Unveiling the Impact of Organic Fertilizer on Rice (Oryza sativa L.) Salinity Tolerance: Insights from the Integration of NDVI and Metabolomics
Abstract
1. Introduction
2. Results
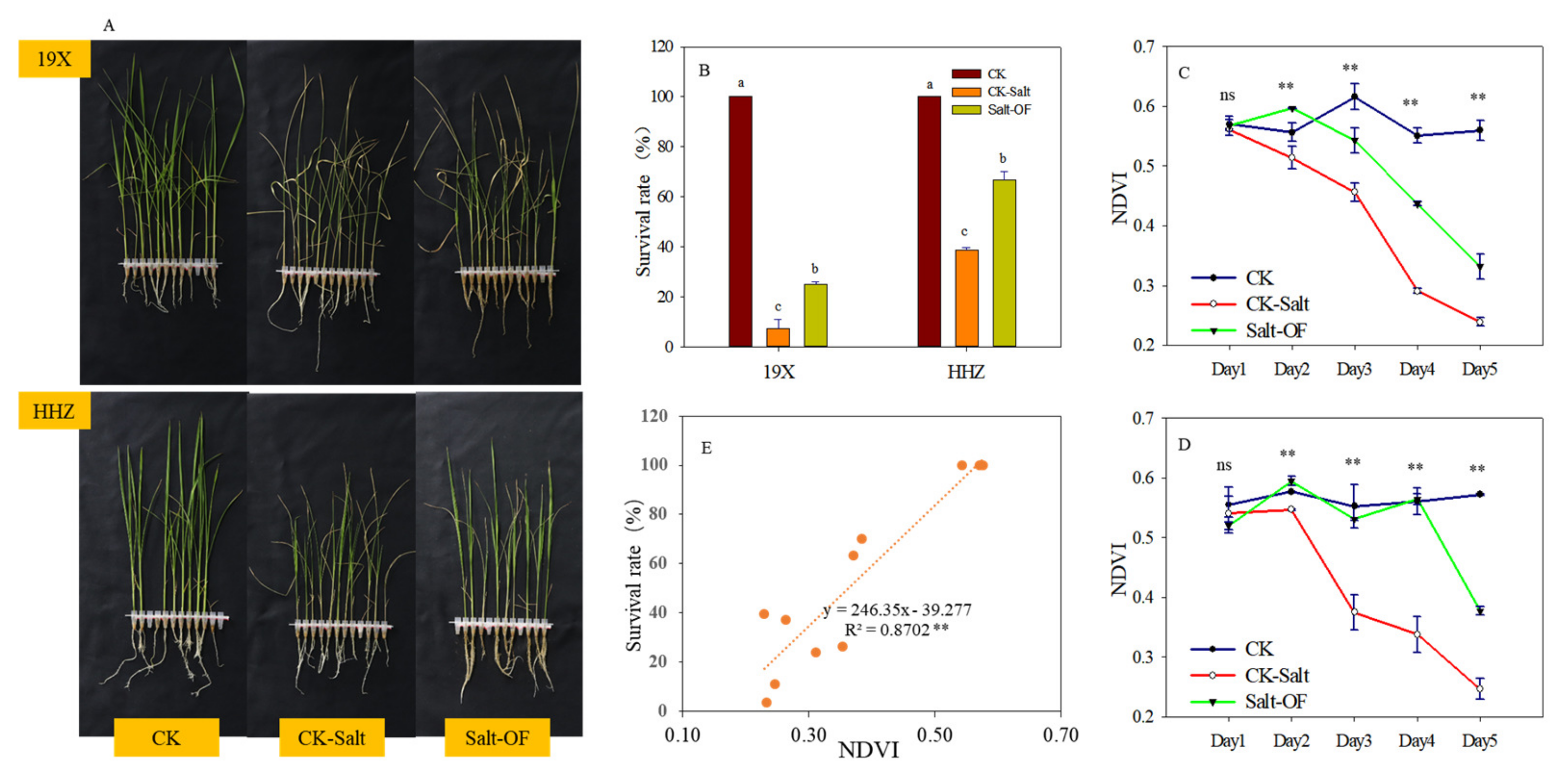
2.1. Survival Rates and NDVI Dynamics of Rice (Oryza sativa L.) Cultivars Under Different Treatments
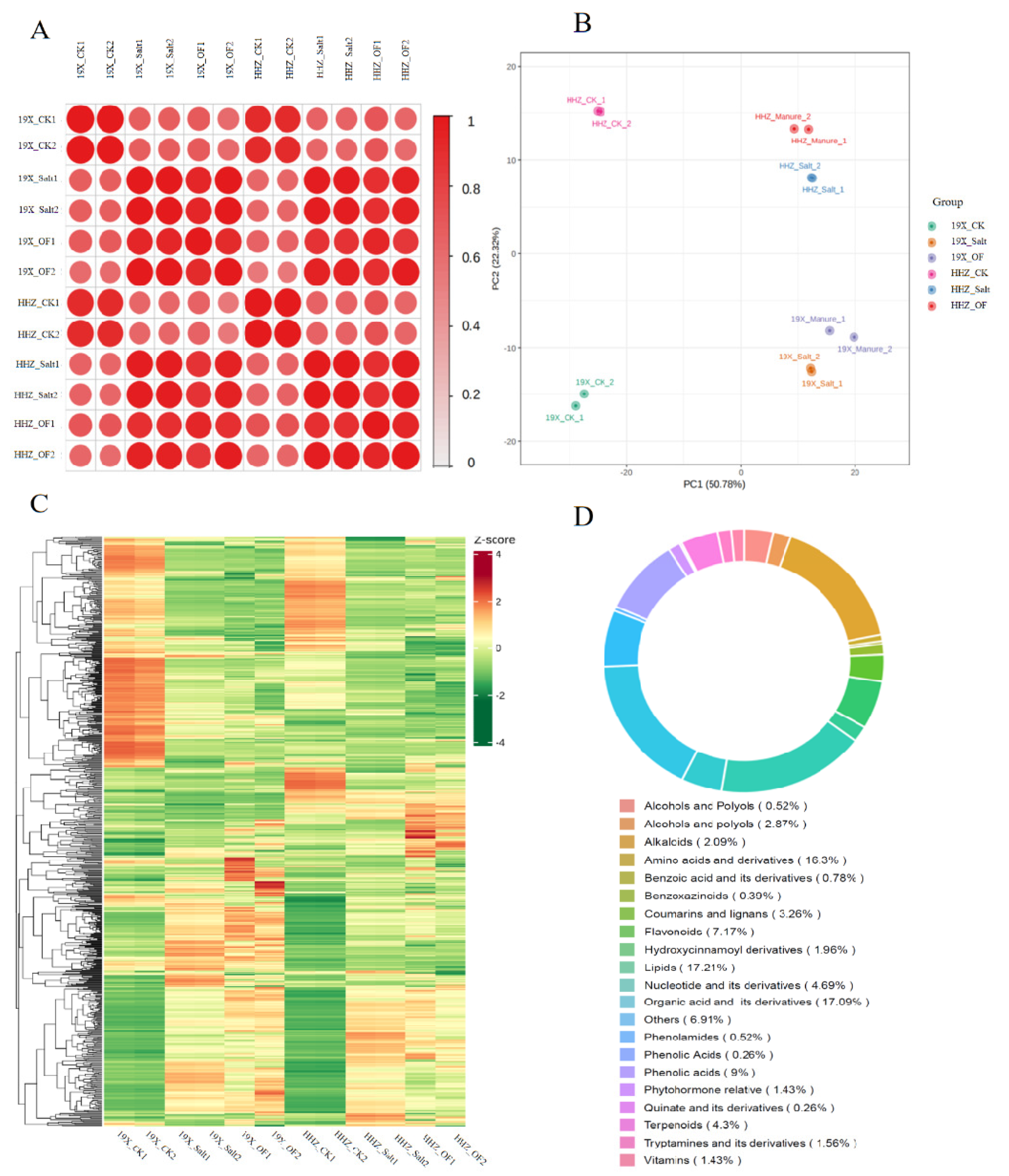
2.2. Metabolomic Profiling of Two Rice Cultivars Under Different Treatments
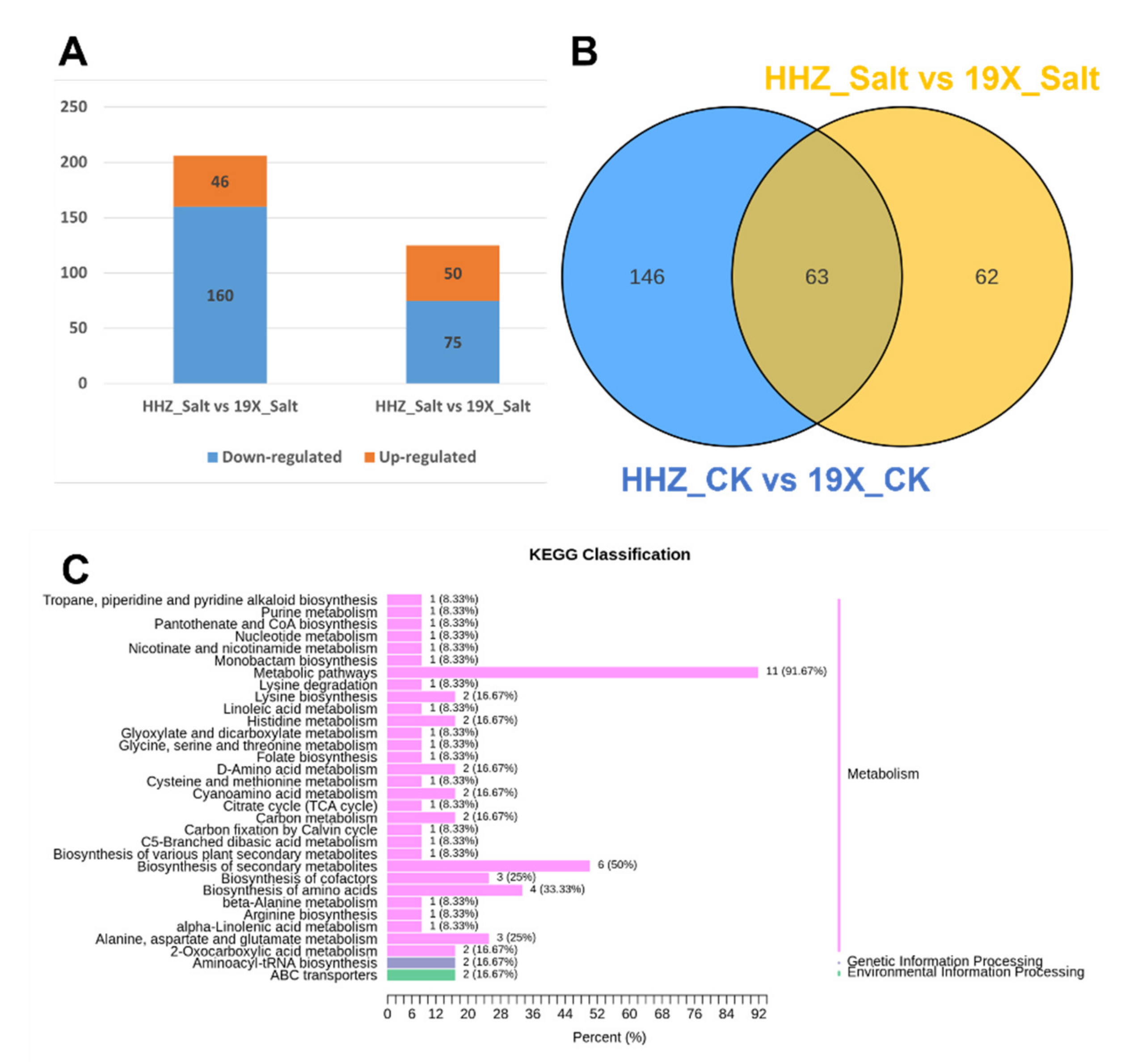
2.3. Salt Tolerance Differences Between Two Rice Cultivars
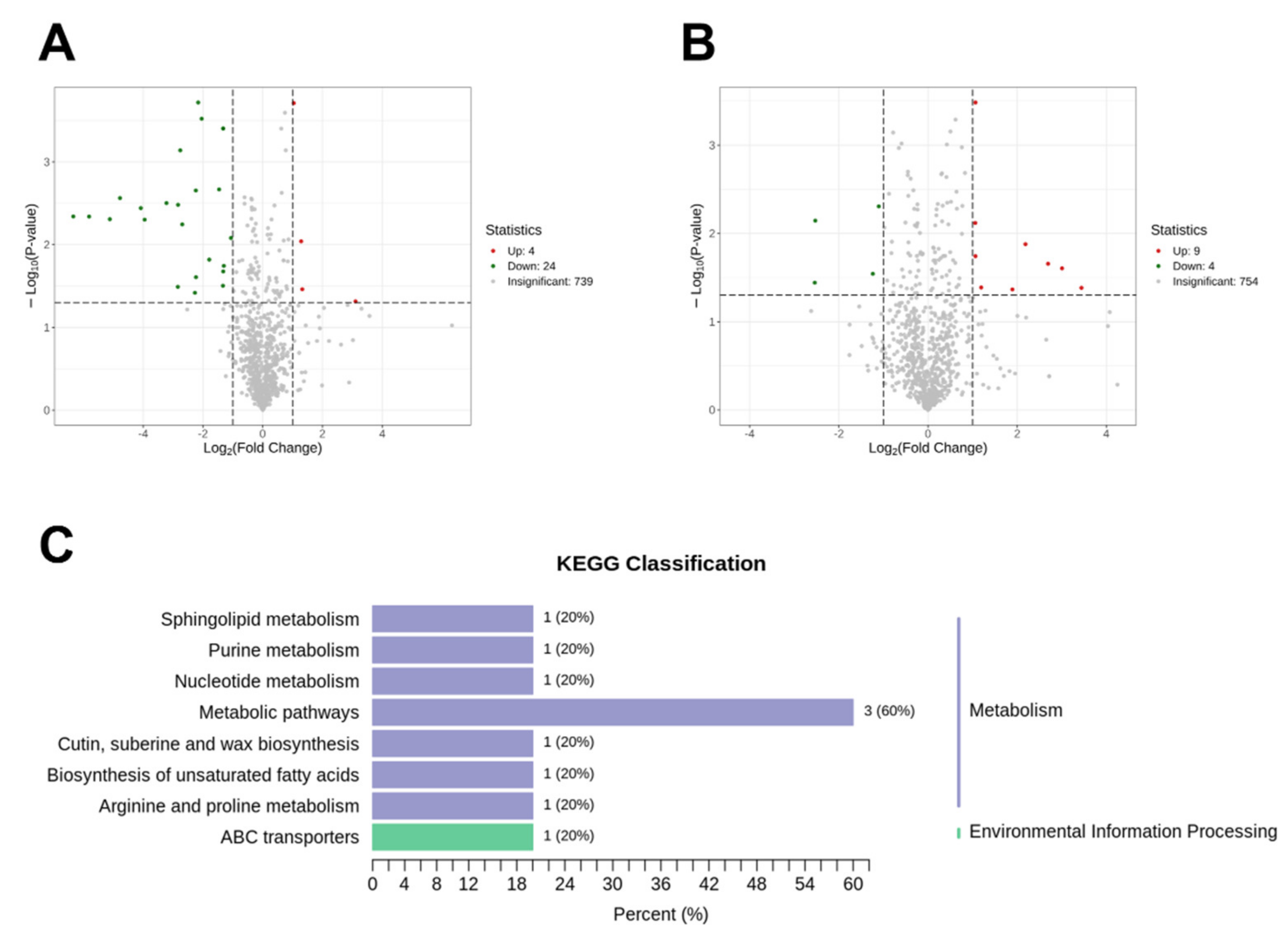
2.4. Effects of OF on Salt Tolerance in 19X and HHZ
2.5. Correlation Analysis Between NDVI and Key Differential Metabolites
3. Discussion
3.1. Effects of Salt Stress on Different Rice Cultivars
3.2. The Role of Organic Fertilizers in Mitigating Salt Stress
3.3. Effects of Salt Stress and Organic Fertilizer on Metabolites
3.4. Integrating NDVI and Metabolomics for Assessing Rice Responses to Salt Stress
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Measurement Indicators
4.3.1. NDVI Measurements
4.3.2. Plant Sampling and Metabolomic Analysis
4.3.3. Survival Rate Observation
4.3.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qadir, M.; Tubeileh, A.; Akhtar, J.; Larbi, A.; Minhas, P.S.; Khan, M.A. Productivity enhancement of salt-affected environments through crop diversification. Land Degrad. Dev. 2008, 19, 429–453. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistical Databases. 2022. Available online: https://www.fao.org (accessed on 12 December 2024).
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, M.; Han, X.; Lu, X.; Chen, X.; Feng, H.; Wu, Z.; Liu, C.; Yan, J.; Zou, W. Evaluation of the soil aggregate stability under long term manure and chemical fertilizer applications: Insights from organic carbon and humic acid structure in aggregates. Agric. Ecosyst. Environ. 2024, 376, 109217. [Google Scholar] [CrossRef]
