Root Ethylene and Abscisic Acid Responses to Flooding Stress in Styrax japonicus: A Transcriptomic Perspective
Abstract
1. Introduction
2. Results
2.1. Changes in the Content of Endogenous Plant Hormones in S. japonicus Roots Under Flooding Stress
2.1.1. ACC
2.1.2. ABA, GA1, SA and T-Zeatin
2.2. De Novo Assembly and Quality Control
2.3. PCA Analysis
2.4. KEGG Analysis of DEGs
2.5. DEGs Screened from Plant Hormone Signal Transduction
2.6. DEG Selected from Plant Hormone Biosynthesis Process
2.6.1. Ethylene Biosynthesis
2.6.2. ABA Biosynthesis
3. Discussion
3.1. Ethylene, ABA Biosynthesis and Regulation of Related Genes
3.2. Interactions Among Ethylene, ABA and GA Under Flooding Stress
3.3. The Role of Other Hormones in Plants’ Response to Flooding Stress
4. Materials and Methods
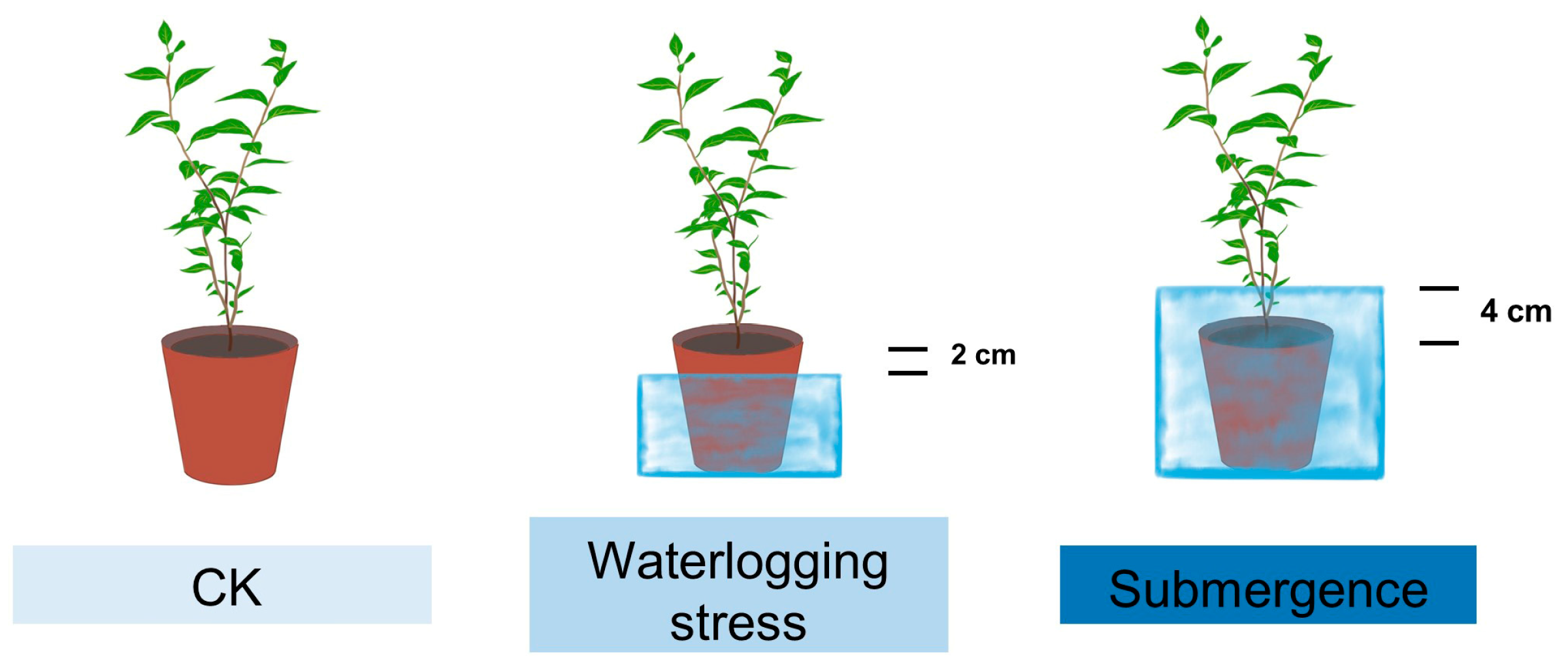
4.1. Materials and Experimental Design
4.2. Determination of Endogenous Hormone Content in Roots
4.3. RNA Extraction and Library Construction
4.4. Quality Control and de Novo Assembly
4.5. KEGG Enrichment Analysis
4.6. qRT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| GA | gibberellic acid |
| SA | salicylic acid |
| JA | jasmonic acid |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ACO | 1-aminocyclopropane-1-carboxylic acid oxidase |
| SAM | S-adenosylmethionine |
| NCED | 9-cis-epoxy carotenoid dioxygenase |
| DEGs | differentially expressed Unigenes |
| PCA | Principal Components Analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Flora of China; Science Press: Beijing, China, 1987; Volume 60, p. 89.
