Plants from Bulgarian Botanical Gardens: Some Selected Species with Potential for Health Food and Medical Applications
Abstract
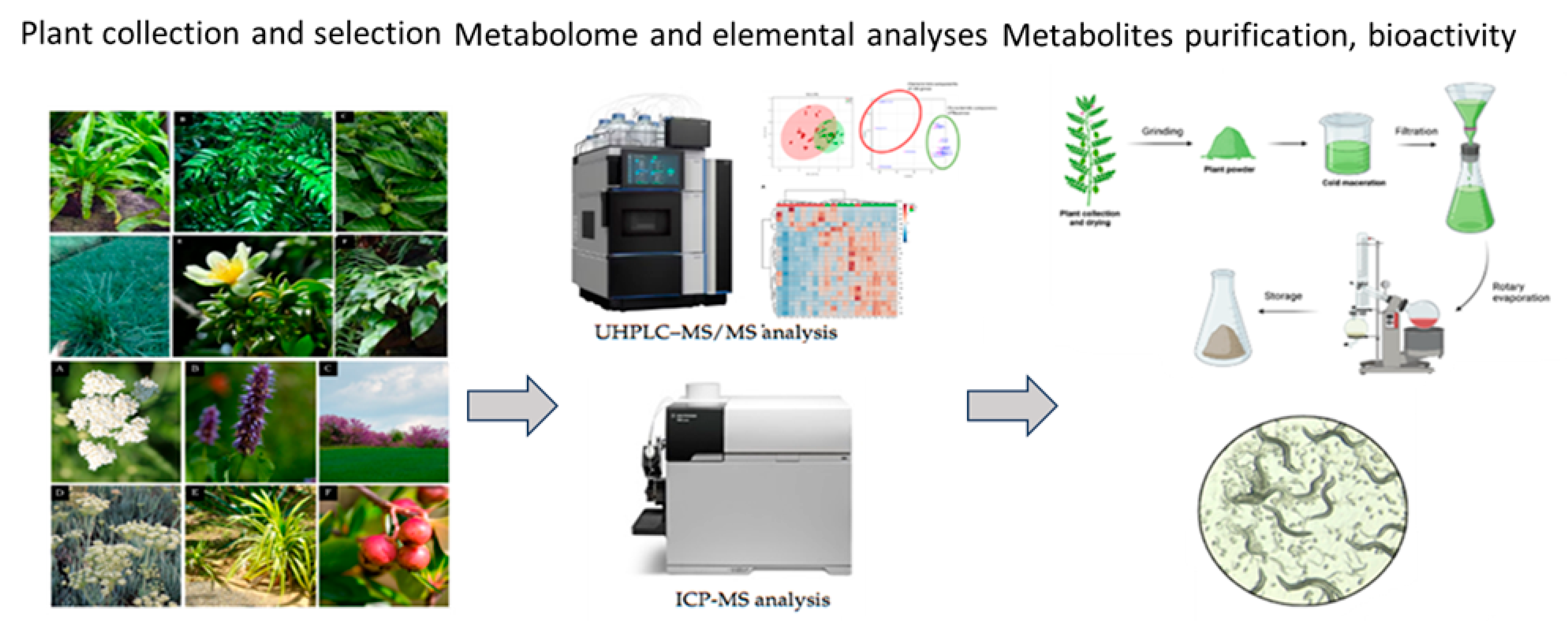
1. Introduction
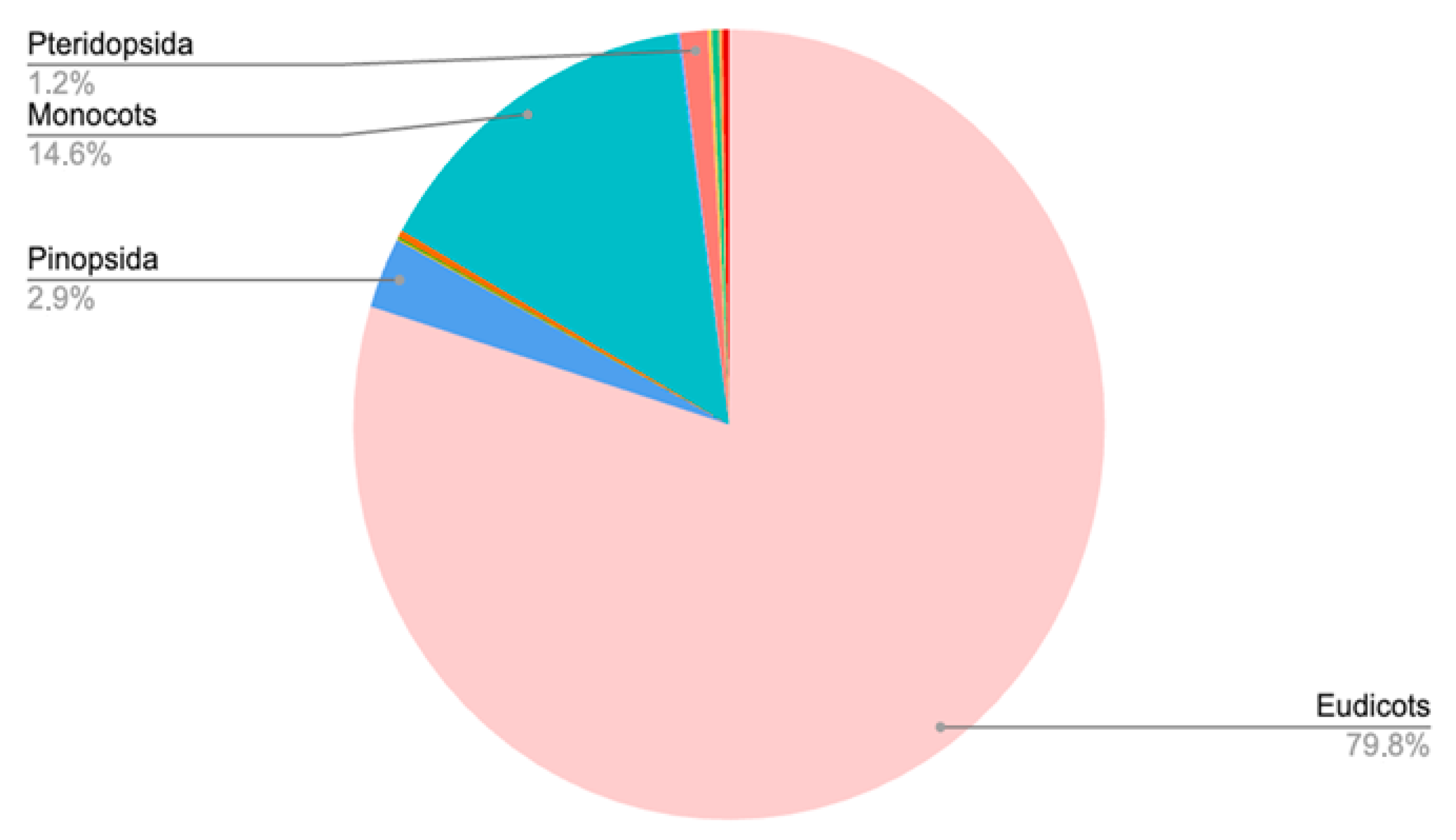
2. Overview of the Plants in the Bulgarian Botanical Gardens
| City | Year of Foundation | Area | Location | Altitude | Average Rainfall |
|---|---|---|---|---|---|
| Sofia | 1892 | 0.5 ha | 42°36′ N; 23°25′ E | 549 m | 650–700 mm |
| Varna | 1977 | 36 ha | 43°14′ N; 28°00′ E | 28–85 m | 450–550 mm |
| Balchik | 1955 | 19 ha | 43°24′ N; 28°01′ E | 1–35 m | 350–400 mm |
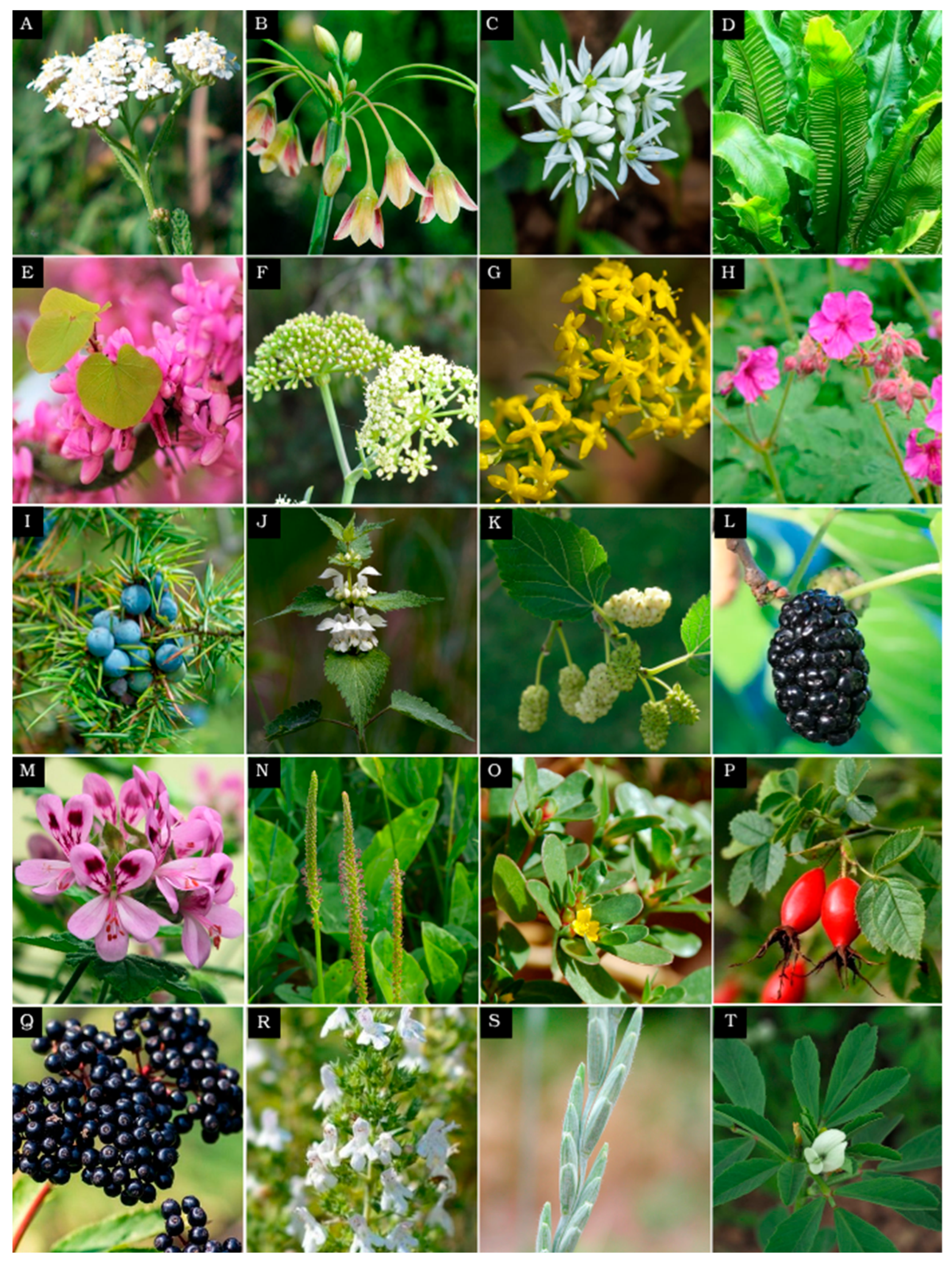
3. Underutilized Crops with Potential to Diversify Food Systems
| Species Latin and English Names | Traditional Use, Used Parts, Major Nutrients/Valuable Metabolites, and Potential Applications | References |
|---|---|---|
| Achillea millefolium L. Common yarrow | Flowers and leaves are used in herbal infusions and cooking. Rich in essential oils, terpenes, and alkaloids, it exhibits strong antimicrobial and antioxidant properties, promising natural preservative. | [33,88,89,90,91] |
| Allium siculum subsp. dioscoridis (Sm.) K. Richt. Bulgarian honey garlic | Culinary spice. Similar to garlic, it contains thiosulfinates. Leaves are used to prepare the Bulgarian dish “sarmi”. | [6,7,8,9,10] |
| Allium ursinum Wild garlic | Both leaves and bulbs are used as salads, boiled as vegetables in dishes, pesto, soups, pasta, cheese, etc. | [11,12,13,14,15,16,17] |
| Asplenium nidus L. Bird’s nest fern | Used in folk medicine, the leaves and aerial parts contain flavonoids, phenolic acids, and xanthones, showing antioxidant, antimicrobial, and anticancer potential for drug development. | [92,93,94] |
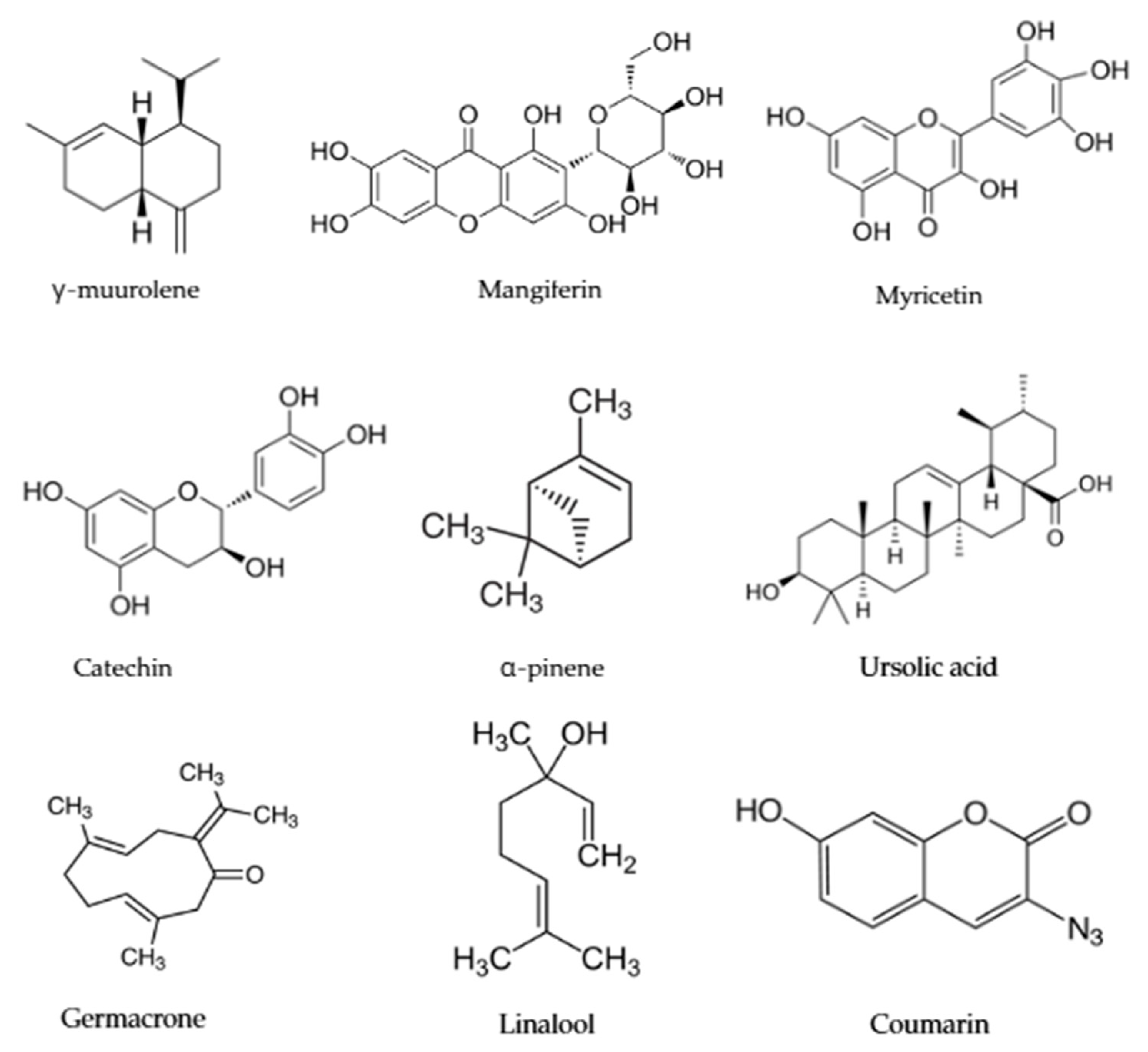
| Cercis siliquastrum L. Judas tree | Flowers in folk medicine are used against anemia, malaria, and stress. Contains aldehydes, terpenoids, and flavonoids (catechin and myricetin). Used in cosmetics, agriculture, and potentially in cancer therapy. | [95,96,97,98,99] |
| Crithmum maritimum, Sea fennel | Emerging crop for biosaline agriculture. Leaves are rich in omega-3 and omega-6 fatty acids. Rich in essential minerals. | [18,19,20,21,22,23,24,25] |
| Galium verum Yellow bedstraw | Aerial parts are used in traditional Bulgarian medicine as an analgesic, laxative, astringent, diuretic, and local hemostatic. It has antioxidant and anti-inflammatory properties. | [100,101,102,103] |
| Geranium macrorrhizum L. Bulgarian geranium | Leaves, flowers, and roots are used in folk medicine for insomnia, high blood pressure, ulcers, wounds, inflammation, and nervous tension, with antibacterial and antiviral effects. | [104,105,106,107,108] |
| Juniperus communis Common juniper | Fruits are edible and are used for treating diabetes, arthritis, and digestive issues, with diuretic, anti-inflammatory, and antimicrobial effects. | [109,110,111,112,113,114] |
| Lamium album White dead-nettle | Whole plants are used in Bulgarian cuisine; they can be eaten raw or cooked. Known for its antiviral, antibacterial, antioxidant, and antidiabetic properties. | [26,27,28,29,30,31,32,33,34,35] |
| Morus alba White mulberry | Fruits are eaten raw or used for jams, while root bark and leaves have antimicrobial, antiviral, and antioxidant properties. | [36,37,38,39,40,41,42,43,44] |
| Morus nigra Black mulberry | Fruits are eaten raw or used in teas and jams. Leaves are valued for their anti-inflammatory properties. Leaves, fruits, and roots exhibit antinociceptive, antimicrobial, antidiabetic, and anti-obesity activities. | [45,46,47,48,49,50,51] |