- Wang, L.; Blanchy, G.; Cornelis, W.; Garré, S. Changes in soil hydraulic and physio-chemical under treated wastewater irrigation: A meta-analysis. Agric. Water Manag. 2024, 295, 108752. [Google Scholar] [CrossRef]
- Bello, S.K.; Alayafi, A.H.; Al-Solaimani, S.G.; Abo-Elyousr, K.A. Mitigating soil salinity stress with gypsum and bio-organic amendments: A review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Mahdy, A.M. Comparative effects of different soil amendments on amelioration of saline-sodic soils. Soil Water Res. 2011, 6, 205–216. [Google Scholar] [CrossRef]
- Faisal, M.; Raza, A.; Batool, A.; Zeshan, M.; Ismail, M.; Farman, M.; Dilshad, M. Unveiling the Impacts of Soil Salinity: Challenges, Mechanisms, and Sustainable Solutions. Sch. J. Agric. Vet. Sci. 2024, 8, 152–162. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Thomas, C.L.; Acquah, G.E.; Whitmore, A.P.; McGrath, S.P.; Haefele, S.M. The effect of different organic fertilizers on yield and soil and crop nutrient concentrations. Agronomy 2019, 9, 776. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, Y.; Yin, J.; Li, D.; Wang, B.; Zhang, K.; Liu, Y. Productivity and quality of banana in response to chemical fertilizer reduction with bio-organic fertilizer: Insight into soil properties and microbial ecology. Agric. Ecosyst. Environ. 2021, 322, 107659. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.; Ali, O.A.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Seleiman, M.F. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, L. Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotoxicol. Environ. Saf. 2021, 216, 112195. