Physiological and Yield Responses of Pepper (Capsicum annuum L.) Genotypes to Drought Stress
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Growing the Seedlings
4.3. Cultivation Practice
4.4. Nutrient Solution Formula
4.5. Sampling of Leaves and Fruits for Macro- and Micronutrient Evaluation
4.6. Assessment of Crop Yield
4.7. Nutrient Content in Leaves and Fruit
4.8. Organoleptic Characteristics
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Redden, R. New approaches for crop genetic adaptation to the abiotic stresses predicted with climate change. Agronomy 2013, 3, 419–432. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2022, Leveraging Automation in Agriculture for Transforming Agrifood Systems; FAO: Rome, Italy, 2022; ISBN 9789251360439. [Google Scholar]
- Hoerling, M.; Eischeid, J.; Perlwitz, J.; Quan, X.; Zhang, T.; Pegion, P. On the increased frequency of mediterranean drought. J. Clim. 2012, 25, 2146–2161. [Google Scholar] [CrossRef]
- Wach, D.; Skowron, P. An overview of plant responses to the drought stress at morphological, physiological and biochemical levels. Pol. J. Agron. 2022, 50, 25–34. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Qadir, T.; Khaliq, A.; Ashraf, U.; Parveen, A.; Saqib, M.; Rafiq, M. Drought stress in plants: An overview on implications, tolerance mechanisms and agronomic mitigation strategies. Plant Sci. Today 2019, 6, 389–402. [Google Scholar] [CrossRef]
- Li, J.; Abbas, K.; Wang, W.; Gong, B.; Wang, L.; Hou, S.; Xia, H.; Wu, X.; Chen, L.; Gao, H. Drought Tolerance Evaluation and Verification of Fifty Pakchoi (Brassica rapa ssp. chinensis) Varieties under Water Deficit Condition. Agronomy 2023, 13, 2087. [Google Scholar] [CrossRef]
- Dhiman, S.; Singh, D.B.; Singh, S.P.; Sohi, A. Abiotic Stress in Vegetable Crops: Challenges and Strategies. Agri Artic. 2024, 4, 582–586. [Google Scholar]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Ahmad, N.; Saleem, B.A. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 2009, 195, 237–246. [Google Scholar] [CrossRef]
