.; Leakey, A.D.B.; Siebke, K.; Demetriades-Shah, T.; Bonnage, S.; Peloe, T.; Lawson, T.; et al. High C3 photosynthetic capacity and high intrinsic water use efficiency underlies the high productivity of the bioenergy grass Arundo donax. Sci. Rep. 2016, 6, 20694. [Google Scholar] [CrossRef]
- Idris, S.M.; Jones, P.L.; Salzman, S.A.; Croatto, G.; Allinson, G. Evaluation of the giant reed (Arundo donax) in horizontal subsurface flow wetlands for the treatment of dairy processing factory wastewater. Environ. Sci. Pollut. Res. 2012, 19, 3525–3537. [Google Scholar] [CrossRef]
- Spencer, D.F.; Liow, P.S.; Chan, W.K.; Ksander, G.G.; Getsinger, K.D. Estimating Arundo donax shoot biomass. Aquat. Bot. 2006, 84, 272–276. [Google Scholar] [CrossRef]
- Kering, M.K.; Butler, T.J.; Biermacher, J.T.; Guretzky, J.A. Biomass yield and nutrient removal rates of perennial grasses under nitrogen fertilization. Bioenergy Res. 2012, 5, 61–70. [Google Scholar] [CrossRef]
- Acharya, M.; Burner, D.M.; Ashworth, A.J.; Fritschi, F.B.; Adams, T.C. Growth rates of giant miscanthus (Miscanthus× giganteus) and giant reed (Arundo donax) in a low-input system in Arkansas, USA. Am. J. Plant Sci. 2018, 9, 2371. [Google Scholar] [CrossRef]
- CAL-IPC-2023, California Invasive Plant Council, Central Valley Arundo donax: Distribution, Impacts, and Management. Available online: https://www.cal-ipc.org/solutions/research/central-valley-arundo/ (accessed on 11 June 2025).
- Sharma, K.P.; Kushwaha, S.P.S.; Gopal, B. A comparative study of stand structure and standing crops of two wetland species Arundo donax and Phragmites karka, and primary production in Arundo donax with observations on the effect of clipping. Trop. Ecol. 1998, 39, 3–14. [Google Scholar]
- Eid, E.M.; Youssef, M.S.; Shaltout, K.H. Population characteristics of giant reed (Arundo donax L.) in cultivated and naturalized habitats. Aquat. Bot. 2016, 129, 1–8. [Google Scholar] [CrossRef]
- Curt, M.D.; Sanz, M.; Mauri, P.V.; Plaza, A.; Cano-Ruiz, J.; Sánchez, J.; Aguado, P.L.; Chaya, C.; Fernández, J. Effect of water regime change in a mature Arundo donax crop under a Xeric Mediterranean climate. Biomass Bioenergy 2018, 115, 203–209. [Google Scholar] [CrossRef]
- Hidalgo, M.; Fernandez, J. Biomass production of ten populations of giant reed (Arundo donax L.) under the environmental conditions of Madrid (Spain). In Biomass for Energy and Industry, Proceeding of the First World Conference, Sevilla, Spain, 5–9 June 2000; Kyritsis, S., Ed.; Routledge: London, UK, 2001; p. 1811. [Google Scholar]
- Angelini, L.G.; Ceccarini, L.; Bonari, E. Biomass yield and energy balance of giant reed (Arundo donax L.) cropped in Central Italy as related to different management practices. Eur. J. Agron. 2005, 22, 375–389. [Google Scholar] [CrossRef]
- Fagnano, M.; Impagliazzo, A.; Mori, M.; Fiorentino, N. Agronomic and environmental impacts of giant reed (Arundo donax L.): Results from a long-term field experiment in hilly areas subject to soil erosion. Bioenergy Res. 2015, 8, 415–422. [Google Scholar] [CrossRef]
- Amaducci, S.; Perego, A. Field evaluation of Arundo donax clones for bioenergy production. Ind. Crop. Prod. 2015, 75, 122–128. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Nutrient removal and biomass production in land treatment systems receiving domestic effluent. Ecol. Eng. 2008, 35, 1485–1492. [Google Scholar] [CrossRef]
- Günes, K.; Saygin, Ö. Productivity of the energy crops: Giant reed and sweet sorghum in Turkey. Fresenius Environ. Bull. 1996, 5, 756–761. [Google Scholar]
- Nazli, R.I.; Tansi, V.; Öztürk, H.H.; Kusvuran, A. Miscanthus, switchgrass, giant reed and bulbous canary grass as potential bioenergy crops in a semi-arid Mediterranean environment. Ind. Crop. Prod. 2018, 125, 9–23. [Google Scholar] [CrossRef]
- Mantineo, M.; D’Agosta, G.M.; Copani, V.; Patanè, C.; Cosentino, S.L. Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crops Res. 2009, 114, 204–213. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L.: A non-food crop for bioenergy and bio-compound production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Johnson, M.; Dudley, T.; Burns, C. Seed production in Arundo donax. Cal-IPC News 2006, 14, 12–13. [Google Scholar]
- Saltonstall, K.; Lambert, A.; Meyerson, L.A. Genetics and reproduction of common (Phragmites australis) and giant reed (Arundo donax). Invas. Plant Sci. Manag. 2010, 3, 495–505. [Google Scholar] [CrossRef]
- Nikhade, C.; Makde, K. Reproductive behavior of Arundo donax L. Int. J. Res. Biosci. Agric. Technol. 2014, 2, 670–679. [Google Scholar]