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Li, S.; Yang, C.; Chen, C.; Yun, W. Changes in Vegetation Resistance and Resilience under Different Drought Disturbances Based on NDVI and SPEI Time Series Data in Jilin Province, China. Remote Sens. 2023, 15, 3280. [Google Scholar] [CrossRef]
- Tang, H.; Fang, J.; Li, Y.; Yuan, J. Increased Sensitivity and Accelerated Response of Vegetation to Water Variability in China from 1982 to 2022. Water 2024, 16, 2677. [Google Scholar] [CrossRef]
- Prusty, N.; Pradhan, B.; Chattopadhyay, K.; Patra, B.C.; Sarkar, R.K. Novel rice (Oryza sativa L.) genotypes tolerant to combined effect of submergence and salt stress. Indian J. Plant Genet. Resour. 2018, 31, 260–269. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Singh, S. Comparative effects of Organic and Inorganic Fertilization on the Morphological and Physiological traits of Sweet Corn (Zea mays var. saccharata). J. Cereal Res. 2024, 16, 287–295. [Google Scholar]
- Wu, H.; Liu, X.; You, L.; Zhang, L.; Zhou, D.; Feng, J. Effects of salinity on metabolic profiles, gene expressions, and antioxidant enzymes in halophyte Suaeda salsa. J. Plant Growth Regul. 2012, 31, 379–388. [Google Scholar] [CrossRef]
- Zaghloul, E.A.; Awad, E.S.A.; Mohamed, I.R.; El-Hameed, A.M.A.; Feng, D.; Desoky, E.S.M.; El-Tarabily, K. Co-application of organic amendments and natural biostimulants on plants enhances wheat production and defense system under salt-alkali stress. Sci. Rep. 2024, 14, 29742. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.K.; Yoon, I.S.; Kim, K.H.; Kwon, T.R. Salt tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Senadheera, P.; Singh, R.K.; Maathuis, F.J. Differentially expressed membrane transporters in rice roots may contribute to cultivar dependent salt tolerance. J. Exp. Bot. 2009, 60, 2553–2563. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, X.; Sun, M.; Zhang, J.; Ding, L.; Sun, Z.; Liu, W. Mitigation Effect of Exogenous Nano-Silicon on Salt Stress Damage of Rice Seedlings. Int. J. Mol. Sci. 2024, 26, 85. [Google Scholar] [CrossRef]
- Jiang, S.; Lan, Z.; Zhang, Y.; Kang, X.; Zhao, L.; Wu, X.; Gao, H. Mechanisms by Which Exogenous Substances Enhance Plant Salt Tolerance through the Modulation of Ion Membrane Transport and Reactive Oxygen Species Metabolism. Antioxidants 2024, 13, 1050. [Google Scholar] [CrossRef]
- Adamiec, M.; Pietrowska-Borek, M.; Luciński, R. The influence of environmental conditions on chloroplast functioning and development. Front. Plant Sci. 2024, 15, 1517094. [Google Scholar] [CrossRef]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Murata, Y. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- Ali, N.; Rafiq, R.; Wijaya, L.; Ahmad, A.; Kaushik, P. Exogenous citric acid improves growth and yield by concerted modulation of antioxidant defense system in brinjal (Solanum melongena L.) under salt-stress. J. King Saud Univ. Sci. 2024, 36, 103012. [Google Scholar] [CrossRef]
- Chakrobortty, J.; Imran, S.; Mahamud, M.A.; Sarker, P.; Paul, N.C. Effect of citric acid (CA) priming and exogenous application on germination and early seedling growth of okra (Abelmoschus esculentus L.) plants under salinity stress condition. Arch. Agric. Environ. Sci. 2022, 7, 318–326. [Google Scholar] [CrossRef]
- Braga, Í.D.O.; Carvalho da Silva, T.L.; Belo Silva, V.N.; Rodrigues Neto, J.C.; Ribeiro, J.A.D.A.; Abdelnur, P.V.; Souza, M.T., Jr. Deep Untargeted Metabolomics Analysis to Further Characterize the Adaptation Response of Gliricidia sepium (Jacq) Walp. to Very High Salinity Stress. Front. Plant Sci. 2022, 13, 869105. [Google Scholar]