| Pelargonium roseum Rose geranium | Leaves are used as a culinary spice. Essential oils support respiratory, digestive, and hormonal health, liver detox, and wound healing, with antioxidant and antibacterial properties. | [52,53,54,55,56] |
| Plantago major Broadleaf plantain | Leaves are used traditionally for wound healing, respiratory, digestive, reproductive issues, pain, and infections, with anti-inflammatory, analgesic, and antioxidant effects. | [115,116,117,118,119] |
| Portulaca oleracea Purslane | Stem and leaves are eaten raw or in salads; traditionally used for digestive, respiratory, liver, and inflammatory issues. Shows antioxidant, analgesic, anti-inflammatory, and neuroactive effects. | [57,58,59,60,61,62] |
| Rosa canina Dog rose | Fruits used in Bulgarian cuisine for jams and teas, traditionally for heart, metabolic, urinary, respiratory, digestive, hormonal, and wound healing support. Exhibits antioxidant and anti-inflammatory properties. | [63,64,65,66] |
| Sambucus nigra L. Elderberry | Fruits eaten cooked; flowers and fruits used for immune support, flu, cough, diuretic, laxative, anti-inflammatory, respiratory, and antidiabetic effects. | [67,68,69,70] |
| Satureja montana Winter savory | One of the most commonly used spices in Bulgaria. Also, used in traditional medicine for treating digestive and respiratory issues. | [71,72,73,74,75,76,77,78] |
| Thinopyrum intermedium Intermediate wheatgrass | Resilient perennial grain that is high in protein, fiber, vitamins, and antioxidants. Used in food products and baking, improves soil health, and reduces tillage. | [79,80,81,82,83,84] |
| Trigonella foenum-graecum Fenugreek | Traditional Bulgarian spice made of seeds; also used in traditional medicine. Rich in proteins, carbohydrates, lipids, vitamins, and minerals. | [85,86,87] |
4. Medicinal Plants and Their Secondary Metabolites with Medical Applications
5. Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, T.W.N.; Schrodt, F.; Allard, P.-M.; Defossez, E.; Jassey, V.E.J.; Schuman, M.C.; Alexander, J.M.; Baines, O.; Baldy, V.; Bardgett, R.D.; et al. Leaf Metabolic Traits Reveal Hidden Dimensions of Plant Form and Function. Sci. Adv. 2023, 9, eadi4029. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Faraji, L.; Karimi, M. Botanical Gardens as Valuable Resources in Plant Sciences. Biodivers. Conserv. 2024, 31, 2905–2926. [Google Scholar] [CrossRef]
- Todorova, M.N.; Savova, M.S.; Mihaylova, L.V.; Georgiev, M.I. Icariin Improves Stress Resistance and Extends Lifespan in Caenorhabditis elegans through Hsf-1 and Daf-2-driven Hormesis. Int. J. Mol. Sci. 2024, 25, 352. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Corral, M.F.; Oliveira, P.G.; Jiménez-López, C. Culinary and Nutritional Value of Edible Wild Plants from the Northern Spain Rich in Phenolic Compounds with Potential Health Benefits. Food Funct. 2025, 11, 8493–8515. [Google Scholar] [CrossRef]
- Mihaylova, D. Comparative Study on the Antioxidant Activity of Selected Culinary Plants Growing in Bulgaria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 436–444. [Google Scholar]
- Vrancheva, R.Z.; Dincheva, I.N.; Aneva, I.Y.; Pavlov, A.I. Metabolite Profiling by Means of GC-MS Combined with Principal Component Analyses of Natural Populations of Nectaroscordum siculum ssp. bulgaricum (Janka) Stearn. Z. Für Naturforsch. C 2020, 75, 451–457. [Google Scholar] [CrossRef]
