Taxus baccata L. Under Changing Climate Conditions in the Steppe Zone of the East European Plain
Abstract
1. Introduction
2. Materials and Methods
2.1. Object of Study
- A clump of common yew in the steppe zone of the arboretum of the botanical garden was selected. The age of the plant is 48–50 years (according to archival data and cuts from the xylotheque of the scientific laboratory for monitoring and forecasting the ecosystems of Donbass), and it grows without care and artificial watering, in conditions of relative control.
- Common yew grown by cuttings from mother plants from a clump of the botanical garden. Selected in 2011 and planted as a seedling in 2013. Age 15 years. It is in a private collection, and grows under relative control conditions.
- The studies were conducted on yew plants growing without the influence of the human factor and only observing the behavior of clumps in natural conditions.
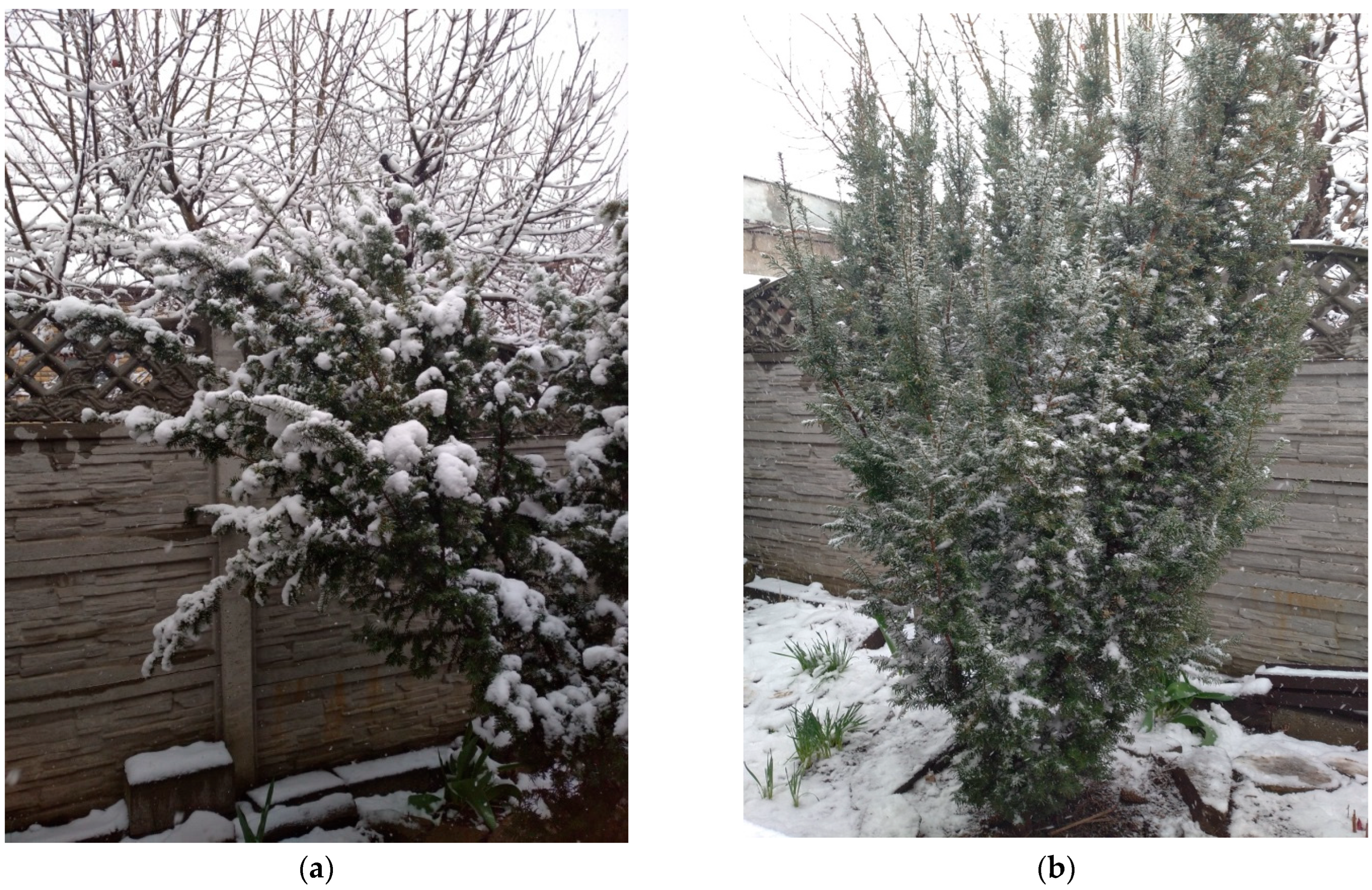
- A study of wood memory under the constant influence of an observer was conducted. When loading skeletal branches with snow cover, we artificially removed part of the load to prevent the action of the critical mass on the organs of T. baccata, thereby disrupting the program for developing the shape memory effect in the body. Depending on the date of the study (meaning, for example, 2017, 2018, 2019, 2022, 2023 years with significant snowstorms and precipitation), the number of load/unload cycles ranged from 2 to 3 to 5 per field observation per day.
