Regulatory Effects of Companion Plants (Maize (Zea mays) and Perilla frutescens) on American Ginseng Growth and Microbiome in Root Rot-Infested Field
Abstract
1. Introduction
2. Results
2.1. Effects of Companion Plants on the Survival Rate and Growth of American Ginseng
2.2. Effects of Companion Plants on Soil Physicochemical Properties and Enzymatic Activities
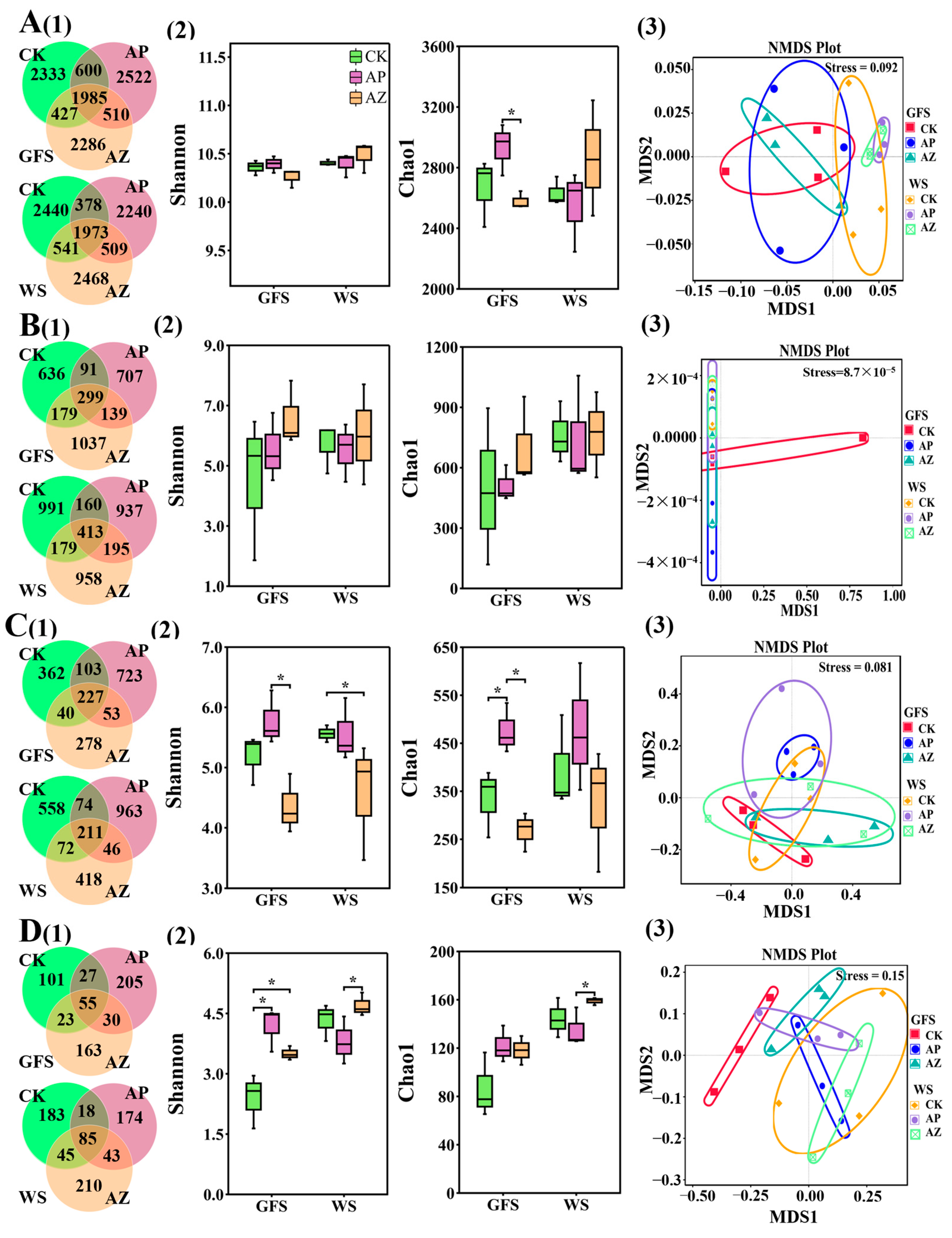
2.3. Effects of Companion Plants on the Diversity of Microbial Community in AG Rhizosphere and Root
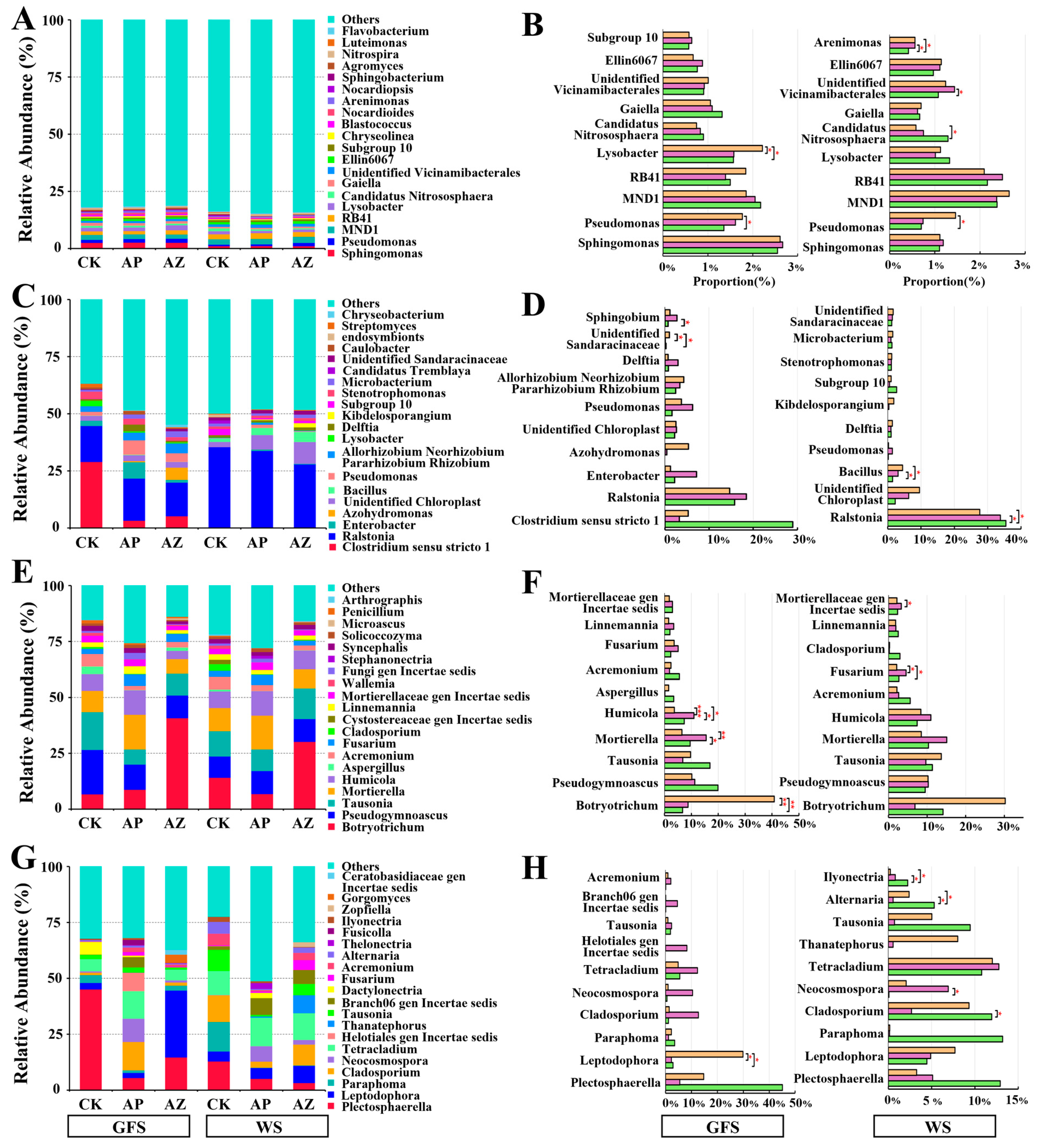
2.4. Effects of Companion Plants on Microbial Composition in AG Rhizosphere and Root
2.5. Effects of Companion Plants on Microbial Co-Occurrence Networks in AG Rhizosphere and Root
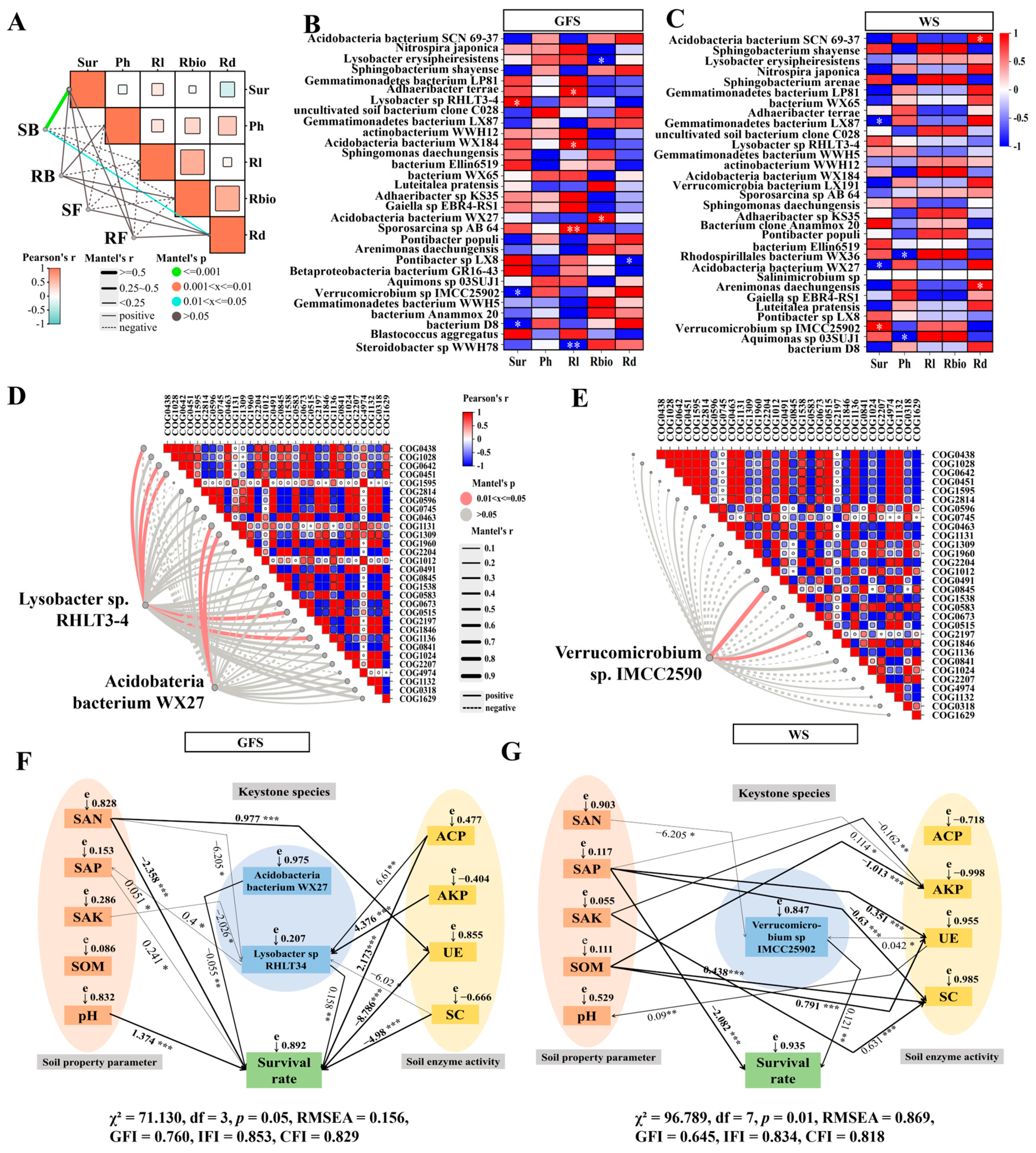
2.6. Relationship of Microbial Communities and Environmental Factors
2.7. Keystone Microbacterial Species Associated with Survival and Growth of AG Plant
3. Discussion
4. Materials and Methods
4.1. Plant Growth
4.2. Survival Rate of American Ginseng Plants
4.3. Plant and Soil Sampling
4.4. Soil Physicochemical Property Determination
4.5. Soil Enzymatic Activity Assay
4.6. DNA Extraction, PCR and Illumina Miseq Sequencing
4.7. Bioinformatics Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, D.; Jia, C.; Sun, P.; Qi, J.; Li, X. Quality evaluation of Panax quinquefolium from different cultivation regions based on their ginsenoside content and radioprotective effects on irradiated mice. Sci. Rep. 2019, 9, 1079. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Dong, Q.; Wu, F.; Liu, Y.; Li, P.; Liu, J. Non-targeted metabolomic analysis of methanolic extracts of wild-simulated and field-grown American ginseng. Molecules 2019, 24, 1053. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Construction Planning of Weihai Wendeng American Ginseng Science and Technology Industry Park. Master’s Thesis, Shandong University of Technology, Zibo, China, 2021. [Google Scholar]
