Comparative Effects of Non-Composted and Composted Sewage Sludge from Wastewater Treatment Plants on the Physiological and Antioxidative Responses of Maize
Abstract
1. Introduction
2. Results
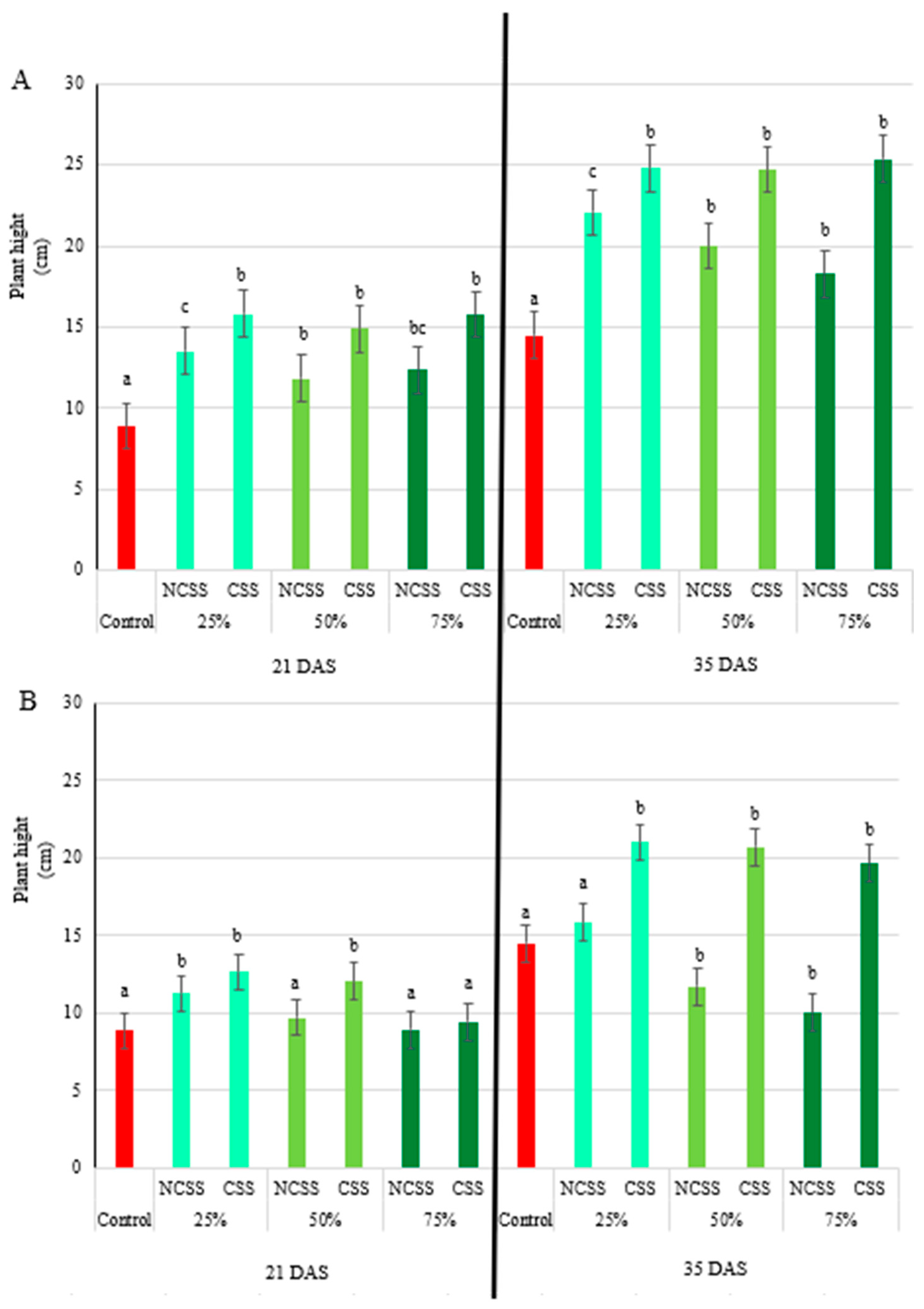
2.1. Plant Height
2.2. Photosynthetic Pigments Content
2.3. Photosynthetic Efficiency Parameters
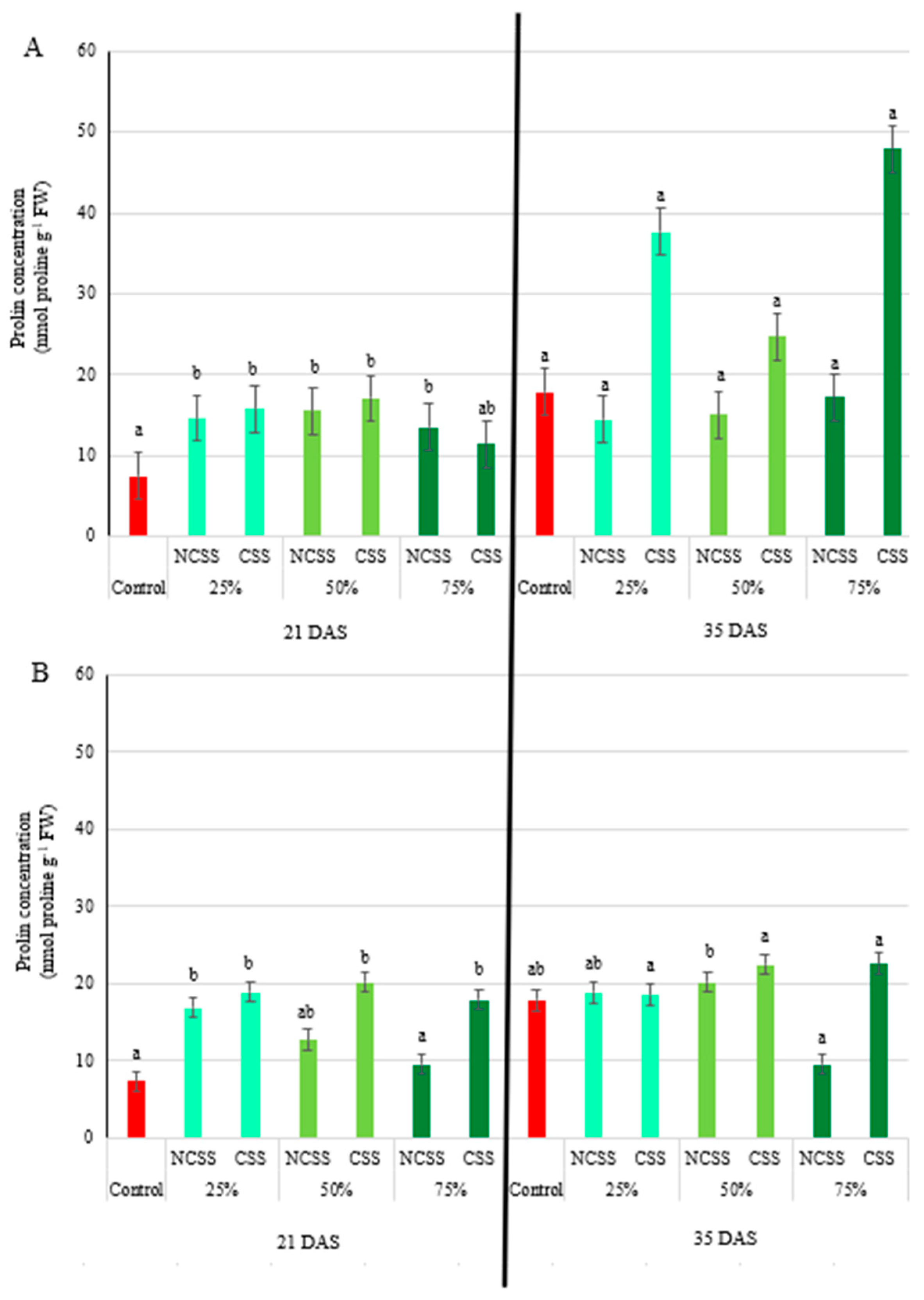
2.4. Proline Concentration
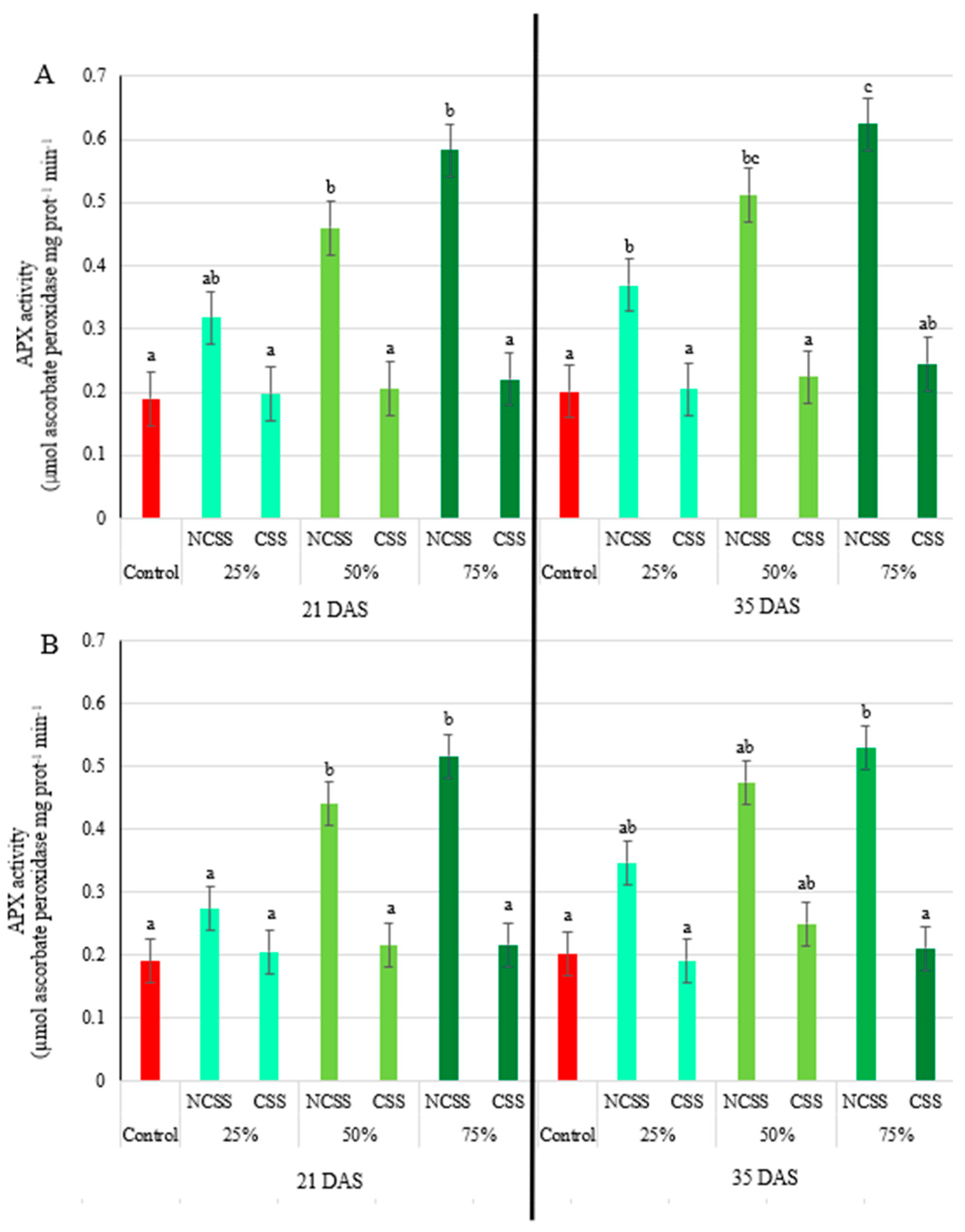