2.2. Dendrological Research
2.3. Conducting Biochemical Studies
3. Results
3.1. Biomechanical Studies
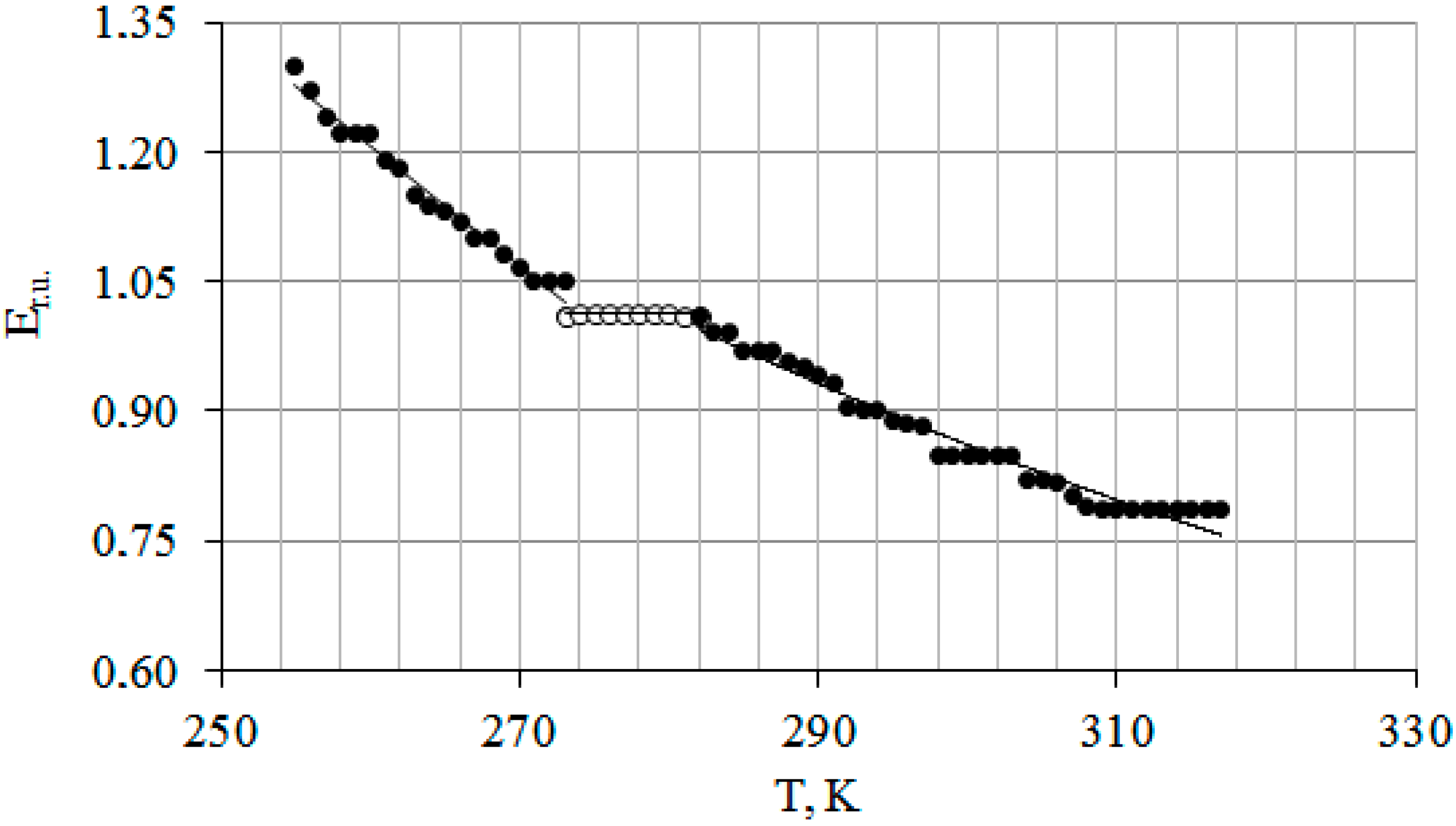
3.1.1. Effect of Temperature on the Elastic Modulus (E) of Wood Tissues of T. baccata In Vitro (Laboratory Studies)
3.1.2. The Influence of Temperature on the Mechanical Stability of Woody Plants T. baccata In Situ
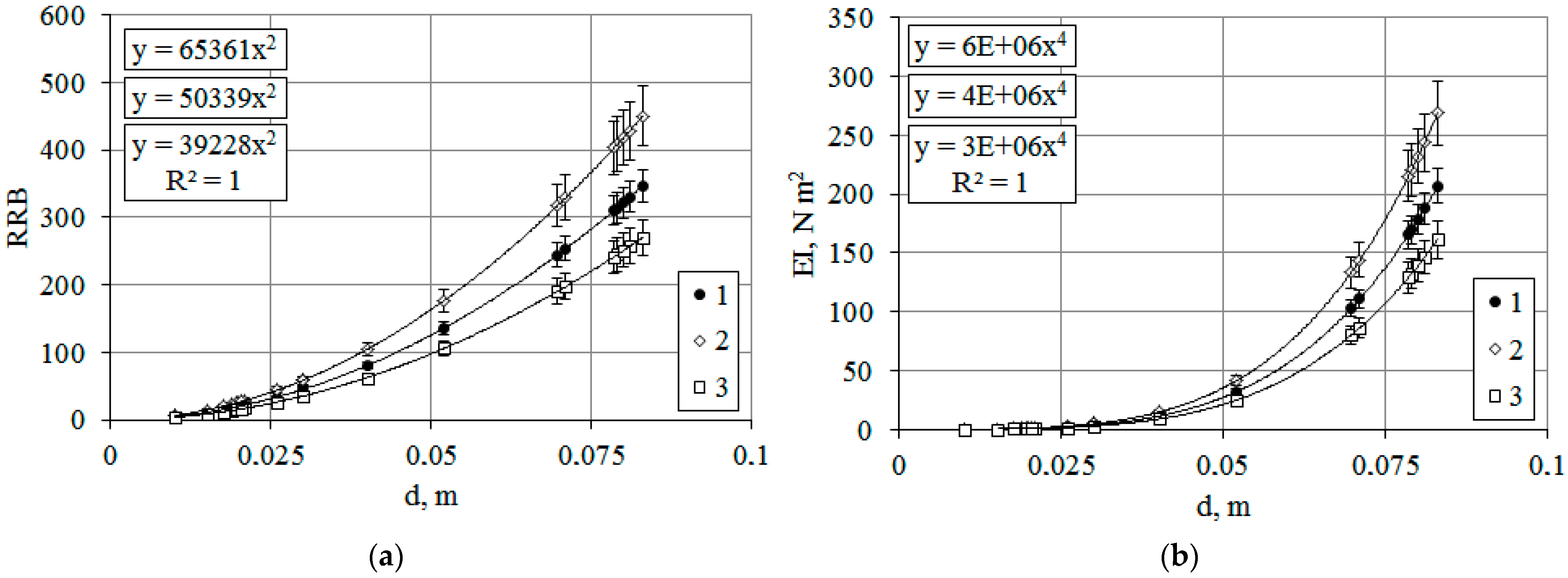
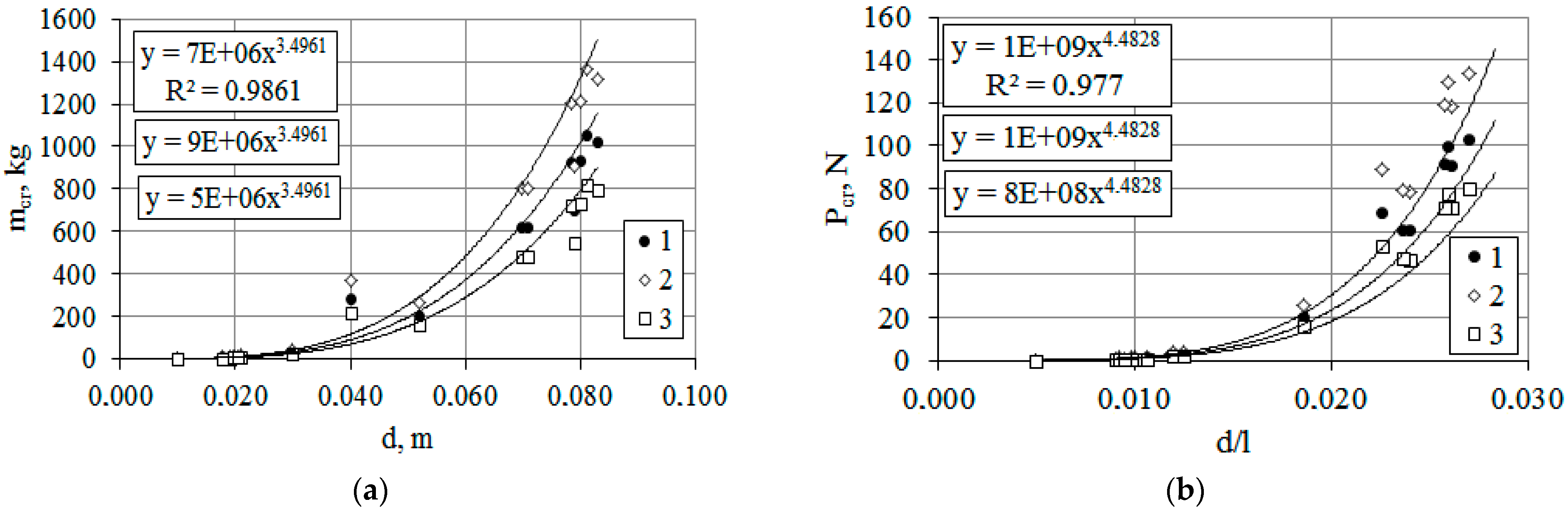
3.1.3. The Influence of Cyclic Processes on the Mechanical Stability of Woody Plants T. baccata
4. Discussion
5. Conclusions
- We recommend introducing Taxus baccata L. into the practice of the landscaping of industrial cities of the steppe zone of the Donetsk ridge as an obligatory species that has passed introduction tests for resistance to climate change in the region.
- We recommend forming both single highly decorative plantings in park zones and forest artificial arrays in order to increase the forest cover of the territory.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| E | Modulus of elasticity |
| MC | Moisture content |
| RRB | Relative bending resistance |
| SME | Shape memory effect |
References
- Dahle, G.A.; Grabosky, J.C. Variation in modulus of elasticity (E) along Acer platanoides L. (Aceraceae) branches. Urban For. Urban Green. 2010, 9, 227–233. [Google Scholar] [CrossRef]
- James, K.R.; Dahle, G.A.; Grabosky, J.; Kane, B.C.; Detter, A. Tree biomechanics literature review: Dynamics. Arboric. Urban For. 2014, 40, 1–15. [Google Scholar] [CrossRef]
- Chen, R.; Ran, J.; Hu, W.; Dong, L.; Ji, M.; Jia, X.; Lu, J.; Gong, H.; Aqeel, M.; Yao, S.; et al. Effects of biotic and abiotic factors on forest biomass fractions. Natl. Sci. Rev. 2021, 8, nwab025. [Google Scholar] [CrossRef] [PubMed]
- Dahle, G.A.; James, K.; Kane, B.C.; Grabosky, J.; Detter, A. A Review of Factors That Affect the Static Load-Bearing Capacity of Urban Trees. Arboric. Urban For. 2017, 43, 89–106. [Google Scholar] [CrossRef]
- Jelonek, T.; Tomczak, A.; Karaszewski, Z.; Jakubowski, M.; Arasimowicz-Jelonek, M.; Grzywiński, W.; Kopaczyk, J.; Klimek, K. The biomechanical formation of trees. Drewno 2019, 62, 5–22. [Google Scholar] [CrossRef]