- Auerswald, H.; Schwarz, D.; Kornelson, C.; Krumbein, A.; Brückner, B. Sensory analysis, sugar and acid content of tomato at different EC values of the nutrient solution. Sci. Hortic. 1999, 82, 227–242. [Google Scholar] [CrossRef]
- Penella, C.; Nebauer, S.G.; Bautista, A.S.; López-Galarza, S.; Calatayud, Á. Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: Physiological responses. J. Plant Physiol. 2014, 171, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]
- Alvino, A.; Centritto, M.; De Lorenzi, F. Photosynthesis response of sunlit and shade pepper (Capsicum annuum) leaves at different positions in the canopy under two water regimes. Aust. J. Plant Physiol. 1994, 21, 377–391. [Google Scholar] [CrossRef]
- Campos, H.; Trejo, C.; Peña-Valdivia, C.B.; García-Nava, R.; Conde-Martínez, F.V.; Cruz-Ortega, M.R. Stomatal and non-stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: Delayed restoration of photosynthesis during recovery. Environ. Exp. Bot. 2014, 98, 56–64. [Google Scholar] [CrossRef]
- Ficiciyan, A.M.; Loos, J.; Tscharntke, T. Similar Yield Benefits of Hybrid, Conventional, and Organic Tomato and Sweet Pepper Varieties Under Well-Watered and Drought-Stressed Conditions. Front. Sustain. Food Syst. 2021, 5, 628537. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Martín-García, R.; Bažon Zidarić, I.; Pascual-Seva, N.; Pascual, B.; Padilla, Y.G.; Calatayud, Á.; López-Galarza, S. A Water Stress-Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation. Horticulturae 2023, 9, 362. [Google Scholar] [CrossRef]
- Conti, V.; Mareri, L.; Faleri, C.; Nepi, M.; Romi, M.; Cai, G.; Cantini, C. Drought stress affects the response of italian local tomato (Solanum lycopersicum L.) varieties in a genotype-dependent manner. Plants 2019, 8, 336. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Kumar, D.; Gautam, N.; Dogra, R.K.; Mehta, D.K.; Sharma, H.D.; Kansal, S. Parthenocarpic Gynoecious Parental Lines of Cucumber Introduced from Netherlands for Developing High-Yielding, Quality Hybrids. J. Crop Improv. 2016, 30, 352–369. [Google Scholar] [CrossRef]