- Li, C.; Chen, G.; Zhang, J.; Zhu, P.; Bai, X.; Hou, Y.; Zhang, X. The comprehensive changes in soil properties are continuous cropping obstacles associated with American ginseng (Panax quinquefolius) cultivation. Sci. Rep. 2021, 11, 5068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bi, Y.; Li, J.; Shao, H.; Jiao, X.L.; Gao, W. First report of root rot caused by Fusarium armeniacum on American ginseng in China. Plant Dis. 2021, 105, 1223. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, J.; Hu, H.; Zhao, F.; Zhang, J.; Gao, L.; Shang, W. Survey of American ginseng diseases in Qinba mountains. Shaanxi J. Agric. Sci. 2021, 67, 81–84. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, F.; Zhang, J.; Shang, W.; Hu, X. American ginseng root rot caused by Fusarium redolens in China. Plant Dis. 2021, 105, 2734. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, L.; Ma, Y.; Zhang, Y.; Zhang, Y. First report of anthracnose of American ginseng caused by Colletotrichum sojae in Northeast China. Plant Dis. 2021, 105, 3755. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Yang, J.; Liu, D. A study on the rotation of crops among Panax quinquefolium, Perilla frutescens and Coix lacryma-jobi. China J. Chin. Mater. Medica 2005, 30, 12–15. [Google Scholar]
- Jiang, J.; Yu, M.; Hou, R.; Li, L.; Ren, X.; Jiao, C.; Yang, L.; Xu, H. Changes in the soil microbial community are associated with the occurrence of Panax quinquefolius L. root rot diseases. Plant Soil. 2019, 438, 143–156. [Google Scholar] [CrossRef]
- Ji, L.; Nasir, F.; Tian, L.; Chang, J.; Sun, Y.; Zhang, J.; Li, X.; Tian, C. Outbreaks of root rot disease in different aged American ginseng plants are associated with field microbial dynamics. Front. Microbiol. 2021, 12, 676880. [Google Scholar] [CrossRef]
- Chen, G.; Xue, Y.; Yu, X.; Li, C.; Hou, Y.; Zhu, H.; Jiang, L.; Zheng, W.; Feng, Z.; Li, Y.; et al. The structure and function of microbial community in rhizospheric soil of American ginseng (Panax quinquefolius L.) changed with planting years. Curr. Microbiol. 2022, 79, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Y.; Li, H.; Hu, J.; Zhao, Z.; Wu, Y.; Yang, H.; Li, J.; Zhou, Y. Rhizosphere microbiome and phenolic acid exudation of the healthy and diseased American ginseng were modulated by the cropping history. Plants 2023, 12, 2993. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, S.; Li, S.; Zhang, T. Report on survey of American ginseng cultivation techniques in Wisconsin, American. J. Chin. Med. Mater. 1988, 11, 53–55. [Google Scholar]