- Cantaluppi, E.; Cassani, E.; Puglisi, D.; Corno, L.; Munaro, M.; Landoni, M.; Adani, F.; Pilu, R. Study on the inflorescences of Arundo donax L. clones sampled in Italy. Braz. J. Bot. 2016, 39, 275–285. [Google Scholar] [CrossRef]
- Perdue Jr, R.E. Arundo donax—Source of musical reeds and industrial cellulose. Econ. Bot. 1958, 12, 368–404. [Google Scholar] [CrossRef]
- Wen, S. Influence of sewage on seed germination of 12 kinds of plants in wetlands. Wetland Sci. 2015, 13, 80–86. [Google Scholar]
- Cao, C.; Huang, J.; Wang, N.; Yan, C.N.; Peng, C. Impact of zinc oxide nanoparticles on seed germination of wetland plant. J. Southeast Univ. Nat. Sci. Ed. 2017, 47, 5. [Google Scholar]
- Colin, R.; Aguirre-Planter, E.; Eguiarte, L.E. Genetic and ecological characterization of the giant reed (Arundo donax) in Central Mexico. PLoS ONE 2025, 20, e0319214. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.M. The importance of layering in the rapid spread of Arundo donax (giant reed). Madrono 2006, 53, 303–312. [Google Scholar] [CrossRef]
- Boose, A.B.; Holt, J.S. Environmental effects on asexual reproduction in Arundo donax. Weed Res. 1999, 39, 117–127. [Google Scholar] [CrossRef]
- Kui, L.; Li, F.; Moore, G.; West, J. Can the riparian invader, Arundo donax, benefit from clonal integration? Weed Res. 2013, 53, 370–377. [Google Scholar] [CrossRef]
- Calazans, E.; Lopes, A.; Girotto, L.; de Paula, A.L.O.; Franco, A.C.; Ferreira, C.S. Mechanical control inadvertently increases risk of alien plant invasion: Influence of stem fragmentation and inundation regimes on Arundo donax regeneration in Neotropical savanna. Aust. J. Bot. 2023, 71, 223–230. [Google Scholar] [CrossRef]
- Else, J.A.; Zedler, P. Dynamics of the flood disturbed zone of a riparian system: Vegetative establishment and resprouting of woody native species and the exotic, Arundo donax. Bull. Ecol. Soc. Am. 1996, 77, 129. [Google Scholar]
- Khudamrongsawat, J.; Tayyar, R.; Holt, J.S. Genetic diversity of giant reed (Arundo donax) in the Santa Ana River, California. Weed Sci. 2004, 52, 395–405. [Google Scholar] [CrossRef]
- Decruyenaere, J.G.; Holt, J.S. Seasonality of clonal propagation in giant reed. Weed Sci. 2001, 49, 760–767. [Google Scholar] [CrossRef]
- Else, J.A. Post-Flood Establishment of Native Woody Species and an Exotic, Arundo donax, in a Southern California Riparian System. Master’s Thesis, San Diego State University, San Diego, CA, USA, 1996. [Google Scholar]
- PIER-2023, Pacific Islands Ecosystems at Risk: Plant Threats to Pacific Ecosystems; Institute of Pacific Islands Forestry, US Forest Service/HEAR, University of Hawaii: Honolulu, HI, USA, 2023; Available online: http://www.hear.org/pier/index.html (accessed on 11 June 2025).
- USDA-APHIS. Weed Risk Assessment for Arundo donax L. (Poaceae)—Giant Reed; Plant Protection and Quarantine, USDA Animal and Plant Health Inspection Service: Raleigh, NC, USA, 2012. Available online: https://www.aphis.usda.gov/sites/default/files/arundo_donax_wra.pdf (accessed on 11 June 2025).
- Lambert, A.M.; D’Antonio, C.; Dudley, T.L. Invasive species and fire in California. Fremontia 2010, 38, 29–36. [Google Scholar]
- Ambrose, R.F.; Rundel, P.W. Influence of Nutrient Loading on the Invasion of an Alien Plant Species, Giant Reed (Arundo donax), in Southern California Riparian Ecosystems, UC Water Resources Center Technical Completion Report Project No. W-960. 2007. Available online: https://escholarship.org/uc/item/3qt3s5c4 (accessed on 11 June 2025).
- Mann, J.J.; Barney, J.N.; Kyser, G.B.; Di Tomaso, J.M. Miscanthus x giganteus and Arundo donax shoot and rhizome tolerance of extreme moisture stress. GCB Bioenergy 2013, 5, 693–700. [Google Scholar] [CrossRef]
- Ren, L.; Eller, F.; Lambertini, C.; Guo, W.Y.; Brix, H.; Sorrel, B.K. Assessing nutrient responses and biomass quality for selection of appropriate paludiculture crops. Sci. Total Environ. 2019, 664, 1150–1161. [Google Scholar] [CrossRef]
- Walshaw, S. Plant Resources of Tropical Africa; PROTA-2023; University Fund Wageningen: Wageningen, The Netherlands, 2023; Available online: https://prota.prota4u.org/ (accessed on 11 June 2025).
- Csurhes, S. Giant reed, Arundo donax. In Invasive Plant Risk Assessment; Department of Agriculture and Fisheries, Biosecurity Queensland: Brisbane, Australia, 2016. Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0006/59973/IPA-Giant-Reed-Risk-Assessment.pdf (accessed on 11 June 2025).