- Korniyenko, V.O.; Kalaev, V.N. Impact of natural climate factors on mechanical stability and failure rate in silver birch trees in the city of Donetsk. Contemp. Probl. Ecol. 2022, 15, 806–816. [Google Scholar] [CrossRef]
- Kornienko, V.O.; Yaitsky, A.S. Mechanical stability of Fagus sylvatica L. in the conditions of the south of the East European Plain: The theory of loss of stability. Samara J. Sci. 2024, 13, 42–51. [Google Scholar] [CrossRef]
- Kornienko, V.O.; Kalaev, V.N. Viability of pedunculate oak in the conditions of the city of Donetsk. Sib. J. For. Sci. 2024, 4, 95–106. [Google Scholar] [CrossRef]
- Kornienko, V.O.; Kalaev, V.N. Mechanical stability of Virginian juniper trees in steppe zone of the eastern-european plain. Lesovedenie 2024, 1, 70–78. [Google Scholar] [CrossRef]
- James, K.R.; Haritos, N.; Ades, P.K. Mechanical stability of trees under dynamic loads. Am. J. Bot. 2006, 93, 1522–1530. [Google Scholar] [CrossRef]
- Nock, C.A.; Lecigne, B.; Taugourdeau, O.; Greene, D.F.; Dauzat, J.; Delagrange, S.; Messier, C. Linking ice accretion and crown structure: Towards a model of the effect of freezing rain on tree canopies. Ann. Bot. 2016, 117, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Spatz, H.C. Worldwide correlations of mechanical properties and green wood density. Am. J. Bot. 2010, 97, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J. Size- and Age-Dependent Variation in the Properties of Sap- and Heartwood in Black Locust (Robinia pseudoacacia L.). Ann. Bot. 1997, 79, 473–478. [Google Scholar] [CrossRef][Green Version]
- Niklas, K.J. Variations of the mechanical properties of Acer saccharum roots. J. Exp. Bot. 1999, 50, 193–200. [Google Scholar] [CrossRef]
- Spicer, R.A.; Niklas, K.J. Plant biomechanics: An Engineering Approach to Plant Form and Function. J. Ecol. 1993, 81, 592. [Google Scholar] [CrossRef][Green Version]
- Niklas, K.J.; Telewski, F.W. Environmental-Biomechanical Reciprocity and the Evolution of Plant Material Properties. J. Exp. Bot. 2022, 73, 1067–1079. [Google Scholar] [CrossRef]
