Abstract
Plant growth-promoting rhizobacteria (PGPR) are beneficial soil microorganisms that enhance plant growth and stress tolerance through various mechanisms, including phytohormone production, EPS production, phosphate solubilization, and extracellular enzyme production. These bacteria establish endosymbiotic relationships with plants, improving nutrient availability and overall crop productivity. Despite extensive research on PGPR isolation, their practical application in agricultural fields has faced challenges due to environmental stresses and limited survival during storage. To address these limitations, the present study aimed to isolate salt-tolerant bacterial strains and formulate them with organic carriers to enhance their stability and effectiveness under saline conditions. The isolated bacterial strains exhibited high salt tolerance, surviving NaCl concentrations of up to 850 millimolar. These strains demonstrated basic key plant growth-promoting traits, including phosphate solubilization, auxin production, and nitrogen fixation. The application of carrier-based formulations with both strains, Bacillus wiedmannii (RR2) and Bacillus paramobilis (RR3), improved physiological and biochemical parameters in wheat plants subjected to salinity stress. The treated plants, when subjected to salinity stress, showed notable increases in chlorophyll a (73.3% by Peat + RR3), chlorophyll b (41.1% by Compost + RR3), carotenoids (51.1% by Peat + RR3), relative water content (77.7% by Compost + RR2), proline (75.8% by compost + RR3), and total sugar content (12.4% by peat + RR2), as compared to the stressed control. Plant yield parameters such as stem length (35.1% by Peat + RR3), spike length (22.5% by Peat + RR2), number of spikes (67.6% by Peat + RR3), and grain weight (39.8% by Peat + RR3) were also enhanced and compared to the stressed control. These results demonstrate the potential of the selected salt-tolerant PGPR strains (ST-strains) to mitigate salinity stress and improve wheat yield under natural field conditions. The study highlights the significance of carrier-based PGPR applications as an effective and sustainable approach for enhancing crop productivity in saline-affected soils.
1. Introduction
As recent studies show, more than 20% of the world’s soils are affected by salt due to human activities and climate variability, resulting in a 50% decrease in crop production. With the world’s population growing rapidly, we will need to increase food production by 70% by 2050 [1]. Wheat is an essential crop in terms of providing calories, carbohydrates, and essential nutrients that are important for our health, and it can feed around 36% of the world’s population [2]. Wheat production can contribute approximately 1.7% to the country’s GDP, accounting for about 9.1% of the total agricultural production. Notably, Pakistan’s annual wheat production reaches 27 million tons. This substantial output showcases the crucial role of wheat in meeting both domestic consumption and potential export demands [3].
One of the main reasons behind the low crop yield in Pakistan is the use of organic fertilizers and the presence of salinity in the soil [4]. Salinity stress damages proteins, cell walls, and the nucleus of plant cells by excessive production of reactive oxygen species (ROS) that ultimately lowers the yield and will disturb the Na+/K+ ratio, causing osmotic imbalance in plants. Along with this, the presence of Na+ and Cl− ions can cause a deficiency of nutrients and ion toxicity that ultimately affect the physiological conditions of plants [5]. One specific area of focus is the use of plant growth-promoting rhizobacteria (PGPR) to enhance plant growth under salt stress, which is considered an emerging technology that plays a crucial role in inducing salt stress resistance through multifaceted mechanisms. Previous studies have highlighted the positive impact of Gram-positive Bacillus sp. on plant growth under stress by enhancing the production of plant hormones and salt-resistant genes [6]. The real milestone is the gap between the laboratories and the farmers that can be reduced by the application of the PGPR in a portable way that can easily be used in the fields. For this purpose, using organic carriers for bacterial field application is the most affordable, environmentally friendly, and effective approach.
Recent studies have identified Bacillus wiedmannii and Bacillus paramobilis as highly effective bacterial strains that significantly enhance plant physiological and biochemical parameters under salt stress conditions. These bacterial species exhibit multiple plant-beneficial traits, including phosphate solubilization, nitrogen fixation, production of stress-alleviating phytohormones, and secretion of antioxidant enzymes [7].
Bacillus wiedmannii has been reported to stimulate root elongation, enhance nutrient uptake, and improve photosynthetic efficiency by modulating stress-responsive gene expression [8]. Additionally, Bacillus paramobilis contributes to salt stress tolerance by improving osmotic balance, increasing proline accumulation, and reducing ROS-induced oxidative damage. In wheat, applying Bacillus wiedmannii and Bacillus paramobilis has been shown to significantly increase chlorophyll content, relative water content (RWC), and sugar accumulation under saline conditions. These bacteria help maintain cell membrane integrity by reducing electrolyte leakage and promoting the synthesis of compatible solutes, which enhance water retention and sustain metabolic activities during stress [9]. In addition to their role in stress mitigation, Bacillus wiedmannii and Bacillus paramobilis also possess biocontrol properties against soil-borne pathogens. Their ability to produce antimicrobial compounds and induce systemic resistance in plants make PGPR a valuable candidate for sustainable agricultural practices [10]. These findings highlight the dual benefits of ST-PGPR, which not only enhance stress tolerance but also contribute to disease suppression, offering a holistic approach to crop improvement.
Salt-tolerant plant growth-promoting rhizobacteria (ST-PGPR) mitigate salt stress in plants through both direct and indirect regulatory mechanisms. Direct regulation involves the synthesis of phytohormone signals, such as auxin, gibberellins, ethylene, and abscisic acid production [11], chemical processes like nitrogen fixation, and enhanced nutrient uptake. Indirect regulation is achieved by bolstering the plant’s defense system and modulating metabolic pathways that facilitate the accumulation of osmoregulatory and antioxidant substances. These combined mechanisms contribute to the alleviation of salt stress and promote plant growth and resilience [12]. Another important trait possessed by Bacillus sp. is the ability to form biofilms. Biofilm formation serves as a coating around bacteria that enables them to survive under harsh conditions and also helps in the colonization of plants. Recent research has shown that Bacillus sp. forms a lot of biofilm under stress conditions [13].
The direct application of PGPR in the field environment presents several challenges due to harsh and unstable environmental conditions, short shelf life of bacterial inoculants, inadequate processing methods by the farmers, and poor field conditions. To address these issues, a suitable carrier medium is required to assist the application of these PGPR under natural environmental conditions. Many studies use of synthetic carriers like nanoparticles, alginate beads, use of polymer gel, but these techniques are often expensive, labor-intensive, time-consuming, and less practical for large-scale use. In contrast to a synthetic carrier material, the use of an organic carrier is a more adequate, cost-effective, and environmentally friendly approach [14]. The use of organic carriers, i.e., compost and peat, is quite helpful to assist plants with abiotic stresses. This improves soil water-holding capacity, fertility, and nutrient quality and also combats soil salinity. The organic carriers serves as a medium for the inoculation of PGPR and is considered a very cost-effective and eco-friendly fertilizer for plants [15].
The focus of this study is to investigate the potential of Bacillus wiedmannii and Bacillus paramobilis to enhance wheat growth under salt stress conditions. By integrating these beneficial microbes with organic carrier-based formulations, this research seeks to develop a biologically driven and sustainable strategy for mitigating salinity stress in wheat. Understanding the underlying mechanisms of PGPR-mediated stress tolerance will contribute to the advancement of environment-friendly approaches for increasing wheat yield and ensuring food security in salt-affected regions.