- Palermo, G.; Curtin, M.; Boland, T.; Thomas, E. Styrax Japonicus, a New Record for Massachusetts. Rhodora 2020, 122, 53. [Google Scholar] [CrossRef]
- Xia, D.D.; Han, X.Y.; Zhang, Y.; Zhang, N. Chemical Constituents and Their Biological Activities from Genus Styrax. Pharmaceuticals 2023, 16, 1043. [Google Scholar] [CrossRef]
- Ren, J.; Fang, A.; Jiao, S.; Li, R.; Huang, Y.; Ni, X.; Zhang, Y.; Ma, Y.; Li, S.; Li, J. Lignans from the Leaves of Styrax japonicus and Their Anti-Inflammatory Activity. Fitoterapia 2024, 172, 105774. [Google Scholar] [CrossRef] [PubMed]
- Styrax Japonicus; Bulletin of popular information—Arnold Arboretum, Harvard University: Boston, MA, USA, 1916; Volume 2, p. 35. [CrossRef]
- Kholibrina, C.R.; Aswandi, A. Increasing Added-Value of Styrax Resin through Post-Harvesting Techniques Improvement and Essential Oil Based Product Innovation. IOP Conf. Ser. Earth Environ. Sci. 2021, 713, 012045. [Google Scholar] [CrossRef]
- Wu, Q.K.; Fei, X.R.; Gao, Y.; Chen, C.; Cao, Y.Y.; Yu, F.Y. Comparative analysis of fuel properties of eight biodiesel plants species of Styrax spp. China Oils Fats 2019, 44, 27–30. (In Chinese) [Google Scholar]
- Zhang, W.; Zhou, T.; Wu, P. Anthropogenic Amplification of Precipitation Variability over the Past Century. Science 2024, 385, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, C.; Liu, Z.; Guy, R.D.; Yu, F. Physiological and Biochemical Response Analysis of Styrax Tonkinensis Seedlings to Waterlogging Stress. Plant Physiol. Biochem. 2024, 210, 108587. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-Mediated Acclimations to Flooding Stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A.C.J. The Role of Ethylene in Metabolic Acclimations to Low Oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef]
- Leeggangers, H.A.C.F.; Rodriguez-Granados, N.Y.; Macias-Honti, M.G.; Sasidharan, R. A Helping Hand When Drowning: The Versatile Role of Ethylene in Root Flooding Resilience. Environ. Exp. Bot. 2023, 213, 105422. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Chandrasekaran, U.; Luo, X.; Zheng, C.; Shu, K. ABA Biosynthesis and Signaling Cascades Under Hypoxia Stress. Front. Plant Sci. 2021, 12, 661228. [Google Scholar] [CrossRef]
- Wang, X.; Komatsu, S. The Role of Phytohormones in Plant Response to Flooding. Int. J. Mol. Sci. 2022, 23, 6383. [Google Scholar] [CrossRef]
