Abstract
This research examines a detailed metabolomic and comparative analysis of bioactive substances of soybean varieties: “Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, and “Musson” by the laser confocal microscope CLSM 800 and the mass spectrometry of bioactive compounds by tandem mass spectrometry. The laser microscopy allowed us to clarify in detail the spatial arrangement of phenolic acids, flavonols, and anthocyanin contents in soybeans. Research has convincingly shown that the polyphenolic content of soybeans, and, in particular, the anthocyanins, are spatially localized mainly in the seed coat of soybeans. Tandem mass spectrometry was used to identify chemical constituents in soybean extracts. The results of initial studies revealed the presence of one hundred and fourteen compounds; sixty-nine of the target analytes were tentatively identified as compounds from polyphenol groups.
1. Introduction
Currently, the nutritional qualities of agricultural crops have received much more attention in terms of the quality of life and health of potential consumers [1]. Antioxidant activity has been widely discussed regarding the nutritional value of various crops as it plays a crucial role in the prevention of several chronic diseases [2]. Antioxidant activity is largely determined by the type and content of different compounds of the polyphenol group, such as anthocyanins, tannins, flavonoids, etc. [3,4]. The study of phytochemical antioxidants can help improve the nutritional properties of crops to meet human health needs. Long-term crop breeding for high-yielding traits has significantly reduced the diversity of genes associated with nutritional quality [5]. Soybeans, a staple food worldwide, are not only a valuable source of oil and protein but are also rich in health-promoting polyphenolic compounds and soya saponins [6]. Isoflavonoids and soy saponins are well-known phytochemicals in soybeans with a wide range of biological activities against oxidative stress-related disorders [7]. Taken together, soybean is a good model crop to study the diversity of functional antioxidants and identify genes associated with chemical synthesis and decoration. During soybean domestication, one of the most obvious changes is the difference in seed coat pigmentation [8]. Genes and transcription factors associated with anthocyanin biosynthesis contributed to the formation of pigmented seed coats in soybean [9]. These data suggest that pigmented wild soybeans have a high metabolic diversity of polyphenolic anthocyanin precursors and products. Anthocyanins are a well-studied class of flavonoids [10]. Flavonoids are a large class of polyphenols and can be subdivided into flavones, flavonols, flavanones, flavanols, chalcones, aurones, isoflavones, anthocyanidins, etc. [11]. The multiple phenolic hydroxyl groups in the backbone of flavonoids contribute to their potent antioxidant activity [12]. In addition to the well-studied isoflavones and anthocyanidins, the emerging characterization of the effects of domestication of other flavonoid subclasses can facilitate the use of more polyphenolic antioxidants in both the food and dietary supplement industries. Natural chemical modifications such as glycosylation and acylation alter the polarity, solubility, stability, bioavailability, and biological activity of polyphenolic antioxidants [13,14]. Due to the significant impact of modifications on the functional properties of polyphenolic antioxidants, it is important to characterize modifications of polyphenols to achieve functional improvement in various soy food products.
It should also be noted that soybean Glycine max (L.) Merr. and soybean-based foods are the main natural sources of saponins in dietary and functional nutrition [15,16]. Soy saponins belong to the group of triterpene glycosides and are divided into three main groups based on the differences in the substitution of the C-22 and C-23 positions of the aglycone (or soyasapogenol): group A, B, and E soy saponins. Group A soy saponins have a glycosyl chain attached to the C-3 and C-22 positions of the aglycone. Group B soy saponins have only one glycosyl chain (attached to the C-3 position) and can be conjugated with 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) at the C-22 position [17]. The E group of soybean saponins is the least abundant and is considered to be photooxidation products of the B group of soybean saponins [18]. In soybeans, the A group of soybean saponins is considered to be fully acetylated [18,19]. Soybean saponins are important bioactive components with significant beneficial effects on human health. The biological effects of soybean saponins have been widely described and include hepatoprotective, antitumor, immunostimulatory, antiviral, and hypocholesterolemic activities [20,21]. The soybean saponin group is considered to be responsible for the astringent and bitter taste of soybean foods, mainly due to the presence of acetyl groups [22]. The exact mechanisms underlying the biological properties of soybean saponins remain to be elucidated due to the lack of purified test compounds and limited information on the content and composition of soybean saponins in soybeans and soybean products. The quantitative determination of individual soybean saponins has always been a difficult task, partly due to the difficulties in isolating authentic standards and the structural complexity of this group of phytochemicals. The covalent bonds linking the acetyl groups, and especially the DDMP groups, to the saponin molecule are relatively weak, even under relatively mild extraction conditions, making it difficult to obtain saponins in their native form [23].
In addition, new advanced research methods are becoming more widespread, such as laser microscopy, a method that exploits the ability of chemicals to fluoresce when excited by a laser. This can be used to solve various visualization problems. Previous autofluorescence-based microscopic studies of soybean plants have focused more on the visualization of anatomical features: the three-dimensional (3D) internal structure of a soybean seed [24] and leaf anatomy of Glycine max (L.) Merr. [25]. We propose the autofluorescence-based study of the spatial distribution of some groups of phytochemicals in the seed tissue using confocal laser microscopy.
This study represents a comparative analysis of eight soybean varieties cultivated in the N.I. Vavilov All-Russian Institute of Plant Genetic Resources and the A.K. Chaika Federal Scientific Centre of Agrobiotechnologies of the Far East. A detailed metabolomic analysis was carried out using tandem mass spectrometry.
Detailed metabolomic and comparative analyses of bioactive substances of soybean varieties were carried out: “Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, and “Musson”, cultivated in the Federal Scientific Centre of Agrobiotechnologies of the Far East named by A.K. Chaika and in N.I. Vavilov All-Russian Institute of Plant Genetic Resources by means of the laser confocal microscope CLSM 800 and the mass spectrometry of bioactive compounds by ion trap amaZon SL.
2. Materials and Methods
2.1. Plant Material
Eight soybean varieties were evaluated. The varieties (“Primorskaya 4”, “Primorskaya 86”, “Primorskaya 96”, “Locus”, “Sphere”, “Breeze”, “Namul”, “Musson”) were collected and grown in the Far East Federal Scientific Centre of Agrobiotechnologies named after A.K. Chaika, following standard agronomic practices. The soybeans were harvested at the end of September 2022. Triplicate (250 g each) samples were taken for each variety. Only completely healthy seed samples were considered for further analysis. Samples were washed with distilled water, dried at room temperature, and stored at −80 °C until processing. All samples conformed morphologically to the pharmacopoeial standards of the State Pharmacopoeia of the Russian Federation [26].
2.2. Chemicals and Reagents and Fractional Maceration
Analytical grade reagents and ultrapure water were used for liquid chromatography (LC) and mass spectrometry (MS).
Highly concentrated extracts were prepared using fractional maceration, as reported earlier [27]. For each replicate of the varieties, 50 g of seeds were macerated and extracted with ethanol (95%). Infusions were prepared as per the methods described in our earlier report [27]. Moreover, the extraction was carried out in triplicate, followed by filtering through a Whatman filter paper. Finally, we used acetonitrile for preparing the final working concentration for LC and MS analyses.
2.3. Liquid Chromatography and Mass Spectrometry
Mass spectrometry analysis was performed on an ion trap amaZon SL (BRUKER DALTONIKS, Bremen, Germany) equipped with an ESI source in positive or/and negative ion modes. The optimized parameters were obtained as follows: ionization source temperature: 70 °C, gas flow: 4 L/min, nebulizer gas (atomizer): 7.3 psi, capillary voltage: 4500 V, end plate bend voltage: 1500 V, fragmentary: 280 V, collision energy: 60 eV. An ion trap was used in the scan range of m/z 100–1.700 for MS and MS/MS. The chemical constituents were identified by comparing their retention index, mass spectra, and MS fragmentation with an in-house self-built database (Biotechnology, Bioengineering and Food Systems Laboratory, Far-Eastern Federal University, Vladivostok, Russia). The in-house self-built database was based on data from other spectroscopic techniques, such as nuclear magnetic resonance, ultraviolet spectroscopy, and MS, as well as data from the literature, which is continuously updated and revised. The capture rate was one spectrum for MS and two spectra for MS/MS. Data acquisition was controlled by Windows software for BRUKER DALTONIKS. All experiments were repeated three times. A four-stage ion separation mode (MS/MS mode) was implemented.
2.4. Optical Microscopy
Optical microscopy was carried out according to the method described earlier [27]. Briefly, soybean seeds were dissected, their autofluorescence parameters were determined using the laser confocal microscope CLSM 800 (Zeiss, Germany), and fluorescence maxima were registered by excitation with violet and blue lasers at respective emission ranges. After the excitation, the images were taken using ZEN 2.1 software (Carl Zeiss Microscopy GmbH, Jena, Germany).
2.5. Statistical Analysis
The upset plot was prepared using an online tool ChiPlot (https://www.chiplot.online/; accessed on 1 December 2024). Principal component analysis, based on covariance, was carried out online at Statistics Kingdom (https://www.statskingdom.com/pca-calculator.html; accessed on 1 December 2024). The Jaccard index was computed as reported earlier [28].
3. Results
3.1. Optical Microscopy of Soybean Components
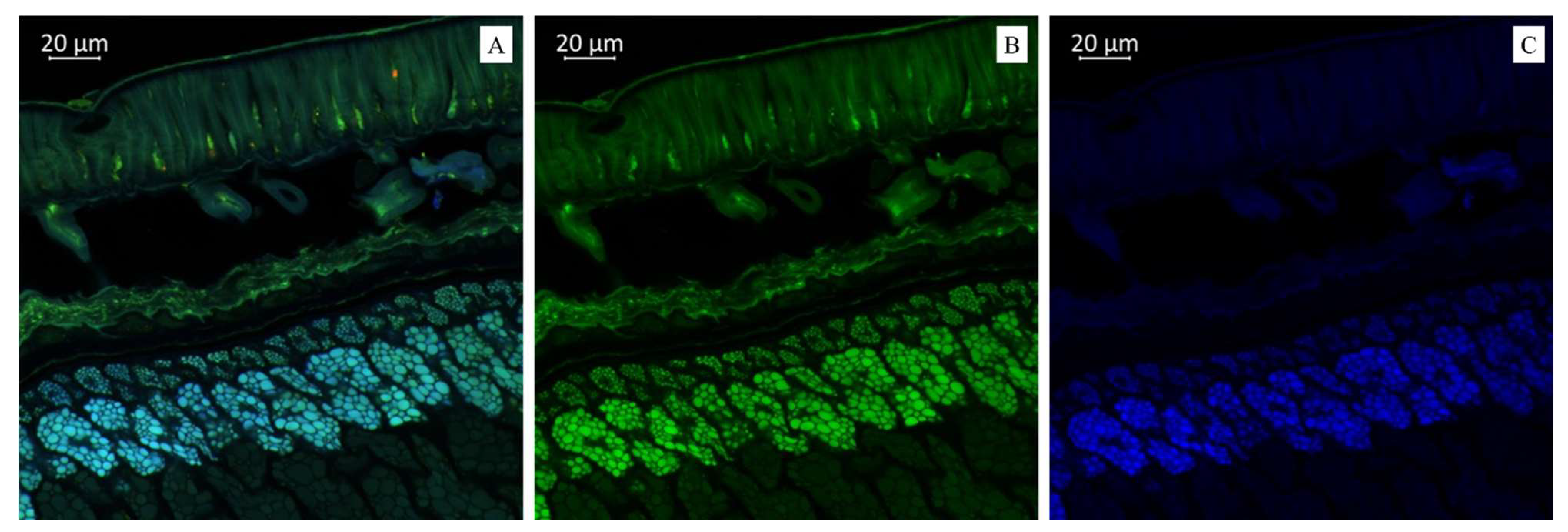
Imaging the distribution of chemical constituents in soybeans by optical microscopy requires prior knowledge of the spectra of pure soybean constituents. Different biochemical substances can be visualized differently under microscopy according to the autofluorescence. Our results showed that the transverse sections of soybean seeds were highly fluorescent under the laser confocal microscope, indicating that several substances with autofluorescence were present in the observed varieties (Figure 1, Figure 2 and Figure 3). We propose that the blue fluorescence in the observed soybean varieties’ seeds was due to the presence of phenolic compounds such as hydroxycinnamic acids [29]. Within this class of compounds, ferulic acid was the major contributor to the blue fluorescence; however, other compounds in this class, such as p-coumaric and caffeic acids, have also been associated with such fluorescence [30]. Other than hydroxycinnamic acids, lignin has also been associated with blue fluorescence in plant tissues [31]; however, the observed blue fluorescence is mostly due to hydroxycinnamic acids. This is because of the fact that legume seed coats generally contain low lignin contents [32,33]. However, this is not strictly associated with seed coats as their cotyledons are also poorly lignified [34]. On the other hand, the soybean seeds were rich in secondary metabolites such as flavonoids, alkaloids, phenols, and others. However, flavonols were characterized by green rather than blue autofluorescence [35,36], as noted in our results when the soybean samples were excited with 500 to 545 nm. Finally, we also noted the red fluorescence (Figure 2), which was associated with anthocyanins and anthocyanidins [37,38]. These results were further confirmed by MS, as presented in the next sections (Table 1 and Table 2). Fluorescent flavonoids or their oxidation products, e.g., Lignum nephriticum (matlaline), have long been reported [39]. However, they also emit yellow and orange autofluorescence. Considering their importance in the health industry as well as for plants’ resistance to biotic and abiotic stresses, our results are important for their detection. Particularly in plants, they play an important role in auxin transport, root and shoot development, the control of reactive oxygen species, pollination, symbiotic nodule formation, and acting as protective compounds [40]. Together with hydroxycinnamic acids and other weakly autofluorescent phenolic substances, flavonoids are responsible for the fluorescence of the leaf epidermis [41,42]. Therefore, consistent with our earlier work on three Glycine species [27] and other reports on, e.g., paprika [36] and Arabidopsis [43,44], the utility of optical microscopy in the detection and distribution of these compounds in different plant tissues is valuable.