- Pompeiano, A.; Vita, F.; Miele, S.; Guglielminetti, L. Freeze tolerance and physiological changes during cold acclimation of giant reed [Arundo donax (L.)]. Grass Forage Sci. 2015, 70, 168–175. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Ruedy, R.; Lacis, A.; Oinas, V. Global warming in the twenty-first century: An alternative scenario. Proc. Nat. Acad. Sci. USA 2000, 97, 9875–9880. [Google Scholar] [CrossRef]
- Mack, R.N. Evaluating the credits and debits of a proposed biofuel species: Giant reed (Arundo donax). Weed Sci. 2008, 56, 883–888. [Google Scholar] [CrossRef]
- Sánchez, E.; Scordia, D.; Lino, G.; Arias, C.; Cosentino, S.L.; Nogués, S. Salinity and water stress effects on biomass production in different Arundo donax L. clones. Bioenergy Res. 2015, 8, 1461–1479. [Google Scholar] [CrossRef]
- Romero-Munar, A.; Baraza, E.; Cifre, J.; Achir, C.; Gulías, J. Leaf plasticity and stomatal regulation determines the ability of Arundo donax plantlets to cope with water stress. Photosynthetica 2018, 56, 698–706. [Google Scholar] [CrossRef]
- Anjum, S.; Xie, X.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Meier, I.C.; Leuschner, C. Leaf size and leaf area index in Fagus sylvatica forests: Competing effects of precipitation, temperature, and nitrogen availability. Ecosystems 2008, 11, 655–669. [Google Scholar] [CrossRef]
- Haworth, M.; Centritto, M.; Giovannelli, A.; Marino, G.; Proietti, N.; Capitani, D.; De Carlo, A.; Loreto, F. Xylem morphology determines the drought response of two Arundo donax ecotypes from contrasting habitats. GCB Bioenergy 2017, 9, 119–131. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic adjustment and plant adaptation to drought stress. In Drought Stress Tolerance in Plants, Vol. 1: Physiology and Biochemistry; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–143. [Google Scholar]
- Ali, O.; Cheddadi, I.; Landrein, B.; Long, Y. Revisiting the relationship between turgor pressure and plant cell growth. New Phytol. 2023, 238, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Cosentino, S.L.; Brunetti, C.; De Carlo, A.; Avola, G.; Riggi, E.; Loreto, F.; Centritto, M. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behavior in fast growing giant reed (Arundo donax L.). Environ. Exp. Bot. 2018, 147, 116–124. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Riggi, E.; Avola, G.; Brunetti, C.; Scordia, D.; Testa, G.; Gomes, M.T.G.; Loreto, F.; Cosentino, S.L.; et al. The effect of summer drought on the yield of Arundo donax is reduced by the retention of photosynthetic capacity and leaf growth later in the growing season. Ann. Bot. 2019, 124, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Tshapa, L.; Naidoo, K.; Naidoo, G. Morphological and physiological responses of Arundo donax and Phragmites australis to waterlogging stress. Flora 2021, 279, 151816. [Google Scholar] [CrossRef]
- Pompeiano, A.; Vita, F.; Alpi, A.; Guglielminetti, L. Arundo donax L. response to low oxygen stress. Environ. Exp. Bot. 2015, 111, 147–154. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Pyruvate metabolism in rice coleoptiles under anaerobiosis. Plant Growth Regul. 2006, 50, 41–46. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Submergence tolerance and ethanolic fermentation in rice coleoptiles. Plant Prod. Sci. 2001, 4, 62–65. [Google Scholar] [CrossRef]
- Armstrong, W.; Beckett, P.M.; Colmer, T.D.; Setter, T.L.; Greenway, H. Tolerance of roots to low oxygen: ‘Anoxic’ cores, the phytoglobin nitric oxide cycle and energy or oxygen sensing. J. Plant Physiol. 2019, 239, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Banti, V.; Giuntoli, B.; Gonzali, S.; Loreti, E.; Magneschi, L.; Novi, G.; Paparelli, E.; Parlanti, S.; Pucciariello, C.; Santaniello, A.; et al. Low oxygen response mechanisms in green organisms. Int. J. Mol. Sci. 2013, 14, 4734–4761. [Google Scholar] [CrossRef]
- Pompeiano, A.; Reyes, H.T.; Moles, T.M.; Guglielminetti, L.; Scartazza, A. Photosynthetic and growth responses of Arundo donax L. plantlets under different oxygen deficiency stresses and re-oxygenation. Front. Plant Sci. 2019, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Speck, O.; Spatz, H.C. Mechanical properties of the rhizome of Arundo donax L. Plant Biol. 2003, 5, 661–669. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Yamauchi, T.; Shimamura, S.; Nakazono, M.; Mochizuki, T. Aerenchyma formation in crop species: A review. Field Crops Res. 2013, 152, 8–16. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M.J.A.O.B. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Sánchez, E.; Gil, S.; Azcón-Bieto, J.; Nogués, S. The response of Arundo donax L. (C3) and Panicum virgatum (C4) to different stresses. Biomass Bioenergy 2016, 85, 335–345. [Google Scholar] [CrossRef]
- Andreu-Rodríguez, J.; Pérez-Espinosa, A.; Pérez-Murcia, M.D.; Moral, R.; Agulló, E.; Ferrández-Villena, M.; Bustamante, M.A. Near infrared reflectance spectroscopy (NIRS) for the assessment of biomass production and C sequestration by Arundo donax L. in salt-affected environments. Agric. Water Manag. 2017, 183, 94–100. [Google Scholar] [CrossRef]
- Pompeiano, A.; Landi, M.; Meloni, G.; Vita, F.; Guglielminetti, L.; Guidi, L. Allocation pattern, ion partitioning, and chlorophyll a fluorescence in Arundo donax L. in responses to salinity stress. Plant Biosyst. 2017, 151, 613–622. [Google Scholar] [CrossRef]