- Kessler, A. Inducible plant defences and the environmental context. Funct. Ecol. 2016, 30, 1738–1739. [Google Scholar] [CrossRef]
- Quartey, E.K.; Nunekpeku, W.; Owusu-Ansah, M.; Appiah, A.S.; Ofori, E.S.K.; Amoatey, H.M. Agronomic Evaluation of Eight Genotypes of Hot Pepper (Capsicum spp. L.) in a Coastal Savanna Zone of Ghana. J. Biol. Agric. Healthc. 2014, 4, 16–28. [Google Scholar]
- Ilić, Z.S.; Kevrešan, Ž.; Mastilović, J.; Zorić, L.; Tomšik, A.; Belović, M.; Pestorić, M.; Karanović, D.; Luković, J. Evaluation of Mineral Profile, Texture, Sensory and Structural Characteristics of Old Pepper Landraces. J. Food Process. Preserv. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A. Impacts of Drought and/or Heat Stress on Physiological, Developmental, Growth, and Yield Processes of Crop Plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; Advances in Agricultural System Modeling; American Society of Agronomy: Madison, WI, USA, 2008; pp. 301–355. [Google Scholar]
- Earl, H.J.; Davis, R.F. Effect of drought stress on leaf and whole canopy radiation use efficiency and yield of maize. Agron. J. 2003, 95, 688–696. [Google Scholar] [CrossRef]
- Dorji, K.; Behboudian, M.H.; Zegbe-Domínguez, J.A. Water relations, growth, yield, and fruit quality of hot pepper under deficit irrigation and partial rootzone drying. Sci. Hortic. 2005, 104, 137–149. [Google Scholar] [CrossRef]
- Moustafa, K.A.; Saleh, M.; Al-Doss, A.A.; Elshafei, A.A.; Salem, A.K.; Al-Qurainy, F.H.; Barakat, M.N. Identification of TRAP and SRAP markers linked with yield components under drought stress in wheat (Triticum aestivum L.). Plant Omics 2014, 7, 253–259. [Google Scholar]
- Namaki, A.; Ghahremani, Z.; Aelaei, M.; Barzegar, T.; Ranjbar, M.E. The First Report of Drought Tolerance Assessment of Iranian Asparagus. Gesunde Pflanz. 2022, 74, 141–149. [Google Scholar] [CrossRef]
