Microbial-Mediated Soil Nutrient Enhancement in Moso Bamboo–Liquidambar formosana vs. Phoebe chekiangensis Mixed Plantings
Abstract
1. Introduction
2. Study Site
3. Results
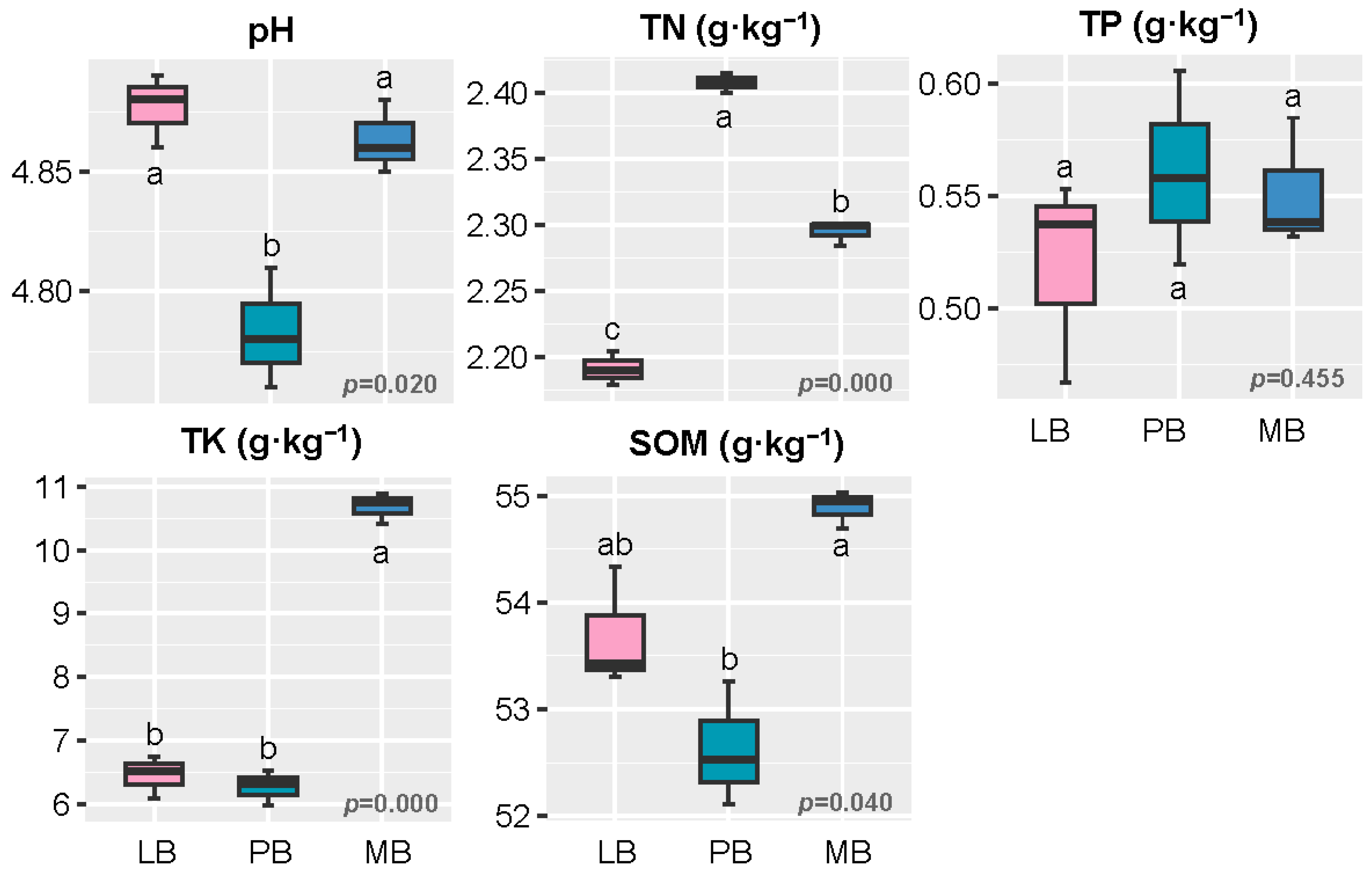
3.1. Effects of Moso Bamboo–Broadleaf Mixed Forests on Soil Chemical Properties
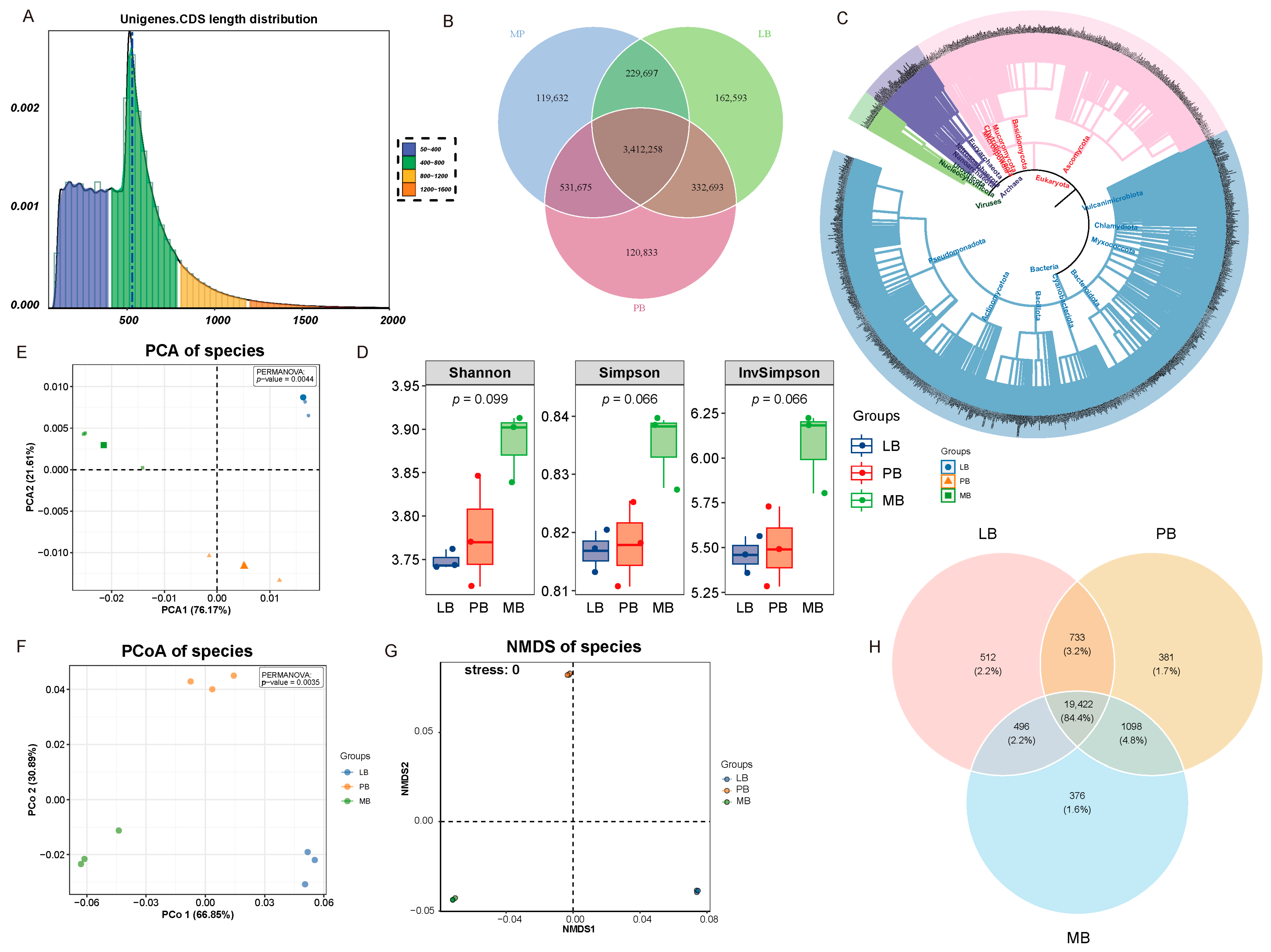
3.2. Effects of Moso Bamboo–Broadleaf Mixed Forests on Soil Microbial Community Structure and Diversity
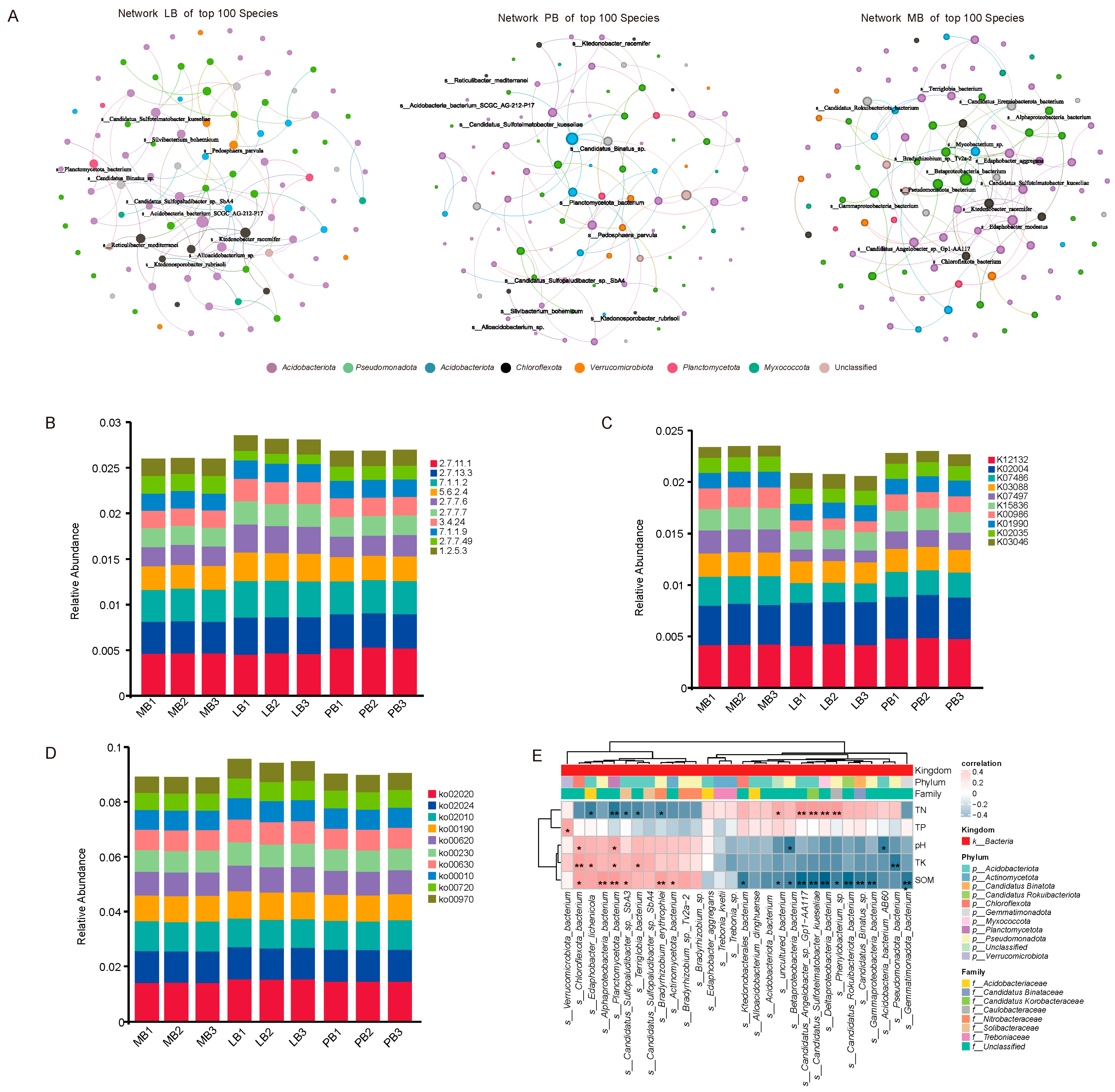
3.3. Effects of Moso Bamboo–Broadleaf Mixed Forests on Soil Microbial Community Structure
3.4. Effects of Moso Bamboo–Broadleaf Mixed Forests on Soil Microbial Functional Structure and Its Coupling Relationship with Soil Nutrients
4. Discussion
5. Materials and Methods
5.1. Experimental Design
5.2. Soil DNA Extraction
5.3. Metagenomic Sequencing and Data Analysis
5.4. Soil Nutrient Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A Review on Effective Soil Health Bio-Indicators for Ecosystem Restoration and Sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef] [PubMed]
- Hüblová, L.; Frouz, J. Contrasting Effect of Coniferous and Broadleaf Trees on Soil Carbon Storage during Reforestation of Forest Soils and Afforestation of Agricultural and Post-Mining Soils. J. Environ. Manag. 2021, 290, 112567. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root Exudate Cocktails: The Link between Plant Diversity and Soil Microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Zheng, T.; Zhu, X.; Bao, X.; Lynch, L.; Liang, C. Bacterial Community Structure and Assembly Dynamics Hinge on Plant Litter Quality. FEMS Microbiol. Ecol. 2023, 99, fiad118. [Google Scholar] [CrossRef]
- Zhou, F.C. Bamboo Cultivation; China Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Zhang, W.; Guo, Z.; Chen, S.; Wang, S.; Li, Y.; Fan, L. Impact of Abandonment on Leaf Morphology Traits and Nutrient Utilization Strategies of Dominant Tree Seedlings in Moso Bamboo Forests. Glob. Ecol. Conserv. 2024, 52, e02969. [Google Scholar] [CrossRef]