- Jia, Y.; Fan, Y.; Chen, T.; Duan, Z.; Liu, S.; Gao, X. Leaf plasticity and biomass allocation of Arundo donax under combined irrigation and nitrogen conditions in Salinized soil. Agriculture 2025, 15, 1166. [Google Scholar] [CrossRef]
- Nackley, L.L.; Kim, S.H. A salt on the bioenergy and biological invasions debate: Salinity tolerance of the invasive biomass feedstock Arundo donax. Glob. Chang. Biol. Bioenergy 2015, 7, 752–762. [Google Scholar] [CrossRef]
- Michela, J.; Claudia, C.; Federico, B.; Sara, P.; Filippo, V.; Nicola, C.; Manuele, B.; Davide, C.; Loreto, F.; Zappettini, A. Real-time monitoring of Arundo donax response to saline stress through the application of in vivo sensing technology. Sci. Rep. 2021, 11, 18598. [Google Scholar] [CrossRef]
- Tyler, R.H.; Boyer, T.P.; Minami, T.; Zweng, M.M.; Reagan, J.R. Electrical conductivity of the global ocean. Earth Planets Space 2017, 69, 1–10. [Google Scholar] [CrossRef]
- Cocozza, C.; Brilli, F.; Miozzi, L.; Pignattelli, S.; Rotunno, S.; Brunetti, C.; Giordano, C.; Pollastri, S.; Centritto, M.; Accotto, G.P.; et al. Impact of high or low levels of phosphorus and high sodium in soils on productivity and stress tolerance of Arundo donax plants. Plant Sci. 2019, 289, 110260. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef]
- Müller, B.; Arcoverde Cerveira Sterner, V.; Papp, L.; May, Z.; Orlóci, L.; Gyuricza, C.; Sági, L.; Solti, Á.; Fodor, F. Alkaline salt tolerance of the biomass plant Arundo donax. Agronomy 2022, 12, 1589. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, X.; Liu, B.; Lin, H.; Luo, H.; Liu, F.; Su, D.; Lv, S.; Lin, Z.; Lin, D. Saline-alkali tolerance evaluation of giant reed (Arundo donax) genotypes under saline–alkali stress at seedling stage. Agronomy 2023, 9, e15521. [Google Scholar] [CrossRef]
- Lino, G.; Espigul, P.; Nogués, S.; Serrat, X. Arundo donax L. growth potential under different abiotic stress. Heliyon 2023, 9, e15521. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Defensive compounds Involved in the invasiveness of Tithonia diversifolia. Molecules 2025, 30, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Evolution of the defense compounds against biotic stressors in the invasive plant species Leucaena leucocephala. Molecules 2025, 30, 2453. [Google Scholar] [CrossRef]
- Karban, R.; Myers, J.H. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 1989, 20, 331–348. [Google Scholar] [CrossRef]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. R. Soc. B Biol. Sci. 2006, 273, 2575–2584. [Google Scholar] [CrossRef]
- Gong, B.; Zhang, G. Interactions between plants and herbivores: A review of plant defense. Acta Ecol. Sin. 2014, 34, 325–336. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Compounds involved in the invasive characteristics of Lantana camara. Molecules 2025, 30, 411. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef]
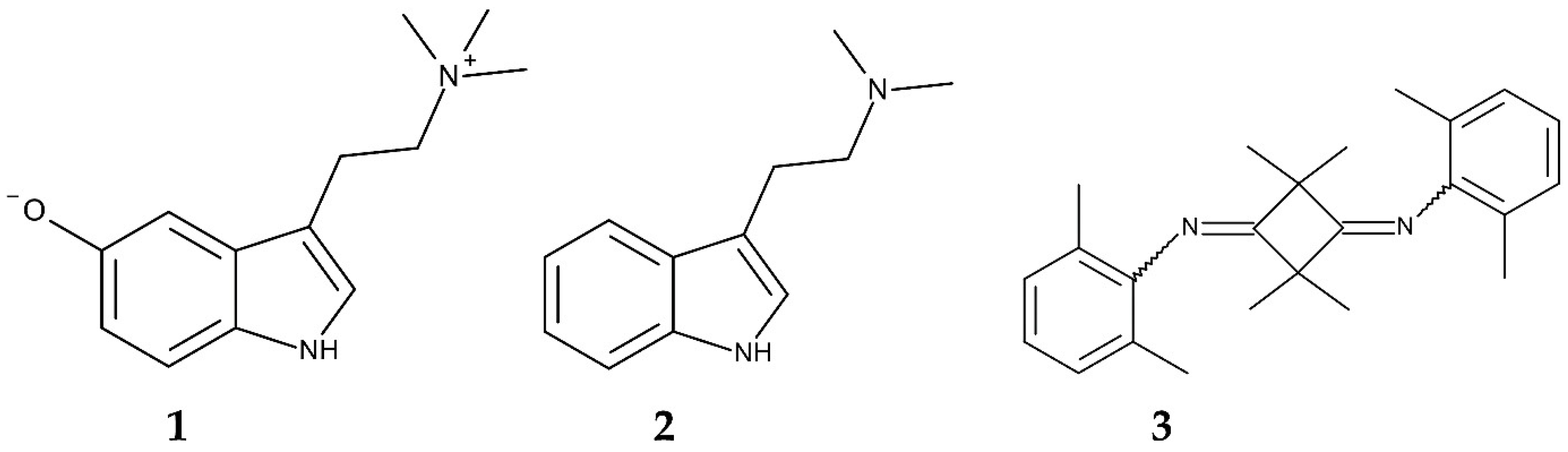
- Ghosal, S.; Dutta, S.K.; Sanyal, A.K.; Bhattacharya, S.K. Arundo donax L. (Graminae). Phytochemical and pharmacological evaluation. J. Med. Chem. 1969, 12, 480–483. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Sanyal, A.K. Neuromuscular blocking activity of bufotenidine isolated from Arundo donax L. Naturwissenschaften 1972, 59, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.W.; Jiang, X.L.; Winter, J.C.; Yu, A.M. Psychedelic 5-methoxy-N,N-dimethyltryptamine: Metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr. Drug Metab. 2010, 11, 659–666. [Google Scholar] [CrossRef]
- Roth, B.; Choudhary, M.; Khan, N.; Uluer, A. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine 2A receptors: Evidence in favor of a modified ternary complex model. J. Pharmacol. Exp. Ther. 1997, 280, 576–583. [Google Scholar] [CrossRef]
- Qi, J.; Zulfiker, A.H.M.; Li, C.; Good, D.; Wei, M.Q. The development of toad toxins as potential therapeutic agents. Toxins 2018, 10, 336. [Google Scholar] [CrossRef]
- Ghosal, S.; Chaudhuri, R.K.; Dutta, S.K. Alkaloids of the flowers of Arundo donax. Phytochemistry 1971, 10, 2852–2853. [Google Scholar] [CrossRef]
- Khuzhaev, V.U. Alkaloids of the flora of Uzbekistan, Arundo donax. Chem. Nat. Compd. 2004, 40, 160–162. [Google Scholar] [CrossRef]
- Pastuszewska, B.; Smulikowska, S.; Wasilewko, J.; Buraczewska, L.; Ochtabinska, A.; Mieczkowska, A.; Lechowski, R.; Bielecki, W. Response of animals to dietary gramine. I. Performance and selected hematological, biochemical and histological parameters in growing chicken, rats and pigs. Arch. Anim. Nutr. 2001, 55, 1–16. [Google Scholar] [CrossRef]
- Sun, X.Q.; Zhang, M.X.; Yu, J.Y.; Jin, Y.; Ling, B.; Du, J.P.; Li, G.H.; Qin, Q.M.; Cai, Q.N. Glutathione S-transferase of brown planthoppers (Nilaparvata lugens) is essential for their adaptation to gramine-containing host plants. PLoS ONE 2013, 8, e64026. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.N.; Zhang, Q.W.; Cheo, M. Contribution of indole alkaloids to Sitobion avenae (F.) resistance in wheat. J. Appl. Entomol. 2004, 128, 517–521. [Google Scholar] [CrossRef]