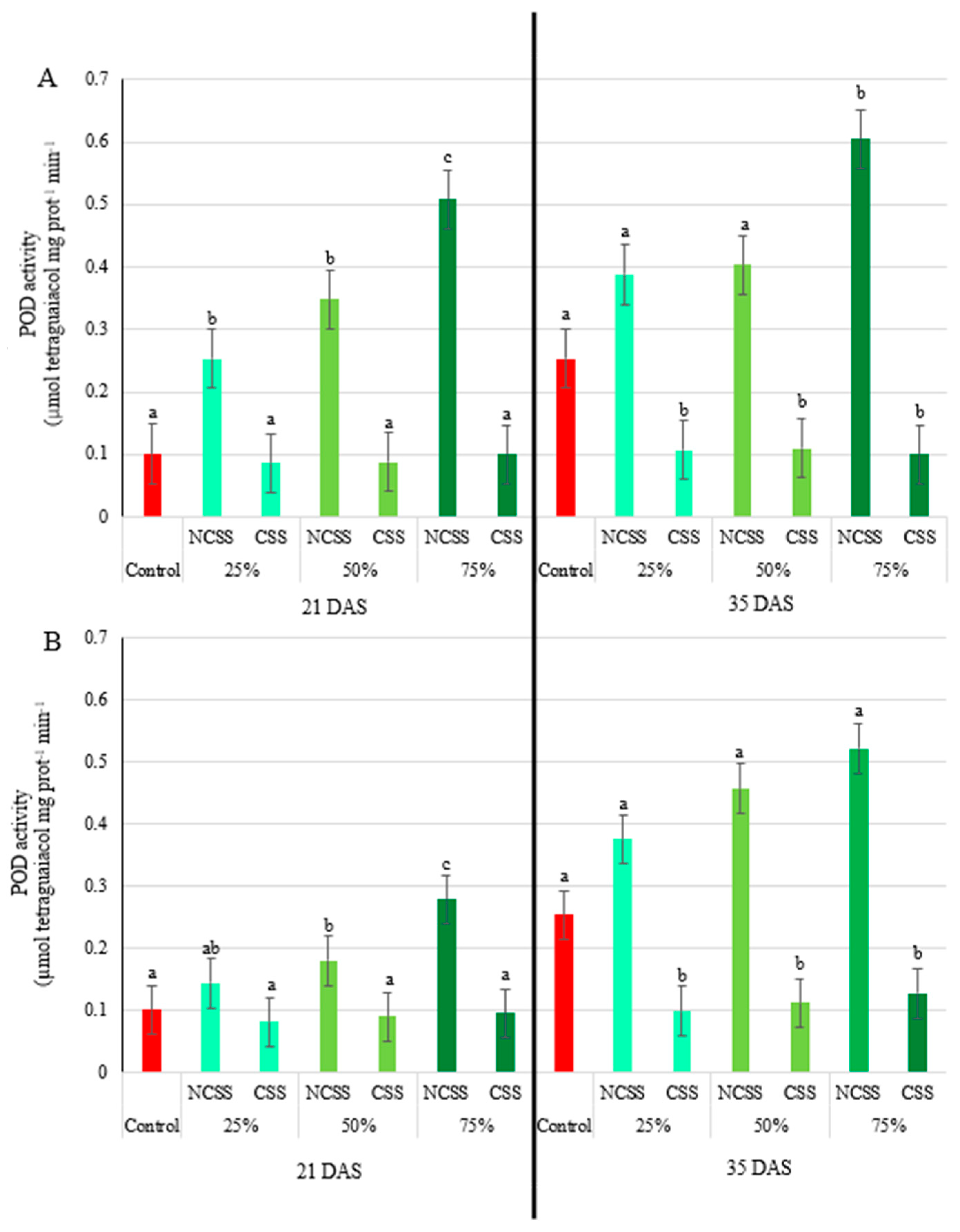
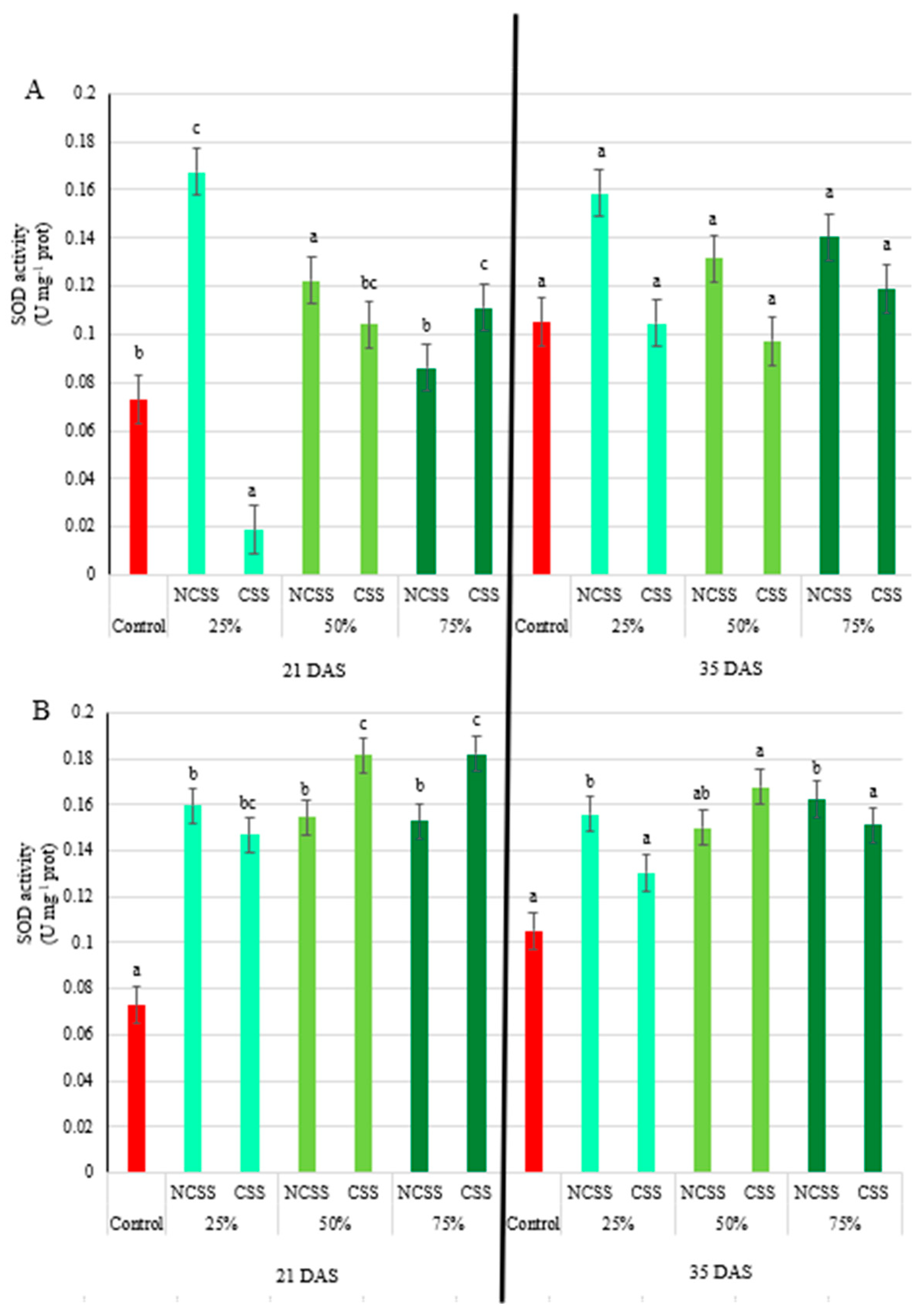
2.5. Antioxidative Enzymes Activity
3. Discussion
4. Materials and Methods
4.1. Experimental Conditions and Treatments
4.2. Composting Process Explanation
4.2.1. Debrecen
4.2.2. Kecskemét
4.3. Determination of Plant Height
4.4. Measurement of the Photosynthetic Pigments
4.5. Measurement of Photochemical Efficiency
4.6. Measurement of the Antioxidant Enzyme Activities
4.7. Proline Determination
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNEP: United Nations Environment Programme’s “Global Waste Management Outlook 2024. Available online: https://www.unep.org/resources/global-waste-management-outlook-2024#:~:text=on%20waste%20management-,Making%20rubbish%20a%20resource%20to%20end%20wasteful%20culture,economic%20disparities%20in%20affected%20communities (accessed on 14 April 2025).