- Li, R.; Liu, J.; Zhang, J.; Chen, J. Root rot and control of Panax quinquefolium: A review. China J. Chin. Mater. Medica 2025, 50, 2317–2323. [Google Scholar] [CrossRef]
- Tian, G.; Bi, Y.; Jiao, X.; Zhang, X.; Li, J.; Niu, F.; Gao, W. Application of vermicompost and biochar suppresses Fusarium root rot of replanted American ginseng. Appl. Microbiol. Biotechnol. 2021, 105, 6977–6991. [Google Scholar] [CrossRef]
- Zou, N. The Effect of Different Nitrogen Forms and Ratios on the Growth and Disease Resistance of American Ginseng Seedlings. Master’s Thesis, Lunan University, Zaozhuang, China, 2024. [Google Scholar]
- Jiao, X.; Zhang, X.; Lu, X.; Qin, R.; Bi, Y.; Gao, W. Effects of maize rotation on the physicochemical properties and microbial communities of American ginseng cultivated soil. Sci. Rep. 2019, 9, 8615. [Google Scholar] [CrossRef]
- Duan, W.; Chen, X.; Ding, Y.; Mao, X.; Song, Z.; Bao, J.; Fang, L.; Guo, L.; Zhou, J. Intricate microbe-plant-metabolic remodeling mediated by intercropping enhances the quality of Panax quinquefolius L. Physiol. Plant 2024, 176, e14499. [Google Scholar] [CrossRef]
- Guo, R.; Guan, R.; Lin, H. Diversity analysis and characteristic of fungal community in rhizosphere soil of Panax quinquefolium L. in different cropping years. China J. Tradit. Chin. Med. Pharm. 2022, 37, 4731–4736. [Google Scholar]
- Hao, F.; Yu, T.; Gao, K.; Xiong, M.; An, H. Production performance and stability of mixed forage grasslands improved by planting proportion and mode in Horqin sandy land, China. Sci. Rep. 2025, 15, 14683. [Google Scholar] [CrossRef]
- Levionnois, S.; Pradal, C.; Fournier, C.; Sanner, J.; Robert, C. Modeling the impact of proportion, sowing date, and architectural traits of a companion crop on foliar fungal pathogens of wheat in crop mixtures. Phytopathology 2023, 113, 1876–1889. [Google Scholar] [CrossRef]