- Hurng, W.P.; Lur, H.S.; Liao, C.K.; Kao, C.H. Role of Abscisic Acid, Ethylene and Polyamines in Flooding-Promoted Senescence of Tobacco Leaves. J. Plant Physiol. 1994, 143, 102–105. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X. Can Early Wilting of Old Leaves Account for Much of the ABA Accumulation in Flooded Pea Plants? J. Exp. Bot. 1994, 45, 1335–1342. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Shishova, M.F. The Role of Phytohormones in the Control of Plant Adaptation to Oxygen Depletion. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Nazar, R., Iqbal, N., Anjum, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 229–248. ISBN 978-3-642-25828-2. [Google Scholar]
- Arbona, V.; Zandalinas, S.I.; Manzi, M.; González-Guzmán, M.; Rodriguez, P.L.; Gómez-Cadenas, A. Depletion of Abscisic Acid Levels in Roots of Flooded Carrizo Citrange (Poncirus Trifoliata L. Raf. × Citrus Sinensis L. Osb.) Plants Is a Stress-Specific Response Associated to the Differential Expression of PYR/PYL/RCAR Receptors. Plant Mol. Biol. 2017, 93, 623–640. [Google Scholar] [CrossRef] [PubMed]
- De Ollas, C.; González-Guzmán, M.; Pitarch, Z.; Matus, J.T.; Candela, H.; Rambla, J.L.; Granell, A.; Gómez-Cadenas, A.; Arbona, V. Identification of ABA-Mediated Genetic and Metabolic Responses to Soil Flooding in Tomato (Solanum Lycopersicum L. Mill). Front. Plant Sci. 2021, 12, 613059. [Google Scholar] [CrossRef]
- Su, S.; Zhu, T.; Su, J.; Li, J.; Zhao, Q.; Kang, X.; Zheng, R. Transcriptome Analysis of Gibberellins and Abscisic Acid during the Flooding Response in Fokienia hodginsii. PLoS ONE 2022, 17, e0263530. [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and Submergence Stress: Affects and Acclimation. Crit. Rev. Biotechnol. 2016, 36, 956–966. [Google Scholar] [CrossRef]
- Benschop, J.J.; Bou, J.; Peeters, A.J.M.; Wagemaker, N.; Gühl, K.; Ward, D.; Hedden, P.; Moritz, T.; Voesenek, L.A.C.J. Long-Term Submergence-Induced Elongation in Rumex Palustris Requires Abscisic Acid-Dependent Biosynthesis of Gibberellin1. Plant Physiol. 2006, 141, 1644–1652. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Saify, S.; Iqbal, N.; Khan, N.A. The Ethylene Biosynthetic Enzymes, 1-Aminocyclopropane-1-Carboxylate (ACC) Synthase (ACS) and ACC Oxidase (ACO): The Less Explored Players in Abiotic Stress Tolerance. Biomolecules 2024, 14, 90. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of Drought Tolerance by Gene Manipulation of 9-cis-epoxycarotenoid Dioxygenase, a Key Enzyme in Abscisic Acid Biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Trivellini, A.; Chhillar, H.; Chopra, P.; Ferrante, A.; Khan, N.A.; Ismail, A.M. The Significance and Functions of Ethylene in Flooding Stress Tolerance in Plants. Environ. Exp. Bot. 2020, 179, 104188. [Google Scholar] [CrossRef]
- McDonnell, L.; Plett, J.M.; Andersson-Gunnerås, S.; Kozela, C.; Dugardeyn, J.; Van Der Straeten, D.; Glick, B.R.; Sundberg, B.; Regan, S. Ethylene Levels Are Regulated by a Plant Encoded 1-Aminocyclopropane-1-Carboxylic Acid Deaminase. Physiol. Plant. 2009, 136, 94–109. [Google Scholar] [CrossRef]