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. NRF 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Pratap, B.; Kumar, S.; Nand, S.; Azad, I.; Bharagava, R.N.; Ferreira, L.F.; Dutta, V. Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety. Chemosphere 2024, 313, 137547. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Yue, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. Efficiency of sewage sludge biochar in improving urban soil properties and promoting grass growth. Chemosphere 2017, 173, 551–556. [Google Scholar] [CrossRef]
- Hao, J.; Tan, J.; Zhang, Y.; Gu, X.; Zhu, G.; Wang, S. Sewage sludge-derived nutrients and biostimulants stimulate rice leaf photosynthesis and root metabolism to enhance carbohydrate, nitrogene and antioxidants accumulation. Chemosphere 2024, 352, 141335. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of Heavy Metal Pollution and Potential Ecological Risk in Sewage Sludge from Municipal Wastewater Treatment Plant Located in the Most Industrialized Region in Poland—Case Study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef]
- EPA 2024. Available online: https://www.epa.gov/biosolids/and-polyfluoroalkyl-substances-pfas-sewage-sludge (accessed on 10 April 2025).
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Moneim, D.A.E.; Ahmed, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Antolín, M.C.; Muro, I.; Sánchez-Diáz, M. Sewage sludge application can induce changes in antioxidant status of nodulated alfalfa plants. Ecotoxicol. Environ. Saf. 2010, 73, 436–442. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfigar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defence in plants under abiotic stress: Revisiting the crucial role of a universal defence regulator. Antioxidants 2020, 29, 681. [Google Scholar] [CrossRef]
- Liu, Q.; Zou, X.; Xiao, C.; Luo, L. Effects of Sewage Sludge Application on Growth of Maize Grown in Aluminum-Toxic Soils. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Lakhdar, A.; Iannelli, M.A.; Debez, A.; Massacci, A.; Jedidi, N.; Abdelly, C. Effect of municipal solid waste compost and sewage sludge use on wheat (Triticum durum): Growth, heavy metal accumulation, and antioxidant activity. J. Sci. Food Agric. 2010, 90, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Ghosh, N.; Mandal, C.; Das, K.; Dey, N.; Adak, M.K. Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz. J. Plant Physiol. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Dhanya, G.; Vinod, G.V.; Radhamany, P.M. Effect of sewage sludge application on growth and photosynthetic pigment in vetiver grass (Vetiveria zizanioides L. Nash). Int. J. Adv. Biochem. Res. 2021, 5, 48–53. [Google Scholar]
- Vráblová, M.; Smutná, K.; Chamrádová, K.; Vrábl, D.; Koutník, I.; Rusín, J.; Bouchalová, M.; Gavlová, A.; Sezimová, H.; Navrátil, M.; et al. Co-composting of sewage sludge as an effective technology for the production of substrates with reduced content of pharmaceutical residues. Sci. Total Environ. 2024, 915, 169818. [Google Scholar] [CrossRef] [PubMed]
- Boutasknit, A.; Benaffari, W.; Anli, M.; Ouamnina, A.; Assouguem, A.; Lahlali, R.; Meddich, A. Comparative Effects of Compost and Arbuscular Mycorrhizal Fungi Versus NPK on Agro-Physiological, Biochemical and Tolerance Responses of Tomatoes to Drought. Phyton-IJEB 2024, 93, 3589–3616. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Effects of heavy metals on soil, plants, human health and aquatic life. Intern. J. Res. Chem. Environ. 2011, 2, 15–21. [Google Scholar]
- Wu, S.; Tursenjan, D.; Sun, Y. Impact of compost methods on humification and heavy metal passivation during chicken manure composting. J. Environ. Manag. 2023, 325, 116573. [Google Scholar] [CrossRef]
- Oksanen, J.; Pöykiö, R.; Dahl, O. Comparison of untreated, lime-stabilised and composted wastewater sludges from a pulp, board and paper mill integrate as a fertilise product. J. Ecol. Eng. 2021, 22, 47–58. [Google Scholar] [CrossRef]
- Cao, X.; Williams, P.N.; Zhan, Y.; Coughlin, S.A.; McGrath, J.W.; Chin, J.P.; Xu, Y. Municipal solid waste compost: Global trends and biogeochemical cycling. SEH 2023, 1, 100038. [Google Scholar] [CrossRef]
- Kelessidis, V.C.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.Y.; Liu, S.D.; Zhang, S.P.; Ma, H.J.; Chen, J.; Ge, C.W.; Shen, Q.; Zhang, X.M.; Zhao, X.H.; Zhang, Y.J.; et al. Transcriptome analysis and identification of genes associated with fruiting branch internode elongation in upland cotton. BMC Plant Biol. 2019, 19, 415. [Google Scholar] [CrossRef] [PubMed]