- Charles, A.K. Pipecolic acid receptors in rat cerebral cortex. Neurochem. Res. 1986, 11, 521–525. [Google Scholar] [CrossRef]
- Moulin, M.; Deleu, C.; Larher, F.; Bouchereau, A. The lysine-ketoglutarate reductase–saccharopine dehydrogenase is involved in the osmo-induced synthesis of pipecolic acid in rapeseed leaf tissues. Plant Physiol. Biochem. 2006, 44, 474–482. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Zorbekova, A.N.; Korbozova, N.K.; Erbay, M.; Mamirova, A. Impact of abiotic stressors on oleic acid accumulation in the leaves of young quinoa plants. Bulletin of the Karaganda University. Biol. Med. Geogr. Ser. 2024, 11529, 79–85. [Google Scholar]
- Biswas, S.; Biswas, A.K.; De, B. Influence of sodium chloride on growth and metabolic reprogramming in nonprimed and haloprimed seedlings of blackgram (Vigna mungo L.). Protoplasma 2020, 257, 1559–1583. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Mittler, R.; Shabala, S. Useful or merely convenient? On the issue of a suitability of enzymatic antioxidant activity as a proxy for abiotic stress tolerance. J. Exp. Bot. 2024. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Rupasinghe, T.W.T.; Roessner, U. Salt stress alters membrane lipid content and lipid biosynthesis pathways in the plasma membrane and tonoplast. Plant Physiol. 2022, 189, 805–819. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, X.; Hou, H.; Ji, J.; Liu, X.; Lv, Z. Multifaceted Ability of Organic Fertilizers to Improve Crop Productivity and Abiotic Stress Tolerance: Review and Perspectives. Agronomy 2024, 14, 1141. [Google Scholar] [CrossRef]