- Ge, Z.; Yu, T.; Tian, X.; Chen, X.; Yao, Y.; Mao, L. Analysis of Understory Plant Community Assembly Differences in Moso Bamboo Forests in the Subtropical Evergreen Broad-Leaved Forest Region of Eastern China. Forests 2025, 16, 478. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, P.; Hui, C.; Wang, F.; Liu, G.; Li, B. An Optimal Proportion of Mixing Broad-leaved Forest for Enhancing the Effective Productivity of Moso Bamboo. Ecol. Evol. 2015, 5, 1576–1584. [Google Scholar] [CrossRef]
- Bai, S.; Conant, R.T.; Zhou, G.; Wang, Y.; Wang, N.; Li, Y.; Zhang, K. Effects of Moso Bamboo Encroachment into Native, Broad-Leaved Forests on Soil Carbon and Nitrogen Pools. Sci. Rep. 2016, 6, 31480. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Huang, S.; Fang, D. Impacts of Moso Bamboo (Phyllostachys pubescens) Invasion on Species Diversity and Aboveground Biomass of Secondary Coniferous and Broad-Leaved Mixed Forest. Front. Plant Sci. 2022, 13, 1001785. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Chang, S.X.; Xu, Q.; Li, Y.; Ma, Z.; Qin, H.; Cai, Y. Linking Enhanced Soil Nitrogen Mineralization to Increased Fungal Decomposition Capacity with Moso Bamboo Invasion of Broadleaf Forests. Sci. Total Environ. 2021, 771, 144779. [Google Scholar] [CrossRef]
- Sardar, M.F.; Chen, Z.; Tang, C.; Zhang, S.; Fang, L.; Miao, D.; Li, Y.; Zhang, Q.; Li, Y. Seasonal Linkages between Soil Nitrogen Mineralization and the Microbial Community in Broadleaf Forests with Moso Bamboo (Phyllostachys edulis) Invasion. Sci. Total Environ. 2023, 899, 165557. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, M.; Wang, T.; Fu, X. Evaluation of Ecological Service Function of Liquidambar Formosana Plantations. Int. J. Environ. Res. Public Health 2022, 19, 15317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Song, M.; Qiao, Y.; Liu, M.; Ma, L.; Fu, S. Long-Term Water Use Efficiency and Non-Structural Carbohydrates of Dominant Tree Species in Response to Nitrogen and Water Additions in a Warm Temperate Forest. Front. Plant Sci. 2022, 13, 1025162. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yi, L.; Wang, J.; Li, X.; Xu, B.; Liu, M. Nitrogen Addition Affected the Root Competition in Cunninghamia Lanceolata –Phoebe Chekiangensis Mixed Plantation. Physiol. Plant. 2024, 176, e14268. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Korpelainen, H.; Zhang, H.; Wang, J.; Huang, H.; Yi, L. Nitrogen Addition Affects Eco-Physiological Interactions between Two Tree Species Dominating in Subtropical Forests. Plant Physiol. Biochem. 2021, 162, 150–160. [Google Scholar] [CrossRef]
- Christel, H.; Bruelheide, H.; Cesarz, S.; Eisenhauer, N.; Hähn, G.J.A.; Beugnon, R. The Spatial Distribution of Tree–Tree Interaction Effects on Soil Microbial Biomass and Respiration. Ecol. Evol. 2024, 14, e11530. [Google Scholar] [CrossRef]
- Li, K.; Lu, Y.; Wang, Q.-W.; Ni, R.; Han, R.; Li, C.; Zhang, C.; Shen, W.; Yao, Q.; Gao, Y.; et al. Leaf Litter Mixtures Alter Decomposition Rate, Nutrient Retention, and Bacterial Community Composition in a Temperate Forest. For. Res. 2023, 3, 22. [Google Scholar] [CrossRef]
- Yin, J.; Ge, Z.; Deng, X.; Shi, Y.; Xu, L.; Zhou, G.; Ye, Y.; Zhang, F.; Zhou, Y.; Li, C.; et al. Abandonment Lead to Structural Degradation and Changes in Carbon Allocation Patterns in Moso Bamboo Forests. For. Ecol. Manag. 2019, 449, 117449. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Relationship between Photosynthetic Phosphorus-use Efficiency and Foliar Phosphorus Fractions in Tropical Tree Species. Ecol. Evol. 2013, 3, 4872–4880. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Zhong, Z.; Li, Q.; Bian, F. Forest Management Impacts on Soil Phosphorus Cycling: Insights from Metagenomics in Moso Bamboo Plantations. J. Environ. Manag. 