- Cai, Q.N.; Han, Y.; Cao, Y.Z.; Hu, Y.; Zhao, X.; Bi, J.L. Detoxification of gramine by the cereal aphid Sitobion avenae. J. Chem. Ecol. 2009, 35, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, Q.N.; Zhang, Q.W.; Han, Y. Effect of the secondary substances from wheat on the growth and digestive physiology of cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae). Eur. J. Entomol. 2006, 103, 255–258. [Google Scholar] [CrossRef]
- Schreiber, K.J.; Nasmith, C.G.; Allard, G.; Singh, J.; Subramaniam, R.; Desveaux, D. Found in translation: High-throughput chemical screening in Arabidopsis thaliana identifies small molecules that reduce fusarium head blight disease in wheat. Mol. Plant Microbe Interact. 2011, 24, 640–648. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.Y.; Xie, X.; Sakoda, A.; Sagehashi, M.; Li, F.M. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium microcystis aeruginosa. Aquat. Toxicol. 2008, 91, 262–269. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.Y.; Sakoda, A.; Sagehashi, M. Isolation and characterization of antialgal allelochemicals from Arundo donax L. Allelopathy J. 2010, 25, 357–368. [Google Scholar]
- Kaneko, T.; Nakajima, N.; Okamoto, S.; Suzuki, I.; Tanabe, Y.; Tamaoki, M.; Nakamura, Y.; Kasai, F.; Watanabe, A.; Kawashima, K.; et al. Complete genomic structure of the bloom-forming toxic cyanobacterium Microcystis aeruginosa NIES-843. DNA Res. 2007, 14, 247–256. [Google Scholar] [CrossRef]
- Wilson, A.E.; Sarnelle, O.; Neilan, B.A.; Salmon, T.P.; Gehringer, M.M.; Hay, M.E. Genetic variation of the bloom-forming cyanobacterium Microcystis aeruginosa within and among lakes: Implications for harmful algal blooms. Appl. Environ. Microbiol. 2005, 71, 6126–6133. [Google Scholar] [CrossRef]
- Kokubo, Y.; Nishizaka, M.; Ube, N.; Yabuta, Y.; Tebayashi, S.I.; Ueno, K.; Taketam, S.; Ishihara, A. Distribution of the tryptophan pathway-derived defensive secondary metabolites gramine and benzoxazinones in Poaceae. Biosci. Biotechnol. Biochem. 2017, 81, 431–440. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The constituents and biological effects of Arundo donax. A review. Int. J. Phytopharm. Res. 2015, 6, 34–40. [Google Scholar]
- Kumar, P.; Singh, S.; Sharma, A.; Kaur, G.; Kaur, R.; Singh, A.N. Arundo donax L.: An overview on its traditional and ethnomedicinal importance, phytochemistry, and pharmacological aspects. J. Herb. Med. Pharmacol. 2021, 10, 269–280. [Google Scholar] [CrossRef]
- Miles, D.H.; Tunsuwan, K.; Chittawong, V.; Kokpol, U.; Choudhary, M.I.; Clardy, J. Boll weevil antifeedants from Arundo donax. Phytochemistry 1993, 34, 1277–1279. [Google Scholar] [CrossRef]
- Mochizuki, K.; Takikawa, H.; Mori, K. Synthesis of 2,2,4,4-tetramethyl-N, N′-bis(2, 6-dimethylphenyl) cyclobutane-1, 3-diimine, a unique compound from Arundo donax, and its analogues to test their antifeedant activity against the boll weevil, Anthonomus grandis. Biosci. Biotechnol. Biochem. 2000, 64, 647–651. [Google Scholar] [CrossRef]
- Going, B.M.; Dudley, T.L. Invasive riparian plant litter alters aquatic insect growth. Biol. Invasions 2008, 10, 1041–1051. [Google Scholar] [CrossRef]
- Badar, S.N.; Iqbal, Z.; Sajid, M.S.; Rizwan, H.M.; Shareef, M.; Malik, M.A.; Khan, M.N. Comparative anthelmintic efficacy of Arundo donax, Areca catechu, and Ferula assa-foetida against Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2021, 30, e001221. [Google Scholar] [CrossRef] [PubMed]
- Galletti, S.; Cianchetta, S.; Righini, H.; Roberti, R. A lignin-rich extract of giant reed (Arundo donax L.) as a possible tool to manage soilborne pathogens in horticulture: A preliminary study on a model pathosystem. Horticulturae 2022, 8, 589. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Wilkinson, H.T. Host specificity of pathogenic Pythium species: Implications for tree species diversity. Biotropica 2007, 39, 702–708. [Google Scholar] [CrossRef]
- Lévesque, C.A.; Brouwer, H.; Cano, L.; Hamilton, J.P.; Holt, C.; Huitema, E.; Raffaele, S.; Robideau, G.P.; Thines, M.; Win, J.; et al. Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol. 2010, 11, R73. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Tripathi, D.K.; Kumar, D.; Kumar, Y. Diversity, distribution and frequency based attributes of phytolith in Arundo donax L. Int. J. Innov. Biol. Chem. Sci. 2011, 1, 22–27. [Google Scholar]
- Shakoor, S.; Soodan, A.S.; Kumar, K. Morphological diversity and frequency of phytolith types in giant reed Arundo donax (L.). World Appl. Sci. J. 2014, 29, 926–932. [Google Scholar]
- Perry, C.C.; Keeling-Tucker, T. Biosilicification: The role of the organic matrix in structure control. J. Biol. Inorganic Chem. 2000, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Piperno, D.R. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists; Rowman Altamira Press: Lanham, MD, USA, 2006; pp. 1–248. [Google Scholar]
- Strömberg, C.A.; Di Stilio, V.S.; Song, Z. Functions of phytoliths in vascular plants: An evolutionary perspective. Funct. Ecol. 2016, 30, 1286–1297. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Zakharenko, A.M.; Zemchenko, I.V.; Haider, M.S.; Ali, M.A.; Imtiaz, M.; Chung, G.; Tsatsakis, A.; Sun, S.; Golokhvast, K.S. Phytolith formation in plants: From soil to cell. Plants 2019, 8, 249. [Google Scholar] [CrossRef]
- Arundo donax; EPPO-2023; EPPO Global Database: Paris, France, 2023; Available online: https://gd.eppo.int/taxon/ABKDO (accessed on 11 June 2025).