- Ivanova, T.; Chervenkov, M.; Stoeva, T.; Chervenkov, S.; Bosseva, Y.; Georgieva, A.; Tsvetanova, E.; Alexandrova, A.; Dimitrova, D. Samardala: Specificities and Changes in the Ethnobotanical Knowledge about Allium siculum Subsp. dioscoridis (Sm.) K. Richt. in Bulgaria. Genet. Resour. Crop Evol. 2018, 65, 1349–1357. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Corona-España, A.M.; García-Ramírez, M.A.; Rodríguez-Buenfil, I.M.; Delgado-Saucedo, J.I.; González-Reynoso, O. Synthesis Mechanism and Therapeutic Effects of Thiosulfinates and Polysulfides of Different Species of Garlic from the Allium Genus. Pharmaceutics 2025, 17, 437. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical Composition and Antimicrobial Activity of Wild Garlic Allium ursinum of Bulgarian Origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, Z.; Sobolewska, D.; Podolak, I. Pregnadienolone Glycoside from Wild Garlic Allium ursinum L. Acta Pol. Pharm. Drug Res. 2000, 57, 131–134. [Google Scholar]
- Kubiak-Martens, L. New Evidence for the Use of Root Foods in Pre-Agrarian Subsistence Recovered from the Late Mesolithic Site at Halsskov, Denmark. Veg. Hist. Archaeobotany 2002, 11, 23–32. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ivancheva, S. Preliminary Comparative Analysis of Medicinal Plants Used in the Traditional Medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Wąs, J. Allium ursinum: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef]
- Schmitt, B.; Schulz, H.; Storsberg, J.; Keusgen, M. Chemical Characterization of Allium ursinum L. Depending on Harvesting Time. J. Agric. Food Chem. 2005, 53, 7288–7294. [Google Scholar] [CrossRef]
- Lupoae, M.; Bounegru, A.V.; Dinică, R.M.; Cârâc, G. Exploring In Vitro Antioxidant Activity of Allium ursinum and Alliaria petiolata through Various Analytical Methods. Rev. Roum. De Chim. 2025, 70, 223–233. [Google Scholar] [CrossRef]
- Martins-Noguerol, R.; Matías, L.; Pérez-Ramos, I.M.; Moreira, X.; Francisco, M.; Pedroche, J.; DeAndrés-Gil, C.; Gutiérrez, E.; Salas, J.J.; Moreno-Pérez, A.J.; et al. Soil Physicochemical Properties Associated with the Yield and Phytochemical Composition of the Edible Halophyte Crithmum maritimum. Sci. Total Environ. 2023, 869, 161806. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Cavaleiro, C.; Cardoso, O.; Donato, M.M.; Salgueiro, L.; Ramos, F. Exploiting the Crithmum maritimum L. Aqueous Extracts and Essential Oil as Potential Preservatives in Food, Feed, Pharmaceutical and Cosmetic Industries. Antioxidants 2023, 12, 252. [Google Scholar] [CrossRef]
- Politeo, O.; Popović, M.; Veršić Bratinčević, M.; Kovačević, K.; Urlić, B.; Generalić Mekinić, I. Chemical Profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils and Their Isolation Residual Waste-Waters. Plants 2023, 12, 214. [Google Scholar] [CrossRef]
- Correia, I.; Antunes, M.; Tecelão, C.; Neves, M.; Pires, C.L.; Cruz, P.F.; Rodrigues, M.; Peralta, C.C.; Pereira, C.D.; Reboredo, F.; et al. Nutritive Value and Bioactivities of a Halophyte Edible Plant: Crithmum maritimum L. (Sea Fennel). Plants 2024, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, D.; Nikolic, D.; Paparella, R.R.; Sabbà, C.; Mazzocca, A. Crithmum maritimum Extract Restores Lipid Homeostasis and Metabolic Profile of Liver Cancer Cells to a Normal Phenotype. Plant Foods Hum. Nutr. 2024, 79, 417–424. [Google Scholar] [CrossRef]