- Farsang, A.; Babcsányi, I.; Ladányi Zs Perei, K.; Bodor, A.; Csányi, K.T.; Barta, K. Evaluating the effects of sewage sludge compost applications on the microbial activity, the nutrient and heavy metal content of a Chernozem soil in a field survey. Arab. J. Geosci. 2020, 13, 982. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigment in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Phytosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Temizgül, A. Determination of Heavy-Metal Concentration with Chlorophyll Contents of Wheat (Triticum aestivum) Exposed to Municipal Sewage Sludge Doses. Comm. Soil Sci. Plant Anal. 2014, 45, 2754–2766. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef]
- Arena, M.E.; Pastur, G.M.; Lencinas, M.V.; Soler, R.; Bustamante, G. Changes in the leaf nutrient and pigment contents of Berberis microhylla G. Forst. in relation to irridance and fertilization. Heliyon 2020, 6, e03264. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Zhang, H.; Liu, W.; Zhang, M. Photosynthetic pigment changes and oxidative stress responses in plants exposed to heavy metals: A review of mechanisms and applications in environmental monitoring. Environ. Exp. Bot. 2022, 200, 104937. [Google Scholar] [CrossRef]
- Parida, S.; Dash, G.K.; Barik, M.; Sahoo, S.K.; Panda, R.K.; Baig, M.J.; Swain, P. Photosystem II photochemistry and chlorophyll intensity in rice (Oryza sativa L.) under drought at flowering stage. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 746–756. [Google Scholar]
- Rouillon, R.; Piletsky, S.A.; Breton, F.; Piletska, E.V.; Carpentier, R. Photosystem II Biosensors for Heavy Metals Monitoring. In Biotechnological Applications of Photosynthetic Proteins: Biochips, Biosensors and Biodevices; Biotechnology Intelligence Unit; Springer: Boston, MA, USA, 2006. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.X.; Wei, J.P.; Shen, C. Phytochrome-interacting factor 4 enhances heat tolerance via modulation of reactive oxygen species metabolism and photosynthetic efficiency in tomato. BMC Plant Biol. 2021, 21, 258. [Google Scholar]
- Yaish, M.W. Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.). Genet. Mol. Res. 2015, 14, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman, M.; Maqbool, A.; Rashid, N.; Ashraf, M. Role of proline under heavy metal stress in plants: A review. Environ. Exp. Bot. 2023, 206, 105174. [Google Scholar]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Nadarajah, K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 10829. [Google Scholar] [CrossRef]
- Li, Q.; Niu, C.; Guo, J.; Chen, G.; Li, J.; Sun, L.; Li, W.; Li, T. Physiological regulation underlying the alleviation of cadmium stress in maize seedlings by exogenous glycerol. Sci. Rep. 2025, 15, 11156. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Effects of sewage sludge application on soil microbial biomass and activity. Enrivon. Sci. Pollut. Res. 2016, 23, 5054–5063. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 403. [Google Scholar]
- Qasim, M.; Javed, N.; Himayatullah, M.; Subhan, M. Effect of Sewage Sludge on the Growth of Maize Crop. J. Biol. Sci. 2001, 1, 52–53. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Burducea, M.; Lobiuc, A.; Asandulesa, M.; Zaltariov, M.-F.; Burducea, I.; Popescu, S.M.; Zheljazkov, V.D. Effects of Sewage Sludge Amendments on the Growth and Physiology of Sweet Basil. Agronomy 2019, 9, 548. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Damalas, C.A.; Fotiadis, S.; Markopoulos, T. Species, Cultivar and Seasonal Effects on Nodulation and Nitrogen Utilization of Spring Mediterranean Grain Legumes. J. Soil Sci. Plant Nutr. 2023, 23, 4463–4473. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plants. Ecol. Eng. 2010, 36, 969–972. [Google Scholar] [CrossRef]
- Elsalam, H.E.A.; El-Sharnouby, M.E.; Mohamed, A.E.; Raafat, B.M.; El-Gamal, E.H. Effect of sewage sludge compost usage on corn and faba bean growth, carbon and nitrogen buedforms in plants and soil. Agronomy 2021, 11, 628. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Santos, T.; Palma, P.; Sengo, J.; Morais, M.C.; Cunha-Queda, C. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: Benefits versus limiting factors. Waste Manag. 2015, 40, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Natal-da-Luz, T.; Tidona, S.; Jesus, B.; Morais, P.V.; Sousa, J.P. The use of sewage sludge as soil amendment. The need for an ecotoxicological evaluation. J. Soil Sediment. 2009, 9, 246–260. [Google Scholar] [CrossRef]
- He, Y.; Yadav, V.; Bai, S.; Wu, J.; Zhou, X.; Zhang, W.; Han, S.; Wang, M.; Zeng, B.; Wu, X.; et al. Performance Evaluation of New Table Grape Varieties under High Light Intensity Conditions Based on the Photosynthetic and Chlorophyll Fluorescence Characteristics. Horticulturae 2023, 9, 1035. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Chen, D.; Huang, Y.; Kong, W.; Yuan, L.; Ye, H.; Huang, W. Assessment of Leaf Chlorophyll Content Models for Winter Wheat Using Landsat-8 Multispectral Remote Sensing Data. Remote Sens. 2020, 12, 2574. [Google Scholar] [CrossRef]