- Olson, D.C.; Oetiker, J.H.; Yang, S.F. Analysis of LE-ACS3, a 1-Aminocyclopropane-1-Carboxylic Acid Synthase Gene Expressed during Flooding in the Roots of Tomato Plants. J. Biol. Chem. 1995, 270, 14056–14061. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Q.; Ni, M.; Chen, C.; Han, C.; Yu, F. Transcriptome Analysis of Endogenous Hormone Response Mechanism in Roots of Styrax tonkinensis Under Waterlogging. Front. Plant Sci. 2022, 13, 896850. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, J.; Long, W.; He, P.; Zhou, Q.; Hu, X.; Zhou, Y.; Zheng, Y. Transcriptomic and Metabolomic Analyses of Seedlings of Two Grape Cultivars with Distinct Tolerance Responses to Flooding and Post-Flooding Stress Conditions. Horticulturae 2023, 9, 980. [Google Scholar] [CrossRef]
- Pan, R.; Buitrago, S.; Feng, X.; Hu, A.; Zhou, M.; Zhang, W. Ethylene Regulates Aerenchyma Formation in Cotton under Hypoxia Stress by Inducing the Accumulation of Reactive Oxygen Species. Environ. Exp. Bot. 2022, 197, 104826. [Google Scholar] [CrossRef]
- The Arabidopsis Genome Initiative. Analysis of the Genome Sequence of the Flowering Plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [PubMed]
- Houben, M.; Van De Poel, B. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef]
- Minami, A.; Yano, K.; Gamuyao, R.; Nagai, K.; Kuroha, T.; Ayano, M.; Nakamori, M.; Koike, M.; Kondo, Y.; Niimi, Y.; et al. Time-Course Transcriptomics Analysis Reveals Key Responses of Submerged Deepwater Rice to Flooding. Plant Physiol. 2018, 176, 3081–3102. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Kherawat, B.S.; Ram, C.; Singh, A.; Dey, P.; Gora, J.S.; Misra, N.; Chung, S.-M.; Kumar, M. Genome-Wide Identification and Expression Profiling of Aconitase Gene Family Members Reveals Their Roles in Plant Development and Adaptation to Diverse Stress in Triticum aestivum L. Plants 2022, 11, 3475. [Google Scholar] [CrossRef]
- Li, J.; Cui, C.; Han, F.; Liu, J. Genome-Wide Identification and Analysis of the UBA2 Gene Family in Wheat (Triticum Aestivum L.). BMC Genom. 2025, 26, 180. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Benning, S.; Kende, H. On the Role of Abscisic Acid and Gibberellin in the Regulation of Growth in Rice. Plant Physiol. 1992, 99, 1156–1161. [Google Scholar] [CrossRef]