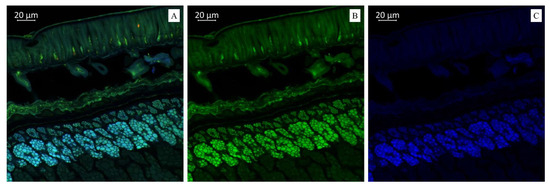
Figure 1.
(A). Multispectral image of a cross section of soybean variety “Breeze” (Russia), presented in all measured spectra. Excitation at 405 nm with emission in the range of 400–475 nm (blue); excitation at 488 nm with emission in the range of 500–545 nm (green) and 620–700 nm (red). (B). Presence of flavonols (green color). (C). Presence of hydroxycinnamic acids (blue color).
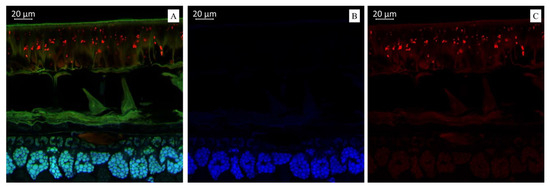
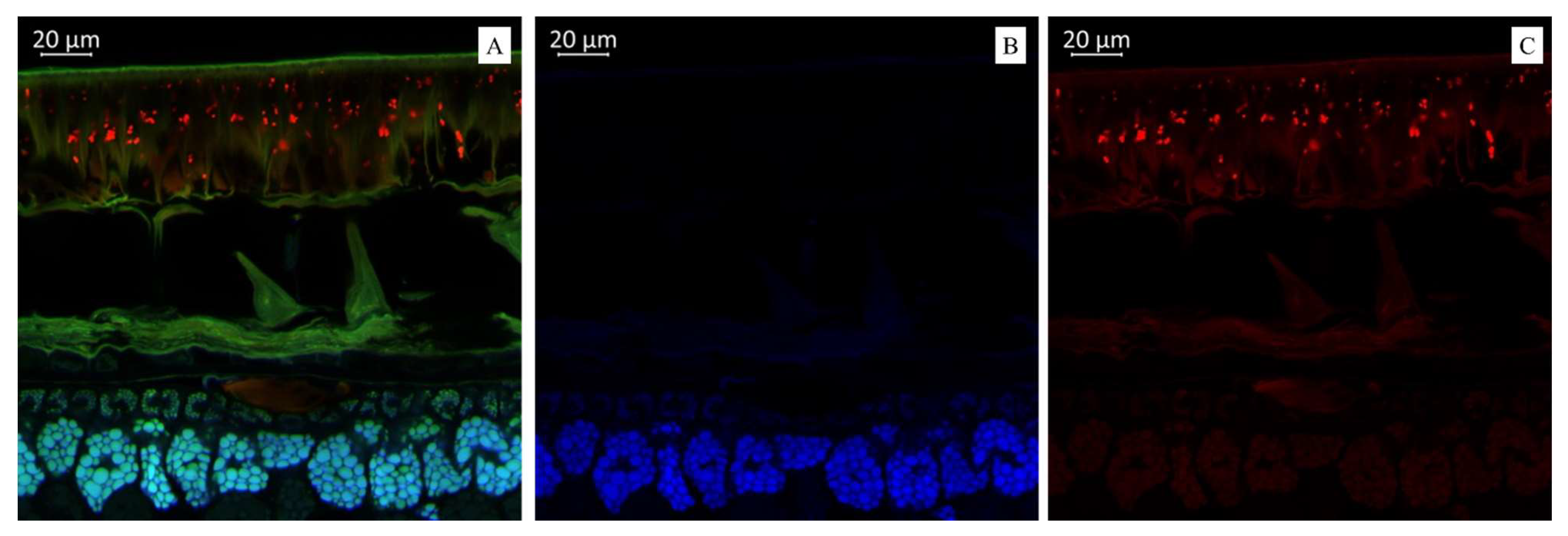
Figure 2.
(A). Multispectral image of transverse section of the soybean variety “Locus” (Russia), presented in all measured spectra. Excitation at 405 nm with the emission in the range of 400–475 nm (blue); excitation at 488 nm with the emission in the range of 500–545 nm (green) and 620–700 nm (red). (B). Presence of hydroxycinnamic acids (blue color). (C). Presence of anthocyanin content (red color).
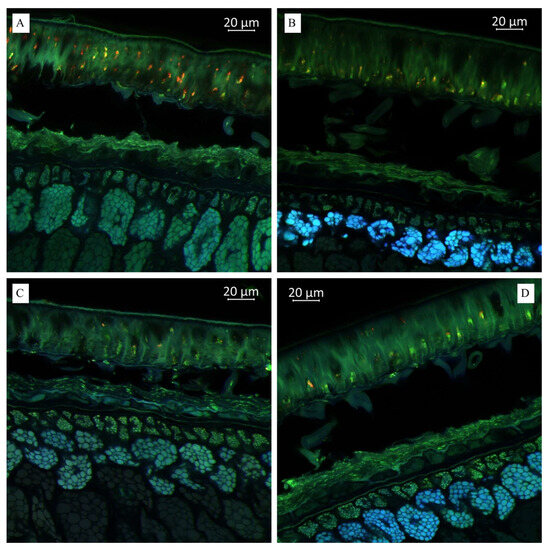
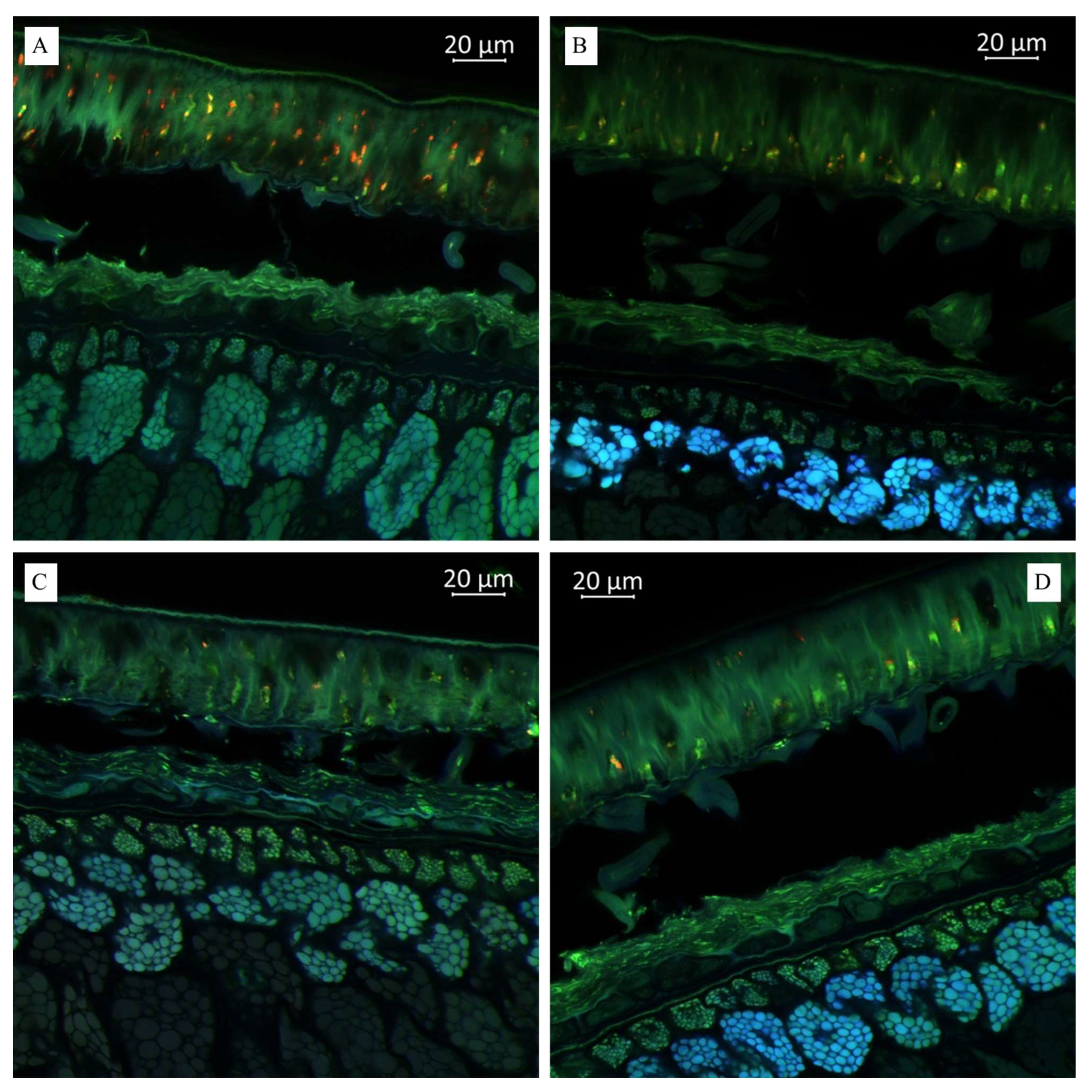
Figure 3.
(A). Multispectral image of transverse section of the soybean variety “Musson” (Russia), presented in all measured spectra. Excitation at 405 nm with the emission in the range of 400–475 nm (blue); excitation at 488 nm with the emission in the range of 500–545 nm (green) and 620–700 nm (red). (B). Multispectral image of transverse section of the soybean variety “Namul” (Russia), presented in all measured spectra. (C). Multispectral image of transverse section of the soybean variety “Primorskaya 4” (Russia). (D). Multispectral image of transverse section of the soybean variety “Primorskaya 86” (Russia).

Table 1.
Jaccard index for eight soybean varieties of the polyphenol group (“Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, “Musson”).

Table 2.
The occurrence of identified chemical substances in the studied soybean varieties (“Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, “Musson”).
Figure 2A illustrates a multispectral image of the transverse section of the soybean variety “Locus” (Russia), displayed across all measured spectra. Figure 2B illustrates a spectral image in a blue color that indicates the presence of hydroxycinnamic acids in the soybean variety “Locus” (Russia). The spectral image in the red color indicates the presence of anthocyanin content in the soybean variety “Locus” (Russia) (Figure 2C). The microscopic analysis showed that the seed coat of this black-seeded variety had the brightest red fluorescence. Interestingly, it has been previously reported that the black color of the seed coat in legumes is due to the accumulation of anthocyanins [45]. This confirms that bright red fluorescence is caused by such compounds.
As shown in Figure 3B, we observed a much more pronounced presence of hydroxycinnamic acids in the “Namul” soybean variety than in the “Musson” soybean variety. A lighter blue fluorescence with more pronounced green, as shown in Figure 3C, indicated relatively lower hydroxycinnamic acids and lignin in “Primorskaya-4” compared with “Namul” and “Primorskaya 86” (Figure 3C). Thus, the autofluorescence enabled us to predict and determine their distribution across different sections of the observed tissue.
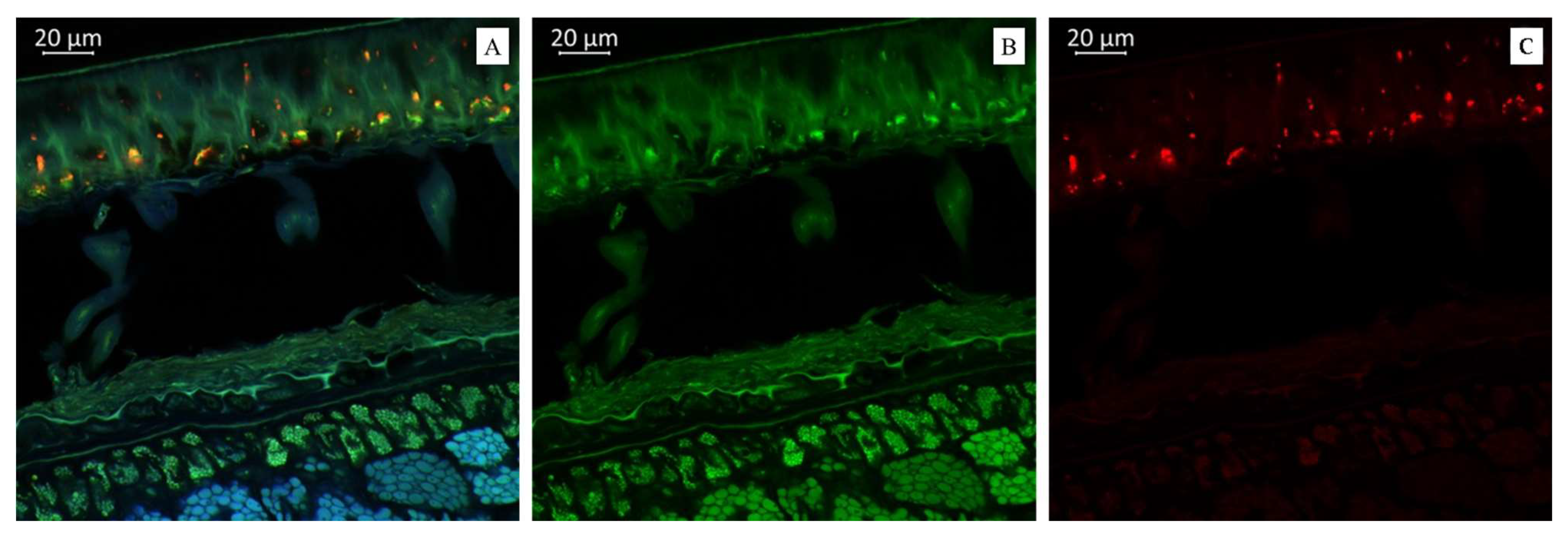
Figure 4A demonstrates that the soybean variety “Sphere” was much richer in anthocyanin content than the soybean varieties “Primorskaya 4” and “Primorskaya-86”. Figure 4B represents a spectral image in a green color that indicates the presence of flavonols in the soybean variety “Sphere”. Figure 4C represents a spectral image in a red color that indicates the presence of anthocyanin content in the soybean variety “Sphere”.