- Spencer, D.F. Response of Arundo donax L. (giant reed) to leaf damage and partial defoliation. J. Freshw. Ecol. 2012, 27, 77–87. [Google Scholar]
- Kato-Noguchi, H. Invasive mechanisms of one of the world’s worst alien plant species Mimosa pigra and its management. Plants 2023, 12, 1960. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Invasive Characteristics and Impacts of Ambrosia trifida. Agronomy 2024, 14, 2868. [Google Scholar] [CrossRef]
- Muller-Scharer, H.; Schaffner, U.; Steinger, T. Evolution in invasive plants: Implications for biological control. Trends Ecol. Evol. 2004, 19, 417–422. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Fushimi, Y.; Shigemori, H. An allelopathic substance in red pine needles (Pinus densiflora). J. Plant Physiol. 2009, 166, 442–446. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ota, K.; Ino, T. Release of momilactone A and B from rice plants into the rhizosphere and its bioactivities. Allelopathy J. 2008, 22, 321–328. [Google Scholar]
- Kato-Noguchi, H.; Nakamura, K.; Ohno, O.; Suenaga, K.; Okuda, N. Asparagus decline: Autotoxicity and autotoxic compounds in asparagus rhizomes. Plant Physiol. 2017, 213, 23–29. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Suzuki, M.; Noguchi, K.; Suenaga, K.; Laosinwattana, C. A potent phytotoxic substance in Aglaia odorata Lour. Chem. Biodiversity 2016, 13, 549–554. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Mizutani, J.; Hasegawa, K. Allelopathy of oats. II. Allelochemical effect of L-tryptophan and its concentration in oat root exudates. J. Chem. Ecol. 1994, 20, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Convergent or parallel molecular evolution of momilactone A and B: Potent allelochemicals, momilactones have been found only in rice and the moss Hypnum plumaeforme. J. Plant Physiol. 2011, 168, 1511–1516. [Google Scholar] [CrossRef]
- Abu-Romman, S.; Ammari, T. Allelopathic effect of Arundo donax, a Mediterranean invasive grass. Plant Omics 2015, 8, 287–291. [Google Scholar]
- Girotto, L.; Franco, A.C.; Nunez, C.V.; Oliveira, S.C.; De Souza, M.C.S.; Fachin-Espinar, M.T.; Ferreira, C.S. Phytotoxicity and allelopathic potential of extracts from rhizomes and leaves of Arundo donax, an invasive grass in neotropical savannas. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12440. [Google Scholar] [CrossRef]
- Patiño, R.; Rashel, R.H.; Rubio, A.; Longing, S. Growth-suppressing and algicidal properties of an extract from Arundo donax, an invasive riparian plant, against Prymnesium parvum, an invasive harmful alga. Harmful Algae 2018, 71, 1–9. [Google Scholar] [CrossRef]
- Manning, S.R.; La Claire, J.W. Prymnesins: Toxic metabolites of the golden alga, Prymnesium parvum Carter (Haptophyta). Marine Drugs 2010, 8, 678–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Wang, W.W.; Liu, F.; Li, H.Y.; Liu, J.S.; Yang, W.D. Removal of two species of harmful algae using gramine modified montmorillonite. Bull. Environ. Contam. Toxicol. 2016, 96, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Laue, P.; Bährs, H.; Chakrabarti, S.; Steinberg, C.E. Natural xenobiotics to prevent cyanobacterial and algal growth in freshwater: Contrasting efficacy of tannic acid, gallic acid, and gramine. Chemosphere 2014, 104, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.A.; Moore, G.W. Water-use dynamics of an invasive reed, Arundo donax, from leaf to stand. Wetlands 2011, 31, 725–734. [Google Scholar] [CrossRef]
- Jain, S.; Ale, S.; Munster, C.L.; Ansley, R.J.; Kiniry, J.R. Simulating the hydrologic impact of Arundo donax invasion on the headwaters of the Nueces River in Texas. Hydrology 2015, 2, 134–147. [Google Scholar] [CrossRef]
- Spencer, D.F.; Tan, W.; Liow, P.S.; Ksander, G.G.; Whitehand, L.C.; Weaver, S.; Olson, J.; Newhouser, M. Evaluation of glyphosate for managing giant reed (Arundo donax). Invasive Plant Sci. Manag. 2008, 1, 248–254. [Google Scholar] [CrossRef]
- Cushman, J.H.; Gaffney, K.A. Community-level consequences of invasion: Impacts of exotic clonal plants on riparian vegetation. Biol. Invasions 2010, 12, 2765–2776. [Google Scholar] [CrossRef]
- Stover, J.E.; Keller, E.A.; Dudley, T.L.; Langendoen, E.J. Fluvial geomorphology, root distribution, and tensile strength of the invasive giant reed, Arundo donax and its role on stream bank stability in the Santa Clara River, Southern California. Geosciences 2018, 8, 304. [Google Scholar] [CrossRef]