- Adams, P.; Thomas, J.C.; Vernon, D.M.; Bohnert, H.J.; Jensen, R.G. Distinct cellular and organismic responses to salt stress. Plant Cell Physiol. 1992, 33, 1215–1223. [Google Scholar]
- Rady, M.M. A novel organo-mineral fertilizer can mitigate salinity stress effects for tomato production on reclaimed saline soil. S. Afr. J. Bot. 2012, 81, 8–14. [Google Scholar] [CrossRef]
- Thompson, J.E.; Froese, C.D.; Madey, E.; Smith, M.D.; Hong, Y. Lipid metabolism during plant senescence. Prog. Lipid Res. 1998, 37, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kim, S.H.; Rhee, S.; Witte, C.P. A plastid nucleoside kinase is involved in inosine salvage and control of purine nucleotide biosynthesis. Plant Cell 2023, 35, 510–528. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Impa, S.M.; Krassovskaya, I.; Vennapusa, A.R.; Gill, K.S.; Obata, T.; Jagadish, S.K. Enhanced N-metabolites, ABA and IAA-conjugate in anthers instigate heat sensitivity in spring wheat. Physiol. Plant. 2020, 169, 501–514. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.; Mohamed, G.A. Summary of natural products ameliorate concanavalin a-induced liver injury: Structures, sources, pharmacological effects, and mechanisms of action. Plants 2021, 10, 228. [Google Scholar] [CrossRef]
- Gokce, A.; Sekmen Cetinel, A.H.; Turkan, I. Carnitine modulates antioxidative defense in ABI2 mutant under salt stress. Plant Growth Regul. 2024, 104, 359–376. [Google Scholar] [CrossRef]
- Rong, Y.; Li, B.; Hou, Y.; Zhang, L.; Jia, R.; Zhu, J. Influences of Stocking Density on Antioxidant Status, Nutrients Composition, and Lipid Metabolism in the Muscles of Cyprinus carpio under Rice–Fish Co-Culture. Antioxidants 2024, 13, 849. [Google Scholar] [CrossRef]