- Kurunc, A.; Unlukara, A.; Cemek, B. Salinity and drought affect yield response of bell pepper similarly. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 514–522. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.; Douthe, C.; El Aou-ouad, H.; Ribas-Carbó, M.; Galmés, J. Tomato landraces as a source to minimize yield losses and improve fruit quality under water deficit conditions. Agric. Water Manag. 2019, 223, 105722. [Google Scholar] [CrossRef]
- Techawongstien, S.; Nawata, E.; Shigenaga, S. After-effect of short-term water stress at the pre-anthesis stage on physiological characteristics in four chilli pepper cultivars. Jpn. J. Trop. Agric. 1992, 36, 88–93. [Google Scholar]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The physiology of channel-mediated K+ acquisition in roots of higher plants. Physiol. Plant. 2014, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kirnak, H.; Kaya, C.; Higgs, D.; Tas, I. Responses of drip irrigated bell pepper to water stress and different nitrogen levels with or without mulch cover. J. Plant Nutr. 2011, 26, 263–277. [Google Scholar] [CrossRef]
- Sadak, A.; Akkopru, A.; Sensoy, S. Effects of Endophytic Bacteria on Some Physiological Traits and Nutrient Contents in Pepper Seedlings under Drought Stress. Yuz. Yil Univ. J. Agric. Sci. 2021, 31, 237–245. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Delfine, S.; Tognetti, R.; Loreto, F.; Alvino, A. Physiological and growth responses to water stress in field-grown bell pepper (Capsicum annuum L.). J. Hortic. Sci. Biotechnol. 2002, 77, 697–704. [Google Scholar] [CrossRef]
- Ntanasi, T.; Savvas, D.; Karavidas, I.; Papadopoulou, E.A.; Mazahrirh, N.; Fotopoulos, V.; Aliferis, K.A.; Sabatino, L.; Ntatsi, G. Assessing Salinity Tolerance and Fruit Quality of Pepper Landraces. Agronomy 2024, 14, 309. [Google Scholar] [CrossRef]
- Martins, B.L.R.; Ferreira, K.N.; Rocha, J.L.A.; Araujo, R.H.C.R.; Lopes, G.; Santos, L.C.d.; Bezerra Neto, F.; Sá, F.V.d.S.; Silva, T.I.d.; da Silva, W.I.; et al. Nano ZnO and Bioinoculants Mitigate Effects of Deficit Irrigation on Nutritional Quality of Green Peppers. Horticulturae 2024, 10, 969. [Google Scholar] [CrossRef]