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Jikumaru, Y.; Fujimoto, M.; Arikawa, T.; Takahashi, H.; Ando, M.; et al. Ethylene Promotes Submergence-Induced Expression of OsABA8ox1, a Gene That Encodes ABA 8′-Hydroxylase in Rice. Plant Cell Physiol. 2007, 48, 287–298. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Chen, L.; Zhao, G.; Li, S.; Zhou, H.; Dai, Y.; Sun, N.; Xie, Y.; Gao, J.; et al. Genome-Wide Identification and Expression Analysis of the NCED Family in Cotton (Gossypium Hirsutum L.). PLoS ONE 2021, 16, e0246021. [Google Scholar] [CrossRef]
- De Oliveira, F.K.; Da-Silva, C.J.; Garcia, N.; Agualongo, D.A.P.; de Oliveira, A.C.B.; Kanamori, N.; Takasaki, H.; Urano, K.; Shinozaki, K.; Nakashima, K.; et al. The overexpression of NCED results in waterlogging sensitivity in soybean. Plant Stress 2022, 3, 100047. [Google Scholar] [CrossRef]
- de Bruxelles, G.L.; Peacock, W.J.; Dennis, E.S.; Dolferus, R. Abscisic Acid Induces the Alcohol Dehydrogenase Gene in Arabidopsis. Plant Physiol. 1996, 111, 381–391. [Google Scholar] [CrossRef]
- Papdi, C.; Pérez-Salamó, I.; Joseph, M.P.; Giuntoli, B.; Bögre, L.; Koncz, C.; Szabados, L. The Low Oxygen, Oxidative and Osmotic Stress Responses Synergistically Act through the Ethylene Response Factor VII Genes RAP 2.12, RAP 2.2 and RAP 2.3. Plant J. 2015, 82, 772–784. [Google Scholar] [CrossRef]
- Peng, H.P.; Chan, C.S.; Shih, M.C.; Yang, S.F. Signaling Events in the Hypoxic Induction of Alcohol Dehydrogenase Gene in Arabidopsis. Plant Physiol. 2001, 126, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Ni, D.; Song, L.; Zhang, Z. Isolation of an Alcohol Dehydrogenase cDNA from and Characterization of Its Expression in Chrysanthemum under Waterlogging. Plant Sci. 2013, 212, 48–54. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant Waterlogging/Flooding Stress Responses: From Seed Germination to Maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Gómez-Cadenas, A.; Arbona, V. Abscisic Acid as an Emerging Modulator of the Responses of Plants to Low Oxygen Conditions. Front. Plant Sci. 2021, 12, 661789. [Google Scholar] [CrossRef]
- Benschop, J.J.; Jackson, M.B.; Gühl, K.; Vreeburg, R.A.M.; Croker, S.J.; Peeters, A.J.M.; Voesenek, L.A.C.J. Contrasting Interactions between Ethylene and Abscisic Acid in Rumex Species Differing in Submergence Tolerance. Plant J. 2005, 44, 756–768. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Tetsuo, K.; Masanori, O.; Kazumi, N.; Kazutoshi, Y.; Sayaka, K.; Tadao, A.; Nobuhiro, H.; Tomokazu, K.; Yuji, K.; Eiji, N. The Arabidopsis Cytochrome P450 CYP707A Encodes ABA 8′-Hydroxylases: Key Enzymes in ABA Catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef]