- Atanasov, A.I.; Mihova, G.M.; Atanasov, A.Z.; Vlăduț, V. Long-Term Assessment of NDVI Dynamics in Winter Wheat (Triticum aestivum) Using a Small Unmanned Aerial Vehicle. Agriculture 2025, 15, 394. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Groli, E.L.; Frascaroli, E.; Maccaferri, M.; Ammar, K.; Tuberosa, R. Dissecting the effect of heat stress on durum wheat under field conditions. Front. Plant Sci. 2024, 15, 1393349. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Li, Q.; Zhang, J.; Yang, R.; Wang, H.; Li, X. Combining UAV-Based Multispectral and Thermal Images to Diagnosing Dryness Under Different Crop Areas on the Loess Plateau. Agriculture 2025, 15, 126. [Google Scholar] [CrossRef]
- Neupane, K.; Witcher, A.; Baysal-Gurel, F. An Evaluation of the Effect of Fertilizer Rate on Tree Growth and the Detection of Nutrient Stress in Different Irrigation Systems. Horticulturae 2024, 10, 767. [Google Scholar] [CrossRef]
- Yu, R.; Luo, Y.; Ren, L. Detection of pine wood nematode infestation using hyperspectral drone images. Ecol. Indic. 2024, 162, 112034. [Google Scholar] [CrossRef]
- Carella, A.; Massenti, R.; Marra, F.P.; Catania, P.; Roma, E.; Lo Bianco, R. Combining proximal and remote sensing to assess ‘Calatina’olive water status. Front. Plant Sci. 2024, 15, 1448656. [Google Scholar] [CrossRef]
- Sapkota, A.; Roby, M.; Peddinti, S.R.; Fulton, A.; Kisekka, I. Comparative analysis of evapotranspiration (ET), crop water stress index (CWSI), and normalized difference vegetation index (NDVI) to delineate site-specific irrigation management zones in almond orchards. Sci. Hortic. 2025, 339, 113860. [Google Scholar] [CrossRef]

| Substance Name | Class | HHZ_Salt_vs._CK | HHZ_Salt_vs._19X_Salt |
|---|---|---|---|
| (±) 13-HpODE | Lipids | up | up |
| (±) 8,9-DiHETE | Lipids | down | down |