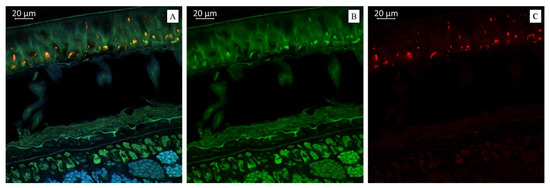
Figure 4.
(A). Multispectral image of transverse section of the soybean variety “Sphere” (Russia), presented in all measured spectra. Excitation at 405 nm with the emission in the range of 400–475 nm (blue); excitation at 488 nm with the emission in the range of 500–545 nm (green) and 620–700 nm (red). (B). Presence of flavonols. (C). Presence of anthocyanin content in the soybean variety “Sphere” (Russia).
3.2. Tandem Mass Spectrometry Analysis
We further analyzed the soybean seed extracts by tandem mass spectrometry to better capture the diversity of phytochemicals. Our results revealed that all the studied soybean varieties were rich in bioactive compounds; sixty-nine polyphenolic compounds were tentatively identified and characterized by comparing fragmentation patterns and retention times. The chemical constituents were identified by comparing their retention indices, mass spectra, and MS fragmentation with an in-house self-built database (Biotechnology, Bioengineering and Food Systems Laboratory, Far-Eastern Federal University, Russia). The in-house self-built database was based on data from other spectroscopic techniques, such as nuclear magnetic resonance, ultraviolet spectroscopy, and MS, as well as data from the literature, which is continuously updated and revised. The capture rate was one spectrum for MS and two spectra for MS/MS. Data acquisition was controlled by Windows software for BRUKER DALTONIKS. All experiments were repeated three times. A four-stage ion separation mode (MS/MS mode) was implemented.
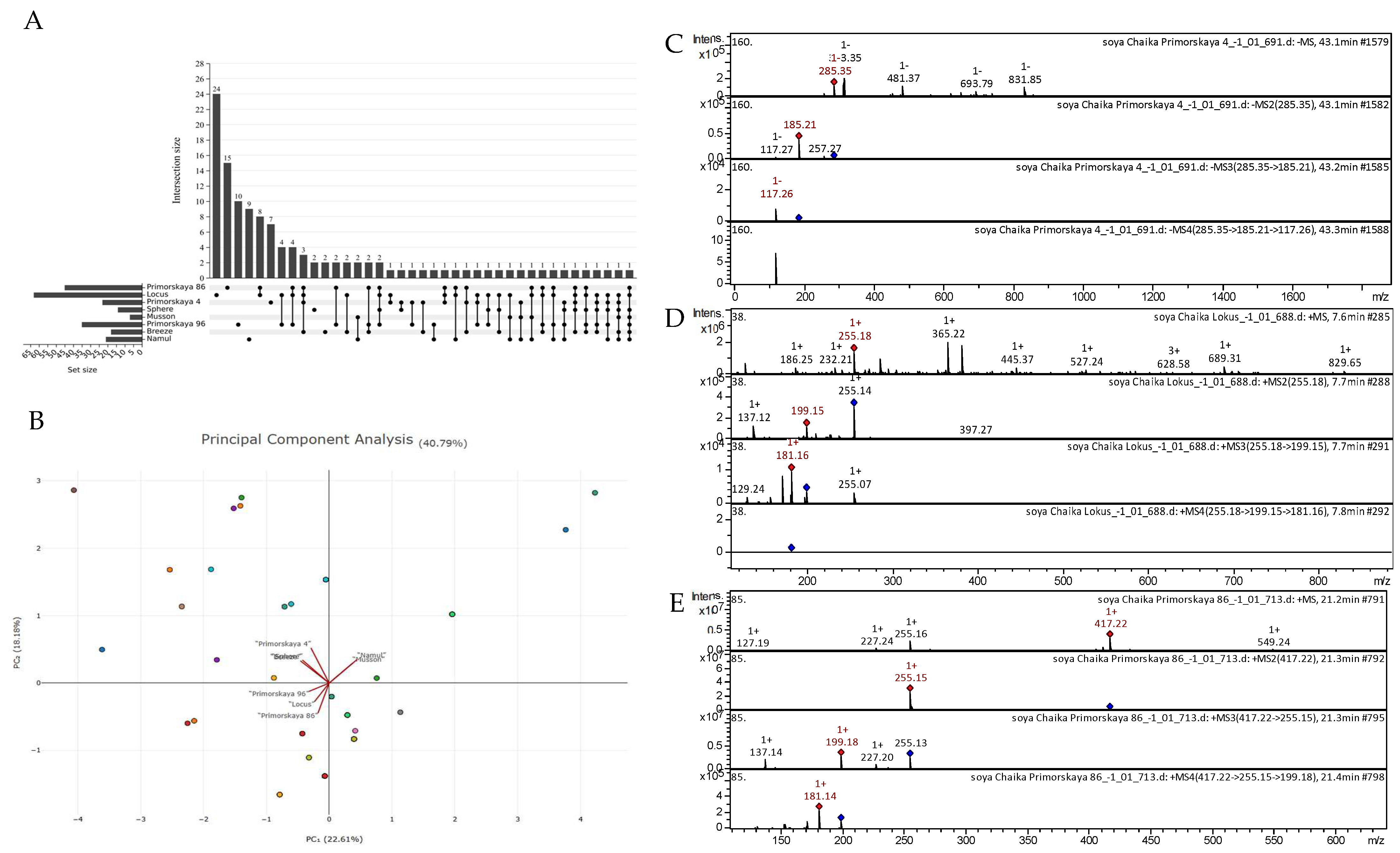
All the tentatively identified compounds along with molecular formulas, m/z calculated and observed, MS/MS data, and their comparative profiles for soybeans (eight varieties) are summarized in Appendix A, Table A1. Overall, one hundred and fourteen compounds belonging to different compound classes were detected from the eight soybean varieties. There were no commonly detected metabolites between the eight soybean varieties (Figure 5A). Principal component analysis indicated that the “Namul” and “Musson” varieties were quite similar, whereas “Primorskaya-4”, “Sphere”, and “Breeze” were grouped closer to each other. Similarly, “Primorskaya-86”, “Primorskaya-96”, and “Locus” were grouped closer to each other (Figure 5B). Several tentatively identified CID spectra (collision-induced spectrum) of chemical compounds in the soybean varieties “Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, and “Musson” are presented below (Figure 5C–E).
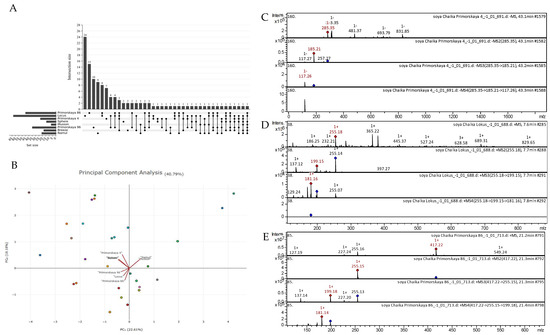
Figure 5.
(A) Upset plot showing similarities and differences in the presence of polyphenol group in soybean varieties. (B) Principal component analysis based on the presence/absence of the detected metabolites in studied soybean varieties. (C) CID spectrum of kaempferol from extracts of soybean variety “Primorskaya-4”, m/z 285.35. At the top is an MS scan in the range of 100–1700 m/z; at the bottom are fragmentation spectra (from top to bottom): MS2 of the protonated kaempferol ion (285.35 m/z, red diamond), MS3 of the fragment 285.35→185.21 m/z, and MS4 of the fragment 285.35→185.21 →117.26 m/z. (D) CID spectrum of daidzein from extracts of soybean variety “Locus”, m/z 255.18. At the top is an MS scan in the range of 100–1700 m/z; at the bottom are fragmentation spectra (from top to bottom): MS2 of the protonated daidzein ion (255.18 m/z, red diamond), MS3 of the fragment 255.18→199.15 m/z, and MS4 of the fragment 255.18→199.15 →181.16 m/z. (E) CID spectrum of daidzin from extracts of soybean variety “Primorskaya-86”, m/z 417.22. At the top is an MS scan in the range of 100–1700 m/z; at the bottom are fragmentation spectra (from top to bottom): MS2 of the protonated daidzin ion (417.22 m/z, red diamond), MS3 of the fragment 417.22→255.15 m/z, and MS4 of the fragment 417.22→255.15 →199.18 m/z.
The highest number of tentatively identified compounds from the polifenolic class were classified as flavones (32), followed by phenolic acids (10), flavonols (9), flavan-3-ols (5), anthocyanidins (4), lignans (4), condensed tannins (2), coumarins (2), dihydrochalcone, and a stilbene. Moreover, fifty chemical compounds of other classes were identified, some of which were identified for the first time, e.g., 9,10-dihydroxy-8-oxooctadec-12-enoic acid and 13-trihydroxy-octadecenoic acid and the compound sterol class desmosterol. Among the studied soybean varieties, “Locus” contained the richest polyphenolic content. Next, “Primorskaya-86” was the second richest in polyphenols, with twenty-eight compounds (Figure 5A). Under the same experimental conditions, we could identify only nine polyphenolic compounds from the “Sphere” variety. Among the identified polyphenolic compounds in the studied soybean varieties, seventeen (flavones, flavonols, anthocyanins, phenolic acids, etc.) were commonly found in all soybean varieties. Principal component analysis indicated that there was 22.61% and 18.18% variability, as displayed by principal components 1 and 2, respectively (Figure 5B). Generally, we observed that “Namul” and “Musson” were grouped together. “Primorskaya-4”, “Sphere”, and “Breeze” were grouped together. The varieties “Primorskaya-96”, “Locus”, and “Primorskaya-86” were grouped together, indicating that they possibly have similar polyphenol compositions.
Figure 5C–E show examples of the decoding spectra (collision-induced dissociation (CID) spectrum) of the ion chromatogram obtained using tandem MS. The mass spectrum in negative ion mode of kaempferol from extracts of the soybean variety “Primorskaya 4” is shown in Figure 5C. The [M − H]− ion produced three fragment ions at m/z 257.27, m/z 185.21, and m/z 117.27 (Figure 5C). The fragment ion with m/z 185.21 yielded one daughter ion at m/z 117.26. Mass spectrometry of kaempferol is presented in detail in scientific studies on Juglans mandshurica [46], Polygala sibirica [47], Rhus coriaria [48], Lonicera japonica [49], Ribes meyeri [50], andean blueberry [51], potato [52], and potato leaves [53].
The mass spectrum in positive ion mode of daidzein from extracts of the soybean variety “Locus” is shown in Figure 5D. The [M + H]+ ion produced two fragment ions at m/z 199.15 and m/z 137.12 (Figure 5D). The fragment ion with m/z 199.15 yielded two daughter ions at m/z 181.16, and m/z 129.24. Mass spectrometry of daidzein is presented in detail in scientific studies on black soya [54], soybean [55], Hedyotis diffusa [56], and Loropetalum chinense [57].
The mass spectrum in positive ion mode of daidzin from extracts of the soybean variety “Primorskaya-86” is shown in Figure 5E. The [M + H]+ ion produced one fragment ion at m/z 255.15 (Figure 5E). The fragment ion with m/z 255.15 yielded three daughter ions at m/z 199.18, m/z 227.20, and m/z 137.14. This bioactive substance was identified in mass spectrometric studies of extracts of black soya [54] and Malus toringoides [58].
Also, to present the similarities and differences in bioactive substances in different varieties of soybeans, we used the Jaccard index. Table 1 below presents the Jaccard index calculated for the sum of chemical compounds present in the soybean varieties.
Table 2 below presents the occurrence of identified chemical substances in the studied soybean varieties (“Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, “Musson”).
4. Discussion
Soybean is an important legume and oil crop in the world because of the provision of food, feed, and industrial products and co-products. Soybean seeds are rich in secondary metabolites such as flavonoids, isoflavones, saponins, amino acids and their derivatives, anthocyanins, phenolic acids, hydroxycinnamic acids, lignans and coumarins, nucleic acids and their derivatives, carbohydrates, and lipids [59,60,61]. Due to the presence of such a diverse range of secondary metabolites, research on their beneficial health effects has significantly improved our understanding of their utility as functional foods [62]. With the increasing number of soybean varieties suitable for the specific agricultural zones of Russia, it is important to understand the secondary metabolite composition of the varieties so that consumers are well informed. The detection of these compounds by traditional biochemical techniques, such as HPLC and MS, should also be supplemented with affordable and reliable techniques that can provide quick and basic knowledge about the possible secondary metabolite composition of soybean seeds. In this regard, our results obtained in this study highly suggest that optical microscopy followed by the use of HPLC and MS are useful for both local soybean breeders, farmers, industrialists, and consumers.