2. Results
2.1. Isolation and Purification of Bacteria
A total of 50 bacterial strains were isolated from 10 rhizospheric soil samples, out of which 27 were halotolerant (Table 1). Five strains showed the best growth at 850 mM salt concentration and were selected for further characterization [16].

Table 1.
Sampling sites, soil texture, and plant source of rhizospheric samples.
2.2. PGP Characterization of Salt-Tolerant Rhizobacteria
To access the plant growth-promoting potential of isolated salt-tolerant rhizobacterial strains, several experiments were conducted to select the strains to be used as a biofertilizer. Initial tests include auxin production, phosphate solubilization, nitrogen fixation, EPS production, HCN production, and ammonia production [17].
All five strains showed positive results for auxin production and phosphate solubilization (Table 2). Auxin production ranged from 36.5 µg/mL (RR6) to 455.7 µg/mL (RR8). The lowest results were shown by RR1 (41.0 µg/mL) and RR6 (36.5 µg/mL), while the other three, RR2 (428.7 µg/mL), RR3 (351.1 µg/mL), and RR6 (455.7 µg/mL), showed maximum production with a sharp contrast to the others (Figure 1).

Table 2.
Plant growth-promoting (PGP) attributes of salt-tolerant rhizobacterial strains.
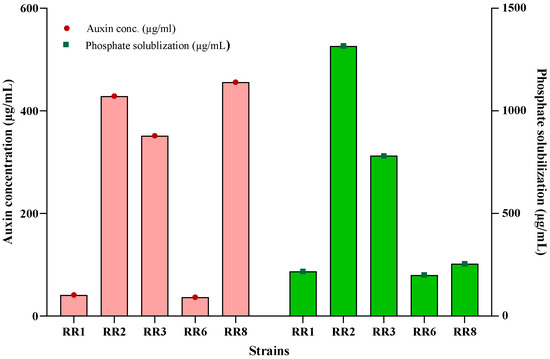
Figure 1.
Auxin production and phosphate solubilization by ST-PGPR. Bars represent standard deviation.
Phosphate solubilization ranged from 200.1 µg/mL (RR6) to 1316.1 µg/mL (RR2). RR1, RR6, and RR8 showed lower phosphate solubilization indexes (PSIs), that is, 217.2 µg/mL, 200.1 µg/mL, and 255.9 µg/mL, respectively, while RR2 and RR3 showed contrastingly higher PSIs, 1316.1 µg/mL and 780.6 µg/mL, respectively (Figure 1).
All five strains showed positive zones for nitrogen fixation, ranging from 0.86 cm to 2.43 cm (Table 2). The larger zones were shown by RR2 (1.31 cm), RR3 (2.13 cm), and RR8 (2.43 cm), while RR1 (1.13 cm) showed a smaller zone, and the smallest zone was produced by RR6 (0.86 cm). Moreover, 80% of ST-strains were positive for HCN production, 40% showed positive results for ammonia production, and 60% showed positive results for EPS production (Table 2).
Salt-tolerant PGPRs were also evaluated in terms of their extracellular enzymes, such as chitinase, pectinase, catalase, protease, and amylase-producing abilities (Table 3). Concerning chitinase and pectinase, about 80% and 20% of strains showed production, respectively, while none of the strains produced the catalase enzyme. About 60% of strains show reproducibility for protease and amylase enzymes.

Table 3.
Extracellular enzyme production by PGPR.
The two most potent strains (RR2) and (RR3) regarding plant growth-promoting attributes were selected for further application on wheat to assess the efficiency of their plant–microbe interaction potential when combined with the selected carriers. Moreover, the GC-MS analysis elaborates on the presence of indole compounds and several secondary metabolites in salt-tolerant PGPR (Table 4).

Table 4.
IAA and its derivatives found in salt-tolerant PGPR.
2.3. Molecular Characterization of Bacterial Strains
The most effective strains, having plant growth-promoting attributes and being salt tolerant, were RR2 and RR3. These strains appeared as damp, opaque, mucoid colonies, were rod-shaped and endospore forming, and were positive for citrate and catalase production. After biochemical analysis, these strains were subjected to 16S rRNA-based identification. RR2 and RR3 were identified as Bacillus wiedmannii and Bacillus paramobilis with 98% similarity, and their GeneBank accession numbers were PQ057000 and PQ057001, respectively. The phylogenetic tree was constructed using the nucleotide sequence according to the nearest neighboring strains to show an evolutionary relationship (Figure 2).

Figure 2.
Phylogenetic tree of salt-tolerant strains RR2 and RR3 and neighboring plant growth-promoting bacteria.
2.4. Characterization of the Carrier and Survival Percentage of PGPR in Carriers
The physiochemical analysis of compost and peat showed electrical conductivities 2.15 and 1.93 dS−1, pH 5.6 and 5.28, nitrogen 1.14% and 0.92%, phosphorus 0.97% and 0.64%, potassium 87.61% and 73.56%, moisture 3.89% and 3.37%, and water-holding capacity 28.4% and 31.12%, respectively (Table 5).

Table 5.
Physiochemical analysis of the carrier’s compost and peat.
The stability of the strains in both carriers was evaluated by storing at room temperature, ranging from 25 °C to 30 °C, for 180 days (6 months). The CFU of RR2 and RR3 decreased gradually over 90 days, from 35.1 × 107 and 30.4 × 107 to 21.0 × 107 and 18.2 × 107, respectively, in compost and from 31.8 × 107 and 29.2 × 107 to 15.9 × 107 and 14.3 × 107, respectively, in peat. However, a sudden decline in the CFU of RR2 and RR3 was noticed during the time interval of 150 days to 180 days, i.e., 7.9 × 107 and 8.3 × 107 in compost and 7.4 × 107 and 5.2 × 107 in peat (Table 6).

Table 6.
Bacterial population (1 × 107 CFU mg−1) in compost and peat at 25 °C to 30 °C.
2.5. Soil Analysis
Soil analysis showed the texture of the soil as sandy loam, with electrical conductivity 2.37 dS−1, pH 8.6, available nitrogen, phosphorus, and potassium 91, 4.42, and 98 mg/kg, organic matter 0.25%, and saturation 11% (Table 7).

Table 7.
Nutrient analysis of soil.
2.6. Seedling Characteristics of Wheat Under Laboratory Conditions
Salinity adversely affects wheat growth, resulting in a decrease in productivity and biomass. However, the application of salt-tolerant strains, both with and without carriers, significantly improved the yield. There was a visible increase in root length, shoot length, fresh root weight, fresh shoot weight, dry root weight, and dry shoot weight when bacteria along the carrier were applied, as shown in Figure 3. Under unstressed conditions among the tested combinations, the application of peat + Bacillus paramobilis (RR3) and peat + Bacillus weidmannii (RR2) increased the root length by 58.1% and 48.3%, respectively. However, the other treatments—C + RR2, C + RR3, compost, peat, RR2, and RR3—increased root length by 39.5%, 43.0%, 36.3%, 37.1%, 26.8%, and 23.4%, respectively, as compared to the control. The same trend was observed for shoot length. Maximum shoot length was obtained with the application of peat + RR3 (55.3%) and compost + RR2 (54.8%), respectively, as compared to the control. Other treatments, such as P + RR2, peat, C + RR3, compost, RR2, and RR3, increased the shoot length by 53.9%, 51.2%, 47.7%, 25.1, 42%, and 38.9%, respectively, as compared to the control (Figure 3A). Under stressed conditions, all treatments performed better than under unstressed conditions. The maximum root length was observed by the application of P + RR3 (63.5%) and P + RR2 (62.5%). Other treatments increased root length by 40% (for RR2), 42.6% (for RR3), 31.5% (for compost), 54.6% (for C + RR2), 48% (for C + RR3), and 39% (for peat). The maximum increase in shoot lengths observed by P + RR2 and P + RR3 were 59.4% and 55.7%, respectively. Other treatments increased shoot length—37.6% for RR2, 44.2% for RR3, 22.2% for compost, 51.8% for C + RR2, 47% for C + RR3, and 41.8% for peat—as compared to the stressed control (Figure 3B).