- Radman, S.; Mastelić, L.; Ljubenkov, I.; Lazarevski, S.; Politeo, O.; Podrug, R.; Prga, I.; Čorić, I.; Popović, M.; Bratinčević, M.V.; et al. Sea Fennel (Crithmum maritimum L.) Flowers as an Emerging Source of Bioactive Compounds. Pol. J. Food Nutr. Sci. 2024, 74, 221–231. [Google Scholar] [CrossRef]
- Prajapati, D. Plant-Based Nanoemulsions for Agricultural Application. In Bio-Based Nanoemulsions for Agri-Food Applications; Abd-Elsalam, K.A., Murugan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 154–164. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea Fennel (Crithmum maritimum L.): From Underutilized Crop to New Dried Product for Food Use. Genet. Resour. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]
- Shah, T.; Khan, F.; Bule, M.; Niaz, K. White Dead-Nettle (Lamium album). In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 455–459. ISBN 978-0-12-812491-8. [Google Scholar]
- Shevchenko, A.; Drobot, V.; Litvynchuk, S.; Galenko, O. Application of Lamium album Leaves Powder in Wheat Bread Technology. Plant Foods Hum. Nutr. 2025, 80, 106. [Google Scholar] [CrossRef]
- Salehi, B.; Armstrong, L.; Rescigno, A.; Yeskaliyeva, B.; Seitimova, G.; Beyatli, A.; Sharmeen, J.; Mahomoodally, M.F.; Sharopov, F.; Durazzo, A.; et al. Lamium Plants—A Comprehensive Review on Health Benefits and Biological Activities. Molecules 2019, 24, 1913. [Google Scholar] [CrossRef]
- Paduch, R.; Wójciak-Kosior, M.; Matysik, G. Investigation of Biological Activity of Lamii albi Flos Extracts. J. Ethnopharmacol. 2007, 110, 69–75. [Google Scholar] [CrossRef]
- Yordanova, Z.P.; Zhiponova, M.K.; Iakimova, E.T.; Dimitrova, M.A.; Kapchina-Toteva, V.M. Revealing the Reviving Secret of the White Dead Nettle (Lamium album L.). Phytochem. Rev. 2014, 13, 375–389. [Google Scholar] [CrossRef]
- Sulborska, A.; Konarska, A.; Matysik-Woźniak, A.; Dmitruk, M.; Weryszko-Chmielewska, E.; Skalska-Kamińska, A.; Rejdak, R. Phenolic Constituents of Lamium album L. subsp. album Flowers: Anatomical, Histochemical, and Phytochemical Study. Molecules 2020, 25, 6025. [Google Scholar] [CrossRef]
- Chipeva, V.A.; Petrova, D.C.; Geneva, M.E.; Dimitrova, M.A.; Moncheva, P.A.; Kapchina-Toteva, V.M. Antimicrobial Activity of Extracts from in vivo and in vitro Propagated Lamium album L. Plants. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 559–562. [Google Scholar] [CrossRef]
- Shah, R.; Peethambaran, B. Anti-Inflammatory and Anti-Microbial Properties of Achillea millefolium in Acne Treatment. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 241–248. ISBN 978-0-12-805417-8. [Google Scholar]
- Zhang, H.; Rothwangl, K.; Mesecar, A.D.; Sabahi, A.; Rong, L.; Fong, H.H.S. Lamiridosins, Hepatitis C Virus Entry Inhibitors from Lamium album. J. Nat. Prod. 2009, 72, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Todorov, D.; Dimitrova, M.; Shishkova, K.; Yordanova, Z.P. Comparative Anti-Herpes Effects of the Chloroform in vitro and in vivo Extracts, Derived from Lamium album L. Bulgar. J. Agric. Sci. 2013, 19, 190–193. [Google Scholar]
- Ercisli, S.; Orhan, E. Chemical Composition of White (Morus alba), Red (Morus rubra) and Black (Morus nigra) Mulberry Fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. Nature’s Functional Tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Sohn, H.-Y.; Son, K.H.; Kwon, C.-S.; Kwon, G.-S.; Kang, S.S. Antimicrobial and Cytotoxic Activity of 18 Prenylated Flavonoids Isolated from Medicinal Plants: Morus alba L., Morus mongolica Schneider, Broussonetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef]