- Sun, J.W.; Zhu, Y.A.; Pang, Y.; Liu, C.X.; Sun, J.H.; Zhang, W.P.; Li, L.; Liu, Y.X. The alteration of interspecific interaction responded to various relative sowing time in wheat/maize intercropping. Front Plant Sci 2024, 15, 1470293. [Google Scholar] [CrossRef] [PubMed]
- Bijarnia, A.; Tetarwal, J.P.; Yadav, R.K.; Bijrania, A.L.; Singh, D.; Saini, Y. Effect of fertility levels and stress mitigating chemicals on nutrient content, uptake, intercropping advantage and competition effect in cowpea-baby corn intercropping. Heliyon 2024, 10, e38194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zhang, J.; Li, K.; Wu, F.Z.; Hu, Z.P.; Mao, T.Y.; Yu, X.H.; Jiang, X.M. Effect of Perilla (Perilla frutescens) intercropping density on controlling pepper bight (Phytophthora capsica) and plant growth. China Veg. 2022, 4, 77–83. [Google Scholar]
- Wu, D.; Austin, R.S.; Zhou, S.; Brown, D. The root transcriptome for North American ginseng assembled and profiled across seasonal development. BMC Genom. 2013, 14, 564. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Tan, J.; Wang, C.; Dong, Q.; Wu, F.; Wang, H.; Liu, J.; Li, P.; Liu, J. Comprehensive investigation on metabolites of wild-simulated American ginseng root based on ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2019, 67, 5801–5819. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Hu, J.; Jiao, H.; Jin, Y.; Sun, J.; Nan, T.; Zhao, Y.; Liu, Y.; Huang, L.; et al. Comparisons of wild and cultivated American ginseng (Panax quinquefolius L.) genomes provide insights into changes in root growth and metabolism during domestication. Plant Biotechnol. J. 2024, 22, 1963–1965. [Google Scholar] [CrossRef]
- Bi, Y.M.; Zhang, X.M.; Jiao, X.L.; Li, J.F.; Peng, N.; Tian, G.L.; Wang, Y.; Gao, W.W. The relationship between shifts in the rhizosphere microbial community and root rot disease in a continuous cropping American ginseng system. Front. Microbiol. 2023, 14, 1097742. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Xie, Z.; Zhang, Y.; Malhi, S.S.; Guo, Z.; Qiu, Y.; Wang, L. Effects of lily/maize intercropping on rhizosphere microbial community and yield of Lilium davidii var. unicolor. J. Basic. Microbiol. 2018, 58, 892–901. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I. Isolation, functional characterization and efficacy of biofilm-forming rhizobacteria under abiotic stress conditions. Antonie Van Leeuwenhoek 2019, 112, 1827–1839. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.; Zang, P.; Li, X.; Ji, Q.; He, Z.; Zhao, Y.; Yang, H.; Zhao, X.; Zhang, L. An endophytic bacterium isolated from Panax ginseng C.A. Meyer enhances growth, reduces morbidity, and stimulates ginsenoside biosynthesis. Phytochem. Lett. 2015, 11, 132–138. [Google Scholar] [CrossRef]
- Martineau, C.; Mauffrey, F.; Villemur, R. Comparative analysis of denitrifying activities of Hyphomicrobium nitrativorans, Hyphomicrobium denitrificans, and Hyphomicrobium zavarzinii. Appl. Environ. Microbiol. 2015, 81, 5003–5014. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhao, Y.; Zhuang, L.; Yu, Y.; Wang, M.; Liu, J.; Wang, Q. A microbial consortium-based product promotes potato yield by recruiting rhizosphere bacteria involved in nitrogen and carbon metabolisms. Microb. Biotechnol. 2021, 14, 1961–1975. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhang, L.; Xue, H.; Chen, Y.; Liu, X.; Del Pozo, J.C.; Zhao, C.; Lozano-Duran, R.; Macho, A.P. Cell wall-mediated root development is targeted by a soil-borne bacterial pathogen to promote infection. Cell Rep. 2024, 43, 114179. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Zhang, X.; Liu, Z.; Yang, S.; Gao, W. Biological characteristics and fungicide sensitivity of four Ilyonectria species causing root rot on American ginseng. Acta Phytopathol. Sin. 2022, 52, 215–222. [Google Scholar] [CrossRef]
- Ebrahim, W.; Ebada, S. Antimicrobial metabolites from extremophilic fungus Botryotrichum piluliferum strain wesh19. Chem. Nat. Compd. 2021, 57, 654–658. [Google Scholar] [CrossRef]