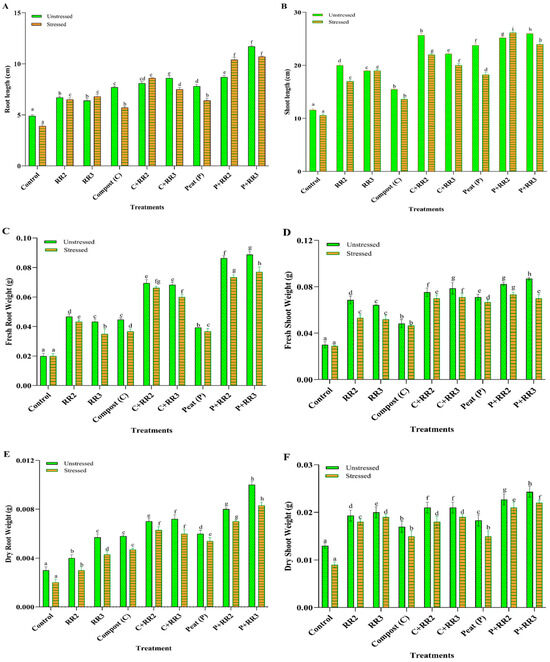
Figure 3.
Vegetative parameters of wheat under laboratory conditions. (A) Root length, (B) Shoot length, (C) Fresh root weight, (D) Fresh shoot weight, (E) Dry root weight, (F) Dry shoot weight in stressed and unstressed conditions. All data represented are the means of triplicate values. Bars represent the standard deviation, and those that do not share a letter are significantly different (p ≤ 0.05, Tukey’s test).
2.7. Root Colonization
The scanning electron microscopic images show the colonization of RR2 and RR3 on the roots of wheat. There is no colonization present in the control (Figure 4A), while RR2 shows clear colonization (Figure 4B,C) as compared to RR3 (Figure 4D). The selected strains developed very good associations with the roots.
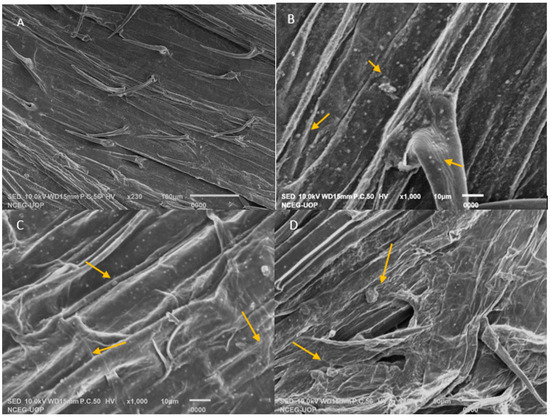
Figure 4.
Scanning electron microscopy images of roots of wheat adhering to salt-tolerant PGPRs. (A) An uninoculated control has no bacterial colonization; (B,C) Root colonization RR2 (D) Root colonization by RR3. Yellow arrows show the bacterial colonization.
2.8. Physiological and Biochemical Parameters of Wheat Under Natural Conditions
Chlorophyll a levels significantly increased in plants treated with bacterial strains Bacillus wiedmanni (RR2) and Bacillus paramobilis (RR3) and the carriers compost (C) and peat (P). Under stressed conditions, the highest level of chlorophyll a was observed in plants treated with P + RR3, P + RR2, and C + RR2 by 73.3%, 67.2%, and 66.1%, respectively, as compared to control. While under unstressed conditions, maximum chlorophyll a was observed in plants treated with P + RR3 and C + RR3 by 64.2% and 62.0%, respectively, as compared control (Figure 5A). A similar trend was observed for chlorophyll b in wheat leaves; the application of PGPR combined with peat and compost increased the chlorophyll b levels in both stressed and unstressed conditions. Under unstressed conditions, maximum chlorophyll b was observed in P + RR2 by 36.1%, followed by compost, C + RR2, C + RR3, RR2, P + RR3, RR3, and peat by 32.5%, 31.8%, 27.7%, 26.3%, 25.1%, 22.0%, and 16.6%, respectively, as compared to the control. In stressed conditions, the maximum chlorophyll b was observed in C + RR3, P + RR2, P + RR3, and C + RR2 by 41.1%, 40.4%, 39.7%, and 39.0%, respectively, as compared to untreated stressed plants (Figure 5B). The application of both strains in combination with the carriers C + RR2 and P + RR3 had maximum chlorophyll b production as compared to bacterial application alone under stressed condition (Figure 5B). The maximum carotenoids were produced by P + RR3 and C + RR2 by 47.6%, 42.0%, respectively, under unstressed conditions, as compared to control. P + RR3 showed a maximum increase of 51.1% in the presence of salt, as compared to the untreated stressed control. Under stressed conditions, both strains in combination with peat (P + RR2 and P + RR3) gave better carotenoid production than RR2 and RR3 (Figure 5C). A similar trend was observed under unstressed conditions. Peat gave better results compared to individual applications of bacterial strains for carotenoid production (Figure 5D). C + RR2 and C + RR3 performed well under unstressed conditions; however, under stressed conditions, the highest results were observed for P + RR3 for total chlorophyll content (Figure 5D).
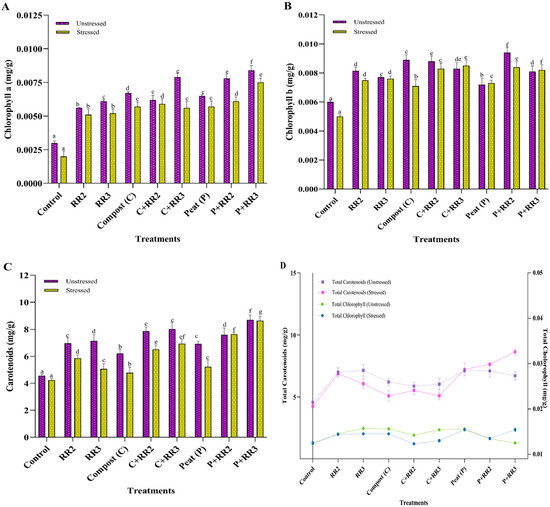
Figure 5.
Physiological parameters of wheat under natural conditions. (A) Chlorophyll a; (B) Chlorophyll b; (C) Carotenoids; (D) Total chlorophyll and total carotenoids. All data presented in the graph are the means of triplicate values. Bars represent the standard error, and those that do not share a letter are significantly different (p ≤ 0.05, Tukey’s test).