- Du, J.; He, Z.-D.; Jiang, R.-W.; Ye, W.-C.; Xu, H.-X.; But, P.P.-H. Antiviral Flavonoids from the Root Bark of Morus alba L. Phytochemistry 2003, 62, 1235–1238. [Google Scholar] [CrossRef]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.D.C.A.; Freitas, K.D.C. Nutraceutical and Medicinal Potential of the Morus Species in Metabolic Dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef]
- Yan, Z.; Alimujiang, S.; Zhang, Y.; Zhao, J.; Hu, Y.; Li, W. Recent Advances on the Chemical Composition, Pharmacological Properties, and Product Development of Morus alba. Phytochem. Anal. 2025, 36, 1301–1332. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Al-Snafi, A.E.; Thuwaini, M.M.; Teibo, J.O.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; et al. Morus alba: A Comprehensive Phytochemical and Pharmacological Review. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 1399–1413. [Google Scholar] [CrossRef]
- Kadam, R.A.; Dhumal, N.D.; Khyade, V.B. The Mulberry, Morus alba (L.): The Medicinal Herbal Source for Human Health. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2941–2964. [Google Scholar] [CrossRef]
- Yimam, M.; Jiao, P.; Hong, M.; Brownell, L.; Lee, Y.-C.; Kim, H.-J.; Nam, J.-B.; Kim, M.-R.; Jia, Q. Morus alba, a Medicinal Plant for Appetite Suppression and Weight Loss. J. Med. Food 2019, 22, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Martínez, J.J.; Hernández, F. Phytochemical Evaluation of White (Morus alba L.) and Black (Morus nigra L.) Mulberry Fruits, a Starting Point for the Assessment of Their Beneficial Properties. J. Funct. Foods 2015, 12, 399–408. [Google Scholar] [CrossRef]
- Vijayan, K. The Emerging Role of Genomic Tools in Mulberry (Morus) Genetic Improvement. Tree Genet. Genomes 2010, 6, 613–625. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Chapter 9—Nutraceuticals in Alternative and Underutilized Fruits as Functional Food Ingredients: Ancient Species for New Health Needs. In Alternative and Replacement Foods; Holban, A.M., Grumezescu, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 261–282. ISBN 978-0-12-811446-9. [Google Scholar]
- Lim, S.H.; Choi, C.-I. Pharmacological Properties of Morus nigra L. (Black Mulberry) as a Promising Nutraceutical Resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef]
- Padilha, M.M.; Vilela, F.C.; Rocha, C.Q.; Dias, M.J.; Soncini, R.; dos Santos, M.H.; Alves-da-Silva, G.; Giusti-Paiva, A. Antiinflammatory Properties of Morus nigra Leaves. Phytother. Res. 2010, 24, 1496–1500. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Martínez-Nicolás, J.J.; Munera-Picazo, S.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Bioactive Compounds and Sensory Quality of Black and White Mulberries Grown in Spain. Plant Foods Hum. Nutr. 2013, 68, 370–377. [Google Scholar] [CrossRef]
- Ferraz, A.P.C.R.; Figueiredo, P.d.O.; Yoshida, N.C. Black Mulberry (Morus nigra L.): A Review of Attributes as an Anticancer Agent to Encourage Pharmaceutical Development. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 3784092. [Google Scholar] [CrossRef]
- Cocoș, D.I.; Earar, K.; Dinu, M.; Lungu, I.; Bazbanela, C.; Galea, C. Perspectives on the Use of Geranium Essential Oil: Pelargonium graveolens and Pelargonium roseum, in Dental Medicine. Rom. J. Med. Dent. Educ. 2023, 12. Available online: https://journal.adre.ro/perspectives-on-the-use-of-geranium-essential-oil-pelargonium-graveolens-and-pelargonium-roseum-in-dental-medicine/ (accessed on 10 July 2025).