Molecules in living tissues of plant matrices will produce fluorescent radiation when excited by appropriate wavelengths. This has been successfully used in the development of spectral markers to understand the metabolic composition of important crops [63]. Some researchers use this technique to understand whether plant tissues are under biotic stress by imaging the increase or decrease of specific metabolites, such as lignin, during infection by Pythium ultimum in apple roots [64]. Our results in soybean are quite useful for the development of fluorescent markers to understand the metabolomic composition of seeds. In particular, the results that we detected hydroxycinnamic acids and lignin (blue fluorescence), flavonols and their derivatives (green fluorescence), anthocyanins, anthocyanidins (red fluorescence), kaempferol, and quercetin (Figure 1, Figure 2 and Figure 3; Table 1). These results are based on previous findings that phenolic hydroxycinnamic acids such as hydroxycinnamic acids (e.g., p-coumaric and caffeic acids) are responsible for the fluorescence [29,30]. Similarly, the fact that lignin produces blue fluorescence in plant tissues [31] suggests that the fluorescence microscopy of soybean seeds can be used as a kind of marker to quickly estimate the possible lignin content in seeds. The presence of different lignin contents can also be revealed by such markers, even if the seed lignin content is low [32,33,34]. Soybeans are mainly consumed because of their high flavonoid content, including isoflavonoids, flavonols, flavanols, and others. Therefore, our microscopic results can become a useful tool in soybean-related industries to detect the estimates of these secondary metabolites [35,36]. In addition to these major metabolites in soy, some soybean varieties are rich in pigment compounds such as anthocyanins or anthocyanidins, which are also associated with a number of health benefits. The red spectrum due to these metabolites may therefore be further developed as a useful detection strategy [37,38].
Nevertheless, for the detailed metabolomic composition of secondary metabolites in soybean seeds, techniques such as HPLC and tandem MS are still preferred.
Many publications have shown that the structures of polyphenolic compounds correlate with their bioactivity [65,66]. Therefore, it is of utmost importance to correctly identify the molecular structures using a time-saving method such as mass spectrometry. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) has been widely used for the structural characterization of both flavonoids and other chemical compounds, including the characterization of the aglycone structure and glycan sequence [67,68,69]. Flavonoids have been extensively analyzed for isomeric differentiation and structural characterization using ESI-MS/MS methods in positive and/or negative ion modes [70,71,72]. Electrospray ionization (ESI) coupled with tandem mass spectrometry (MS/MS) has proven to be a valuable method with high sensitivity and high resolution. So far, there have been many reports on the fragmentation characteristics of flavonoid group isomers using tandem mass spectrometry [73]. Rich structural information can be obtained by collision induced dissociation (CID) during MS/MS analysis. The elemental composition of both precursor and product ions can be obtained from high-resolution mass spectrometry (HRMS/MS) analysis. Thus, the product ion structures obtained from MS/MS experiments can be confirmed.
Our results that the studied Russian soybean varieties contained one hundred and fourteen different secondary metabolites indicate their richness and potential use as functional foods. Although the number of detected compounds in the studied varieties was lower than that reported for several Chinese soybean varieties [74], our results are still valuable for local soybean breeders to further improve these varieties by manipulating specific genes/pathways. Due to differences in their genetic background, the environment in which they are grown, and how the seeds are handled after harvest, the observed variation in the soybean varieties studied could have been due to any of these factors. Also, since we grew these soybean varieties under similar agronomic conditions, it is understandable that the observed differences are mainly due to their genetic background. Studies by other researchers using multiple soybean cultivars have shown that the metabolomic composition differs between cultivars. For example, a study using 29 Chinese soybean varieties detected 169 metabolites and reported that 104 of them showed intervarietal variation [75]. The metabotyping of different Korean soybean varieties showed that the metabolomic profiles of cultivated, wild, and semi-wild soybeans differed. Even within cultivated soybeans, the metabolomic composition differed. The authors related these differences to the distinct adaptation of the studied plant material to its respective environmental conditions [76]. Overall, we conclude that the studied cultivars differ in their metabolomic composition due to their genetic background and can therefore be used differently due to the composition and presence of certain health-promoting compounds.
5. Conclusions
The present study addressed the important question of the relative contribution of genotype to light anthocyanin color in soybeans. Such important traits as soybean color and anthocyanin content are tightly controlled by genotype, allowing for a wide range of selection. Our results based on metabotyping and fluorescence microscopy highlight that the “Locus” variety is good in terms of polyphenolic composition, followed by the variety “Primorskaya-86”. Our results are highly relevant for the development of fluorescent markers for the early detection of secondary metabolites. In addition, our results on the detection of health-promoting compounds (secondary metabolites) from these eight soybean varieties are highly relevant to the ongoing soybean breeding programs aimed at the development of nutritionally rich varieties.
Author Contributions
Conceptualization, M.P.R., A.N.E., S.E., M.A.N. and K.S.G.; methodology, M.P.R., M.A.N. and S.E.; software, M.P.R.; validation, M.P.R. and K.S.G.; formal analysis, M.A.N. and M.P.R.; investigation, K.S.G. and M.P.R.; resources, K.S.G. and M.P.R.; data curation, E.S.B., L.M.L. and O.A.C.; writing—original draft preparation, M.A.N. and M.P.R.; writing—review and editing M.A.N., M.P.R. and E.S.B.; visualization, M.P.R. and M.A.N.; supervision, K.S.G.; project administration, A.N.E., K.S.G. and M.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out at the N.I. Vavilov All-Russian Institute of Plant Genetic Resources at the expense of the budget project of VIR no. 0481-2022-0002, “Revealing the possibilities of legumes gene pool to optimize their breeding and diversify their use in various sectors of the national economy”.
Data Availability Statement
All datasets produced as a result of this work are given in the main manuscript.
Acknowledgments
This work was supported financially project No. 0481-2022-0002 “Revealing the possibilities of legumes gene pool to optimize their breeding and diversify their use in various sectors of the national economy”. Muhammad Amjad Nawaz and Kirill S. Golokhvast were supported by The Ministry of Science and Higher Education of the Russian Federation, project No FSWM-2024-0009.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Chemical compounds tentatively identified from the extracts of eight soybean varieties: “Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, and “Musson” in positive and negative ionization modes by HPLC-ion trap-MS/MS.
Table A1.
Chemical compounds tentatively identified from the extracts of eight soybean varieties: “Primorskaya-4”, “Primorskaya-86”, “Primorskaya-96”, “Locus”, “Sphere”, “Breeze”, “Namul”, and “Musson” in positive and negative ionization modes by HPLC-ion trap-MS/MS.
| № | Class of Compounds | Identification | Formula | Calculated Mass | Observed Mass [M − H]− | Observed Mass [M + H]+ | MS/MS Stage 1 Fragmentation | MS/MS Stage 2 Fragmentation | MS/MS Stage 3 Fragmentation | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Flavone | Formononetin [Biochanin B; Formononetol] | C16H12O4 | 268.2641 | 269.26 | 254.24 | 239.22; 110.29 | 196.24 | Dracocephalum jacutense [77]; Maackia amurensis [78]; Chinese herbal formula Jian-Pi-Yi-Shen pill [79] | |
| 2 | Flavone | Daidzein [4′,7-Dihydroxyisoflavone; Daidzeol] | C15H10O4 | 254.2375 | 255.18 | 199.15; 137.12 | 181.16; 129.24 | Soybean [55]; Black soya [54] | ||
| 3 | Flavone | Apigenin | C15H10O5 | 270.2369 | 271.18 | 153.10; 215.17 | 170.77 | Ribes meyeri [50]; Lonicera japonica [49] | ||
| 4 | Flavone | Trihydroxy(iso)flavone | C15H10O5 | 270.2369 | 271.15 | 215.15; 145.18 | 197.14; 169.13 | Propolis [80] | ||
| 5 | Flavone | Genistein [Pruneton; 4′,5,5-Trihydroxyisoflavone; Sophoricol] | C15H10O5 | 270.2369 | 271.27 | 253.12; 215; 153 | 210; 181; 133 | Black soya [54]; Mexican lupine species [81] | ||
| 6 | Flavone | Acacetin [Linarigenin; Buddleoflavonol] | C16H12O5 | 284.2635 | 285.16 | 270.12; 167.15; | 242.15; 152.15 | 214.10; 125.16 | Mexican lupine species [81]; Propolis [80] | |
| 7 | Flavone | Glycitein [7,4′-Dihydroxy-6-Methoxyisoflavone] | C16H12O5 | 284.2635 | 285.19 | 270.13; 229.15; 145.19 | 242.08 | 213.15; 168.18 | Black soya [54] | |
| 8 | Flavone | Chrysoeriol [Chryseriol] | C16H12O6 | 300.2629 | 301.31 | 284.28; 200.27 | 252.24; 196.17; 168.20 | 196.13; 167.12 | Rhus coriaria [48]; Propolis [80] | |
| 9 | Flavone | Hispidulin | C16H12O6 | 300.2629 | 301.29 | 284.28; 200.20 | 252.24; 168.22 | 223.13; 195.25; 168.18 | Artemisia argyl [82]; Mentha [83] | |
| 10 | Flavone | 5,7-Dimethoxyluteolin | C17H14O6 | 314.2895 | 313.34 | 285.21; 213.18; 113.22 | 185.20; 113.19 | Syzygium aromaticum [84]; Rosa rugosa [85] | ||
| 11 | Flavone | Cirsimaritin [Scrophulein; 4′,5-Dihydroxy-6,7-Dimethoxyflavone] | C17H14O6 | 314.2895 | 315.20 | 300.12 | 272.11 | 229.16 | Artemisia annua [86]; Rosmarinus officinalis [87] | |
| 12 | Flavone | Dimethoxy-trihydroxy(iso)flavone | C17H14O7 | 330.2889 | 331.16 | 303.15; 221.06 | 203.05 | Propolis [80]; Jatropha [88] | ||
| 13 | Flavone | Daidzin [Daidzoside; Daidzein 7-O-Glucoside] | C21H20O9 | 416.3781 | 417.22 | 255.15 | 199.18; 227.20; 137.14 | 181.14 | Malus toringoides [58]; Black soya [54] | |
| 14 | Flavone | Apigenin-7-O-glucoside [Apigetrin; Cosmosiin] | C21H20O10 | 432.3775 | 433.22 | 271.14 | 153.14; 215.14 | Grataegi Fructus [89]; Mexican lupine species [81] | ||
| 15 | Flavone | Vitexin [Apigenin 8-C-Glucoside] | C21H20O10 | 432.3775 | 433.40 | 415.30; 271.11 | 133.22; 177.19; 221.23 | Aspalathus linearis [90]; Lemon, Passion fruit [91] | ||
| 16 | Flavone | Genistin [Genistoside; Genistein 7-Glucoside] | C21H20O10 | 432.3775 | 433.25 | 271.13; 127.18; 397.02 | 127.17 | Isoflavones [92] | ||
| 17 | Flavone | Glycitin [Glycitein 7-O-glucoside] | C22H22O10 | 446.4041 | 447.21 | 285.15 | 270.13; 225.15; 197.11 | 242.10; 214.18; 152.12 | Black soya [54]; Rhus coriaria [48] | |
| 18 | Flavone | Luteolin 7-O-glucoside [Cynaroside] | C21H20O11 | 448.3769 | 449.19 | 287.14 | 213.05; 137.15 | 170.96 | Lonicera japonica [49] | |