- De Pascale, S.; Ruggiero, C.; Barbieri, G.; Maggio, A. Physiological responses of pepper to salinity and drought. J. Am. Soc. Hortic. Sci. 2003, 128, 48–54. [Google Scholar] [CrossRef]
- Birgin, Ö.; Akhoundnejad, Y.; Dasgan, H.Y. The effect of foliar calcium application in tomato (Solanum lycopersicum L.) under drought stress in greenhouse conditions. Appl. Ecol. Environ. Res. 2021, 19, 2971–2982. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits-making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef] [PubMed]
- Belakbir, A.; Ruiz, J.M.; Romero, L. Yield and fruit quality of pepper (Capsicum annuum L.) in response to bioregulators. HortScience 1998, 33, 85–87. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Pascual, B.; Nájera, I.; Domene, M.A.; Baixauli, C.; Pascual-Seva, N. Effects of deficit irrigation on the yield and irrigation water use efficiency of drip-irrigated sweet pepper (Capsicum annuum L.) under Mediterranean conditions. Irrig. Sci. 2020, 38, 89–104. [Google Scholar] [CrossRef]
- Maduranga Bandara Rathnayaka, R.M.S.; Minami, M.; Nemoto, K.; Prabandaka, S.S.; Matsushima, K. Relationship between water supply and sugar and capsaicinoids contents in fruit of Chili pepper (Capsicum Annuum L.). Hortic. J. 2021, 90, 58–67. [Google Scholar] [CrossRef]
- Demir, Z.; Özbahçe, A. Evaluating the effects of different irrigation and nitrogen applications on soil water content and yield quality parameters of pepper using surface and subsurface drip irrigation. Irrig. Drain. 2021, 70, 1039–1055. [Google Scholar] [CrossRef]
- Ehret, D.L.; Ho, L.C. The effects of salinity on dry matter partitioning and fruit growth in tomatoes grown in nutrient film culture. J. Hortic. Sci. 1986, 61, 361–367. [Google Scholar] [CrossRef]
- Bakr, J.; Daood, H.G.; Pék, Z.; Helyes, L.; Posta, K. Yield and quality of mycorrhized processing tomato under water scarcity. Appl. Ecol. Environ. Res. 2017, 15, 401–413. [Google Scholar] [CrossRef]
- Renquist, A.R.; Reid, J.B. Processing tomato fruit quality: Influence of soil water deficits at flowering and ripening. Aust. J. Agric. Res. 2001, 52, 793–799. [Google Scholar] [CrossRef]