- Pruyn, M.L.; Ewers, B.J.; Telewski, F.W. Thigmomorphogensesis: Changes in the morphology and mechanical properties of two Populus hybrid in response to mechanical perturbation. Tree Physiol. 2000, 20, 535–540. [Google Scholar] [CrossRef]
- Kern, K.A.; Ewers, F.W.; Telewski, F.W.; Koehler, L. Mechanical perturbation affects conductivity, mechanical properties, and aboveground biomass of hybrid poplars. Tree Physiol. 2005, 25, 1243–1251. [Google Scholar] [CrossRef]
- Green, D.W.; Evans, J.W.; Logan, J.D.; Nelson, W.J. Adjusting modulus of elasticity of lumber for changes in temperature. For. Prod. J. 1999, 49, 82–94. [Google Scholar]
- Szmutku, M.B.; Campean, M.; Laurenzi, W. Influence of cyclic freezing and thawing upon spruce wood properties. Pro Ligno. 2012, 8, 35–43. [Google Scholar]
- Szmutku, M.B.; Campean, M.; Sandu, A.V. Microstructure Modifications Induced in Spruce Wood by Freezing. Pro Ligno. 2011, 7, 26–31. [Google Scholar]
- Gao, S.; Wang, X.; Wang, L. Modeling temperature effect on dynamic modulus of elasticity of red pine (Pinus resinosa) in frozen and non-frozen states. Holzforschung 2015, 69, 233–240. [Google Scholar] [CrossRef]
- Green, D.W.; Evans, J.W. The Immediate Effect of Temperature on the Modulus of Elasticity of Green and Dry Lumber 1. Wood Fiber Sci. 2008, 40, 374–383. [Google Scholar]
- Zhao, L.; Lu, J.; Zhou, Y.; Jiang, J. Effect of Low Temperature Cyclic Treatments on Modulus of Elasticity of Birch Wood. Bioresources 2015, 10, 2318–2327. [Google Scholar] [CrossRef]
- Netsvetov, M.; Sergeyev, M.; Nikulina, V.; Korniyenko, V.; Prokopuk, Y. The climate to growth relationships of pedunculate oak in steppe. Dendrochronologia 2017, 44, 31–38. [Google Scholar] [CrossRef]
- Golovin, Y.I.; Tyurin, A.I.; Gusev, A.A.; Matveev, S.M.; Golovin, D.Y.; Vasyukova, I.A. Distribution of mechanical properties in annual growth rings of deciduous trees measured using scanning nanoindentation. Tech. Phys. Lett. 2024, 50, 110–114. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant Allometry: The Scaling of Form and Process; University of Chicago Press: Chicago, IL, USA, 1994; p. 396. [Google Scholar]