The REL in the plants increased when subjected to stress for all treatments; however, if a comparison was made between the stressed and unstressed group, less REL was observed in the unstressed group for all treatments. The REL was reduced to 165.5% and 144.8% for C + RR3 and P + RR2 under a saline environment, as compared to untreated salt stress (Figure 6A). The RWC of the stressed plants significantly increased with the application of Bacillus wiedmannii (RR2) and Bacillus paramobilis (RR3) in combination with compost by 77.7% to 65.5%, respectively, as compared to the control (Figure 6B). The estimated increases for both strains RR2 and RR3 in combination with peat were 62.4% and 64.2%, respectively, compared to the control (Figure 6B).
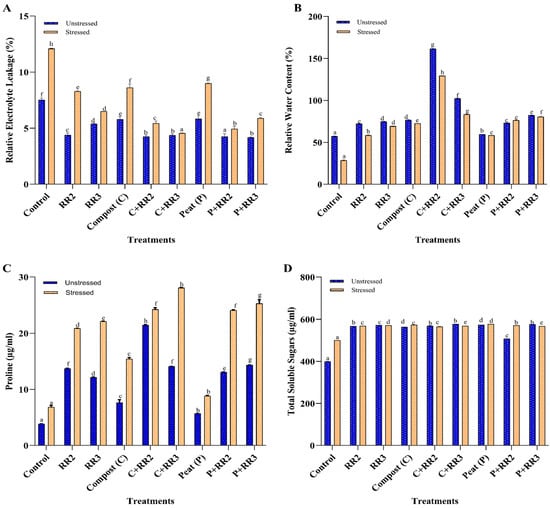
Figure 6.
Effect of biochemical parameters on wheat under natural conditions. (A) Relative electrolyte leakage; (B) Relative water content; (C) Proline content; (D) Total soluble sugar. All data presented in the graph are means of triplicate values. Bars represent the standard error and those that do not share a letter are significantly different (p ≤ 0.05, Tukey’s test).
Proline content and total soluble sugar content in the leaves also increased significantly by the application of strains with carriers. Proline production was increased to 82.5%, 73.9%, and 72.6% for C + RR2, P + RR3, and C + RR3, and 70.62%, 71.9%, and 68.3% for P + RR2, RR2, and RR3, respectively, under unstressed conditions, as compared to the control. However, under salt stress, the maximum proline content was observed in C + RR3, P + RR3, C + RR2, and P + RR2 and was 75.8%, 73.6%, 71.8%, and 71.6% respectively, as compared to untreated stressed plants. Under stressed conditions, RR2 and RR3 in combination with compost gave more proline production as compared to individual applications of RR2 and RR3. Similarly, RR2 and RR3 in combination with peat had better proline production as compared to individual bacterial application (Figure 6C). The total soluble sugar content (TSS) in the leaves showed a constant increase in all treatments as compared to the control. The maximum TSS produced by C + RR3, P + RR3, and peat was 30.8%, 30.6%, and 30.4%, as compared to the unstressed control; however, in the case of stressed plants, the maximum TSS observed by the application of peat, C + RR3, and P + RR2 was 13.4%, 12.3%, and 12.0%, respectively, as compared to the control (Figure 6D).
2.9. Yield Parameters of Wheat Under Natural Conditions
Yield components of wheat, including shoot length, spike length, no. of spikes, and weight per 100 grains, were measured, and a clear decrease in these parameters was noticed under stressed conditions. However, treatment with compost and peat combined with PGPR significantly increased the yield parameters in both stressed and unstressed conditions. In the case of yield attributes, the maximum shoot length was observed by P + RR2, C + RR3, and P + RR3 in both stressed and unstressed conditions. The maximum spike length was observed in P + RR3 in an unstressed environment, while, under stress, C + RR2 yielded the maximum results. For the no. of spikes, P + RR2 gave the maximum results in unstressed conditions, while P + RR3 gave the maximum no. of spikes in stressed conditions. In the case of grain weight, P + RR3 gave the maximum results in both stressed and unstressed conditions (Table 8).

Table 8.
Yield attributes of wheat in the natural environment.
3. Discussion
Around 36% of the global population is dependent on wheat as a staple crop, and it is ranked the most grain-producing crop [18]. However, due to harsh climate fluctuations, the temperature of the Earth is rising 2–4 °C annually, resulting in different types of environmental stresses, including drought, salinity, and global warming [19]. The climate of Pakistan has been dynamically changing for the past few decades. This instability in the environment causes several abiotic stresses on crops. Salt stress is one of the major stresses that drastically affects crop productivity [20]. This study aimed to find a novel approach that can improve the production of wheat facing salt stress. After conducting a series of experiments, the findings revealed that bacterial application, in combination with carriers, could be a suitable approach for improving wheat growth under salt stress. The experiments conducted in this study showed that the use of plant growth-promoting rhizobacterial strains (Bacillus wiedmannii and Bacillus paramobilis) combined with peat and compost effectively helped plants cope with salt stress and increase the yield of wheat. These are the same findings as reported by other studies using other strains of Bacillus, including Bacillus thuringiensis, B. subtilis, B. amyloliquefaciens, and B. velezensis [21,22]. The application of Bacillus spp. against salinity to improve wheat growth has been investigated, but this study showed that the use of carriers like peat and compost makes these strains even more accessible and effective in both natural and field environments [23].
The most significant improvements were observed, on average, in the case of peat, rather than compost, in combination with RR3. However, RR2 gave better results with compost in most of the experiments. If comparisons were made between all the treatments and the control, then all the treatments gave better results as compared to the control. The halotolerant rhizobacterial strains have great potential to improve plant growth under a saline environment. This is consistent with other research showing that halotolerant bacteria have advantageous plant–microbe interactions and are well-suited to saline settings [24,25]. The improved growth and yield parameters and resistance toward salinity and drought by Bacillus sp. have been reported in the study [26]. As shown in this research, the Bacillus spp. have been reported to have many plant growth-promoting attributes such as the production of auxin, siderophores, and extracellular enzymes, phosphate solubilization, nitrogen fixation, metal resistance, and biocontrol activities [27,28,29,30].
These results are consistent with research showing that PGPR can improve plant development by producing phytohormones and solubilizing phosphate, which are essential for nutrient availability in environments. The strains (RR2) and (RR3) with plant growth-promoting attributes were selected for application on wheat to assess the efficiency of their plant–microbe interaction potential when combined with the selected carriers. These treatments improved the vegetative parameters, including root length, shoot length, fresh and dry root weight, and fresh and dry shoot weight, as compared to the control. Similar or greater effects produced by Bacillus subtilis, Bacillus velenzensis, and other Bacillus strains have been reported [31,32,33].
Moreover, salinity affects the chlorophyll content by damaging photosystem II and causing a decrease in the rate of photosynthesis in wheat through the closing of stomata due to imbalances of Na+ ions [34]. A recent study showed that different varieties of wheat are affected by salinity, resulting in the reduction of several photosynthetic parameters such as REL content, RWC content, rate of transpiration, and temperature. Salinity also affects the chlorophyll and carotenoid content of plants by the production of excessive reactive oxygen species and H2O2 [35]. However, the exogenous application of Bacillus paramobilis in combination with peat significantly increased the chlorophyll level and reduced the production of ROS. These results have been reported in several plants, including cucumber, rice, and tobacco [36,37].