| 19 | Flavone | Eriodictyol-O-hexoside | C21H22O11 | 450.3928 | 449.38 | 287.19; 259.25 | 259.18; 243.27; 201.28 | 215.22; 200.22; 173.23 | F. glaucescens; F. pottsii [93]; Rhus coriaria [48] | |
| 20 | Isoflavone | Acetyl daidzin | C23H22O10 | 458.4148 | 459.25 | 255.16 | 199.16; 227.18 | 181.14 | Black soya [54] | |
| 21 | Isoflavone | Apigenin-O-rhamnoside | C22H22O11 | 462.4035 | 461.44 | 415.31; 253.23 | 225.24 | Passion fruit [91]; Punica granatum [94] | ||
| 22 | Flavone | Acetyl genistin | C23H22O11 | 474.4142 | 475.21 | 271.14 | 215.18 | 197.12 | Black soya [54] | |
| 23 | Isoflavone | Malonyl daidzin | C24H22O12 | 502.4243 | 503.23 | 255.15 | 227.16; 199.20; 157.24 | 199.21; 181.17 | Black soya [54] | |
| 24 | Flavone | Dihydroxy-trimethoxyflavone-O-hexoside | C24H26O12 | 506.456 | 507.31 | 345.15; 198.13 | 198.05 | Citrus species [95] | ||
| 25 | Flavone | Genistein C-glucoside malonylated | C24H22O13 | 518.4237 | 519.22 | 271.14 | 215.12; 187.17; 153.15 | 197.10 | Black soya [54]; Mexican lupine species [81] | |
| 26 | Flavone | Apigenin O-glucoside malonylated | C24H22O13 | 518.4237 | 519.25 | 271.11; 164.17 | 152.19 | Mexican lupine species [81] | ||
| 27 | Flavone | Chrysoeriol 8-C-glucoside malonylated | C25H24O14 | 548.4497 | 549.46 | 531.38; 485.39; 367.25; 235.26 | 485.36; 429.36; 323.30; 235.23; 191.11 | 146.88 | Mexican lupine species [81] | |
| 28 | Flavone | Malonyl glycitin | C25H24O13 | 532.4503 | 533.32 | 362.20; 281.13; 191.13 | 281.12; 191.10 | 272.06; 200.09 | Black soya [54] | |
| 29 | Flavone | Apiin II | C26H28O14 | 564.4921 | 565.26 | 433.18; 403.16; 271.17 | 271.14 | 215.23; 201.02; 153.10 | Rhus coriaria [48] | |
| 30 | Flavone | Chrysin di-O-glucoside | C27H30O14 | 578.5187 | 579.26 | 417.20; 255.18 | 255.15; 137.15 | Passiflora incarnata [96] | ||
| 31 | Flavonol | Kaempferol | C15H10O6 | 286.2363 | 285.35 | 257.27; 185.21; 117.27 | 117.26 | Juglans mandshurica [46]; Polygala sibirica [47]; Rhus coriaria [48] | ||
| 32 | Flavonol | Herbacetin [3,5,7,8-Tetrahydroxy-2-(4-hydro-xyphenyl)-4H-chromen-4-one] | C15H10O7 | 302.2357 | 303.19 | 203.11; 275.14; | 184.71; 127.14 | Lonicera caerulea [97]; Ocimum [98] | ||
| 33 | Flavonol | Dihydroquercetin (Taxifolin; Taxifoliol) | C15H12O7 | 304.2516 | 305.18 | 190.16; 287.15 | 172.13 | 144.14 | Juglans mandshurica [46]; Glycine soja [99] | |
| 34 | Flavonol | Isorhamnetin [Isorhamnetol; Quercetin 3′-Methyl ether] | C16H12O7 | 316.2623 | 315.31 | 283.16 | 255.17 | 227.16 | Spondias purpurea [100]; Rosmarinus officinalis [87] | |
| 35 | Flavonol | Quercetin 3-D-xyloside [Reynoutrin] | C20H18O11 | 434.3503 | 433.41 | 313.21 | 285.23 | 257.22; 123.28 | Embelia [101]; Cranberry [102] | |
| 36 | Flavonol | Dihydrokaempferol-O-hexoside | C21H22O11 | 450.3928 | 449.36 | 287.20 | 259.22 | 215.23 | Rhus coriaria [48] | |
| 37 | Flavonol | Quercetin 3-O-glucoside [Isoquercitrin; Hirsutrin] | C21H20O12 | 464.3763 | 463.37 | 301.18 | 271.16; 179.17 | 151.15 | Ribes meyeri [50]; Lonicera japonica [49]; Spondias purpurea [100] | |
| 38 | Flavonol | Rhamnetin-O-hexoside | C22H22O12 | 478.4029 | 477.56 | 431.26; 269.23 | 268.23 | Artemisia absinthium [86]; Spondias purpurea [100] | ||
| 39 | Flavan-3-ol | Epiafzelechin [(epi)Afzelechin] | C15H14O5 | 274.2687 | 275.31 | 257.22; 159.24 | 212.24 | 195 | A. cordifolia; F. glaucescens; F. herrerae [93] | |
| 40 | Flavan-3-ol | Catechin | C15H14O6 | 290.2687 | 291.00 | 273.21; 217.00 | 237.32; 147.13 | Ribes meyeri [50]; Ribes magellanicum [103] | ||
| 41 | Flavan-3-ol | (Epi)Gallocatechin | C15H14O7 | 306.2675 | 305.26 | 225.24 | 165.19 | 147.20 | Ribes meyeri [50]; Ribes magellanicum [103]; Vaccinium myrtillus [104] | |
| 42 | Flavan-3-ol | Epiafzelechin derivative | C18H16O10 | 392.3136 | 393.13 | 274.39; 149.17 | 131.12 | Zostera marina [105]; Lonicera caerulea [97] | ||
| 43 | Tannin | Procyanidin A-type dimer | C30H24O12 | 576.501 | 577.27 | 425.15; 245.08; 163.13 | 245.09; 289.25; 408.12 | 217.10; 189.23 | Grape juice [106] | |
| 44 | Ellagitannin | Punicalin alpha | C34H22O22 | 782.5253 | 783.73 | 721.60; 597.59; 502.30; 461.02 | 596.64 | Myrtle [107] | ||
| 45 | Flavonoid | 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranoside | C41H32O26 | 940.6772 | 939.88 | 523.63; 455.60 | 421.49 | Rhodiola crenulata [108] | ||
| 46 | Anthocyanin | Cyanidin-3-O-glucoside [Cyanidin 3-O-beta-D-Glucoside; Kuromarin] | C21H21O11+ | 449.3848 | 449.38 | 287.17 | 213; 137 | 170 | Black soybean [54]; Glycine soja [99]; Ribes magellanicum [103] | |
| 47 | Anthocyanin | Pelargonidin-3-glucoside (callistephin) | C21H21O10 | 433.3854 | 433.22 | 271.14 | 253.11; 215.15; 145.14 | 197.11; 173.05 | Black soybean [54]; Black currant, Elderberry [109]; Strawberry [110] | |
| 48 | Anthocyanin | Pelargonidin 3-O-(6-O-malonyl-beta-D-glucoside) | C24H23O13 | 519.4388 | 519.23 | 271.11 | 215.14; 153.16 | 197.13; 147.21 | Strawberry [110]; Lonicera caerulea [97] | |
| 49 | Anthocyanin | Pelargonidin-3-O-acetyl hexoside | C23H23O11 | 475.4221 | 475.21 | 271.14 | 215.18 | 197.12 | Strawberry [91] | |
| 50 | Hydroxybenzoic acid (Phenolic acid) | Protocatechuic acid | C7H6O4 | 154.1201 | 155.18 | 126.27 | Ribes meyeri [50]; Lonicera japonica [49] | |||
| 51 | Hydroxybenzoic acid (Phenolic acid) | Ethyl protocatechuate [3,4-Dihydroxybenzoic Acid Ethyl Ester] | C9H10O4 | 182.1733 | 183.19 | 155.15 | 127.17 | 116.76 | Ocimum [98] | |
| 52 | Methylbenzoic acid | Methylgallic acid [Methyl gallate] | C8H8O5 | 184.1461 | 185 | 168.15; 143.19 | 122.33 | Lonicera caerulea [97]; Ocimum [98]; Papaya [91]; Rhus coriaria [48] | ||
| 53 | Phenolic acid | Ethyl caffeate [Ethyl 3,4-Dihydroxycinnamate] | C11H12O4 | 208.2106 | 207.31 | 179.19 | 135.23 | Ocimum [98]; Lepechinia [111] | ||
| 54 | Phenolic acid | p-Coumaric acid-O-hexoside [Trans-p-Coumaric acid 4-glucoside] | C15H18O8 | 326.2986 | 327.24 | 309.26 | 221.15; 115.20 | 193.24; 137 | Ribes meyeri [50]; Ribes magellanicum [103]; Strawberry [110]; Lemon, Strawberry [91]; G. linguiforme [93]; Rhus coriaria [48] | |
| 55 | Phenolic acid | p-Coumaroylquinic acid | C16H18O8 | 338.3093 | 339 | 303.35; 191.13; 163.25 | 163.06 | Artemisia absinthium [86]; Ribes magellanicum [103]; Ribes meyeri [50] | ||
| 56 | Phenolic acid | Caffeic acid-O-hexoside [Caffeoyl-O-hexoside] | C15H18O9 | 342.298 | 341.39 | 179.19 | 161.08 | Punica granatum [94]; Carpinus betulus [112]; Inula viscosa [113] | ||
| 57 | Hydroxycinnamic acid | Chlorogenic acid [3-O-Caffeoylquinic acid] | C16H18O9 | 354.3088 | 353.32 | 191.24 | 127.26 | Ribes magellanicum [103]; Lonicera japonica [49]; Vaccinium myrtillus [104]; Spondias purpurea [100] | ||
| 58 | Phenolic acid | Caffeic acid derivative 1 | C18H18O9 | 378.3301 | 377.35 | 341.24; 215.20 | 179.18; 131.23 | Embelia [101] | ||
| 59 | Phenolic acid | Ellagic acid pentoside | C19H14O12 | 434.3073 | 433.39 | 313.25; 285.28 | 285.22; 269.33; 241.31 | 257.24; 213.27; 163.20 | Strawberry [110]; Carpinus betulus [112] | |
| 60 | Phenolic acid | Caffeic acid-O-hexoside-O-rhamnoside | C24H24O11 | 488.4408 | 487.49 | 341.21 | 179.16 | Lemon, Papaya, Passion fruit [91] | ||
| 61 | Dihydrochalcone | Phloretin [Dihydronaringenin; Phloretol] | C15H14O5 | 274.2687 | 275.34 | 256.35; 202.15 | 212.44 | Eucalyptus [114]; Malus toringoides [58]; G. linguiforme [93]; Apple [115] | ||
| 62 | Coumarin | Fraxetin | C10H8O5 | 208.1675 | 209.25 | 191.19 | 145.22 | 119.24 | Embelia [101]; Jatropha [88]; Artemisia martjanovii [116] | |
| 63 | Hydroxycoumarin | Fraxidin | C11H10O5 | 222.1941 | 223.19 | 208.11 | 180.13 | 165.18 | Jatropha [88] | |
| 64 | Lignan | Secoisolariciresinol | C20H26O6 | 362.4168 | 361.50 | 343.43; 273.30; 237.36; 201.29; 171.29 | 255.32; 171.27 | 237.31; 197.21; 153.27 | F. pottsii [93]; Lignans [117] | |
| 65 | Lignan | Dimethyl-secoisolariciresinol | C22H30O6 | 390.470 | 391.35 | 373.30; 149.15 | 173.11; 111.11 | 156.24 | Lignans [117] | |
| 66 | Lignan | Medioresinol | C21H24O7 | 388.4111 | 387.44 | 207.30; 369.29; 269.14; 163.28 | 163.26 | Lignans [117] | ||
| 67 | Lignan | Syringaresinol | C22H26O8 | 418.4436 | 419.20 | 326.10; 257.19 | 298.09; 254.10 | 252.11; 154.20 | Magnolia [118]; Annona montana [119]; Lignans [117] | |
| 68 | Stilbene | 3-Hydroxyresveratrol [Piceatannol] | C14H12O4 | 244.2427 | 243.39 | 225.28; 207.29 | 207.28; 181.36 | 163.28; 145.23 | G. linguiforme [93]; Grape [120]; Oenocarpus bataua [121] | |
| 69 | Gallate ester | Pentagalloyl hexose | C41H32O26 | 940.6772 | 939.88 | 921.65; 793.73; 731.70; 613.65; 523.63; 455.60 | 421.49 | Carpinus betulus [112]; Rhus coriaria [48] | ||
| OTHERS | ||||||||||
| 70 | Aliphatic amino acid | L-Threonine [(2S, 3R)-2-Amino-3-Hydroxybutanoic acid] | C4H9NO3 | 119.1192 | 120.25 | 74 | Soybean [55]; Soybean leaves [122] | |||
| 71 | Organic acid | Malic acid [DL-Malic acid] | C4H6O5 | 134.0874 | 135.13 | 116.23 | Soybean [55]; Soybean leaves [122]; Rhus coriaria [48]; Ribes meyeri [50] | |||
| 72 | Amino compound | Tyramine [4-Hydroxyphenethylamine] | C8H11NO | 137.1790 | 138.21 | 119.27 | Hylocereus polyrhizus [123] | |||
| 73 | Oxo dicarboxylate | Alpha-ketoglutaric acid | C5H6O5 | 146.0981 | 147.12 | 137.14 | Soybean [55] | |||
| 74 | Benzaldehyde | Vanillin | C8H8O3 | 152.1473 | 153 | 127 | Solanum tuberosum [52,124]; Triticum [125] | |||
| 75 | Phenylethanoid | Hydroxy tyrosol | C8H10O3 | 154.1632 | 155.17 | 145.15 | G. linguiforme [93] | |||
| 76 | Amino acid | Tryptamine | C10H12N2 | 160.2157 | 161.10 | 143.14 | Hylocereus polyrhizus [123] | |||
| 77 | Amino acid | Phenylalanine | C9H11NO2 | 165.1891 | 166.20 | 120.24 | Soybean [55]; Soybean leaves [122]; Lonicera japonica [49] | |||
| 78 | Sugar | D-glycerol-1-phosphate | C3H9O6P | 172.0737 | 173.18 | 153.52; 145.14 | Soybean [55] | |||
| 79 | Amino acid | L-theanine [Theanine; N-Ethyl-L-glutamine] | C7H14N2O3 | 174.1977 | 175.23 | 157.24 | 112.24 | Camellia kucha [126] | ||
| 80 | Auxin | Indole-3-acetic acid | C10H9NO2 | 175.1840 | 176.16 | 132.16 | Triticum aestivum L. [127] | |||
| 81 | Aromatic amino acid | Tyrosine | C9H11NO3 | 181.1885 | 182 | 154 | 127 | Soybean leaves [122]; Hylocereus polyrhizus [123] | ||
| 82 | Organic acid | Gluconic acid [Gluconate; Dextronic acid; Maltonic acid] | C6H12O7 | 196.1553 | 197.09 | 156.22; 119.21 | 119.18 | Soybean [55]; Soybean leaves [122]; Ribes meyeri [50] | ||
| 83 | Essential amino acid | L-Tryptophan [Tryptophan] | C11H12N2O2 | 204.2252 | 205.16 | 187.16 | 146.20; 118.11 | Rosa acicularis [85]; Passiflora incarnata [96]; Camellia kucha [126]; Hylocereus polyrhizus [123] | ||
| 84 | Organic acid | Glucoheptonic acid | C7H14O8 | 226.1813 | 227.19 | 161.67 | 145.16 | 127.11 | Soybean leaves [122] | |
| 85 | Carboxylic acid | Myristoleic acid [Cis-9-Tetradecanoic acid] | C14H26O2 | 226.3550 | 227.28 | 209.25 | 139.20; 192.21 | F. glaucescens [93]; Maackia amurensis [78]; Artemisia martjanovii [116] | ||
| 86 | Ribonucleoside composite of adenine (purine) | Adenosine | C10H13N5O4 | 267.2413 | 268.18 | 136.21 | 119.17 | Lonicera japonica [49]; Rosa acicularis [85] | ||
| 87 | Ribonucleoside composite of adenine (purine) | Inosine | C10H12N4O5 | 268.2261 | 269.18 | 136.18 | Lonicera japonica [49] | |||