- Song, X.; Chen, G.; Zheng, L.; Shen, J.; Xue, C.; Chang, Y. Microbiota involved in the degradation of tremella fuciformis polysaccharide and microbial enzymatic potential revealed by microbiome and metagenome. Microorganisms 2025, 13, 263. [Google Scholar] [CrossRef]
- Yano, S.; Kanno, H.; Tsuhako, H.; Ogasawara, S.; Suyotha, W.; Konno, H.; Makabe, K.; Uechi, K.; Taira, T. Cloning, expression, and characterization of a GH 19-type chitinase with antifungal activity from Lysobacter sp. MK9-1. J. Biosci. Bioeng. 2021, 131, 348–355. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, T.; Xu, H.; Xu, G.; Qian, G.; Liu, F. Characterization of Lysobacter spp. strains and their potential use as biocontrol agents against pear anthracnose. Microbiol. Res. 2021, 242, 126624. [Google Scholar] [CrossRef]
- Xie, Y.; Wright, S.; Shen, Y.; Du, L. Bioactive natural products from Lysobacter. Nat. Prod. Rep. 2012, 29, 1277–1287. [Google Scholar] [CrossRef]
- Lin, L.; Xu, K.; Shen, D.; Chou, S.H.; Gomelsky, M.; Qian, G. Antifungal weapons of Lysobacter, a mighty biocontrol agent. Environ. Microbiol. 2021, 23, 5704–5715. [Google Scholar] [CrossRef] [PubMed]
- Bünger, W.; Jiang, X.; Müller, J.; Hurek, T.; Reinhold-Hurek, B. Novel cultivated endophytic Verrucomicrobia reveal deep-rooting traits of bacteria to associate with plants. Sci. Rep. 2020, 10, 8692. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Y.B.; Sui, J.H.; Liu, C.S.; Liu, R.; Xu, Z.F.; Han, X.Y.; Zhang, T.; Zhang, Q.H.; Chen, C.B. Response of ginseng rhizosphere microbial communities and soil nutrients to phosphorus addition. Ind. Crops Prod. 2025, 226, 120687. [Google Scholar] [CrossRef]
- Peng, N.; Bi, Y.; Jiao, X.; Zhang, X.; Li, J.; Wang, Y.; Yang, S.; Liu, Z.; Gao, W. A soil fumigant increases American ginseng (Panax quinquefolius L.) survival and growth under continuous cropping by affecting soil microbiome assembly: A 4-year in situ field experiment. Microbiol. Spectr. 2024, 12, e0175723. [Google Scholar] [CrossRef]
- Li, Y.; Guan, Y.; Jiang, Z.; Xie, Q.; Wang, Q.; Yu, C.; Yu, W. soil microbial and metabolomic shifts induced by phosphate-solubilizing bacterial inoculation in Torreya grandis seedlings. Plants 2024, 13, 3209. [Google Scholar] [CrossRef]