- Carmen, G.; Hancu, G. Antimicrobial and Antifungal Activity of Pelargonium roseum Essential Oils. Adv. Pharm. Bull. 2014, 4, 511–514. [Google Scholar] [CrossRef]
- Fan, G.-W.; Wang, P.; Liu, Y.-S.; Sang, Y.-L.; Liu, N.; Hao, Y.-J. Insecticidal Activity of Two Pelargonium Essential Oils and Head Transcriptome Analysis of Stored-Product Pest Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) in Response to Citronellyl Formate Fumigation. Pestic. Biochem. Physiol. 2025, 208, 106278. [Google Scholar] [CrossRef]
- Medeiros, M.T.; Campos, D.R.; Soares, E.F.M.S.; de Assis, J.D.; de Oliveira, G.F.; Santos, L.d.O.; e Silva, T.M.; da Silva, M.P.; Cid, Y.P.; Scott, F.B.; et al. Larvicidal Activity in vitro of Essential Oils Against Cochliomyia hominivorax. Vet. Parasitol. 2023, 322, 110020. [Google Scholar] [CrossRef] [PubMed]
- Galea, C.; Cocoș, D.I.; Forna, N.C.; Păcurar, M.; Earar, K. Phytochemical Study of the Essential Oil of Pelargonium roseum by Gas Chromatographic Method. Rom. J. Oral Rehab. 2024, 16, 644–651. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. Biomed. Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Cantatore, V.; Cavoski, I.; Gobbetti, M. Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury. Nutrients 2019, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.C.L.; Da Silveira, T.F.F.; Rodrigues, M.I.; Ruiz, A.L.T.G.; Neves, D.A.; Duarte, M.C.T.; Cunha-Santos, E.C.E.; Kuhnle, G.; Ribeiro, A.B.; Godoy, H.T. A Study of the Bioactive Potential of Seven Neglected and Underutilized Leaves Consumed in Brazil. Food Chem. 2021, 364, 130350. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A Review of Traditional Uses, Phytochemistry and Pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Napolitano, A.; Heiss, E.H.; Dirsch, V.M.; Piacente, S. Portulaca oleracea, a Rich Source of Polar Lipids: Chemical Profile by LC-ESI/LTQOrbitrap/MS/MSn and in vitro Preliminary Anti-Inflammatory Activity. Food Chem. 2022, 388, 132968. [Google Scholar] [CrossRef]
- Nađpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anačkov, G.T.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Comparative Study of Biological Activities and Phytochemical Composition of Two Rose Hips and Their Preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef]
- Guantario, B.; Nardo, N.; Fascella, G.; Ranaldi, G.; Zinno, P.; Finamore, A.; Pastore, G.; Mammano, M.M.; Baiamonte, I.; Roselli, M. Comparative Study of Bioactive Compounds and Biological Activities of Five Rose Hip Species Grown in Sicily. Plants 2023, 13, 53. [Google Scholar] [CrossRef]
- Winther, K.; Vinther Hansen, A.S.; Campbell-Tofte, J. Bioactive Ingredients of Rose Hips (Rosa canina L) with Special Reference to Antioxidative and Anti-Inflammatory Properties: In vitro Studies. BioTher. Res. Appl. Toxicol. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Jariani, P.; Shahnejat-Bushehri, A.-A.; Naderi, R.; Zargar, M.; Naghavi, M.R. Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.). Molecules 2024, 29, 3145. [Google Scholar] [CrossRef] [PubMed]
- Seixas, N.L.; Paula, V.B.; Dias, T.; Dias, L.G.; Estevinho, L.M. The Effect of Incorporating Fermented Elderberries (Sambucus nigra) into Bread: Quality, Shelf Life, and Biological Enhancement. Foods 2025, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Setz, C.; Rauch, P.; Setz, M.; Breitenberger, S.; Plattner, S.; Schubert, U. Synergistic Antiviral Activity of European Black Elderberry Fruit Extract and Quinine Against SARS-CoV-2 and Influenza A Virusa. Nutrients 2025, 17, 1205. [Google Scholar] [CrossRef]
- Safta, D.A.; Vlase, A.-M.; Pop, A.; Cherfan, J.; Carpa, R.; Iurian, S.; Bogdan, C.; Vlase, L.; Moldovan, M.-L. Optimized Sambucus nigra L., Epilobium hirsutum L., and Lythrum salicaria L. Extracts: Biological Effects Supporting Their Potential in Wound Care. Antioxidants 2025, 14, 521. [Google Scholar] [CrossRef]
- Floares (Oarga), D.; Obistioiu, D.; Hulea, A.; Suleiman, M.A.; Popescu, I.; Berbecea, A.; Samfira, I.; Radulov, I. Antimicrobial and Antioxidant Properties of Sambucus nigra L. (Elderflower) Oil: A Molecular Docking and Biochemical Study. Agronomy 2025, 15, 310. [Google Scholar] [CrossRef]
- Santos, J.D.C.; Coelho, E.; Silva, R.; Passos, C.P.; Teixeira, P.; Henriques, I.; Coimbra, M.A. Chemical Composition and Antimicrobial Activity of Satureja montana Byproducts Essential Oils. Ind. Crops Prod. 2019, 137, 541–548. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E. HERBS | Herbs of the Labiatae. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3082–3090. ISBN 978-0-12-227055-0. [Google Scholar]
- Matejić, J.S.; Stefanović, N.; Ivković, M.; Živanović, N.; Marin, P.D.; Džamić, A.M. Traditional Uses of Autochthonous Medicinal and Ritual Plants and Other Remedies for Health in Eastern and South-Eastern Serbia. J. Ethnopharmacol. 2020, 261, 113186. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Čanadanović-Brunet, J.M.; Djilas, S.M.; Tumbas, V.T.; Markov, S.L.; Cvetković, D.D. Antioxidant Potential, Lipid Peroxidation Inhibition and Antimicrobial Activities of Satureja montana L. Subsp kitaibelii Extracts. Int. J. Mol. Sci. 2007, 8, 1013–1027. [Google Scholar] [CrossRef]
- Aćimović, M.; Šovljanski, O.; Pezo, L.; Travičić, V.; Tomić, A.; Zheljazkov, V.D.; Ćetković, G.; Švarc-Gajić, J.; Brezo-Borjan, T.; Sofrenić, I. Variability in Biological Activities of Satureja montana Subsp. montana and Subsp. variegata Based on Different Extraction Methods. Antibiotics 2022, 11, 1235. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Stojanović-Radić, Z.; Radulović, N.; Nešić, M. Chemical Composition and Antifungal Effect of the Essential Oils of Thymus vulgaris L., Origanum vulgare L., and Satureja montana L. against Clinical Isolates of Candida spp. Chem. Biodivers. 2025, 22, e202500270. [Google Scholar] [CrossRef] [PubMed]