| 88 | Fatty acid methyl ester | Methyl palmitoleate | C17H32O2 | 268.4348 | 269.18 | 255.36; 233.09; 219.82; 194.62; 169.14 | Soybean [55] | |||
| 89 | Omega-3-fatty acid | Linolenic acid | C18H30O2 | 278.4296 | 279.15 | 259.32; 232.21; 186.24 | 204.13; 186.13; 169.20 | 168.17; 142.08 | Jatropha [88]; Maackia amurensis [78] | |
| 90 | Omega-3 fatty acid; octadecatetraenoic acid | Stearidonic acid | C18H28O2 | 276.4137 | 277.12 | 177.14; 231.12; 131.19 | 131.14 | G. linguiforme [93]; Rhus coriaria [48]; Jatropha [88] | ||
| 91 | Jasmonate | 12-Hydroxyjasmonate sulfate | C12H18O7S | 306.3321 | 305.29 | 225.25 | 207.24; 181.27; 147.25 | 163.29 | Arabidopsis [128] | |
| 92 | Oxylipin | 11-Hydroperoxy-octadecatrienoic acid | C18H30O4 | 310.4284 | 311.20 | 182.17 | 165.17 | 147.14 | Potato leaves [53] | |
| 93 | Oxylipin | 9,10-Dihydroxy-8-oxooctadec-12-enoic acid [oxo-DHODE] | C18H32O5 | 328.4437 | 327.43 | 291.31; 229.34; 171.31 | 222.27; 153.28 | Rosa acicularis [85]; Lonicera caerulea [97]; Dracocephalum jacutense [77] | ||
| 94 | Hydroxy fatty acid | Hydroxyoctadecenedioic acid | C18H32O5 | 328.4437 | 327.50 | 239.36; 195.36 | 179.28 | Cyperus laevigatus [129] | ||
| 95 | Oxylipin | 13-Trihydroxy-Octadecenoic acid [THODE] | C18H34O5 | 330.4596 | 329.48 | 229.28; 171 | 210.67 | Jatropha [88] | ||
| 96 | Glyceryl palmitate | Monopalmitin | C19H38O4 | 330.5026 | 331.25 | 227 | 205 | 182 | Soybean [55] | |
| 97 | Dicarboxylic acid | Gibberellin A19 | C20H26O6 | 362.4168 | 361.15 | 273.37; 237.36; 171.32 | 254.69; 171.34 | 235.28; 193.32 | Analysis of gibberellins [130] | |
| 98 | Iridoid glucoside | Harpagide | C15H24O10 | 364.3451 | 365.20 | 337.55; 203.17 | 113.20 | Honey [131] | ||
| 99 | Trehalose dihydrate | C12H26O13 | 378.3270 | 377.39 | 341.29 | 179.21; 113.27 | 113.21 | Pubchem | ||
| 100 | Sterol | Desmosterol | C22H24O6 | 384.4224 | 385.34 | 367.26; 269.25; 213.19; 147.22 | 349.27; 322.83; 279.27; 216.39; 182.18 | 290.27 | A. cordifolia [93] | |
| 101 | Trehalose (+FA adduct) CH2O2 (46.0254) | C13H24O13 | 388.3219 | 387.40 | 341.27 | 179.17; 113.26 | 143.19 | Pubchem | ||
| 102 | Steroid | Vebonol | C30H44O3 | 452.6686 | 453.46 | 435.48; 336.25; 209.26 | 336.26; 226.31 | 209.26 | Rhus coriaria [48]; Hylosereus polyrhizus [123] | |
| 103 | Saponin | Soyasapogenol A | C30H50O4 | 474.5434 | 475.43 | 457.40; 384; 271.14 | 439.41; 341.11; 290.28; 176.98 | 363.11 | Pubchem | |
| 104 | Thromboxane receptor antagonist | Vapiprost | C30H39NO4 | 477.6350 | 478.43 | 337.39 | 121.28; 319.30 | Rhus coriaria [48]; Hylosereus polyrhizus [123] | ||
| 105 | Sugar | Maltotriose [Amylotriose] | C18H32O16 | 504.4371 | 505.11 | 487.26; 441.34; 327.31; 221.23; 177.17 | 441.35; 367.24; 323.23; 235.20; 191.18; 147.15 | 367.27; 322.43; 235.21; 163.11 | Soybean leaves [122] | |
| 106 | Indole sesquiterpene alkaloid | Sespendole | C33H45NO4 | 519.7147 | 520.48 | 184.16 | 125.13 | Rhus coriaria [48] | ||
| 107 | Phytohormone | GA8-hexose gibberellin | C25H34O12 | 526.5303 | 527.32 | 365.14; 347.14; 305.14; 275.11; 245.05 | 305.13; 275.08; 245.09; 203.12 | 245.05; 203.05 | Strawberry [132] | |
| 108 | Saponin | Chikusetsusaponin Iva [Calenduloside F] | C42H66O14 | 794.9650 | 795.23 | 597.47; 439.46; 245.32 | 421.45; 365.23; 245.28 | 403.35; 308.30; 271.18 | Bougainvillea [133]; Leguminous [134] | |
| 109 | Saponin | Soyasaponin Bb′ [Soyasaponin III] | C42H68O14 | 796.4610 | 797.50 | 599.54; 423.43; 247.39 | 581.35; 423.47; 203.20 | 211.36 | Black soya [54] | |
| 110 | Product of chlorophyll degradation | Pheophytin A | C55H74N4O5 | 871.1999 | 872.72 | 593.45 | 533.36 | 461.38 | Physalis peruviana [135]; Capsicum [136] | |
| 111 | Saponin | Soyasaponin Bd | C48H76O19 | 957.1056 | 958.11 | 597.42; 439.47 | Black soya [54]; Leguminous [134]; Soya [16] | |||
| 112 | Saponin | Soyasaponin I [Soyasaponin Bb] | C48H78O18 | 943.1221 | 944.12 | 423.44; 381.68; 281.34 | 202.99 | Leguminous [134]; Soya [16]; Black soya [54] | ||
| 113 | Saponin | Soyasaponin Ba (V) | C48H78O19 | 959.1215 | 960.37 | 599.12; 423.46; 281.32 | 423.51; 271.27 | Black soya [54]; Leguminous [134]; Soya [16] | ||
| 114 | Saponin | Soyasaponin beta g (VI) | C54H84O21 | 1069.2322 | 1070 | 507; 415; 331; 299 | 331; 299 | 185 | Black soya [54]; Leguminous [134]; Soya [16] |
References
- Scharff, L.B.; Saltenis, V.L.R.; Jensen, P.E.; Baekelandt, A.; Burgess, A.J.; Burow, M.; Ceriotti, A.; Cohan, J.; Geu-Flores, F.; Halkier, B.A.; et al. Prospects to Improve the Nutritional Quality of Crops. Food Energy Secur. 2022, 11, e327. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Alseekh, S.; Scossa, F.; Wen, W.; Luo, J.; Yan, J.; Beleggia, R.; Klee, H.J.; Huang, S.; Papa, R.; Fernie, A.R. Domestication of Crop Metabolomes: Desired and Unintended Consequences. Trends Plant Sci. 2021, 26, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Isanga, J.; Zhang, G.-N. Soybean Bioactive Components and Their Implications to Health—A Review. Food Rev. Int. 2008, 24, 252–276. [Google Scholar] [CrossRef]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological Functionality of Soyasaponins and Soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef]
- Gao, R.; Han, T.; Xun, H.; Zeng, X.; Li, P.; Li, Y.; Wang, Y.; Shao, Y.; Cheng, X.; Feng, X.; et al. MYB Transcription Factors GmMYBA2 and GmMYBR Function in a Feedback Loop to Control Pigmentation of Seed Coat in Soybean. J. Exp. Bot. 2021, 72, 4401–4418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.-A.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-Genome of Wild and Cultivated Soybeans. Cell 2020, 182, 162–176.e13. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.P.S.; Borges, R.S.; Neto, A.M.J.C.; de Macedo, L.G.M.; da Silva, A.B.F. The Basic Antioxidant Structure for Flavonoid Derivatives. J. Mol. Model. 2012, 18, 4073–4080. [Google Scholar] [CrossRef] [PubMed]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated Anthocyanins: A Review on Their Bioavailability and Effects on Postprandial Carbohydrate Metabolism and Inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef]
- Raab, T.; Barron, D.; Vera, F.A.; Crespy, V.; Oliveira, M.; Williamson, G. Catechin Glucosides: Occurrence, Synthesis, and Stability. J. Agric. Food Chem. 2010, 58, 2138–2149. [Google Scholar] [CrossRef]
- Fenwick, D.E.; Oakenfull, D. Saponin Content of Food Plants and Some Prepared Foods. J. Sci. Food Agric. 1983, 34, 186–191. [Google Scholar] [CrossRef]
- Decroos, K.; Vincken, J.-P.; Heng, L.; Bakker, R.; Gruppen, H.; Verstraete, W. Simultaneous Quantification of Differently Glycosylated, Acetylated, and 2,3-Dihydro-2,5-Dihydroxy-6-Methyl-4H-Pyran-4-One-Conjugated Soyasaponins Using Reversed-Phase High-Performance Liquid Chromatography with Evaporative Light Scattering Detection. J. Chromatogr. A 2005, 1072, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kudou, S.; Tonomura, M.; Tsukamoto, C.; Uchida, T.; Sakabe, T.; Tamura, N.; Okubo, K. Isolation and Structural Elucidation of DDMP-Conjugated Soyasaponins as Genuine Saponins from Soybean Seeds. Biosci. Biotechnol. Biochem. 1993, 57, 546–550. [Google Scholar] [CrossRef]
- Kitagawa, I.; Wang, H.K.; Taniyama, T.; Yoshikawa, M. Saponin and Sapogenol. XLI. Reinvestigation of the Structures of Soyasapogenols A,B,and E, Oleanene-Sapogenols from Soybean. Structures of Soyasaponins I, II, and III. Chem. Pharm. Bull. 1988, 36, 153–161. [Google Scholar] [CrossRef]
- Kitagawa, I.; Taniyama, T.; Nagahama, Y.; Okubo, K.; Yamauchi, F.; Yoshikawa, M. Saponin and Sapogenol. XLII. Structures of Acetyl-Soyasaponins A1, A2, and A3, Astringent Partially Acetylated Bisdesmosides of Soyasapogenol A, from American Soybean, the Seeds of Glycine Max MERRILL. Chem. Pharm. Bull. 1988, 36, 2819–2828. [Google Scholar] [CrossRef][Green Version]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The Biological Action of Saponins in Animal Systems: A Review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Philbrick, D.J.; Bureau, D.P.; William Collins, F.; Holub, B.J. Evidence That Soyasaponin Bb Retards Disease Progression in a Murine Model of Polycystic Kidney Disease. Kidney Int. 2003, 63, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Iijima, M.; Kobayashi, Y.; Yoshikoshi, M.; Uchida, T.; Kudou, S. Components Responsible for the Undesirable Taste of Soybean Seeds. Biosci. Biotechnol. Biochem. 1992, 56, 99–103. [Google Scholar] [CrossRef]
- Oleszek, W.A. Chromatographic Determination of Plant Saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyashita, K.; Shimizu, H.; Sugiyama, J. Three-Dimensional Internal Structure of a Soybean Seed by Observation of Autofluorescence of Sequential Sections. Nippon. Shokuhin Kagaku Kogaku Kaishi 2003, 50, 213–217. [Google Scholar] [CrossRef][Green Version]
- Pegg, T.J.; Gladish, D.K.; Baker, R.L. Algae to Angiosperms: Autofluorescence for Rapid Visualization of Plant Anatomy among Diverse Taxa. Appl. Plant Sci. 2021, 9, e11437. [Google Scholar] [CrossRef]
- Eurasian Economic Commission. Pharmacopoeia of the Eurasian Economic Union; Approved by Decision of the Board of Eurasian Economic Commission; Eurasian Economic Commission: Moscow, Russia, 2020. [Google Scholar]
- Razgonova, M.P.; Zinchenko, Y.N.; Kozak, D.K.; Kuznetsova, V.A.; Zakharenko, A.M.; Ercisli, S.; Golokhvast, K.S. Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach. Molecules 2022, 27, 8228. [Google Scholar] [CrossRef]
- Chung, N.C.; Miasojedow, B.; Startek, M.; Gambin, A. Jaccard/Tanimoto Similarity Test and Estimation Methods for Biological Presence-Absence Data. BMC Bioinform. 2019, 20, 644. [Google Scholar] [CrossRef] [PubMed]
- Corcel, M.; Devaux, M.-F.; Guillon, F.; Barron, C. Identification of Tissular Origin of Particles Based on Autofluorescence Multispectral Image Analysis at the Macroscopic Scale. EPJ Web Conf. 2017, 140, 05012. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Schweiger, J. Cell Wall Bound Ferulic Acid, the Major Substance of the Blue-Green Fluorescence Emission of Plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Donaldson, L. Softwood and Hardwood Lignin Fluorescence Spectra of Wood Cell Walls in Different Mounting Media. IAWA J. 2013, 34, 3–19. [Google Scholar] [CrossRef]
- Brillouet, J.; Riochet, D. Cell Wall Polysaccharides and Lignin in Cotyledons and Hulls of Seeds from Various Lupin (Lupinus L.) Species. J. Sci. Food Agric. 1983, 34, 861–868. [Google Scholar] [CrossRef]
- Krzyzanowski, F.C.; Franca Neto, J.d.B.; Mandarino, J.M.G.; Kaster, M. Evaluation of Lignin Content of Soybean Seed Coat Stored in a Controlled Environment. Rev. Bras. Sementes 2008, 30, 220–223. [Google Scholar] [CrossRef]
- Brillouet, J.-M.; Carré, B. Composition of Cell Walls from Cotyledons of Pisum Sativum, Vicia Faba and Glycine Max. Phytochemistry 1983, 22, 841–847. [Google Scholar] [CrossRef]
- Sudo, E.; Teranishi, M.; Hidema, J.; Taniuchi, T. Visualization of Flavonol Distribution in the Abaxial Epidermis of Onion Scales via Detection of Its Autofluorescence in the Absence of Chemical Processes. Biosci. Biotechnol. Biochem. 2009, 73, 2107–2109. [Google Scholar] [CrossRef] [PubMed]