2025, 373, 123735. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, Y.; Yang, Z.; Wang, Q.C.; Lü, P.P.; Hu, H.W.; Yang, Y.S.; He, J.Z. Bacterial Communities in the Phyllosphere Are Distinct from Those in Root and Soil, and Sensitive to Plant Species Changes in Subtropical Tree Plantations. FEMS Microbiol. Ecol. 2023, 99, fiad033. [Google Scholar] [CrossRef] [PubMed]
- Khlifa, R.; Paquette, A.; Messier, C.; Reich, P.B.; Munson, A.D. Do Temperate Tree Species Diversity and Identity Influence Soil Microbial Community Function and Composition? Ecol. Evol. 2017, 7, 7965–7974. [Google Scholar] [CrossRef] [PubMed]
- Messyasz, A.; Männistö, M.K.; Kerkhof, L.J.; Häggblom, M.M. Genome Analysis and Description of Tunturibacte Gen. Nov. Expands the Diversity of Terriglobia in Tundra Soils. Environ. Microbiol. 2024, 26, e16640. [Google Scholar] [CrossRef] [PubMed]
- White, D.C.; Sutton, S.D.; Ringelberg, D.B. The Genus Sphingomonas: Physiology and Ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, C.; Wan, R.; Li, S.; Yang, Y.; Lv, C.; Li, Y.; Yang, G. Influence of Weeding Methods on Rhizosphere Soil and Root Endophytic Microbial Communities in Tea Plants. Front. Microbiol. 2024, 15, 1334711. [Google Scholar] [CrossRef]
- Wen, Z.L.; Yang, M.K.; Chen, X.Y.; Hao, C.Y.; Ren, Y.; Chu, S.J.; Han, H.W.; Lin, H.Y.; Lu, G.H.; Qi, J.L.; et al. Bacterial Composition, Function and the Enrichment of Plant Growth Promoting Rhizobacteria (PGPR) in Differential Rhizosphere Compartments of Al-Tolerant Soybean in Acidic Soil. Hereditas 2021, 43, 487–500. [Google Scholar]
- Watson, S.W.; Valois, F.W.; Waterbury, J.B. The Family Nitrobacteraceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Ormeño-Orrillo, E.; Martínez-Romero, E. A Genomotaxonomy View of the Bradyrhizobium Genus. Front. Microbiol. 2019, 10, 1334. [Google Scholar] [CrossRef]