- Kulić, M.; Drakul, D.; Sokolović, D.; Kordić-Bojinović, J.; Milovanović, S.; Blagojević, D. Essential Oil of Satureja montana L. from Herzegovina: Assessment of Composition, Antispasmodic, and Antidiarrheal Effects. Rec. Nat. Prod. 2023, 17, 536–548. [Google Scholar] [CrossRef]
- Vrancheva, R.; Dincheva, I.; Aneva, I.; Georgiev, V.; Pavlov, A. GC-MS-Based Metabolite Profiling of Wild and in vitro Growing Plants of Satureja montana L. Proc. Bulg. Acad. Sci. 2022, 75, 150–158. [Google Scholar] [CrossRef]
- Craine, E.B.; DeHaan, L.R. Nutritional Quality of Early-Generation Kernza Perennial Grain. Agriculture 2024, 14, 919. [Google Scholar] [CrossRef]
- Soto-Gómez, D.; Pérez-Rodríguez, P. Sustainable Agriculture through Perennial Grains: Wheat, Rice, Maize, and Other Species. A Review. Agric. Ecosyst. Environ. 2022, 325, 107747. [Google Scholar] [CrossRef]
- de Oliveira, G.; Brunsell, N.A.; Crews, T.E.; DeHaan, L.R.; Vico, G. Carbon and Water Relations in Perennial Kernza (Thinopyrum intermedium): An Overview. Plant Sci. 2020, 295, 110279. [Google Scholar] [CrossRef]
- Dobbratz, M.; Jungers, J.M.; Gutknecht, J.L.M. Seasonal Plant Nitrogen Use and Soil N Pools in Intermediate Wheatgrass (Thinopyrum intermedium). Agriculture 2023, 13, 468. [Google Scholar] [CrossRef]
- Cetiner, B.; Shamanin, V.P.; Tekin-Cakmak, Z.H.; Pototskaya, I.V.; Koksel, F.; Shepelev, S.S.; Aydarov, A.N.; Ozdemir, B.; Morgounov, A.I.; Koksel, H. Utilization of Intermediate Wheatgrass (Thinopyrum intermedium) as an Innovative Ingredient in Bread Making. Foods 2023, 12, 2109. [Google Scholar] [CrossRef]
- Rahardjo, C.P.; Gajadeera, C.S.; Simsek, S.; Annor, G.; Schoenfuss, T.C.; Marti, A.; Ismail, B.P. Chemical Characterization, Functionality, and Baking Quality of Intermediate Wheatgrass (Thinopyrum intermedium). J. Cereal Sci. 2018, 83, 266–274. [Google Scholar] [CrossRef]
- Ouzir, M.; El Bairi, K.; Amzazi, S. Toxicological Properties of Fenugreek (Trigonella foenum-graecum). Food Chem. Toxicol. 2016, 96, 145–154. [Google Scholar] [CrossRef]
- Nathiya, S.; Durga, M.; Devasena, T. Therapeutic Role of Trigonella foenum-graecum [Fenugreek]—A Review. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 74–80. [Google Scholar]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M.; et al. Moroccan Antidiabetic Medicinal Plants: Ethnobotanical Studies, Phytochemical Bioactive Compounds, Preclinical Investigations, Toxicological Validations and Clinical Evidences; Challenges, Guidance and Perspectives for Future Management of Diabetes Worldwide. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar] [CrossRef]
- Garzoli, S.; Cicaloni, V.; Salvini, L.; Trespidi, G.; Iriti, M.; Vitalini, S. SPME-GC-MS Analysis of the Volatile Profile of Three Fresh Yarrow (Achillea millefolium L.) Morphotypes from Different Regions of Northern Italy. Separations 2023, 10, 51. [Google Scholar] [CrossRef]
- Shabih, S.; Hajdari, A.; Mustafa, B.; Quave, C.L. Chapter 3—Medicinal Plants in the Balkans with Antimicrobial Properties. In Medicinal Plants as Anti-Infectives; Chassagne, F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 103–138. ISBN 978-0-323-90999-0. [Google Scholar]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Cvetković, S.; Ignjatijević, A.; Kukić-Marković, J.; Vuletić, S.; Ušjak, L.; Milutinović, V.; Mitić-Ćulafić, D.; Petrović, S.; Nikolić, B. Further Insights into Antimicrobial and Cytotoxic Potential of Achillea millefolium Herb Methanol and Dichloromethane Extracts. Ind. Crops Prod. 2025, 225, 120553. [Google Scholar] [CrossRef]
- Živković, S.; Milutinović, M.; Maksimović, V.; Ćirić, A.; Ivanov, M.; Božunović, J.; Banjanac, T.; Mišić, D. Antioxidant and Antimicrobial Activity of Two Asplenium Species. S. Afr. J. Bot. 2020, 132, 180–187. [Google Scholar] [CrossRef]
- Jarial, R.; Thakur, S.; Sakinah, M.; Zularisam, A.W.; Sharad, A.; Kanwar, S.S.; Singh, L. Potent Anticancer, Antioxidant and Antibacterial Activities of Isolated Flavonoids from Asplenium nidus. J. King Saud Univ. Sci. 2018, 30, 185–192. [Google Scholar] [CrossRef]
- Al-Assar, N.B.; Khattak, M.N.K.; Mashwani, Z.-U.-R.; Kanan, S.; Ullah, I.; Ali, U.; Khan, A.A. Phytochemical Profile and Antiproliferative Activities of Acetone Extracts of Asplenium polypodioides Blume and A. dalhousiae Hook. in MDA-MB-231 Breast Cancer Cells. Saudi J. Biol. Sci. 2021, 28, 6324–6331. [Google Scholar] [CrossRef]
- Caño, L.; Serrano, M.; Fernández, J.E.; Rodríguez, A. Vicariance Between Cercis siliquastrum L. and Ceratonia siliqua L. Unveiled by the Physical–Chemical Properties of the Leaves’ Epicuticular Waxes. Front. Plant Sci. 2022, 13, 890647. [Google Scholar] [CrossRef]
- Amer, J.; Jaradat, N.; Hattab, S.; Al-hihi, S.; Juma’a, R. Traditional Palestinian Medicinal Plant Cercis siliquastrum (Judas Tree) Inhibits the DNA Cell Cycle of Breast Cancer—Antimicrobial and Antioxidant Characteristics. Eur. J. Integr. Med. 2019, 27, 90–96. [Google Scholar] [CrossRef]
- Moghaddam, M.; Stegemann, T.; Zidorn, C. Flavonoids and Volatile Compounds of Cercis siliquastrum (Fabaceae, Cercideae). Biochem. Syst. Ecol. 2025, 120, 104954. [Google Scholar] [CrossRef]
- Babyn, O.; Pinchuk, A.; Derii, A.; Boyko, O.; Likhanov, A. Influence of Urban Environment Factors on Morphometric Parameters and Accumulation of Secondary Metabolites in Cercis canadensis L. and Cercis siliquastrum ‘Alba’. Ukr. J. For. Wood Sci. 2024, 15, 8–24. [Google Scholar] [CrossRef]
- Yaşar, Ü.; Ozyigit, I.I.; Serin, M. Judas Tree (Cercis siliquastrum L. subsp siliquastrum) as a Possible Biomonitor for Cr, Fe and Ni in Istanbul (Turkey). Rom. Biotechnol. Lett. 2010, 15, 4979–4989. [Google Scholar]