| 14,16-dihydroxy-3-methyl-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecine-1,7-dione | Benzoxazinoids | up | up |
| Citric acid | Organic acids and derivatives | down | down |
| Cyclopentadecanolide | Lipids | down | down |
| (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinic acid (HTPA) | Amino acids and derivatives | down | down |
| Maleamic acid | Organic acids and derivatives | down | down |
| Myristoleic acid | Lipids | down | down |
| Octadeca penten-3-one | Lipids | up | up |
| Pipecolic acid | Amino acids and derivatives | down | down |
| Propentofylline | Others | down | down |
| Tridecylic acid | Organic acids and derivatives | down | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, Y.; Xu, Q.; Niu, X.; Cao, G.; Liu, H. Unveiling the Impact of Organic Fertilizer on Rice (Oryza sativa L.) Salinity Tolerance: Insights from the Integration of NDVI and Metabolomics. Plants 2025, 14, 902. https://doi.org/10.3390/plants14060902
Li J, Li Y, Xu Q, Niu X, Cao G, Liu H. Unveiling the Impact of Organic Fertilizer on Rice (Oryza sativa L.) Salinity Tolerance: Insights from the Integration of NDVI and Metabolomics. Plants. 2025; 14(6):902. https://doi.org/10.3390/plants14060902
Chicago/Turabian StyleLi, Jiaolong, Yunluo Li, Qiyun Xu, Xiaolei Niu, Guangping Cao, and Hongyan Liu. 2025. "Unveiling the Impact of Organic Fertilizer on Rice (Oryza sativa L.) Salinity Tolerance: Insights from the Integration of NDVI and Metabolomics" Plants 14, no. 6: 902. https://doi.org/10.3390/plants14060902
APA StyleLi, J., Li, Y., Xu, Q., Niu, X., Cao, G., & Liu, H. (2025). Unveiling the Impact of Organic Fertilizer on Rice (Oryza sativa L.) Salinity Tolerance: Insights from the Integration of NDVI and Metabolomics. Plants, 14(6), 902. https://doi.org/10.3390/plants14060902