- Zhang, R.; Rong, L.; Zhang, L. Soil Nutrient Variability Mediates the Effects of Erosion on Soil Microbial Communities: Results from a Modified Topsoil Removal Method in an Agricultural Field in Yunnan Plateau, China. Environ. Sci. Pollut. Res. 2022, 29, 3659–3671. [Google Scholar] [CrossRef]
- Willms, I.M.; Bolz, S.H.; Yuan, J.; Krafft, L.; Schneider, D.; Schöning, I.; Schrumpf, M.; Nacke, H. The Ubiquitous Soil Verrucomicrobial Clade ‘Candidatus Udaeobacter’ Shows Preferences for Acidic PH. Environ. Microbiol. Rep. 2021, 13, 878–883. [Google Scholar] [CrossRef]
- Liu, J.; Liao, H.; Fan, M.; Zhou, T.; Peng, S. Comparison of Root Morphology and Rhizosphere Microbial Communities Form Moso-bamboo in Different Forest Types. Ecol. Evol. 2023, 13, e10153. [Google Scholar] [CrossRef]
- Fan, L.; Chen, S.; Guo, Z.; Hu, R.; Yao, L. Soil PH Enhancement and Alterations in Nutrient and Bacterial Community Profiles Following Pleioblastus Amarus Expansion in Tea Plantations. BMC Plant Biol. 2024, 24, 837. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, A.; Lal, S.; Munir, R.; Stott, M.; Henrissat, B.; Polikarpov, I.; Sparling, R.; Levin, D.B. Analysis of Carbohydrate-Active Enzymes in Thermogemmatispora Sp. Strain T81 Reveals Carbohydrate Degradation Ability. Can. J. Microbiol. 2018, 64, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, W.; Dai, Y.; Ai, L.; Wu, M.; Hu, J.; Zuo, Z.; Li, M.; Yang, H.; Ma, J. Moso Bamboo (Phyllostachys edulis (Carrière) J. Houzeau) Invasion Affects Soil Microbial Communities in Adjacent Planted Forests in the Lijiang River Basin, China. Front. Microbiol. 2023, 14, 1111498. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; Arikawa, K.; Saeki, T.; Matsuhashi, A.; Hosokawa, M. High-Quality Draft Single-Cell Genome Sequences of Two Gammaproteobacteria Strains Sampled from Soil in a Strawberry Farm. Microbiol. Resour. Announc. 2020, 9, e00743-20. [Google Scholar] [CrossRef]