- Meliti, D.; Madesis, P.; Magiatis, P. A Review of Phytochemical and Pharmacological Studies on Galium verum L., Rubiaceae. Molecules 2025, 30, 1856. [Google Scholar] [CrossRef]
- Bradic, J.; Petkovic, A.; Tomovic, M. Phytochemical and Pharmacological Properties of Some Species of the Genus Galium L. Galium verum and Mollugo. Serbian J. Exp. Clin. Res. 2021, 22, 187–193. [Google Scholar] [CrossRef]
- Ciotlaus, I.; Pojar-Fenesan, M.; Balea, A. Analysis of Volatile Organic Compounds from the Aerial Parts of Medicinal Plant, Galium verum. Rev. Chim. 2020, 71, 136–144. [Google Scholar] [CrossRef]
- Antoniak, K.; Studzińska-Sroka, E.; Szymański, M.; Dudek-Makuch, M.; Cielecka-Piontek, J.; Korybalska, K. Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root. Molecules 2023, 28, 4966. [Google Scholar] [CrossRef]
- Dimitrova, V.; Tashev, A. Medicinal Plants of Bulgaria. Curr. Perspect. Med. Aromat. Plants 2019, 2, 29–39. [Google Scholar] [CrossRef][Green Version]
- Tzanova, M.T.; Grozeva, N.H.; Gerdzhikova, M.A.; Todorova, M.H. Composition and Antioxidant Potential of Essential Oil of Geranium macrorrhizum L. from Different Regions of Bulgaria. Bulg. Chem. Commun. 2024, 56, 32–37. [Google Scholar] [CrossRef]
- Ivancheva, S.; Manolova, N.; Serkedjieva, J.; Dimov, V.; Ivanovska, N. Polyphenols from Bulgarian Medicinal Plants with Anti-Infectious Activity. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Springer US: Boston, MA, USA, 1992; pp. 717–728. ISBN 978-1-4613-6540-2. [Google Scholar]
- Radulović, N.S.; Stojković, M.B.; Mitić, S.S.; Randjelović, P.J.; Ilić, I.R.; Stojanović, N.M.; Stojanović-Radić, Z.Z. Exploitation of the Antioxidant Potential of Geranium macrorrhizum (Geraniaceae): Hepatoprotective and Antimicrobial Activities. Nat. Prod. Commun. 2012, 7, 121–128. [Google Scholar] [CrossRef]
- Radulović, N.S.; Dekić, M.S.; Stojanović-Radić, Z.Z.; Zoranić, S.K. Geranium macrorrhizum L. (Geraniaceae) Essential Oil: A Potent Agent Against Bacillus subtilis. Chem. Biodivers. 2010, 7, 2783–2800. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L as a Nutraceutical in Human and Veterinary Medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed]
- Pepeljnjak, S.; Kosalec, I.; Kalođera, Z.; Blažević, N. Antimicrobial Activity of Juniper Berry Essential Oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar] [PubMed]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A Phytopharmacological Review on a Medicinal Plant: Juniperus communis. Int. Scholarly Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef]
- Sati, S.C.; Joshi, S. Antibacterial Potential of Leaf Extracts of Juniperus communis L. from Kumaun Himalaya. Int. J. Pharm. Pharm. Sci. 2012, 4, 500–502. [Google Scholar]
- Banerjee, S.; Mukherjee, A.; Chatterjee, T.K. Evaluation of Analgesic Activities of Methanolic Extract of Medicinal Plant Juniperus communis Linn. Int. J. Pharm. Pharm. Sci. 2012, 4, 547–550. [Google Scholar]
- Sytykiewicz, H.; Łukasik, I.; Goławska, S. Chemical Composition, Anti-Tyrosinase and Antioxidant Potential of Essential Oils from Acorus calamus (L.) and Juniperus communis (L.). Molecules 2025, 30, 2417. [Google Scholar] [CrossRef]
- Yernazarova, K.B.; Abdrassulova, Z.T.; Tuleuhanov, S.T.; Tussupbekova, G.A.; Salybekova, N.N.; Isayev, G.; Basim, H. Biological Features of the Medicinal Plant Plantago major L. Int. J. Biol. Chem. 2019, 12, 86–93. [Google Scholar] [CrossRef]
- Nazarizadeh, A.; Mikaili, P.; Moloudizargari, M.; Aghajanshakeri, S.; Javaherypour, S. Therapeutic Uses and Pharmacological Properties of Plantago major L. and Its Active Constituents. J. Basic Appl. Sci. Res. 2013, 3, 212–221. [Google Scholar]
- Samuelsen, A.B. The Traditional Uses, Chemical Constituents and Biological Activities of Plantago major L. A Review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Abdul Kudos, M.B.; Wan Sulaiman, M.W.A.; Sengupta, P.; Susanti, D. Chemical Constituents and Medical Benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.S.M.; Ruiz, L.d.S.; Pereira, A.F.M.; Sani, A.A.; Zapata, T.B.; Monari, G.P.d.M.; da Rosa, C.F.; Junior, A.F.; Rall, V.L.M.; Prado, D.G.; et al. Plantago major Leaf Extract against Bacteria and Fungi of Medical Importance. Braz. J. Pharm. Sci. 2025, 61, e24143. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding Function in Novel Targets: C. Elegans as a Model Organism. Nat. Rev. Drug Discov. 2006, 5, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Barclay, J.W.; Burgoyne, R.D.; Morgan, A. Using C. Elegans to Discover Therapeutic Compounds for Ageing-Associated Neurodegenerative Diseases. Chem. Cent. J. 2015, 9, 65. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, A.; Bogdanova, S.; Petrov, V.; Gechev, T. Plants from Bulgarian Botanical Gardens: Some Selected Species with Potential for Health Food and Medical Applications. Plants 2025, 14, 2176. https://doi.org/10.3390/plants14142176
Ivanova A, Bogdanova S, Petrov V, Gechev T. Plants from Bulgarian Botanical Gardens: Some Selected Species with Potential for Health Food and Medical Applications. Plants. 2025; 14(14):2176. https://doi.org/10.3390/plants14142176
Chicago/Turabian StyleIvanova, Aleksandra, Stefka Bogdanova, Veselin Petrov, and Tsanko Gechev. 2025. "Plants from Bulgarian Botanical Gardens: Some Selected Species with Potential for Health Food and Medical Applications" Plants 14, no. 14: 2176. https://doi.org/10.3390/plants14142176
APA StyleIvanova, A., Bogdanova, S., Petrov, V., & Gechev, T. (2025). Plants from Bulgarian Botanical Gardens: Some Selected Species with Potential for Health Food and Medical Applications. Plants, 14(14), 2176. https://doi.org/10.3390/plants14142176