- Niklas, K.J. The allometry of safety-factors for plant height. Am. J. Bot. 1994, 81, 345–351. [Google Scholar] [CrossRef]
- Sellier, D.; Fourcaud, T. Crown structure and wood properties: Influence on tree sway and response to high winds. Am. J. Bot. 2009, 96, 885–896. [Google Scholar] [CrossRef]
- Zinicovscaia, I.I.; Safonov, A.I.; Yushin, N.S.; Nespirnyi, V.N.; Germonova, E.A. Phytomonitoring in Donbass for identifying new geochemical anomalies. Russ. J. Gen. Chem. 2024, 94, 3472–3482. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Safonov, A.; Kravtsova, A.; Chaligava, O.; Germonova, E. Neutron activation analysis of rare earth elements (Sc, La, Ce, Nd, Sm, Eu, Tb, Dy, Yb) in the diagnosis of ecosystems of Donbass. Phys. Part. Nuclei Lett. 2024, 21, 186–200. [Google Scholar] [CrossRef]
- Safonov, A.I.; Alemasova, A.S.; Zinicovscaia, I.I.; Vergel, K.N.; Yushin, N.S.; Kravtsova, A.V.; Chaligava, O. Morphogenetic abnormalities of bryobionts in geochemically contrasting conditions of Donbass. Geochem. Int. 2023, 61, 1036–1047. [Google Scholar] [CrossRef]
- Kornienko, V.O. A retrospective analysis of anthropogenic pollution of the city of Donetsk. Vibration and acoustic noise. Bull. Donetsk Natl. Univ. Ser. A Nat. Sci. 2024, 1, 93–100. [Google Scholar] [CrossRef]
- Sun, D.; Liddle, M.J. Trampling resistance, stem flexibility and leaf strength in nine Australian grasses and herbs. Biol. Conserv. 1993, 65, 35–41. [Google Scholar] [CrossRef]
- Sanson, G.D. The biomechanics of browsing and grazing. Am. J. Bot. 2006, 93, 1531–1545. [Google Scholar] [CrossRef]
- Read, J.; Stokes, A. Plant biomechanics in an ecological context. Am. J. Bot. 2006, 93, 1546–1565. [Google Scholar] [CrossRef]
- Cocroft, R.B.; Rodriguez, R.L. The behavioral ecology of insect vibrational communication. BioScience 2005, 55, 323–334. [Google Scholar] [CrossRef]
- Bailey, N.W.; Fowler-Finn, K.D.; Rebar, D.; Rodríguez, R.L. Green symphonies or wind in the willows? Testing acoustic communication in plants. Behav. Ecol. 2013, 24, 797–798. [Google Scholar] [CrossRef]