- Gitelson, A.; Arkebauer, T.; Viña, A.; Skakun, S.; Inoue, Y. Evaluating plant photosynthetic traits via absorption coefficient in the photosynthetically active radiation region. RSE 2021, 258, 112401. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, Z.; Han, J.; Zhao, H.; Liu, J.; Yu, Y. Suppression of chorismate synthase, which is localized in chloroplasts and peroxisomes, results in abnormal flower development and anthocyanin reduction in petunia. Sci. Rep. 2020, 10, 10846. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.D.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Banu, N. Extraction and estimation of chlorophyll from medicinal plants. Int. J. Sci. Res. IJSR 2015, 4, 209–212. [Google Scholar]
- Zhu, J.; Liang, Y.; Zhu, Y.; Hao, W.; Lin, X.; Wu, X.; Luo, A. The interactive effects of water and fertilizer on photosynthesis capacity and yield in tomato plants. Aust. J. Crop Sci. 2012, 6, 200–209. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Csiszár, J.; Gallé, Á.; Horváth, E.; Dancsó, P.; Gombos, M.; Váry Zs Erdei, L.; Györgye, J.; Tari, I. Different peroxidase activities and expression of abiotic stress-related peroxidase in apical root segments of wheat genotypes with different drought stress tolerance under osmotic stress. Plant Physiol. Biochem. 2012, 52, 119–129. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Muhammad, A.; Kong, X.; Zheng, S.; Bai, N.; Li, L.; Khan, M.H.U.; Fiaz, S.; Zhang, Z. Exploring plant-microbe interactions in adapting to abiotic stress under climate change: A review. Front. Plant Sci. 2024, 15, 1482739. [Google Scholar] [CrossRef]
- Ying, Z.; Fu, S.; Yang, Y. Singaling and scavenging: Unraveling the complex network of antioxidnat enzyme regulation in plant cold adaptation. Plant Stress 2025, 16, 100833. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L.; Hernandez, M.T.; Garcia, C. Application of different organic wastes in a soil polluted by cadmium: Effects on soil biological properties. Geoderma 2006, 130, 121–129. [Google Scholar] [CrossRef]
- Eriksen-Hamel, N.S.; Whalen, J.K. Compost amendments increase the abundance of soil microarthropods and enhance soil quality. Soil Biol. Biochem. 2007, 39, 291–302. [Google Scholar]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, genlesse expression and antioxidant enzyme activity in Arabidobsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Chen, R.G.; Gong, Z.H.; Yin, Y.X.; Ahmed, S.S.; He, Y.M. Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper (Capsicum annuum) leaves subjected to chilling stress. Genet. Mol. Res. 2012, 11, 4063–4080. [Google Scholar] [CrossRef]
- Miśkowiec, P.; Olech, Z. Searching for the Correlation Between the Activity of Urease and the Content of Nickel in the Soil Samples: The Role of Metal Speciation. J. Soil Sci. Plant Nutr. 2020, 20, 1904–1911. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, Y.; Adeel, M.; Shakoor, N.; Zhao, W.; Liu, Y.; Li, Y.; Li, M.; Azeem, I.; Rui, Y.; et al. Nickel Oxide Nanoparticles Improve Soybean Yield and Enhance Nitrogen Assimilation. Environ. Sci. Technol. 2023, 57, 7547–7558. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. A plant economics spectrum of litter decomposability. Funct. Ecol. 2018, 32, 260–272. [Google Scholar] [CrossRef]
- Prates, A.R.; Kawakami, K.C.; Coscione, A.R.; Filho, M.C.M.T.; Arf, O.; Abreu-Junior, C.H.; Oliveira, F.C.; Moreira, A.; Galindo, F.S.; He, Z.; et al. Composted Sewage Sludge Sustains High Maize Productivity on an Infertile Oxisol in the Brazilian Cerrado. Land 2022, 11, 1246. [Google Scholar] [CrossRef]
- Jaskulak, M.; Grobelak, A.; Grosser, A.; Vandenbulcke, F. Gene expression, DNA damage and other stress markers in Sinapsis alba L. exposed to heavy metals with special reference to sewage sludge application on contaminated sites. Ecotox. Environ. Saf. 2019, 181, 508–517. [Google Scholar] [CrossRef]
- Han, S.-H.; Lee, J.-C.; Jang, S.-S.; Kim, P.-G. Composted sewage sludge can improve the physiological properties of Betula schmidtii grown in tailings. J. Plant Biol. 2004, 47, 99–104. [Google Scholar] [CrossRef]
- Song, U.; Lee, E.J. Ecophysiological Responses of Plants After Sewage Sludge Compost Applications. J. Plant Biol. 2010, 53, 259–267. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll Determination in intact tissues using N, N-dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, R.A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvent with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J. Plant Physiol. 2005, 162, 873–885. [Google Scholar] [CrossRef]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the activities of antioxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef]
- Zeislin, N.; Ben-Zaken, R. Peroxidases, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. Biochem. 1991, 29, 147–151. [Google Scholar]