The application of organic matter, such as compost, combined with PGPR helps in increasing the microbial activity in the soil and facilitates the breakdown and availability of organic matter to the microorganisms. The application of compost also enhances the water-holding capacity of soil, microbial respiration rate, and osmosis. It also balances the ionic concentration by increasing the uptake of K+ ions from the roots to the leaves, resulting in a decrease in the Na+ ion concentration by the production of auxin and exopolysaccharides [38,39]. A sandy loam composition with an alkaline pH of 8.6 and an electrical conductivity of 2.37 dS−1 was found by soil texture analysis. Wheat growth was greatly enhanced by the use of PGPR with both carriers as compared to the control. Another study highlighted the role of peat in balancing the ionic concentrations in soil by regulating the K+/Na+ ion ratio in the soil. The application of peat and another organic carrier like biochar increases K+ uptake in soil and also helps in the reduction of Na+ ions, ultimately coping with salt stress. More concentration of K+ ions facilitates plant growth and germination. The findings of the current research also follow the previous data, proving that the peat application increases the plant yield and helps plants cope with salinity stress, as compared to the control [40].
All of the above findings suggested that the application of PGPR in combination with peat and compost showed a significant improvement in the growth and yield of wheat as compared to the control in both unstressed and stressed conditions. This portable and novel approach could be used in the field for improved wheat crop production in salt-affected areas.
4. Materials and Methodology
4.1. Plant and Bacterial Strains
Seeds of Triticum aestivum (Akbar-19) were purchased from Punjab Seeds Corporation, located in the city of Khanewal, Pakistan. Bacterial strains were isolated and characterized at the Department of Microbiology and Molecular Genetics (MMG) at The Women University, Multan, Pakistan.
4.2. Sampling, Isolation, and Purification of Bacteria
Following the protocol, soil samples were collected from the rhizospheric soil of plants near the Indus basin of Pakistan under sterile conditions [41]. The initial sampling was done from the root zones of Eriobotrya japonica, Citrus limon, Bauhinia variegate, and Syzygium cumini growing in the district of Muzaffargarh (30.0736° N, 71.1805° E) and Multan (30.1864° N, 71.4886° E). The soil samples were carefully transferred into sterile zip lock bags and transported to the laboratory of the MMG department of The Women University, Multan. One gram of the soil sample was dissolved in 9 mL of saline and serially diluted. Dilutions (10−3 and 10−5) were spread on Luria–Bertani (LB) agar medium plates that were incubated at 37 °C for 24 h. Distinct colonies were purified using the quadrant streaking method.
4.3. Screening of Salt-Tolerant Strains
LB agar plates with salt concentrations (0–1500 mM) were used for the screening of salt-tolerant strains. Isolated colonies were picked based on minimum inhibitory conditions (MIC) of salt and re-streaked on LB agar with (850 mM) NaCl and incubated for 24 h at 37 °C [42]. Separated colonies were selected and stored at 4 °C for further study.
4.4. Identification of Salt-Tolerant Strains
Bacterial identification was done by performing basic biochemical tests according to Bergey’s manual system and 16S rRNA sequencing [43].
4.5. PGPR Characterizations
4.5.1. Screening Based on Auxin Production
Salt-tolerant strains were screened for auxin production using Salowski’s reagent and following the protocol with some modifications [44]. The strains were incubated in LB broth with the addition of 0.1% L-tryptophan and incubated for 48 h in a shaker incubator at 120 rpm. The supernatant was extracted by using centrifugation at 10,000 rpm for 10 min. Then, 2 mL of supernatant was mixed with 2 mL of Salowski’s reagent and incubated for 30 min in the dark. The appearance of a pink to red color indicates the production of auxin. The quantification of auxin was done using a spectrophotometer. The optical densities (ODs) were noted at 530 nm. The standard graph method was used for the quantitative analysis using synthetic auxin solutions of different concentrations. The Gas Chromatography–Mass Spectrometry (GC–MS) analysis was done to validate indole compounds and their derivatives specifically [45].
4.5.2. Screening Based on Phosphate Solubilization
The experiment involved assessing the phosphate-solubilizing ability of the isolated rhizobacteria using the Pikovskaya’s agar containing glucose, calcium phosphate (Ca3(PO4)2), ammonium sulfate ((NH4)2SO4), sodium chloride (NaCl), magnesium sulfate (MgSO4.7H2O), potassium chloride (KCL), yeast extract, manganese(II) sulfate monohydrate (MnSO4.H2O), ferrous sulfate heptahydrate (FeSO4.7H2O), agar, bromophenol blue, and distilled water. The bacteria were spot-inoculated on NBRIP agar plates and incubated at 30 °C. The solubilizing index was determined using a formula described in the study [46]. The quantification of solubilized inorganic phosphate was conducted using the standard curve method [47].
4.5.3. Screening Based on Nitrogen Fixation
The ability of bacteria to fix nitrogen was determined by growing strains on Jensen’s media (N2-free media) containing sucrose, dipotassium phosphate, magnesium sulfate, NaCl, ferrous sulfate, sodium molybdate, calcium carbonate, and agar. Halo zones on the plates indicated the presence of N2-fixing bacteria. The N2-fixing capacity of the strains showed positive results determined using Nessler’s reagent and following the protocol [48].
4.5.4. Screening Based on HCN, Ammonia, and Exopolysaccharide Production
A comprehensive screening of the salt-tolerant rhizobacteria was conducted to evaluate their capacity for hydrogen cyanide (HCN) production, ammonia production, and exopolysaccharide production, following the protocol mentioned earlier [49].
4.5.5. Screening Based on the Production of Cell Wall-Degrading Enzymes
The production of chitinase, protease, and pectinase enzymes was evaluated by salt-tolerant rhizobacterial strains [50].
4.6. Characterization of Carrier Material
Peat and compost were selected for the experiment and purchased from Green Enterprises Pk. (GEP: Garden Gallery), located in Multan, Pakistan. The physicochemical analysis of peat and compost was also performed.
Stability of Bacteria in Carriers
A total of 10 mL of bacteria with 108 colony forming units (CFU) per mL was thoroughly mixed with 50 g of autoclaved carrier and packed in ultraviolet [51]-sterilized polythene bags. These bags were prepared in and placed at room temperature (25–30 °C). CFU was evaluated at time intervals of 0 days, 1 month, 3 months, and 6 months.
Using the above formula, CFU was evaluated after each interval to check the stability of the bacteria in the carriers [52].
4.7. Nutrient Analysis of Soil
Nutrient analysis of soil and carrier materials used for the experiments was done to evaluate nitrogen, potassium, and phosphorus concentrations, along with moisture content, electrical conductivity, and organic content, as described in [53].
4.8. Experimental Layout for Plant–Microbe Interaction
The efficiency of the selected strains in the presence of carriers was evaluated through their application to wheat under axenic and natural conditions (wire-house). Seeds of Triticum aestivum (variety Akbar-19) were purchased from Punjab Seed Corporation, Khanewal, Pakistan, sown on 1 November, and harvested in March. The experiment was performed at The Women University, Multan. Plant–microbe interaction was observed in the presence and absence of salinity stress, and 200 mM NaCl stress was applied [54].