- Spencer, D.F.; Colby, L.; Norris, G.R. An evaluation of flooding risks associated with giant reed (Arundo donax). J. Freshw. Ecol. 2013, 28, 397–409. [Google Scholar] [CrossRef]
- Buldrini, F.; Landi, S.; Titti, G.; Parodi, S.; Valente, M.; Borgatti, L.; Bolpagni, R. Invasive alien plant species, riverbank instability and hydraulic risk: What do we know about Amorpha fruticosa, Arundo donax and Reynoutria japonica? J. Limnol. 2024, 83, 2204. [Google Scholar] [CrossRef]
- Scott, G. Fire threat from Arundo donax. In Proceedings of the Arundo Donax Workshop Proceedings, Ontario, QC, Canada, 19 November 1993; Jackson, N.E., Frandsen., P., Douthit, S., Eds.; University of California Cooperative Extension: Imperial, CA, USA, 1994; pp. 17–18. [Google Scholar]
- D’Antonio, C.M. Fire, plant invasions, and global changes. In Invasive Species in a Changing World; Mooney, H.A., Hobbs, R.J., Eds.; Island Press: Washington, DC, USA, 2000; pp. 65–93. [Google Scholar]
- Jasprica, N.; Bogdanović, S.; Dolina, K.; Ruščić, M.; Pandža, M.; Kovačić, S. Syntaxonomy of Arundo stands along the eastern Adriatic coast. Plant Biosyst. 2016, 150, 887–903. [Google Scholar] [CrossRef]
- Coffman, G.C. Factors Influencing Invasion of Giant Reed (Arundo donax) in Riparian Ecosystems of Mediterranean-Type Climate Regions. Doctoral Dissertation, University of California, Los Angeles, CA, USA, 2007. [Google Scholar]
- Guthrie, G. Impacts of the Invasive Reed Arundo donax on Biodiversity at the Community-Ecosystem Level. Doctoral Dissertation, University of the Western Cape, Cape Town, South Africa, 2007. [Google Scholar]
- Gaffney, K.A.; Gledhill, K. Influence of giant reed on floodplain riparian plant communities: Implications for invasive plant control and habitat restoration at the watershed level. In Proceedings of the Riparian Habitat and Floodplains Conference, Sacramento, CA, USA, 12–25 March 2001; Faber, P.M., Ed.; University of California Press: Sacramento, CA, USA, 2003. [Google Scholar]
- Frandsen, P.; Jackson, N. The impact of Arundo donax on flood control and endangered species. In Proceedings of the Arundo donax Workshop, Ontario, QC, Canada, 19 November 1993; Jackson, N.E., Frandsen, P., Duthoit, S., Eds.; California Exotic Pest Plant Council: Riverside, CA, USA, 1993; pp. 13–16. Available online: https://www.cal-ipc.org/docs/symposia/archive/pdf/Arundo_Proceedings_1993.pdf (accessed on 11 June 2025).
- Osbrink, W.L.; Goolsby, J.A.; Thomas, D.B.; Mejorado, A.; Showler, A.T.; Pérez De León, A. Higher ant diversity in native vegetation than in stands of the invasive Arundo, Arundo donax L., along the Rio Grande basin in Texas, USA. Int. J. Insect Sci. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Osbrink, W.L.A.; Thomas, D.B.; Goolsby, J.A.; Showler, A.T.; Leal, B. Higher beetle diversity in native vegetation than in stands of the invasive Arundo, Arundo donax L., along the Rio Grande basin in Texas, USA. J. Insect Sci. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.M.; Dudley, T.L. Reduction of riparian arthropod abundance and diversity as a consequence of giant reed (Arundo donax) invasion. Biol. Invasions 2003, 5, 167–177. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Basas, H.; Lanzaco, G.; Sala, M.; De Sostoa, A.; Serra, A. Impacts of the invader giant reed (Arundo donax) on riparian habitats and ground arthropod communities. Biol. Invasions 2016, 18, 731–749. [Google Scholar] [CrossRef]
- Lovich, R.E.; Ervin, E.L.; Fisher, R.N. Surface-dwelling and subterranean invertebrate fauna associated with giant reed (Arundo donax Poaceae) in Southern California. Bull. South. Calif. Acad. Sci. 2009, 108, 29–35. [Google Scholar] [CrossRef]
- Kisner, D.A. The Effect of Giant Reed (Arundo donax) on the Southern California Riparian Bird Community. Master’s Thesis, San Diego State University, San Diego, CA, USA, 2004. [Google Scholar]
- Orr, D.A. Avian Response to Arundo donax Invasion on the Lower Santa Clara River. California Invasive Plant Council Symposium, Ventura, CA, USA, poster No. 30. 2010. Available online: https://www.cal-ipc.org/wp-content/uploads/2017/12/2010Cal-IPCFinalProgram.pdf (accessed on 11 June 2025).