- Mohammed, S.; Hussen, A. Influence of deficit irrigation levels on agronomic performance of pepper (Capsicum annuum L.) under drip at alage, central rift valley of Ethiopia. PLoS ONE 2023, 18, e0280639. [Google Scholar] [CrossRef]
- Mardani, S.; Tabatabaei, S.H.; Pessarakli, M.; Zareabyaneh, H. Physiological responses of pepper plant (Capsicum annuum L.) to drought stress. J. Plant Nutr. 2017, 40, 1453–1464. [Google Scholar] [CrossRef]
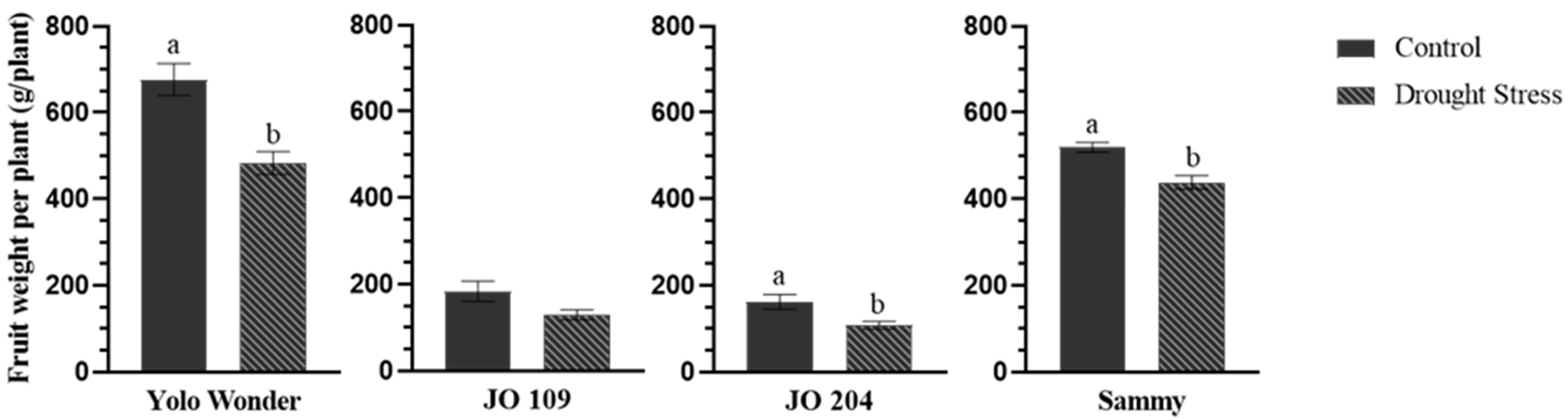
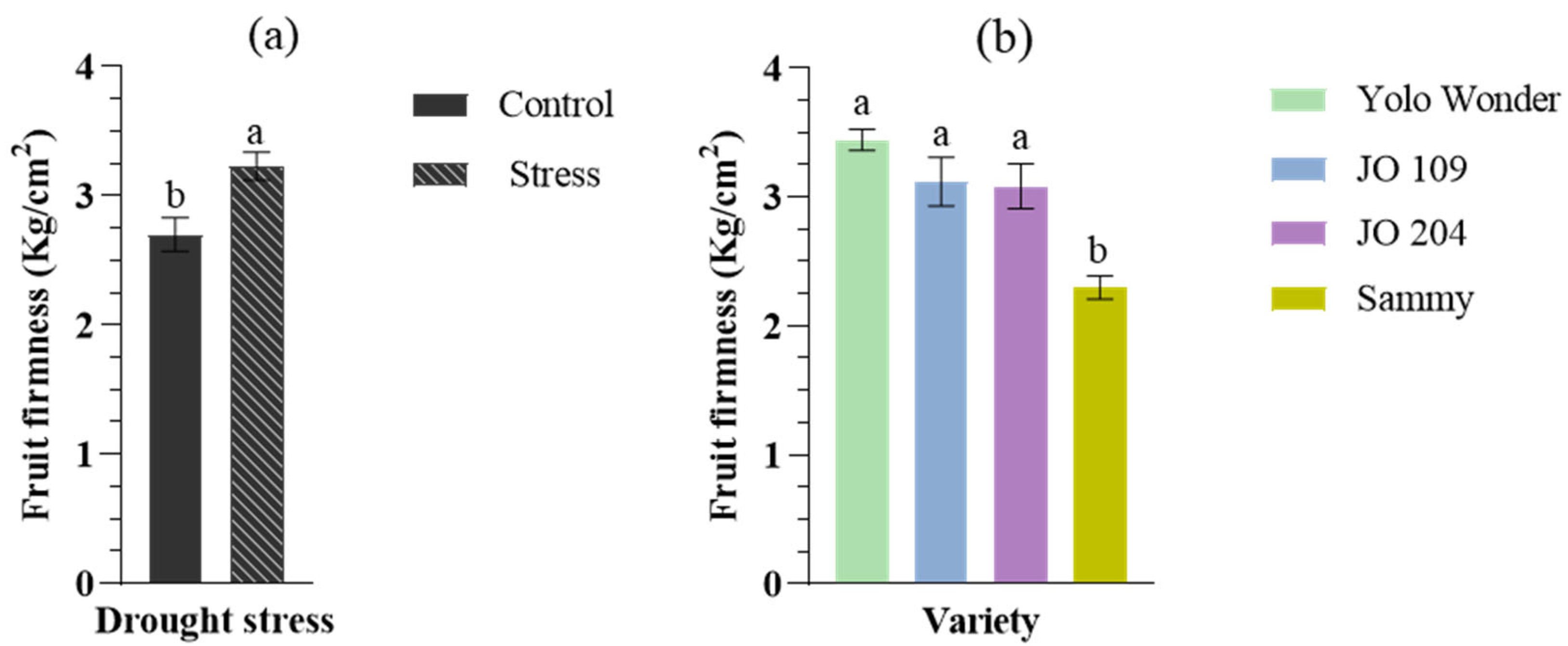
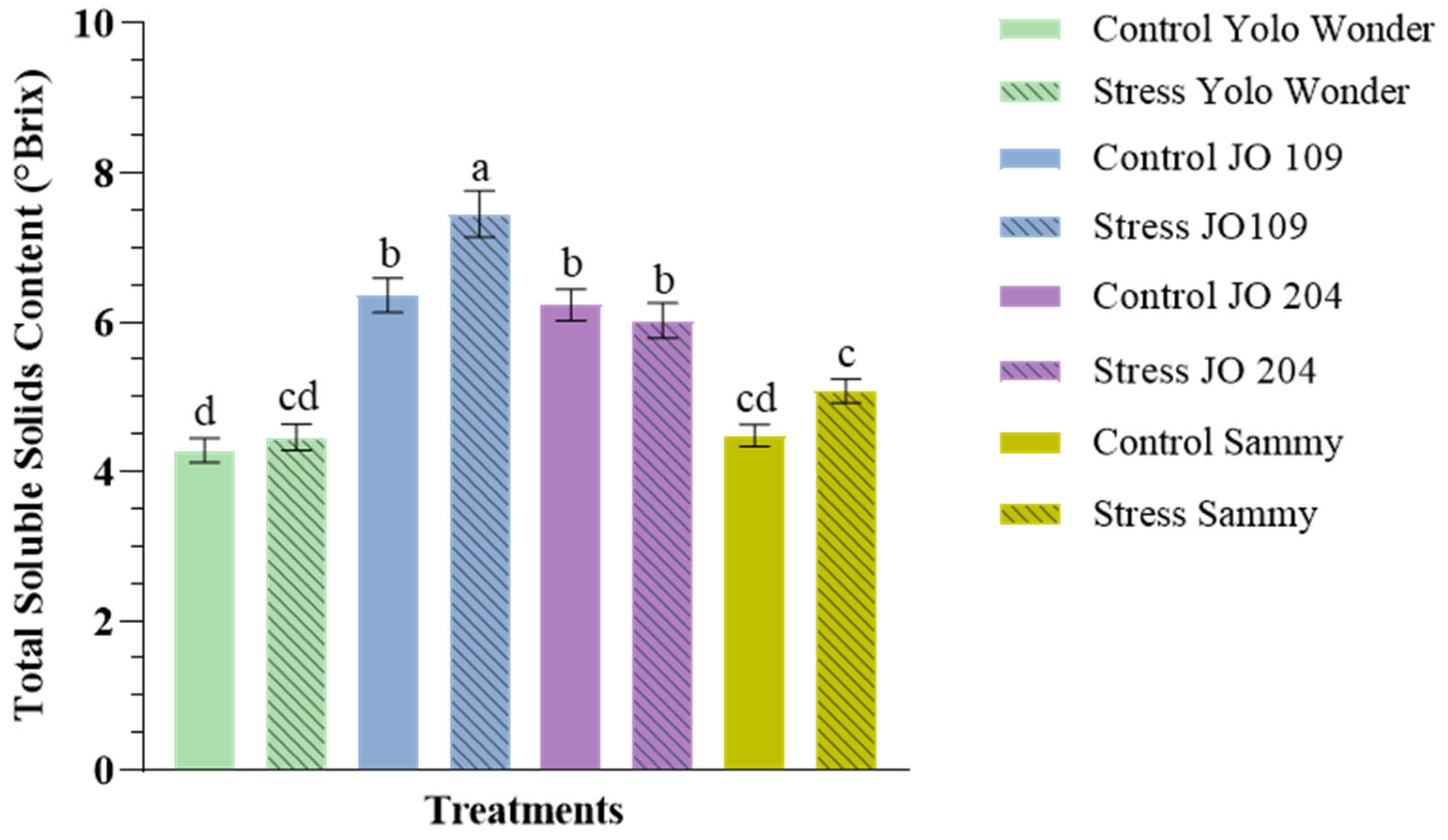
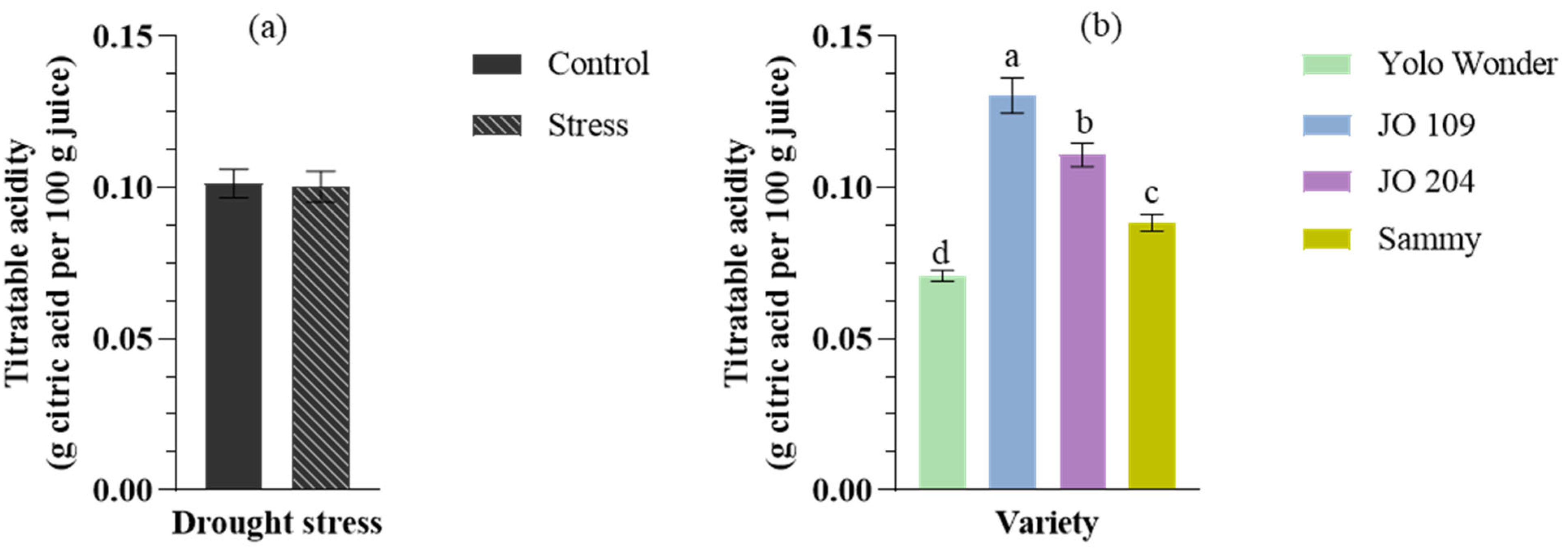
| FN (No Plant−1) | MFW (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Water Availability | Yolo Wonder | JO109 | JO204 | Sammy | Yolo Wonder | JO109 | JO204 | Sammy |
| Control | 4.75 | 14.17 | 13.88 | 12.08 | 142.22 | 12.81 | 11.61 | 43.00 |
| Drought stress | 3.67 | 10.92 | 13.00 | 11.17 | 132.29 | 11.96 | 8.31 | 39.33 |
| Statistical significance | ** | NS | NS | NS | NS | NS | ** | * |
| Leaves | ||||||
|---|---|---|---|---|---|---|
| Water Availability | Variety | K (mg/g) | Na (mg/g) | K/Na | Ca (mg/g) | Mg (mg/g) |
| Main effects (Drought stress) | ||||||
| Control | 43.75 | 0.25 | 176.06 | 30.04 | 9.20 | |
| Drought stress | 38.38 | 0.29 | 134.47 | 35.15 | 10.48 | |
| Main effects (Variety) | ||||||
| Yolo Wonder | 38.75 b | 0.23 c | 171.32 a | 35.34 a | 10.73 | |
| JO 109 | 52.00 a | 0.33 a | 166.86 a | 27.10 b | 9.40 | |
| JO 204 | 39.50 b | 0.29 ab | 140.03 b | 30.03 b | 9.58 | |
| Sammy | 34.00 c | 0.25 bc | 142.84 b | 37.91 a | 9.64 | |
| Interaction | ||||||
| Control | Yolo Wonder | 41.50 c | 0.23 | 180.68 ab | 33.27 | 10.52 |
| JO 109 | 55.00 a | 0.29 | 197.30 a | 24.23 | 8.48 | |
| JO 204 | 39.00 cd | 0.26 | 148.97 bc | 27.13 | 8.36 | |
| Sammy | 39.50 cd | 0.23 | 177.29 ab | 35.54 | 9.42 | |
| Drought stress | Yolo Wonder | 36.00 d | 0.23 | 161.96 bc | 37.40 | 10.94 |
| JO 109 | 49.00 b | 0.38 | 136.43 cd | 29.96 | 10.33 | |
| JO 204 | 40.00 cd | 0.31 | 131.10 cd | 32.93 | 10.79 | |