Plant–Microbe Interaction
The initial experiment was performed to check the efficiency of the prepared treatment on wheat under controlled conditions in the laboratory of the MMG department. A total of 54 small cups (3.5″ × 2.6″) were filled 3/4 full with autoclaved soil and labeled accordingly (Table 9). The seeds were treated with 0.1% mercuric chloride for sterilization. After treatment, the seeds were coated with PGPR and carriers (peat and compost). Control seeds were dipped in autoclaved water for 30 min after sterilization. (T1 and T2) seeds, after sterilization, were dipped in broth for 30 min; the broth had a 24 h growth of the respective strains. (T3, T4, T5, T6, T7, T8) seeds were coated with respective carriers and PGPR + carrier combinations. Ten treated seeds were sown in each cup and placed in a completely randomized manner. A 12 h photoperiod with a (light intensity of 2200 lux) light chamber was used, and the cups were placed there for 15 days at 30 ± 2 °C temperature. After the germination of the seedlings, physiological parameters including shoot length, root length, fresh and dry shoot weight, and fresh and dry root weight were measured [55].

Table 9.
The following are the treatments for the experiment.
In the second phase, the experiment was performed under natural conditions (wire-house). For the wire-house experiment, 54 medium-sized pots were selected and filled with 11 kg of soil. The pots were labeled according to the treatment mentioned above, in triplicate, and placed in a Randomized Complete Block Design (RCBD). The experiment was conducted in two sets, one with no stress application and the other with 200 mM of salt stress. Seeds were sterilized, treated, and coated accordingly, as mentioned above, and sown in November in triplicate. Ten seeds were sown in each pot, and after germination, thinning was done from ten to five seeds per pot. Two weeks after germination, stress was applied. The physiological parameters (chlorophyll content, relative water content, relative electrolyte leakage, proline content, and total soluble sugar content) were evaluated. Yield parameters (shoot length, spike length, no. of spikes, no. of grains, and grain weight) were evaluated at the time of harvest.
4.9. Root Colonization Assay
To examine the root surface colonization by bacterial strains on wheat seedlings, scanning electron microscopy (SEM) analysis was performed [56]. The wheat seedlings were grown under controlled conditions in the laboratory using autoclaved soil. Treated wheat seedlings with PGPR were washed with double deionized water to remove any loosely adhered bacteria from the roots, and then the seedlings were fixed in 2.5% glutaraldehyde (v/v) for up to 4 h and dehydrated with a gradient of ethanol. The dried samples were coated with gold using an automated sputter coater for 3 min and carefully mounted for visualization. Treated samples were then examined under SEM using different magnifications.
4.10. Physiological Parameters
4.10.1. Chlorophyll Content
To calculate the total chlorophyll and carotenoid content, 0.1 g of fresh leaves was mixed with 10 mL of 80% acetone for a night. After centrifugation at 10,000 rpm for 5 min, the optical densities of supernatants were noted at 663 nm, 645 nm, and 470 nm [57].
4.10.2. Relative Electrolyte Leakage (REL)
To determine REL, small parts of leaves from all treatments were cut into discs and dipped in double-distilled water for 4 h. Electrical conductivities were noted (EC1) using a digital device. The samples were then boiled in the water bath for 30 min and then cooled down. Electrical conductivities were noted (EC2), and REL was calculated using the following formula [58].
4.10.3. Relative Water Content (RWC)
For the estimation of RWC, the weight of fresh leaves (FW) was measured immediately after sampling, and, after the saturation of leaves in water for 8 to 12 h at 4 °C, turgid weight (TW) was measured. Leaves were dried at 80 °C in a drying oven for 24 h, and dry weight (DW) was calculated using the following formula [59].
4.11. Biochemical Parameters
For the estimation of proline content from the fresh leaves, the Bates method was used as described in [59]. The total soluble sugar content of leaves in wheat was calculated using the protocol mentioned in [60].
4.12. Yield Parameters
After harvesting the plants of the wire-house, yield parameters including length, spike length, number of spikes, number of grains, and weight of 100 grains per treatment were evaluated as mentioned in [61].
4.13. Statistical Analysis
Data obtained from the above-mentioned experiments was statistically evaluated through analysis of variance [62] using IBM SPSS statistics 25.0 software [62]. Tukey’s test with a significance level of p < 0.05 was used to check the presence of any significant difference between the treatments. All the statistical analyses were performed within the defined groups (stressed and unstressed) [63].
5. Conclusions
This research concludes that the combined application of the salt-tolerant strains Bacillus wiedmannii (RR2) and Bacillus paramobilis (RR3) with organic carriers has significantly enhanced wheat growth under saline conditions and has proven to be a sustainable approach for improving wheat yield. The pot experiments have demonstrated the effectiveness of these strains in promoting plant health in natural environments by increasing chlorophyll a and chlorophyll b content, proline and TSS content, and yield attributes of wheat, reinforcing their potential as viable biofertilizers. Additionally, these formulations have provided an economical and sustainable approach to improve bacterial stability, colonization efficiency, and long-term soil fertility. These findings have underscored the potential of integrating PGPR with organic amendments to develop cost-effective and eco-friendly strategies for enhancing crop resilience in salt-affected soils. Future research should focus on the molecular study of these strains to identify the genes that play a role in phytohormone production and salt tolerance. Moreover, in the future, field-scale validation of these treatments to facilitate their large-scale adoption in sustainable agriculture can be studied.
Author Contributions
Conceptualization, R.R. and A.I.; methodology, R.R. and A.I.; software, H.A.M., S.N., and M.S.; validation, A.I.; formal analysis, M.S., S.N., and H.A.M.; investigation, R.R. and S.N.; writing—original draft preparation, R.R.; writing—review and editing, R.R., S.N., A.I., M.S., and H.A.M.; visualization, A.I.; supervision, A.I. All authors have read and agreed to the published version of the manuscript.
Funding
The Higher Education Commission (HEC) of Pakistan supported the research under the indigenous scholarship program, phase VI, funding number 520-162395-2BS6-206.