- Rebar, D.; Rodriguez, R.L. Trees to treehoppers: Genetic variation in host plants contributes to variation in the mating signals of a plant-feeding insect. Ecol. Lett. 2014, 17, 203–210. [Google Scholar] [CrossRef]
- Rebar, D.; Rodriguez, R.L. Insect mating signal and mate preference phenotypes covary among host plant genotypes. Evolution. 2015, 69, 602–610. [Google Scholar] [CrossRef]
- Fowler-Finn, K.D.; Cruz, D.C.; Rodriguez, R.L. Local population density and group composition influence the signal-preference relationship in Enchenopa treehoppers (Hemiptera: Membracidae). J. Evolut. Biol. 2017, 30, 13–25. [Google Scholar] [CrossRef]
- Netsvetov, M.; Nikulina, V. Seasonal variations of oscillation and vibration parameters of Acer platanoides. Dendrobiology 2010, 64, 37–42. [Google Scholar]
- Tittensor, R.M. Ecological history of yew Taxus baccata L. in Southern England. Biol. Conserv. 1980, 17, 243–265. [Google Scholar] [CrossRef]
- Linares, J.C. Shifting limiting factors for population dynamics and conservation status of the endangered English yew (Taxus baccata L., Taxaceae). Ecol. Manag. 2013, 291, 119–127. [Google Scholar] [CrossRef]
- Thomas, P.A.; Polwart, A. Taxus baccata L. J. Ecol. 2003, 91, 489–524. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Magard, E. Population ecology and conservation status of the last natural population of English yew Taxus baccata in Denmark. Biol. Conserv. 1999, 88, 173–182. [Google Scholar] [CrossRef]
- Alavi, S.J.; Ahmadi, K.; Hosseini, S.M.; Tabari, M.; Nouri, Z. The response of English yew (Taxus baccata L.) to climate change in the Caspian Hyrcanian Mixed Forest ecoregion. Reg. Environ. Change 2019, 19, 1495–1506. [Google Scholar] [CrossRef]
- Koc, D.E.; Svenning, J.-C.; Avci, M. Climate change impacts on the potential distribution of Taxus baccata L. in the Eastern Mediterranean and the Bolkar Mountains (Turkey) from Last Glacial Maximum to the future. Eurasian J. For. Sci. 2018, 6, 69–82. [Google Scholar] [CrossRef]
- Alekseev, V.A. Diagnostics of the vital state of trees and tree stands. Lesovedenie 1989, 4, 51–54. [Google Scholar]