- He, K.; Lai, Y.; Hu, S.; Song, M.; Su, Y.; Li, C.; Wu, X.; Zhang, C.; Hua, Y.; Huang, J.; et al. Assembly Characteristics and Influencing Factors of the Soil Microbial Community in the Typical Forest of Funiu Mountain. Microorganisms 2024, 12, 2355. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Guo, X.; Zeng, Z.; Wang, Y.; Zhang, P.; Gao, D.; Deng, G.; Sun, G.; Yang, Y.; et al. Forest Soil PH and Dissolved Organic Matter Aromaticity Are Distinct Drivers for Soil Microbial Community and Carbon Metabolism Potential. Microb. Ecol. 2024, 87, 177. [Google Scholar] [CrossRef]
- Yao, L.; Jiao, J.; Wu, C.; Jiang, B.; Fan, L. Effects of Thinning on the Structure of Soil Microbial Communities in a Subtropical Secondary Evergreen Broad-Leaved Forest. Front. Plant Sci. 2024, 15, 1465237. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Yang, L.; Huang, P.; Li, W.; Wang, S.; Zhao, G.; Zhang, M.; Pang, X.; Yan, Z.; et al. Structural Modulation of Gut Microbiota in Life-Long Calorie-Restricted Mice. Nat. Commun. 2013, 4, 2163. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Kumar, P.; Dubey, R.C.; Maheshwari, D.K. Bacillus Strains Isolated from Rhizosphere Showed Plant Growth Promoting and Antagonistic Activity against Phytopathogens. Microbiol. Res. 2012, 167, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Alm, E.J. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct Soil Microbial Diversity under Long-Term Organic and Conventional Farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]

| Sample ID | Raw_Base (G) | Clean_Base (G) | Clean_Q20 (%) | Clean_Q30 (%) | Clean_GC (%) | Effective (%) |
|---|---|---|---|---|---|---|
| MB1 | 15.36 | 15.26 | 98.48 | 96.06 | 60.19 | 99.36 |
| MB2 | 14.63 | 14.53 | 98.44 | 95.98 | 60.23 | 99.29 |
| MB3 | 16.91 | 16.81 | 98.5 | 96.04 | 60.19 | 99.37 |
| LB1 | 10.38 | 10.07 | 97.82 | 94.33 | 60.38 | 97.02 |
| LB2 | 13.97 | 13.9 | 98.44 | 95.94 | 60.01 | 99.46 |
| LB3 | 17.38 | 17.27 | 98.44 | 95.97 | 60.22 | 99.39 |