- Giannopolities, C.H.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plant. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. 2011. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline (accessed on 10 September 2024).
| Treatments | Chl-a | Chl-b | Car | Chl a/b | Total chl | Total Chl/car |
|---|---|---|---|---|---|---|
| 21 DAYS AFTER SOWING | ||||||
| Control | 12.72 ± 1.74 a | 4.76 ± 0.65 a | 3.96 ± 0.26 a | 2.71 ± 0.44 a | 19.12 ± 2.66 a | 2.68 ± 0.02 a |
| Debr 25% | 17.93 ± 3.74 a | 5.97 ± 2.25 a | 8.52 ± 1.49 a | 3.21 ± 0.72 a | 23.90 ± 5.85 a | 2.77 ± 0.22 a |
| Debr 50% | 21.14 ± 1.49 a | 9.56 ± 1.90 a | 10.35 ± 0.30 a | 2.30 ± 0.57 a | 30.70 ± 0.57 b | 2.97 ± 0.11 a |
| Debr 75% | 17.32 ± 1.59 a | 4.53 ± 0.49 a | 8.19 ± 0.63 a | 3.53 ± 0.55 a | 22.38 ± 2.71 a | 2.71 ± 0.02 a |
| Kecs 25% | 20.29 ± 0.79 b | 20.28 ± 1.09 b | 6.09 ± 0.70 c | 1.01 ± 0.05 a | 39.51 ± 2.71 b | 6.53 ± 0.95 b |
| Kecs 50% | 17.59 ± 3.68 b | 20.11 ± 0.59 b | 6.08 ± 0.07 c | 0.85 ± 0.12 a | 38.22 ± 5.88 b | 6.31 ± 0.95 b |
| Kecs 75% | 20.16 ± 1.59 b | 21.21 ± 1.89 b | 5.04 ± 0.18 b | 0.97 ± 0.02 a | 41.37 ± 3.28 b | 7.89 ± 0.54 c |
| 35 DAYS AFTER SOWING | ||||||
| Control | 9.51 ± 1.38 a | 3.93 ± 0.33 a | 6.61 ± 0.74 a | 3.82 ± 0.06 a | 13.26 ± 1.65 a | 2.57 ± 0.11 a |
| Debr 25% | 21.64 ± 1.10 a | 9.08 ± 1.04 a | 9.99 ± 0.61 a | 2.38 ± 0.07 a | 30.72 ± 1.60 b | 3.08 ± 0.04 a |
| Debr 50% | 22.64 ± 0.59 a | 8.61 ± 1.05 a | 10.30 ± 0.31 a | 2.46 ± 0.04 a | 29.99 ± 2.19 b | 2.99 ± 0.02 a |
| Debr 75% | 19.38 ± 2.17 a | 8.36 ± 2.41 a | 9.18 ± 0.10 a | 2.52 ± 0.88 a | 27.74 ± 1.64 b | 3.00 ± 0.07 a |
| Kecs 25% | 22.46 ± 0.78 c | 9.96 ± 1.72 c | 12.61 ± 0.56 b | 2.09 ± 0.16 a | 31.96 ± 2.56 c | 2.54 ± 0.19 b |
| Kecs 50% | 19.76 ± 0.94 bc | 7.59 ± 0.74 b | 12.04 ± 0.96 b | 2.52 ± 0.45 ab | 26.49 ± 1.78 bc | 2.13 ± 0.01 a |
| Kecs 75% | 18.11 ± 3.42 b | 5.71 ± 0.94 ab | 11.25 ± 1.64 b | 2.97 ± 0.13 b | 24.85 ± 5.81 b | 2.19 ± 0.26 a |
| Treatments | Chl-a | Chl-b | Car | Chl a/b | Total Chl | Total Chl/car |
|---|---|---|---|---|---|---|
| 21 DAYS AFTER SOWING | ||||||
| Control | 12.72 ± 1.74 a | 4.76 ± 0.65 b | 3.96 ± 0.26 a | 2.71 ± 0.44 a | 19.12 ± 2.66 a | 2.68 ± 0.02 a |
| Debr 25% | 9.93 ± 0.41 a | 3.69 ± 0.39 a | 4.01 ± 0.38 a | 2.71 ± 0.32 a | 13.62 ± 0.55 b | 3.39 ± 0.07 b |
| Debr 50% | 10.83 ± 1.54 a | 3.50 ± 0.20 a | 4.46 ± 0.77 a | 3.08 ± 0.34 a | 14.34 ± 1.69 b | 3.24 ± 0.17 b |
| Debr 75% | 11.96 ± 1.95 a | 4.13 ± 0.57 ab | 4.51 ± 0.92 a | 2.89 ± 0.23 a | 16.10 ± 2.48 ab | 3.60 ± 0.29 b |
| Kecs 25% | 18.99 ± 1.86 b | 7.04 ± 0.02 b | 9.04 ± 0.45 b | 2.80 ± 0.21 a | 26.41 ± 1.34 b | 2.98 ± 0.14 ab |
| Kecs 50% | 20.81 ± 0.33 b | 7.89 ± 1.45 b | 8.40 ± 1.11 b | 2.64 ± 0.35 a | 28.37 ± 2.96 b | 3.42 ± 0.58 b |
| Kecs 75% | 19.43 ± 0.43 b | 6.18 ± 0.37 ab | 8.47 ± 0.99 b | 2.99 ± 0.17 a | 25.49 ± 1.36 b | 2.83 ± 0.07 ab |
| 35 DAYS AFTER SOWING | ||||||
| Control | 9.51 ± 1.38 a | 3.93 ± 0.33 a | 6.61 ± 0.74 a | 3.82 ± 0.06 b | 13.26 ± 1.65 a | 2.57 ± 0.11 a |
| Debr 25% | 19.32 ± 2.59 b | 6.60 ± 1.09 b | 8.56 ± 0.98 b | 2.94 ± 0.11 a | 25.92 ± 1.77 b | 3.02 ± 0.09 b |
| Debr 50% | 18.43 ± 1.71 b | 5.95 ± 0.25 b | 8.17 ± 0.57 ab | 3.04 ± 0.31 a | 24.50 ± 1.77 b | 2.99 ± 0.08 b |
| Debr 75% | 18.12 ± 2.72 b | 5.94 ± 0.24 b | 7.95 ± 1.07 ab | 2.85 ± 0.39 a | 24.52 ± 3.51 b | 3.08 ± 0.04 b |
| Kecs 25% | 19.39 ± 1.78 b | 9.13 ± 0.37 b | 9.47 ± 0.73 b | 2.16 ± 0.10 a | 28.79 ± 3.31 b | 3.03 ± 0.13 b |
| Kecs 50% | 18.52 ± 1.26 b | 7.48 ± 1.14 b | 8.72 ± 0.59 b | 2.52 ± 0.43 ab | 26.00 ± 1.58 b | 2.98 ± 0.07 b |
| Kecs 75% | 20.09 ± 1.36 b | 8.30 ± 1.51 b | 9.05 ± 1.03 b | 2.47 ± 0.39 ab | 28.39 ± 2.39 b | 3.06 ± 0.04 b |
| Treatments | Fo | Fm | Fv | Fv/Fm | Fv/Fo |
|---|---|---|---|---|---|
| 21 DAYS AFTER SOWING | |||||
| Control | 155.00 ± 8.33 a | 767.50 ± 47.09 a | 612.50 ± 38.87 b | 0.78 ± 0.00 a | 3.59 ± 0.05 a |
| Debr 25% | 145.75 ± 2.63 a | 666.40 ± 13.14 a | 523.60 ± 10.45 a | 0.78 ± 0.01 a | 3.67 ± 0.19 a |
| Debr 50% | 143.67 ± 3.21 a | 689.60 ± 56.67 a | 545.00 ± 44.39 a | 0.78 ± 0.01 a | 3.77 ± 0.17 a |
| Debr 75% | 142.50 ± 3.11 a | 691.00 ± 6.25 a | 548.00 ± 8.71 a | 0.79 ± 0.01 a | 3.89 ± 0.16 a |
| Kecs 25% | 160.00 ± 5.32 a | 727.00 ± 38.66 a | 558.25 ± 14.66 ab | 0.78 ± 0.01 c | 3.67 ± 0.18 c |
| Kecs 50% | 164.80 ± 3.82 ab | 705.20 ± 39.97 a | 540.40 ± 37.15 a | 0.76 ± 0.00 b | 3.28 ± 0.18 ab |
| Kecs 75% | 186.40 ± 19.11 b | 752.00 ± 24.07 a | 570.50 ± 12.79 ab | 0.75 ± 0.01 a | 3.14 ± 0.25 b |
| 35 DAYS AFTER SOWING | |||||
| Control | 165.00 ± 9.98 a | 815.80 ± 23.38 a | 650.80 ± 13.44 a | 0.79 ± 0.01 a | 3.95 ± 0.16 a |
| Debr 25% | 161.67 ± 2.52 a | 802.00 ± 43.83 a | 641.20 ± 33.06 a | 0.80 ± 0.00 a | 3.99 ± 0.10 a |
| Debr 50% | 161.25 ± 1.70 a | 801.40 ± 29.45 a | 641.60 ± 27.01 a | 0.80 ± 0.01 a | 4.01 ± 0.13 a |