- Monago-Maraña, O.; Durán-Merás, I.; Galeano-Díaz, T.; Muñoz de la Peña, A. Fluorescence Properties of Flavonoid Compounds. Quantification in Paprika Samples Using Spectrofluorimetry Coupled to Second Order Chemometric Tools. Food Chem. 2016, 196, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Collings, D.A. Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules. Plants 2019, 8, 596. [Google Scholar] [CrossRef]
- Mackon, E.; Ma, Y.; Jeazet Dongho Epse Mackon, G.C.; Li, Q.; Zhou, Q.; Liu, P. Subcellular Localization and Vesicular Structures of Anthocyanin Pigmentation by Fluorescence Imaging of Black Rice (Oryza sativa L.) Stigma Protoplast. Plants 2021, 10, 685. [Google Scholar] [CrossRef]
- Acuña, A.U.; Amat-Guerri, F.; Morcillo, P.; Liras, M.; Rodríguez, B. Structure and Formation of the Fluorescent Compound of Lignum nephriticum. Org. Lett. 2009, 11, 3020–3023. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their Structure, Biosynthesis and Role in the Rhizosphere, Including Allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Donaldson, L.; Williams, N. Imaging and Spectroscopy of Natural Fluorophores in Pine Needles. Plants 2018, 7, 10. [Google Scholar] [CrossRef]
- Berg, R.H. Evaluation of Spectral Imaging for Plant Cell Analysis. J. Microsc. 2004, 214, 174–181. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K. The Transparent Testa4 Mutation Prevents Flavonoid Synthesis and Alters Auxin Transport and the Response of Arabidopsis Roots to Gravity and Light[W]. Plant Cell 2004, 16, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Brown, D.E.; Tague, B.W.; Muday, G.K.; Taiz, L.; Murphy, A.S. Flavonoid Accumulation Patterns of Transparent Testa Mutants of Arabidopsis. Plant Physiol. 2001, 126, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Lee, J.Y.; Cho, H.; Choi, H.J.; Son, C.K.; Bae, J.S.; Bilyeu, K.; Song, J.T.; Lee, J.-D. Genetic Diversity of Soybeans (Glycine max (L.) Merr.) with Black Seed Coats and Green Cotyledons in Korean Germplasm. Agronomy 2021, 11, 581. [Google Scholar] [CrossRef]
- Huo, J.-H.; Du, X.-W.; Sun, G.-D.; Dong, W.-T.; Wang, W.-M. Identification and Characterization of Major Constituents in Juglans Mandshurica Using Ultra Performance Liquid Chromatography Coupled with Time-of-Flight Mass Spectrometry (UPLC-ESI-Q-TOF/MS). Chin. J. Nat. Med. 2018, 16, 525–545. [Google Scholar] [CrossRef]
- Song, Y.-L.; Zhou, G.-S.; Zhou, S.-X.; Jiang, Y.; Tu, P.-F. Polygalins D–G, Four New Flavonol Glycosides from the Aerial Parts of Polygala sibirica L. (Polygalaceae). Nat. Prod. Res. 2013, 27, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS Screening of Bioactive Components from Rhus coriaria L. (Sumac) Fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera Japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Wang, Q.; Liu, H.; Shen, H.; Xu, W.; Ge, J.; He, D. Rapid Qualitative Profiling and Quantitative Analysis of Phenolics in Ribes meyeri Leaves and Their Antioxidant and Antidiabetic Activities by HPLC-QTOF-MS/MS and UHPLC-MS/MS. J. Sep. Sci. 2021, 44, 1404–1420. [Google Scholar] [CrossRef]
- Aita, S.; Capriotti, A.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.; Piovesana, S.; Laganà, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.-P. Metabolite Profiling of Red and Blue Potatoes Revealed Cultivar and Tissue Specific Patterns for Anthocyanins and Other Polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; Guerra-Hernández, E.; Cerretani, L.; García-Villanova, B.; Verardo, V. Comprehensive Metabolite Profiling of Solanum tuberosum L. (Potato) Leaves by HPLC-ESI-QTOF-MS. Food Res. Int. 2018, 112, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Shin, J.-S.; Park, S.-K.; Kang, S.; Jeong, S.-C.; Moon, J.-K.; Choi, Y. Differences in the Metabolic Profiles and Antioxidant Activities of Wild and Cultivated Black Soybeans Evaluated by Correlation Analysis. Food Res. Int. 2017, 100, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic Characteristics and Metabolic Analyses of Two Soybean Genotypes Revealed Adaptive Strategies to Low-Nitrogen Stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, P.; Liu, B.; Wei, L.; Xu, Y. Simultaneous Determination of Fourteen Compounds of Hedyotis Diffusa Willd Extract in Rats by UHPLC–MS/MS Method: Application to Pharmacokinetics and Tissue Distribution Study. J. Pharm. Biomed. Anal. 2018, 159, 490–512. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, D.; Liu, Y.; Lin, L.; Xiong, X.; Zhang, D.; Sun, M.; Cai, M.; Yu, X.; et al. Transcriptomic and Metabolomic Profiling Provides Insights into Flavonoid Biosynthesis and Flower Coloring in Loropetalum chinense and Loropetalum chinense Var. rubrum. Agronomy 2023, 13, 1296. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Yang, M.; Cao, J.; Khan, A.; Cheng, G. UHPLC-ESI-HRMS/MS Analysis on Phenolic Compositions of Different E Se Tea Extracts and Their Antioxidant and Cytoprotective Activities. Food Chem. 2020, 318, 126512. [Google Scholar] [CrossRef]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Yang, S.H.; Chung, G. Functional Characterization of Naturally Occurring Wild Soybean Mutant (Sg-5) Lacking Astringent Saponins Using Whole Genome Sequencing Approach. Plant Sci. 2018, 267, 148–156. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Golokhvast, K.S.; Rehman, H.M.; Tsukamoto, C.; Kim, H.-S.; Yang, S.H.; Chung, G. Soyisoflavone Diversity in Wild Soybeans (Glycine Soja Sieb. & Zucc.) from the Main Centres of Diversity. Biochem. Syst. Ecol. 2018, 77, 16–21. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Luk, C.-Y.; Ludidi, N.; Chung, G.; Chen, S.-P.T.; Lam, H.-M. Soybean Secondary Metabolites and Flavors: The Art of Compromise among Climate, Natural Enemies, and Human Culture. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2022; pp. 295–347. [Google Scholar]
- Tripathi, A.K.; Misra, A.K. Soybean—A Consummate Functional Food: A Review. J. Food Sci. Technol. 2005, 42, 111–119. [Google Scholar]
- Talamond, P.; Verdeil, J.-L.; Conéjéro, G. Secondary Metabolite Localization by Autofluorescence in Living Plant Cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef]
- Zhu, Y. The Feasibility of Using Autofluorescence to Detect Lignin Deposition Pattern during Defense Response in Apple Roots to Pythium Ultimum Infection. Horticulturae 2022, 8, 1085. [Google Scholar] [CrossRef]
- Guo, X.; Yue, Y.; Tang, F.; Wang, J.; Yao, X.; Sun, J. A Comparison of C-Glycosidic Flavonoid Isomers by Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry in Negative and Positive Ion Mode. Int. J. Mass Spectrom. 2013, 333, 59–66. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Qin, Y.; Cheng, Z.; Chen, D. Approach to the Study of Flavone Di-C-glycosides by High Performance Liquid Chromatography-tandem Ion Trap Mass Spectrometry and Its Application to Characterization of Flavonoid Composition in Viola yedoensis. J. Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of Phenolic Compounds in Rhubarbs Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef]
- Lu, L.; Song, F.; Tsao, R.; Jin, Y.; Liu, Z.; Liu, S. Studies on the Homolytic and Heterolytic Cleavage of Kaempferol and Kaempferide Glycosides Using Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 169–172. [Google Scholar] [CrossRef]
- Truchado, P.; Vit, P.; Ferreres, F.; Tomas-Barberan, F. Liquid Chromatography–Tandem Mass Spectrometry Analysis Allows the Simultaneous Characterization of C-Glycosyl and O-Glycosyl Flavonoids in Stingless Bee Honeys. J. Chromatogr. A 2011, 1218, 7601–7607. [Google Scholar] [CrossRef]
- Ma, Y.; Cuyckens, F.; Heuvel, H.V.D.; Claeys, M. Mass Spectrometric Methods for the Characterisation and Differentiation of Isomeric O-diglycosyl Flavonoids. Phytochem. Anal. 2001, 12, 159–165. [Google Scholar] [CrossRef]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. Practical Guidelines for Characterization of O-diglycosyl Flavonoid Isomers by Triple Quadrupole MS and Their Applications for Identification of Some Fruit Juices Flavonoids. J. Mass Spectrom. 2009, 44, 1017–1025. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the Interglycosidic Linkage in Di-, Tri-, Tetra- and Pentaglycosylated Flavonoids and Differentiation of Positional Isomers by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Ablajan, K. A Study of Characteristic Fragmentation of Isoflavonoids by Using Negative Ion ESI-MSn. J. Mass Spectrom. 2011, 46, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Zhao, G.; Zhou, Y.; Xia, X.; Wang, J.; Wang, G.; Lu, S.; He, W.; Bi, T.; Li, J. Metabonomics Analysis of Flavonoids in Seeds and Sprouts of Two Chinese Soybean Cultivars. Sci. Rep. 2022, 12, 5541. [Google Scholar] [CrossRef]
- Lin, H.; Rao, J.; Shi, J.; Hu, C.; Cheng, F.; Wilson, Z.A.; Zhang, D.; Quan, S. Seed Metabolomic Study Reveals Significant Metabolite Variations and Correlations among Different Soybean Cultivars. J. Integr. Plant Biol. 2014, 56, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.-Y.; Kang, Y.-G.; Kim, M.; Kim, D.; Kim, E.-H.; Hong, Y.-S. Metabotyping of Different Soybean Genotypes and Distinct Metabolism in Their Seeds and Leaves. Food Chem. 2020, 330, 127198. [Google Scholar] [CrossRef]
- Okhlopkova, Z.M.; Razgonova, M.P.; Rozhina, Z.G.; Egorova, P.S.; Golokhvast, K.S. Dracocephalum Jacutense Peschkova from Yakutia: Extraction and Mass Spectrometric Characterization of 128 Chemical Compounds. Molecules 2023, 28, 4402. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Cherevach, E.I.; Tekutyeva, L.A.; Fedoreyev, S.A.; Mishchenko, N.P.; Tarbeeva, D.V.; Demidova, E.N.; Kirilenko, N.S.; Golokhvast, K. Maackia Amurensis Rupr. et Maxim.: Supercritical CO2 Extraction and Mass Spectrometric Characterization of Chemical Constituents. Molecules 2023, 28, 2026. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Chen, Q.; Hu, Z.; Li, Z.; Zheng, P.; Liu, X.; Li, S.; Zhang, S.; Chen, J. Chemical Characterisation and Quantification of the Major Constituents in the Chinese Herbal Formula Jian-Pi-Yi-Shen Pill by UPLC-Q-TOF-MS/MS and HPLC-QQQ-MS/MS. Phytochem. Anal. 2020, 31, 915–929. [Google Scholar] [CrossRef]
- Belmehdi, O.; Bouyahya, A.; Jekő, J.; Cziáky, Z.; Zengin, G.; Sotkó, G.; EL Baaboua, A.; Senhaji, N.S.; Abrini, J. Synergistic Interaction between Propolis Extract, Essential Oils, and Antibiotics against Staphylococcus Epidermidis and Methicillin Resistant Staphylococcus Aureus. Int. J. Second. Metab. 2021, 8, 195–213. [Google Scholar] [CrossRef]
- Wojakowska, A.; Piasecka, A.; García-López, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, Ł.; Kachlicki, P.; Stobiecki, M. Structural Analysis and Profiling of Phenolic Secondary Metabolites of Mexican Lupine Species Using LC–MS Techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, D.; Yang, G.; Zheng, Y.; Guo, L. Screening of Anti-Lipase Components of Artemisia Argyi Leaves Based on Spectrum-Effect Relationships and HPLC-MS/MS. Front. Pharmacol. 2021, 12, 675396. [Google Scholar] [CrossRef]
- Xu, L.-L.; Xu, J.-J.; Zhong, K.-R.; Shang, Z.-P.; Wang, F.; Wang, R.-F.; Zhang, L.; Zhang, J.-Y.; Liu, B. Analysis of Non-Volatile Chemical Constituents of Menthae Haplocalycis Herba by Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of Nonvolatile Compounds in Clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862, 030079. [Google Scholar]