- Hardesty-Moore, M.; Orr, D.; McCauley, D.J. Invasive plant Arundo donax alters habitat use by carnivores. Biol. Invasions 2020, 22, 1983–1995. [Google Scholar] [CrossRef]
- Gerhards, R.; Sanchez, D.A.; Hamouz, P.; Peteinatos, G.G.; Christensen, S.; Fernandez-Quintanilla, C. Advances in site-specific weed management in agriculture: A review. Weed Res. 2022, 62, 123–133. [Google Scholar] [CrossRef]
- Korres, N.E.; Burgos, N.R.; Travlos, I.; Vurro, M.; Gitsopoulos, T.K.; Varanasi, V.K.; Duke, S.O.; Kudsk, P.; Brabham, C.; Rouse, C.E.; et al. Chapter six—New directions for integrated weed management: Modern technologies, tools, and knowledge discovery. Adv. Agron. 2019, 155, 243–319. [Google Scholar]
- Monteiro, A.; Santos, S. Sustainable approach to weed management: The role of precision weed management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Sharma, G.; Shrestha, S.; Kunwar, S.; Tseng, T. Crop diversification for improved weed management: A review. Agriculture 2021, 11, 461. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, Z.; Li, N.; He, C.; Sun, T. Review of current robotic approaches for precision weed management. Curr. Robot. Rep. 2022, 3, 139–151. [Google Scholar] [CrossRef] [PubMed]


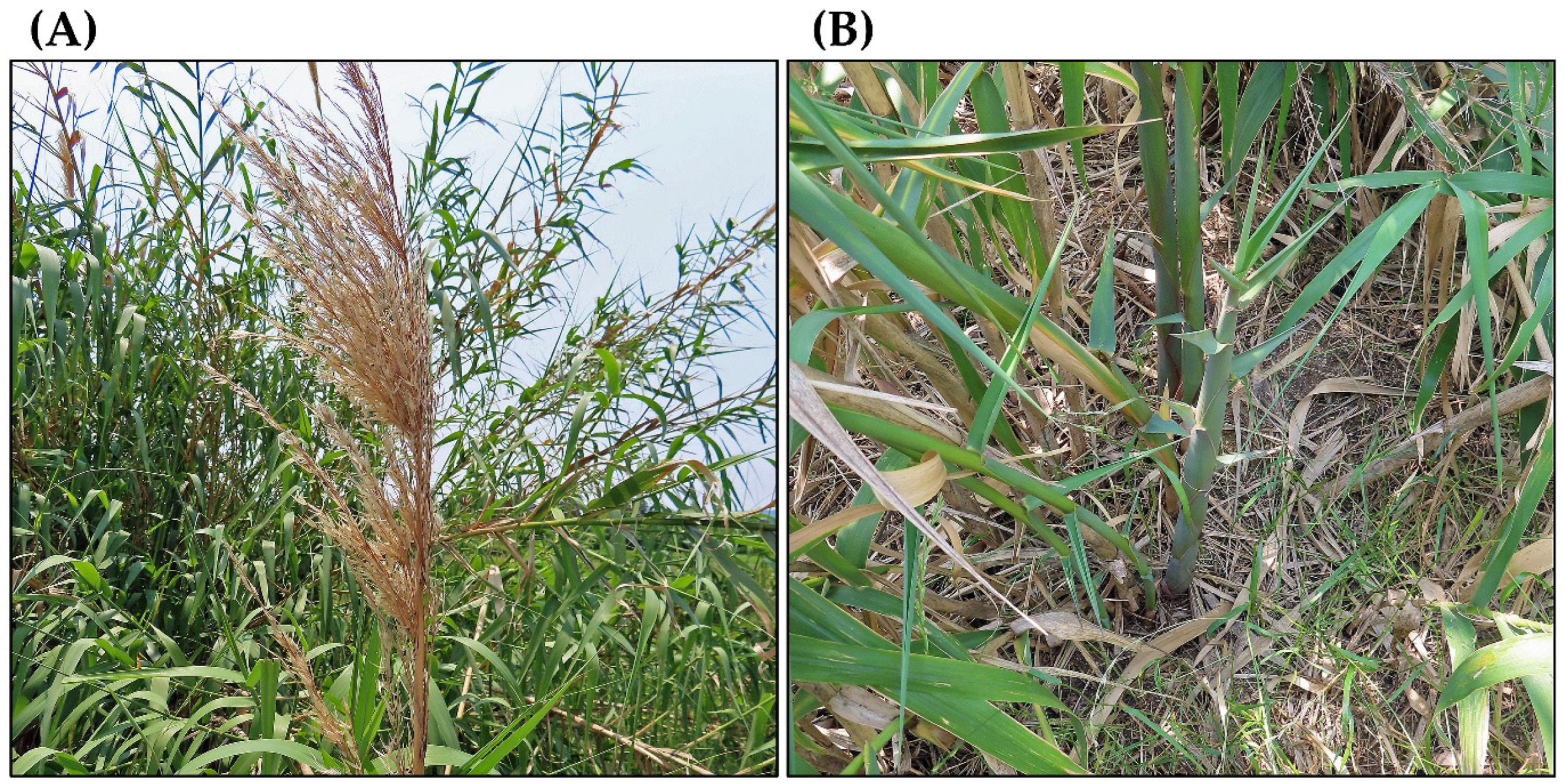
| Invasive Characteristics | References |
|---|---|
| Rapid growth and large biomass production | |
| [48,49,50,51,52,53] |
| High vegetative reproduction | |
| [2,31,83] |
| [16,31,87,88,89,90] |
| High morphological and physiological adaptation to adverse conditions | |
| [2,95,96,97,98] |
| [2,22,31,91,97,99] |
| [102,103,105,106,108,111,112] |
| [113,114,120] |
| [102,126,127,128,129,130,131,133] |
| High defense ability against biotic stressors | |
| [149,154,155,169] |
| [176,177] |
| [197,198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato-Noguchi, H.; Kato, M. The Invasive Mechanism and Impact of Arundo donax, One of the World’s 100 Worst Invasive Alien Species. Plants 2025, 14, 2175. https://doi.org/10.3390/plants14142175
Kato-Noguchi H, Kato M. The Invasive Mechanism and Impact of Arundo donax, One of the World’s 100 Worst Invasive Alien Species. Plants. 2025; 14(14):2175. https://doi.org/10.3390/plants14142175
Chicago/Turabian StyleKato-Noguchi, Hisashi, and Midori Kato. 2025. "The Invasive Mechanism and Impact of Arundo donax, One of the World’s 100 Worst Invasive Alien Species" Plants 14, no. 14: 2175. https://doi.org/10.3390/plants14142175
APA StyleKato-Noguchi, H., & Kato, M. (2025). The Invasive Mechanism and Impact of Arundo donax, One of the World’s 100 Worst Invasive Alien Species. Plants, 14(14), 2175. https://doi.org/10.3390/plants14142175