Data Availability Statement
The original contributions related to the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
I would like to express my special thanks to our mentor, Atia Iqbal, for the time and effort she provided throughout the year. Her useful advice and suggestions were helpful to me during my Ph.D. research work. Thank you to The Women University, Multan, Pakistan, for providing me with the platform for practical work/research work in labs. Thank you to the Higher Education Commission (HEC) of Pakistan, which supported the research under the indigenous scholarship program, phase VI.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seleiman, M.F.; Aslam, M.T.; Alhammad, B.A.; Hassan, M.U.; Maqbool, R.; Chattha, M.U.; Khan, I.; Gitari, H.I.; Uslu, O.S.; Roy, R. Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton (0031-9457) 2022, 91, 667–694. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R. The microbial consortium of indigenous rhizobacteria improving plant health, yield and nutrient content in wheat (Triticum aestivum). J. Plant Nutr. 2021, 44, 1942–1956. [Google Scholar] [CrossRef]
- Zahra, S.T.; Tariq, M.; Abdullah, M.; Azeem, F.; Ashraf, M.A. Dominance of Bacillus species in the wheat (Triticum aestivum L.) rhizosphere and their plant growth promoting potential under salt stress conditions. PeerJ 2023, 11, e14621. [Google Scholar] [CrossRef]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, C.; Feng, Q.; Liou, R.-M.; Lin, Y.-F.; Qiao, J.; Lu, Y.; Chang, Y. The mechanisms of sodium chloride stress mitigation by salt-tolerant plant growth promoting rhizobacteria in wheat. Agronomy 2022, 12, 543. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Jiang, Q.; Wang, R.; Wang, Z.; Mu, G.; Khan, S.A.; Khan, A.R.; Manghwar, H.; Wu, H. Salt tolerant Bacillus strains improve plant growth traits and regulation of phytohormones in wheat under salinity stress. Plants 2022, 11, 2769. [Google Scholar] [CrossRef]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant growth promotion using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef]
- Zhou, Y.; Sang, T.; Tian, M.; Jahan, M.S.; Wang, J.; Li, X.; Guo, S.; Liu, H.; Wang, Y.; Shu, S. Effects of Bacillus cereus on photosynthesis and antioxidant metabolism of cucumber seedlings under salt stress. Horticulturae 2022, 8, 463. [Google Scholar] [CrossRef]
- Jung, H.; Choi, S.; Kim, Y.; Han, J.A.; Lee, H.-S.; Kim, E.Y. Complete genome sequence of Bacillus paramobilis sp. strain IMGN7 from soil. Microbiol. Resour. Announc. 2024, 13, e00540-24. [Google Scholar] [CrossRef]
- July, E.; Gillis, A. Antiviral defence arsenal across members of the Bacillus cereus group. Sci. Rep. 2025, 15, 4958. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Santoyo, G.; Glick, B.R. Recent advances in the bacterial phytohormone modulation of plant growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Yin, Z.-y.; Yuan, Y.-c.; Zhang, R.; Gan, J.-t.; Yu, L.; Qiu, X.-h.; Chen, R.-p.; Wang, Q. Understanding Bacillus response to salt stress: Growth inhibition, enhanced EPS secretion, and molecular adaptation mechanisms. Process Biochem. 2024, 146, 412–422. [Google Scholar] [CrossRef]
- Roy, B.; Maitra, D.; Biswas, A.; Chowdhury, N.; Ganguly, S.; Bera, M.; Dutta, S.; Golder, S.; Roy, S.; Ghosh, J. Efficacy of high-altitude biofilm-forming novel Bacillus subtilis species as plant growth-promoting rhizobacteria on Zea mays L. Appl. Biochem. Biotechnol. 2024, 196, 643–666. [Google Scholar] [CrossRef]
- Perveen, R.; Hussain, A.; Ditta, A.; Dar, A.; Aimen, A.; Ahmad, M.; Alataway, A.; Dewidar, A.Z.; Mattar, M.A. Growth and yield of Okra exposed to a consortium of rhizobacteria with different organic carriers under controlled and natural field conditions. Horticulturae 2022, 9, 8. [Google Scholar] [CrossRef]
- Omara, A.E.-D.; Hafez, E.M.; Osman, H.S.; Rashwan, E.; El-Said, M.A.; Alharbi, K.; Abd El-Moneim, D.; Gowayed, S.M. Collaborative impact of compost and beneficial rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants 2022, 11, 877. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of Salt Stress on Growth, Physiological Parameters, and Ionic Concentration of Water Dropwort (Oenanthe javanica) Cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef]
- Noureen, S.; Iqbal, A.; Muqeet, H.A. Potential of drought tolerant Rhizobacteria amended with biochar on growth promotion in Wheat. Plants 2024, 13, 1183. [Google Scholar] [CrossRef]
- El Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Farooq, A.; Farooq, N.; Akbar, H.; Hassan, Z.U.; Gheewala, S.H. A critical review of climate change impact at a global scale on cereal crop production. Agronomy 2023, 13, 162. [Google Scholar] [CrossRef]
- Alamer, K.H. Alleviatory Role of Boron Supplementation on the Adverse Effects of Salinity Stress in Wheat. J. Plant Growth Regul. 2025, 44, 3179–3192. [Google Scholar] [CrossRef]
- Joshi, S.; Gangola, S.; Jaggi, V.; Sahgal, M. Functional characterization and molecular fingerprinting of potential phosphate solubilizing bacterial candidates from Shisham rhizosphere. Sci. Rep. 2023, 13, 7003. [Google Scholar] [CrossRef] [PubMed]
- Amri, M.; Rjeibi, M.R.; Gatrouni, M.; Mateus, D.M.; Asses, N.; Pinho, H.J.; Abbes, C. Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Tang, S.; Huang, S. Mitigation of salinity stress via improving growth, chlorophyll contents and antioxidants defense in sunflower with Bacillus pumilis and biochar. Sci. Rep. 2025, 15, 9641. [Google Scholar] [CrossRef] [PubMed]
- Haroon, U.; Khizar, M.; Liaquat, F.; Ali, M.; Akbar, M.; Tahir, K.; Batool, S.S.; Kamal, A.; Chaudhary, H.J.; Munis, M.F.H. Halotolerant plant growth-promoting rhizobacteria induce salinity tolerance in wheat by enhancing the expression of SOS genes. J. Plant Growth Regul. 2021, 41, 2435–2448. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; El Houda Rabhi, N.; Belbahri, L. Mitigation of NaCl stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Devi, S.; Sharma, S.; Tiwari, A.; Bhatt, A.K.; Singh, N.K.; Singh, M.; Kaushalendra; Kumar, A. Screening for multifarious plant growth promoting and biocontrol attributes in bacillus strains isolated from indo gangetic soil for enhancing growth of rice crops. Microorganisms 2023, 11, 1085. [Google Scholar] [CrossRef]
- Iqbal, M.; Naveed, M.; Sanaullah, M.; Brtnicky, M.; Hussain, M.I.; Kucerik, J.; Holatko, J.; Mustafa, A. Plant microbe mediated enhancement in growth and yield of canola (Brassica napus L.) plant through auxin production and increased nutrient acquisition. J. Soils Sediments 2023, 23, 1233–1249. [Google Scholar] [CrossRef]
- Jensen, C.N.G.; Pang, J.K.Y.; Gottardi, M.; Kračun, S.K.; Svendsen, B.A.; Nielsen, K.F.; Kovács, Á.T.; Moelbak, L.; Fimognari, L.; Husted, S. Bacillus subtilis promotes plant phosphorus (P) acquisition through P solubilization and stimulation of root and root hair growth. Physiol. Plant. 2024, 176, e14338. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting soil bacteria: Nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Balaji, L.; Chittoor, J.T.; Jayaraman, G. Optimization of extracellular lipase production by halotolerant Bacillus sp. VITL8 using factorial design and applicability of enzyme in pretreatment of food industry effluents. Prep. Biochem. Biotechnol. 2020, 50, 708–716. [Google Scholar] [CrossRef]