| Debr 75% | 157.60 ± 7.37 a | 776.60 ± 39.70 a | 616.33 ± 8.62 a | 0.80 ± 0.01 a | 3.93 ± 0.15 a |
| Kecs 25% | 217.80 ± 7.89 b | 787.80 ± 170.83 a | 570.00 ± 170.93 a | 0.71 ± 0.01 a | 2.62 ± 0.80 a |
| Kecs 50% | 218.20 ± 27.49 b | 735.00 ± 10.15 a | 507.40 ± 68.67 a | 0.70 ± 0.05 a | 2.38 ± 0.57 a |
| Kecs 75% | 240.80 ± 12.78 b | 782.00 ± 36.62 a | 541.20 ± 36.54 a | 0.69 ± 0.02 a | 2.25 ± 0.21 a |
| Treatments | Fo | Fm | Fv | Fv/Fm | Fv/Fo |
|---|---|---|---|---|---|
| 21 DAYS AFTER SOWING | |||||
| Control | 155.00 ± 8.33 b | 767.50 ± 47.09 b | 612.50 ± 38.87 b | 0.78 ± 0.00 a | 3.59 ± 0.05 a |
| Debr 25% | 145.80 ± 5.93 b | 734.40 ± 29.65 ab | 588.60 ± 24.82 ab | 0.80 ± 0.00 b | 4.02 ± 0.03 b |
| Debr 50% | 153.50 ± 4.12 b | 733.80 ± 33.30 ab | 583.20 ± 26.28 ab | 0.80 ± 0.00 b | 3.87 ± 0.06 b |
| Debr 75% | 132.00 ± 7.21 a | 669.80 ± 44.76 ab | 537.80 ± 39.32 a | 0.80 ± 0.00 b | 3.98 ± 0.07 b |
| Kecs 25% | 149.80 ± 13.61 ab | 739.75 ± 18.45 a | 595.00 ± 11.28 a | 0.80 ± 0.01 b | 4.09 ± 0.18 b |
| Kecs 50% | 156.25 ± 3.30 ab | 825.20 ± 53.69 b | 663.40 ± 42.25 b | 0.80 ± 0.01 b | 4.11 ± 0.15 b |
| Kecs 75% | 161.75 ± 4.03 b | 803.60 ± 39.66 ab | 645.00 ± 32.02 ab | 0.80 ± 0.00 b | 4.07 ± 0.05 b |
| 35 DAYS AFTER SOWING | |||||
| Control | 165.00 ± 9.98 a | 815.80 ± 23.38 a | 650.80 ± 13.44 a | 0.79 ± 0.01 a | 3.95 ± 0.16 a |
| Debr 25% | 167.80 ± 7.19 a | 875.40 ± 42.40 ab | 707.60 ± 35.45 ab | 0.81 ± 0.00 ab | 4.22 ± 0.01 ab |
| Debr 50% | 190.75 ± 3.50 b | 937.33 ± 17.24 b | 756.00 ± 10.15 b | 0.81 ± 0.01 ab | 4.25 ± 0.26 ab |
| Debr 75% | 164.80 ± 11.79 a | 897.20 ± 65.23 ab | 732.40 ± 53.48 ab | 0.82 ± 0.00 b | 4.44 ± 0.03 b |
| Kecs 25% | 159.25 ± 10.21 a | 840.00 ± 69.66 a | 680.75 ± 60.19 a | 0.81 ± 0.01 b | 4.27 ± 0.17 b |
| Kecs 50% | 154.20 ± 5.89 a | 809.40 ± 22.18 a | 655.20 ± 17.67 a | 0.81 ± 0.00 b | 4.26 ± 0.02 ab |
| Kecs 75% | 149.00 ± 12.62 a | 782.50 ± 87.41 a | 633.50 ± 74.88 a | 0.81 ± 0.01 ab | 4.24 ± 0.15 ab |
| Element Content (mg kg−1 Dry Material) | ||||||
|---|---|---|---|---|---|---|
| Debrecen | Kecskemét | Element Content Concerning the Permitted Limit | ||||
| Elements | NCSS | CSS | NCSS | CSS | CSS | NCSS |
| Cadmium | 1.00 | <1 | <1 | <1 | 5 | 10 |
| Cobalt | 4.39 | 4.72 | 6.06 | 4.64 | 50 | 50 |
| Chromium | 51.3 | 51.1 | 71.1 | 48.1 | 350 | 1000 |
| Copper | 218 | 139 | 199 | 119 | 750 | 1000 |
| Mercury | 0.76 | 0.91 | 0.25 | 0.28 | 5 | 10 |
| Nickel | 22.4 | 19.5 | 49 | 28 | 100 | 200 |
| Lead | 26.6 | 24 | 14.7 | 28.2 | 400 | 750 |
| Tests Performed | Soil Test Results | Extended Measurement Uncertainty |
|---|---|---|
| pH value (KCl) | 6.3 | ±5% |
| Gold-bonded number | 50 | ±10% |
| Total water soluble salinity [m/m%] | 0.06 | ±10% |
| Hydrochloric acid lime [m/m%] | 0.21 | ±10% |
| Humus content [m/m%] | 2.9 | ±10% |
| (nitrate + nitrite)-N (KCl soluble) [mg/kg] | 4.2 | ±5% |
| Phosphorus pentoxide (AL soluble) [mg/kg] | 211 | ±10% |
| Potassium oxide (AL soluble) [mg/kg] | 419 | ±10% |
| Sodium (AL soluble) [mg/kg] | 445 | ±10% |
| Magnesium (KCl-soluble) [mg/kg] | 647 | ±10% |
| Sulfated sulfur (KCl-soluble) [mg/kg] | 7.9 | ±5% |
| Cadmium (EDTA-Na2 soluble) [mg/kg] | <1 | ±10% |
| Cobalt (EDTA-Na2 soluble) [mg/kg] | <1 | ±10% |
| Chromium (EDTA-Na2 soluble) [mg/kg] | <1 | ±10% |
| Mercury (EDTA-Na2 soluble) [mg/kg] | <1 | ±10% |
| Nickel (EDTA-Na2 soluble) [mg/kg] | <1 | ±10% |
| Lead (EDTA-Na2 soluble) [mg/kg] | <1 | ±10% |
| Zinc (EDTA-Na2 soluble) [mg/kg] | 0.9 | ±10% |
| Copper (EDTA-Na2 soluble) [mg/kg] | 9 | ±10% |
| Manganese (EDTA-Na2 soluble) [mg/kg] | 201 | ±10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczur, D.; Moloi, M.J.; Mousavi, S.M.N.; Tóth, B. Comparative Effects of Non-Composted and Composted Sewage Sludge from Wastewater Treatment Plants on the Physiological and Antioxidative Responses of Maize. Plants 2025, 14, 1955. https://doi.org/10.3390/plants14131955
Kaczur D, Moloi MJ, Mousavi SMN, Tóth B. Comparative Effects of Non-Composted and Composted Sewage Sludge from Wastewater Treatment Plants on the Physiological and Antioxidative Responses of Maize. Plants. 2025; 14(13):1955. https://doi.org/10.3390/plants14131955
Chicago/Turabian StyleKaczur, Dávid, Makoena Joyce Moloi, Seyed Mohammad Nasir Mousavi, and Brigitta Tóth. 2025. "Comparative Effects of Non-Composted and Composted Sewage Sludge from Wastewater Treatment Plants on the Physiological and Antioxidative Responses of Maize" Plants 14, no. 13: 1955. https://doi.org/10.3390/plants14131955
APA StyleKaczur, D., Moloi, M. J., Mousavi, S. M. N., & Tóth, B. (2025). Comparative Effects of Non-Composted and Composted Sewage Sludge from Wastewater Treatment Plants on the Physiological and Antioxidative Responses of Maize. Plants, 14(13), 1955. https://doi.org/10.3390/plants14131955