- Niklas, K.J. Tree biomechanics with special reference to tropical trees in Tropical Tree Physiology. Trop. Tree Physiol. Adapt. Responses A Chang. Environ. 2016, 6, 413–435. [Google Scholar]
- Asare, S.; Stoescu, M.; Balmori, L.; Allena, S.; Kidando, E.; Owusu-Danquah, J. Freeze-thaw Effect on the mechanical properties of different wood species: Impact of moisture content variations, microscopic morphology, and thermal modification in a selected species. J. Infrastruct Preserv. Resil. 2024, 5, 14. [Google Scholar] [CrossRef]
- Gorbacheva, G.A.; Ugolev, B.N.; Sanaev, V.G.; Belkovskiy, S.Y. Characterization of the shape memory effect of beech wood by the method of thermomechanical spectrometry. For. Bull. 2016, 20, 10–14. [Google Scholar]
- Ugolev, B.N. Wood as a natural smart material. Wood Sci. Technol. 2014, 48, 553–568. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornienko, V.; Shkirenko, A.; Reuckaya, V.; Meskhi, B.; Dzhedirov, D.; Olshevskaya, A.; Odabashyan, M.; Shevchenko, V.; Mangasarian, D.; Kulikova, N. Taxus baccata L. Under Changing Climate Conditions in the Steppe Zone of the East European Plain. Plants 2025, 14, 1970. https://doi.org/10.3390/plants14131970
Kornienko V, Shkirenko A, Reuckaya V, Meskhi B, Dzhedirov D, Olshevskaya A, Odabashyan M, Shevchenko V, Mangasarian D, Kulikova N. Taxus baccata L. Under Changing Climate Conditions in the Steppe Zone of the East European Plain. Plants. 2025; 14(13):1970. https://doi.org/10.3390/plants14131970
Chicago/Turabian StyleKornienko, Vladimir, Alyona Shkirenko, Valeriya Reuckaya, Besarion Meskhi, Dmitry Dzhedirov, Anastasiya Olshevskaya, Mary Odabashyan, Victoria Shevchenko, Dzhuletta Mangasarian, and Natalia Kulikova. 2025. "Taxus baccata L. Under Changing Climate Conditions in the Steppe Zone of the East European Plain" Plants 14, no. 13: 1970. https://doi.org/10.3390/plants14131970
APA StyleKornienko, V., Shkirenko, A., Reuckaya, V., Meskhi, B., Dzhedirov, D., Olshevskaya, A., Odabashyan, M., Shevchenko, V., Mangasarian, D., & Kulikova, N. (2025). Taxus baccata L. Under Changing Climate Conditions in the Steppe Zone of the East European Plain. Plants, 14(13), 1970. https://doi.org/10.3390/plants14131970