| Sammy | 28.50 e | 0.27 | 108.39 d | 40.29 | 9.86 | |
| Statistical significance | ||||||
| Drought Stress | *** | ** | *** | *** | *** | |
| Variety | *** | *** | ** | *** | NS | |
| Drought Stress × Variety | ** | NS | * | NS | NS | |
| Fruit | ||||||
|---|---|---|---|---|---|---|
| Drought Stress | Variety | K (mg/g) | Na (mg/g) | K/Na | Ca (mg/g) | Mg (mg/g) |
| Main effects (Drought stress) | ||||||
| Control | 47.88 | 0.18 | 279.56 | 11.28 | 5.59 | |
| Drought stress | 49.75 | 0.19 | 269.59 | 9.66 | 5.58 | |
| Main effects (Variety) | ||||||
| Yolo Wonder | 45.25 c | 0.21 a | 226.47 b | 10.91 b | 5.15 b | |
| JO 109 | 49.50 b | 0.15 b | 341.04 a | 9.33 c | 5.96 a | |
| JO 204 | 59.25 a | 0.20 a | 306.26 a | 9.09 c | 6.23 a | |
| Sammy | 41.25 d | 0.19 a | 224.54 b | 12.56 a | 4.99 b | |
| Interaction | ||||||
| Control | Yolo Wonder | 43.00 | 0.22 ab | 204.65 b | 11.35 abc | 5.27 |
| JO 109 | 49.00 | 0.15 cd | 322.41 a | 9.29 c | 6.07 | |
| JO 204 | 57.50 | 0.16 cd | 358.69 a | 11.37 abc | 5.88 | |
| Sammy | 42.00 | 0.19 bcd | 232.50 b | 13.11 a | 5.13 | |
| Drought stress | Yolo Wonder | 47.50 | 0.20 abc | 248.30 b | 10.46 bc | 5.02 |
| JO 109 | 50.00 | 0.14 d | 359.67 a | 9.37 c | 5.85 | |
| JO 204 | 61.00 | 0.25 a | 253.83 b | 6.82 d | 6.58 | |
| Sammy | 40.50 | 0.19 bcd | 216.58 b | 12.01 ab | 4.85 | |
| Statistical significance | ||||||
| Drought Stress | NS | NS | NS | ** | NS | |
| Variety | *** | ** | *** | *** | *** | |
| Drought Stress × Variety | NS | ** | ** | ** | NS | |
| Water Stress | Variety | TSSC (°Brix) |
|---|---|---|
| Main effects (Drought stress) | ||
| Control | 5.41 | |
| Drought stress | 5.74 | |
| Main effects (Variety) | ||
| Yolo Wonder | 4.37 d | |
| JO 109 | 6.81 a | |
| JO 204 | 6.12 b | |
| Sammy | 4.81 c | |
| Statistical significance | ||
| Drought Stress | ** | |
| Variety | *** | |
| Drought Stress × Variety | * | |
| Variety | Type | Provider |
|---|---|---|
| Yolo Wonder | Reference | INRA 1 |
| JO109 (Capsicum anuum var. grossum) | Landrace | NARC 2 |