- Jabborova, D.P.; Narimanov, A.A.; Enakiev, Y.I.; Davranov, K.D. Effect of Bacillus subtilis 1 strain on the growth and development of wheat (Triticum aestivum L.) under saline condition. Bulg. J. Agric. Sci. 2020, 26, 744–747. [Google Scholar]
- Afzal, A.; Bahader, S.; Ul Hassan, T.; Naz, I.; Din, A.-u.-. Rock phosphate solubilization by plant growth-promoting Bacillus velezensis and its impact on wheat growth and yield. Geomicrobiol. J. 2023, 40, 131–142. [Google Scholar] [CrossRef]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-tolerant Bacillus species as a promising strategy to mitigate the salinity stress in wheat (Triticum turgidum subsp. durum). J. Arid. Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib; Woodrow, P.; Ciarmiello, L.F. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Gul, S.; Javed, S.; Azeem, M.; Aftab, A.; Anwaar, N.; Mehmood, T.; Zeshan, B. Application of Bacillus subtilis for the alleviation of salinity stress in different cultivars of Wheat (Tritium aestivum L.). Agronomy 2023, 13, 437. [Google Scholar] [CrossRef]
- Zapałowska, A.; Jarecki, W.; Skwiercz, A.; Malewski, T. Optimization of Compost and Peat Mixture Ratios for Production of Pepper Seedlings. Int. J. Mol. Sci. 2025, 26, 442. [Google Scholar] [CrossRef]
- Anđelković, J.; Mihajilov Krstev, T.; Dimkić, I.; Unković, N.; Stanković, D.; Joković, N. Growth-Promoting Effects of Ten Soil Bacterial Strains on Maize, Tomato, Cucumber, and Pepper Under Greenhouse Conditions. Plants 2025, 14, 1874. [Google Scholar] [CrossRef]
- Elbagory, M. Reducing the adverse effects of salt stress by utilizing compost tea and effective microorganisms to enhance the growth and yield of wheat (Triticum aestivum L.) plants. Agronomy 2023, 13, 823. [Google Scholar] [CrossRef]
- Sohaib, M.; Zahir, Z.A.; Khan, M.Y.; Ans, M.; Asghar, H.N.; Yasin, S.; Al-Barakah, F.N. Comparative evaluation of different carrier-based multi-strain bacterial formulations to mitigate the salt stress in wheat. Saudi J. Biol. Sci. 2020, 27, 777–787. [Google Scholar] [CrossRef]
- Yang, M.; Yang, R.; Li, Y.; Pan, Y.; Sun, J.; Zhang, Z. Effects of Different Peat Application Methods on Water and Salt Migration in a Coastal Saline Soil. J. Soil. Sci. Plant Nutr. 2022, 22, 791–800. [Google Scholar] [CrossRef]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Iqbal, A.; Ahmed, F.; Ahmad, M. Phytobeneficial and salt stress mitigating efficacy of IAA producing salt tolerant strains in Gossypium hirsutum. Saudi J. Biol. Sci. 2021, 28, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinate Bacteriology; Williams & Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Acuña, J.J.; Rilling, J.I.; Inostroza, N.G.; Zhang, Q.; Wick, L.Y.; Sessitsch, A.; Jorquera, M.A. Variovorax sp. strain P1R9 applied individually or as part of bacterial consortia enhances wheat germination under salt stress conditions. Sci. Rep. 2024, 14, 2070. [Google Scholar] [CrossRef]
- Rico-Jiménez, M.; Muñoz-Mira, S.; Lomas-Martínez, C.; Krell, T.; Matilla, M.A. Regulation of indole-3-acetic acid biosynthesis and consequences of auxin production deficiency in Serratia plymuthica. Microb. Biotechnol. 2023, 16, 1671–1689. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.-l.; Zhou, J.; Zhou, W.; Zhou, S.-Q. Construction of phosphate-solubilizing microbial consortium and its effect on the remediation of saline-alkali soil. Microb. Ecol. 2025, 88, 11. [Google Scholar] [CrossRef]
- Leal, M.; Tovar, D.; Infante, A.; Barriga, O.; Ruíz, E.; Sánchez, J.; Melgarejo, L. Phosphate solubilization by microorganisms in pyroclastic material from Half Moon Island in Antarctica: Perspectives for astrobiology. Polar Biol. 2025, 48, 29. [Google Scholar] [CrossRef]
- Shomi, F.Y.; Uddin, M.B.; Zerin, T. Isolation and characterization of nitrogen-fixing bacteria from soil sample in Dhaka, Bangladesh. Stamford J. Microbiol. 2021, 11, 11–13. [Google Scholar] [CrossRef]
- Batool, S.; Iqbal, A. Phosphate solubilizing rhizobacteria as alternative of chemical fertilizer for growth and yield of Triticum aestivum (Var. Galaxy 2013). Saudi J. Biol. Sci. 2019, 26, 1400–1410. [Google Scholar] [CrossRef]
- Chaiharn, M.; Chunhaleuchanon, S.; Kozo, A.; Lumyong, S. Screening of rhizobacteria for their plant growth promoting activities. Curr. Appl. Sci. Technol. 2008, 8, 18–23. [Google Scholar]
- Thangamanil, P.; Thiruvengadam, R.; Thillaigovindan, K. Morphological characterization and reaction of partial purified toxin of sugarcane red rot pathogen Colletotrichum falcatum collected from Southern India. Int. J. Agric. Sci. 2013, 3, 60–78. [Google Scholar]
- Patil, P.S.; Kotia, P.; Azad, A.; Gedam, S.; Bansal, D.; Tyson, K. In vivo study to evaluate of human breast milk, infant milk formula on cariogenicity in children: A comparative study. Cuest. Fisioter. 2025, 54, 1246–1251. [Google Scholar]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; Van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Bocianowski, J.; Jamshidi, B.; Turkoglu, A. Physiological and molecular responses of wild relatives of wheat possessing the D genome to salinity stress. Genet. Resour. Crop Evol. 2025, 72, 1819–1834. [Google Scholar] [CrossRef]
- Nadeem, M.; Shahbaz, M.; Ahmad, F.; Waraich, E.A. Enhancing wheat resistance to salinity: The role of gibberellic acid and β-Carotene in morphological, yielding and ionic adaptations. J. Ecol. Eng. 2025, 26, 76–94. [Google Scholar] [CrossRef]
- Ansari, F.; Jabeen, M.; Ahmad, I. Pseudomonas azotoformans FAP5, a novel biofilm-forming PGPR strain, alleviates drought stress in wheat plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]
- Morgvan, C.M.; Petrehele, A.I.G.; Fodor, A.; Cipleu, M. Influence of Manganese Ions of Chlorophyl Concentration from Wheat Grass. Fasc. Chim. 2020, XXVII, 32. [Google Scholar]
- Saeed, R.; Mirza, S.; Ahmad, R. Electrolyte leakage and relative water content as affected by organic mulch in okra plant (Abelmoschus esculentus (L.) Moench) grown under salinity. FUUAST J. Biol. 2014, 4, 221. [Google Scholar]
- Bates, L.S.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Afzal, I.; Akram, M.; Rehman, H.; Rashid, S.; Basra, S. Moringa leaf and sorghum water extracts and salicylic acid to alleviate impacts of heat stress in wheat. S. Afr. J. Bot. 2020, 129, 169–174. [Google Scholar] [CrossRef]
- Mustafa, N.; Raja, N.I.; Ilyas, N.; Abasi, F.; Ahmad, M.S.; Ehsan, M.; Mehak, A.; Badshah, I.; Proćków, J. Exogenous application of green titanium dioxide nanoparticles (TiO2 NPs) to improve the germination, physiochemical, and yield parameters of wheat plants under salinity stress. Molecules 2022, 27, 4884. [Google Scholar] [CrossRef]
- İbrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Zivcak, M.; Tahjib-Ul-Arif, M.; Hussain, S.; Abdelhamid, M. Progress in understanding salt stress response in plants using biotechnological tools. J. Biotechnol. 2021, 329, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L.) varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).