- Razgonova, M.P.; Bazhenova, B.A.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa Davurica Pall., Rosa Rugosa Thumb., and Rosa Acicularis Lindl. Originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Three Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Etienne, O.K.; Sharmeen, J.B.; Brunetti, L.; Leone, S.; Di Simone, S.C.; Recinella, L.; et al. Chemical Composition and Biological Properties of Two Jatropha Species: Different Parts and Different Extraction Methods. Antioxidants 2021, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, P.; Leung, K.W.; Wang, H.; Kong, X.P.; Wang, L.; Dong, T.T.X.; Chen, Y.; Tsim, K.W.K. The Yin-Yang Property of Chinese Medicinal Herbs Relates to Chemical Composition but Not Anti-Oxidative Activity: An Illustration Using Spleen-Meridian Herbs. Front. Pharmacol. 2018, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Fantoukh, O.I.; Wang, Y.-H.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations 2022, 9, 159. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and Quantification of Phenolic Compounds of Selected Fruits from Madeira Island by HPLC-DAD–ESI-MSn and Screening for Their Antioxidant Activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Daniela Hanganu, L.V.N.O. LC/MS analysis of isoflavones from fabaceae species extracts. Farmacia 2010, 58, 177–183. [Google Scholar]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS—Bioassay Guided Approach. J. Chromatogr. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamočlija, J.; Ćirić, A.; Soković, M.; Heropoulos, G.; Proestos, C. Antiradical–Antimicrobial Activity and Phenolic Profile of Pomegranate (Punica granatum L.) Juices from Different Cultivars: A Comparative Study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; Chaves, D.S.d.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of Bioactive Compounds Content in Leaf Extracts of Passiflora incarnata, P. caerulea and P. alata and in Vitro Cytotoxic Potential on Leukemia Cell Lines. Rev. Bras. Farmacogn. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Navaz, M.A.; Sabitov, A.S.; Zinchenko, Y.N.; Rusakova, E.A.; Petrusha, E.N.; Golokhvast, K.S.; Tikhonova, N.G. The Global Metabolome Profiles of Four Varieties of Lonicera Caerulea, Established via Tandem Mass Spectrometry. Horticulturae 2023, 9, 1188. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-Based Rapid Screening of Phenolics and Triterpenic Acids in Leaf Extracts of Ocimum Species and Their Interspecies Variation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Wang, J.; Chen, G.; Tao, X.; Xu, S. Metabolomic Analysis Reveals Domestication-Driven Reshaping of Polyphenolic Antioxidants in Soybean Seeds. Antioxidants 2023, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of Phenolic Compounds in Jocote (Spondias purpurea L.) Peels by Ultra High-Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Rini Vijayan, K.P.; Raghu, A.V. Tentative Characterization of Phenolic Compounds in Three Species of the Genus Embelia by Liquid Chromatography Coupled with Mass Spectrometry Analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Wang, Y.; Vorsa, N.; Harrington, P.d.B.; Chen, P. Nontargeted Metabolomic Study on Variation of Phenolics in Different Cranberry Cultivars Using UPLC-IM—HRMS. J. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G. Colonic Fermentation of Polyphenols from Chilean Currants (Ribes Spp.) and Its Effect on Antioxidant Capacity and Metabolic Syndrome-Associated Enzymes. Food Chem. 2018, 258, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.-P.; Yang, B. Characterization of Metabolite Profiles of Leaves of Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Tekutyeva, L.A.; Podvolotskaya, A.B.; Stepochkina, V.D.; Zakharenko, A.M.; Golokhvast, K. Zostera marina L.: Supercritical CO2-Extraction and Mass Spectrometric Characterization of Chemical Constituents Recovered from Seagrass. Separations 2022, 9, 182. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Low Molecular Weight Phenolics of Grape Juice and Winemaking Byproducts: Antioxidant Activities and Inhibition of Oxidation of Human Low-Density Lipoprotein Cholesterol and DNA Strand Breakage. J. Agric. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef]
- D’Urso, G.; Sarais, G.; Lai, C.; Pizza, C.; Montoro, P. LC-MS Based Metabolomics Study of Different Parts of Myrtle Berry from Sardinia (Italy). J. Berry Res. 2017, 7, 217–229. [Google Scholar] [CrossRef]
- Han, F.; Li, Y.; Ma, L.; Liu, T.; Wu, Y.; Xu, R.; Song, A.; Yin, R. A Rapid and Sensitive UHPLC-FT-ICR MS/MS Method for Identification of Chemical Constituents in Rhodiola Crenulata Extract, Rat Plasma and Rat Brain after Oral Administration. Talanta 2016, 160, 183–193. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic Compounds in Strawberry (Fragaria x Ananassa Duch.) Fruits: Composition in 27 Cultivars and Changes during Ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Serrano, C.A.; Villena, G.K.; Rodríguez, E.F. Phytochemical Profile and Rosmarinic Acid Purification from Two Peruvian Lepechinia Willd. Species (Salviinae, Mentheae, Lamiaceae). Sci. Rep. 2021, 11, 7260. [Google Scholar] [CrossRef]
- Felegyi-Tóth, C.A.; Garádi, Z.; Darcsi, A.; Csernák, O.; Boldizsár, I.; Béni, S.; Alberti, Á. Isolation and Quantification of Diarylheptanoids from European Hornbeam (Carpinus betulus L.) and HPLC-ESI-MS/MS Characterization of Its Antioxidative Phenolics. J. Pharm. Biomed. Anal. 2022, 210, 114554. [Google Scholar] [CrossRef]
- Kheyar-Kraouche, N.; da Silva, A.B.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by Liquid Chromatography–Mass Spectrometry and Antioxidant Activity of an Ethanolic Extract of Inula Viscosa Leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Applied to the Identification of Valuable Phenolic Compounds from Eucalyptus Wood. J. Chromatogr. B 2013, 938, 65–74. [Google Scholar] [CrossRef]
- Zielińska, D.; Turemko, M. Electroactive Phenolic Contributors and Antioxidant Capacity of Flesh and Peel of 11 Apple Cultivars Measured by Cyclic Voltammetry and HPLC–DAD–MS/MS. Antioxidants 2020, 9, 1054. [Google Scholar] [CrossRef]
- Okhlopkova, Z.; Ercisli, S.; Razgonova, M.; Ivanova, N.; Antonova, E.; Egorov, Y.; Kucharova, E.; Golokhvast, K. Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia Martjanovii Krasch: Samples from Yakutia. Horticulturae 2023, 9, 1329. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.Å.; Smeds, A.I.; Sjöholm, R.E. Identification of Lignans by Liquid Chromatography-electrospray Ionization Ion-trap Mass Spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Tong, C.; Fu, Q.; Xu, J.; Shi, S.; Xiao, Y. Identification of Minor Lignans, Alkaloids, and Phenylpropanoid Glycosides in Magnolia Officinalis by HPLC–DAD–QTOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 170, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Chang, F.-R.; Chen, S.-L.; Wu, C.-C.; Lee, K.-H.; Wu, Y.-C. Novel Cytotoxic Monotetrahydrofuranic Annonaceous Acetogenins from Annona Montana. Bioorg. Med. Chem. 2005, 13, 4767–4776. [Google Scholar] [CrossRef]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. Int. Sch. Res. Not. 2013, 2013, 813563. [Google Scholar] [CrossRef]
- Rezaire, A.; Robinson, J.-C.; Bereau, D.; Verbaere, A.; Sommerer, N.; Khan, M.K.; Durand, P.; Prost, E.; Fils-Lycaon, B. Amazonian Palm Oenocarpus Bataua (“Patawa”): Chemical and Biological Antioxidant Activity—Phytochemical Composition. Food Chem. 2014, 149, 62–70. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Xu, J.; Liu, X.; Wang, S.; Shi, L. Physiological and Metabolomics Analyses of Young and Old Leaves from Wild and Cultivated Soybean Seedlings under Low-Nitrogen Conditions. BMC Plant Biol. 2019, 19, 389. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and Glycoalkaloid Contents in Potato Cultivars Grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef]
- Sharma, M.; Sandhir, R.; Singh, A.; Kumar, P.; Mishra, A.; Jachak, S.; Singh, S.P.; Singh, J.; Roy, J. Comparative Analysis of Phenolic Compound Characterization and Their Biosynthesis Genes between Two Diverse Bread Wheat (Triticum aestivum) Varieties Differing for Chapatti (Unleavened Flat Bread) Quality. Front. Plant Sci. 2016, 7, 1870. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of Key Metabolites Based on Non-Targeted Metabolomics and Chemometrics Analyses Provides Insights into Bitterness in Kucha [Camellia Kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef]
- Hou, S.; Zhu, J.; Ding, M.; Lv, G. Simultaneous Determination of Gibberellic Acid, Indole-3-Acetic Acid and Abscisic Acid in Wheat Extracts by Solid-Phase Extraction and Liquid Chromatography–Electrospray Tandem Mass Spectrometry. Talanta 2008, 76, 798–802. [Google Scholar] [CrossRef]
- Gidda, S.K.; Miersch, O.; Levitin, A.; Schmidt, J.; Wasternack, C.; Varin, L. Biochemical and Molecular Characterization of a Hydroxyjasmonate Sulfotransferase from Arabidopsis Thaliana. J. Biol. Chem. 2003, 278, 17895–17900. [Google Scholar] [CrossRef]
- Ayoub, I.M.; El-Baset, M.A.; Elghonemy, M.M.; Bashandy, S.A.E.; Ibrahim, F.A.A.; Ahmed-Farid, O.A.H.; El Gendy, A.E.-N.G.; Afifi, S.M.; Esatbeyoglu, T.; Farrag, A.R.H.; et al. Chemical Profile of Cyperus Laevigatus and Its Protective Effects against Thioacetamide-Induced Hepatorenal Toxicity in Rats. Molecules 2022, 27, 6470. [Google Scholar] [CrossRef]
- Urbanová, T.; Tarkowská, D.; Novák, O.; Hedden, P.; Strnad, M. Analysis of Gibberellins as Free Acids by Ultra Performance Liquid Chromatography–Tandem Mass Spectrometry. Talanta 2013, 112, 85–94. [Google Scholar] [CrossRef]
- Chuah, W.C.; Lee, H.H.; Ng, D.H.J.; Ho, A.L.; Sulaiman, M.R.; Chye, F.Y. Antioxidants Discovery for Differentiation of Monofloral Stingless Bee Honeys Using Ambient Mass Spectrometry and Metabolomics Approaches. Foods 2023, 12, 2404. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling Polyphenols of Two Diploid Strawberry (Fragaria Vesca) Inbred Lines Using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef]
- El-sayed, M.; Abbas, F.; Refaat, S.; El-Shafae, A.; Fikry, E. UPLC-ESI-MS/MS Profile of The Ethyl Acetate Fraction of Aerial Parts of Bougainvillea “Scarlett O’Hara” Cultivated in Egypt. Egypt. J. Chem. 2020, 64, 793–806. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, B.W.; Park, K.H.; Jeong, S.H.; Kim, H.-T.; Ko, J.-M.; Baek, I.-Y.; Lee, J.H. Rapid Characterisation and Comparison of Saponin Profiles in the Seeds of Korean Leguminous Species Using Ultra Performance Liquid Chromatography with Photodiode Array Detector and Electrospray Ionisation/Mass Spectrometry (UPLC–PDA–ESI/MS) Analysis. Food Chem. 2014, 146, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of Carotenoid Profiles in Goldenberry (Physalis peruviana L.) Fruits at Various Ripening Stages and in Different Plant Tissues by HPLC-DAD-APCI-MS. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Calvete, D.; Guauque-Medina, J.; Villegas-Torres, M.F.; Montoya, G. Analysis of Triacylglycerides, Carotenoids and Capsaicinoids as Disposable Molecules from Capsicum Agroindustry. Hortic. Environ. Biotechnol. 2019, 60, 227–238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).