| JO204 (Capsicum anuum var. grossum) | Landrace | NARC 2 |
| Sammy RZ (F1-Hybrid) | Reference | Rijk Zwaan 3 |
| Nutrient | Starter Solution (13 November 2021) | Vegetative Phase (14 November 2021) | Reproductive Phase (7 February 2022) | Unit |
|---|---|---|---|---|
| NO3− | 16.05 | 15.79 | 16.64 | mM |
| K+ | 5.70 | 5.86 | 6.94 | mM |
| Ca2+ | 6.15 | 5.60 | 5.55 | mM |
| Mg2+ | 2.50 | 1.63 | 1.66 | mM |
| SO42− | 3.27 | 2.10 | 2.08 | mM |
| H2PO4− | 1.25 | 1.35 | 1.35 | mM |
| NH4+ | 1.05 | 1.22 | 1.00 | mM |
| Fe | 20.00 | 20.00 | 16.20 | μM |
| Mn2+ | 12.00 | 12.00 | 10.80 | μM |
| Zn2+ | 7.00 | 6.00 | 5.40 | μM |
| B | 50.00 | 32.00 | 32.40 | μM |
| Cu2+ | 0.80 | 0.80 | 0.86 | μM |
| Mo | 0.60 | 0.60 | 0.54 | μΜ |
| Cl− | 0.40 | 0.40 | 0.40 | μΜ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntanasi, T.; Karavidas, I.; Savvas, D.; Spyrou, G.P.; Giannothanasis, E.; Consentino, B.B.; Papasotiropoulos, V.; Sabatino, L.; Ntatsi, G. Physiological and Yield Responses of Pepper (Capsicum annuum L.) Genotypes to Drought Stress. Plants 2025, 14, 1934. https://doi.org/10.3390/plants14131934
Ntanasi T, Karavidas I, Savvas D, Spyrou GP, Giannothanasis E, Consentino BB, Papasotiropoulos V, Sabatino L, Ntatsi G. Physiological and Yield Responses of Pepper (Capsicum annuum L.) Genotypes to Drought Stress. Plants. 2025; 14(13):1934. https://doi.org/10.3390/plants14131934
Chicago/Turabian StyleNtanasi, Theodora, Ioannis Karavidas, Dimitrios Savvas, George P. Spyrou, Evangelos Giannothanasis, Beppe Benedetto Consentino, Vasileios Papasotiropoulos, Leo Sabatino, and Georgia Ntatsi. 2025. "Physiological and Yield Responses of Pepper (Capsicum annuum L.) Genotypes to Drought Stress" Plants 14, no. 13: 1934. https://doi.org/10.3390/plants14131934
APA StyleNtanasi, T., Karavidas, I., Savvas, D., Spyrou, G. P., Giannothanasis, E., Consentino, B. B., Papasotiropoulos, V., Sabatino, L., & Ntatsi, G. (2025). Physiological and Yield Responses of Pepper (Capsicum annuum L.) Genotypes to Drought Stress. Plants, 14(13), 1934. https://doi.org/10.3390/plants14131934