| PB1 | 15.96 | 15.89 | 98.5 | 96.09 | 60.18 | 99.56 |
| PB2 | 15.31 | 15.23 | 98.5 | 96.06 | 60.14 | 99.53 |
| PB3 | 15.51 | 15.39 | 98.46 | 95.87 | 60.11 | 99.18 |
| Sample ID | Total Length (bp) | Scaftigs Num | Average Length (bp) | N50 Length (bp) | N90 Length (bp) | Max Length (bp) |
|---|---|---|---|---|---|---|
| MB1 | 730,229,717 | 899,185 | 812.1 | 772 | 533 | 81,211 |
| MB2 | 675,247,105 | 835,002 | 808.68 | 768 | 533 | 105,128 |
| MB3 | 831,806,140 | 1,014,565 | 819.86 | 781 | 534 | 89,391 |
| LB1 | 324,951,331 | 456,067 | 712.51 | 676 | 524 | 18,717 |
| LB2 | 512,751,453 | 697,120 | 735.53 | 699 | 526 | 21,800 |
| LB3 | 714,921,510 | 945,420 | 756.19 | 719 | 528 | 26,918 |
| PB1 | 795,079,007 | 993,465 | 800.31 | 761 | 533 | 46,135 |
| PB2 | 757,034,068 | 940,087 | 805.28 | 765 | 533 | 64,636 |
| PB3 | 717,743,789 | 899,519 | 797.92 | 757 | 533 | 58,615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, A.; Fan, L.; Lu, G.; Yao, L.; Fan, J. Microbial-Mediated Soil Nutrient Enhancement in Moso Bamboo–Liquidambar formosana vs. Phoebe chekiangensis Mixed Plantings. Plants 2025, 14, 1868. https://doi.org/10.3390/plants14121868
Zhu A, Fan L, Lu G, Yao L, Fan J. Microbial-Mediated Soil Nutrient Enhancement in Moso Bamboo–Liquidambar formosana vs. Phoebe chekiangensis Mixed Plantings. Plants. 2025; 14(12):1868. https://doi.org/10.3390/plants14121868
Chicago/Turabian StyleZhu, Anming, Lili Fan, Gang Lu, Liangjin Yao, and Jianzhong Fan. 2025. "Microbial-Mediated Soil Nutrient Enhancement in Moso Bamboo–Liquidambar formosana vs. Phoebe chekiangensis Mixed Plantings" Plants 14, no. 12: 1868. https://doi.org/10.3390/plants14121868
APA StyleZhu, A., Fan, L., Lu, G., Yao, L., & Fan, J. (2025). Microbial-Mediated Soil Nutrient Enhancement in Moso Bamboo–Liquidambar formosana vs. Phoebe chekiangensis Mixed Plantings. Plants, 14(12), 1868. https://doi.org/10.3390/plants14121868