- Yang, L.; Yang, K. Biological function of Klebsiella variicola and its effect on the rhizosphere soil of maize seedlings. Peer J. 2020, 8, e9894. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J. Bioinformatic and Statistical Analysis of Microbiome Data: From Raw Sequences to Advanced Modeling with QIIME 2 and R; Springer International Publishing: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]




| Microbial DNA Type | Amplicon Region | Forward Primer | Reverse Primer |
|---|---|---|---|
| Soil bacteria | 16SV4 | 515F: 5′-GTGCCAGCMGCCGCGGTAA-3′ | 806R: 5′-GGACTACHVGGGTWTCTAAT-3′ |
| Soil fungi | ITS1-5F | ITS5-1737F: 5′-GGAAGTAAAAGTCGTAACAAGG-3′ | ITS2-2043R: 5′-GCTGCGTTCTTCATCGATGC-3′ |
| endophytic bacteria | 16SV57 | 799F: 5′-AACMGGATTAGATACCCKG-3′ | 1193R: 5′-ACGTCATCCCCACCTTCC-3′ |
| endophytic fungi | ITS1-1F | ITS1-1F-F: 5′-CTTGGTCATTTAGAGGAAGTAA-3′ | ITS1-1F-R: 5′-GCTGCGTTCTTCATCGATGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Liao, D.; Han, T.; Ji, C.; He, C.; Li, X. Regulatory Effects of Companion Plants (Maize (Zea mays) and Perilla frutescens) on American Ginseng Growth and Microbiome in Root Rot-Infested Field. Plants 2025, 14, 1871. https://doi.org/10.3390/plants14121871
Luo D, Liao D, Han T, Ji C, He C, Li X. Regulatory Effects of Companion Plants (Maize (Zea mays) and Perilla frutescens) on American Ginseng Growth and Microbiome in Root Rot-Infested Field. Plants. 2025; 14(12):1871. https://doi.org/10.3390/plants14121871
Chicago/Turabian StyleLuo, Dan, Dengqun Liao, Tingting Han, Changhao Ji, Chao He, and Xianen Li. 2025. "Regulatory Effects of Companion Plants (Maize (Zea mays) and Perilla frutescens) on American Ginseng Growth and Microbiome in Root Rot-Infested Field" Plants 14, no. 12: 1871. https://doi.org/10.3390/plants14121871
APA StyleLuo, D., Liao, D., Han, T., Ji, C., He, C., & Li, X. (2025). Regulatory Effects of Companion Plants (Maize (Zea mays) and Perilla frutescens) on American Ginseng Growth and Microbiome in Root Rot-Infested Field. Plants, 14(12), 1871. https://doi.org/10.3390/plants14121871