- Yang, S.H.; Choi, D. Characterization of Genes Encoding ABA 8′-Hydroxylase in Ethylene-Induced Stem Growth of Deepwater Rice (Oryza Sativa L.). Biochem. Biophys. Res. Commun. 2006, 350, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Kende, H.; van der Knaap, E.; Cho, H.-T. Deepwater Rice: A Model Plant to Study Stem Elongation1. Plant Physiol. 1998, 118, 1105–1110. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between Ethylene, Gibberellin and Abscisic Acid Regulate Emergence and Growth Rate of Adventitious Roots in Deepwater Rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-Gibberellin Signaling Underlies Adaptation of Rice to Periodic Flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhang, Z.; Wu, F.; Feng, M.; Sun, Y.; Chen, W.; Cheng, Z.; Zhang, X.; Ren, Y.; Lei, C.; et al. The APC/CTE E3 Ubiquitin Ligase Complex Mediates the Antagonistic Regulation of Root Growth and Tillering by ABA and GA. Plant Cell 2020, 32, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.C.J. Interactions Between Plant Hormones Regulate Submergence-Induced Shoot Elongation in the Flooding-Tolerant Dicot Rumex palustris. Ann. Bot. 2003, 91, 205–211. [Google Scholar] [CrossRef]
- Gu, X.Y.; Liu, Y.; Liu, L.J. Progress on the biosynthesis and signal transduction of phytohormone salicylic acid. Hereditas 2020, 42, 858–869. (In Chinese) [Google Scholar]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in Roles of Salicylic Acid in Plant Tolerance Responses to Biotic and Abiotic Stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Koramutla, M.K.; Tuan, P.A.; Ayele, B.T. Salicylic Acid Enhances Adventitious Root and Aerenchyma Formation in Wheat under Waterlogged Conditions. Int. J. Mol. Sci. 2022, 23, 1243. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, A.K.; Dwivedi, P. Modulating Effect of Salicylic Acid in Tomato Plants in Response to Waterlogging Stress. Int. J. Agric. Environ. Biotechnol. 2017, 10, 31. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Ahmed, N.; Saha, T.; Rahman, M.; Rahman, K.; Alam, M.; Rohman, M.; Nahar, K. Exogenous Salicylic Acid and Kinetin Modulate Reactive Oxygen Species Metabolism and Glyoxalase System to Confer Waterlogging Stress Tolerance in Soybean (Glycine Max L.). Plant Stress 2022, 3, 100057. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Huynh, L.N.; VanToai, T.; Streeter, J.; Banowetz, G. Regulation of Flooding Tolerance of SAG12:Ipt Arabidopsis Plants by Cytokinin. J. Exp. Bot. 2005, 56, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Lo, C.C.; Chain, P.S.G. Rapid Evaluation and Quality Control of next Generation Sequencing Data with FaQCs. BMC Bioinform. 2014, 15, 366. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xu, Q.; Chen, H.; Peng, H.; Yu, F. Transcriptome Analysis Provides Insights into Lignin Biosynthesis in Styrax Tonkinensis Branches. Forests 2024, 15, 601. [Google Scholar] [CrossRef]

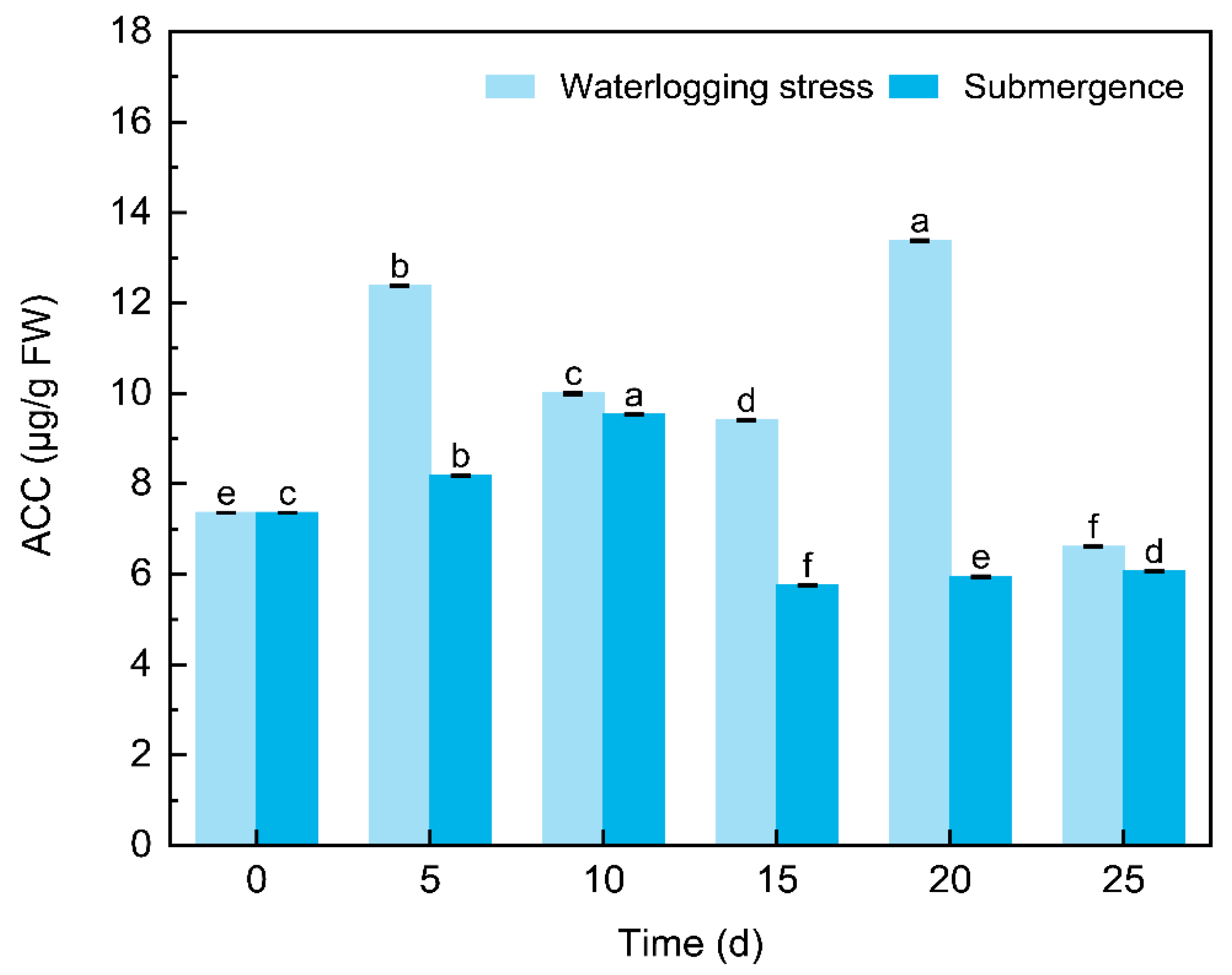

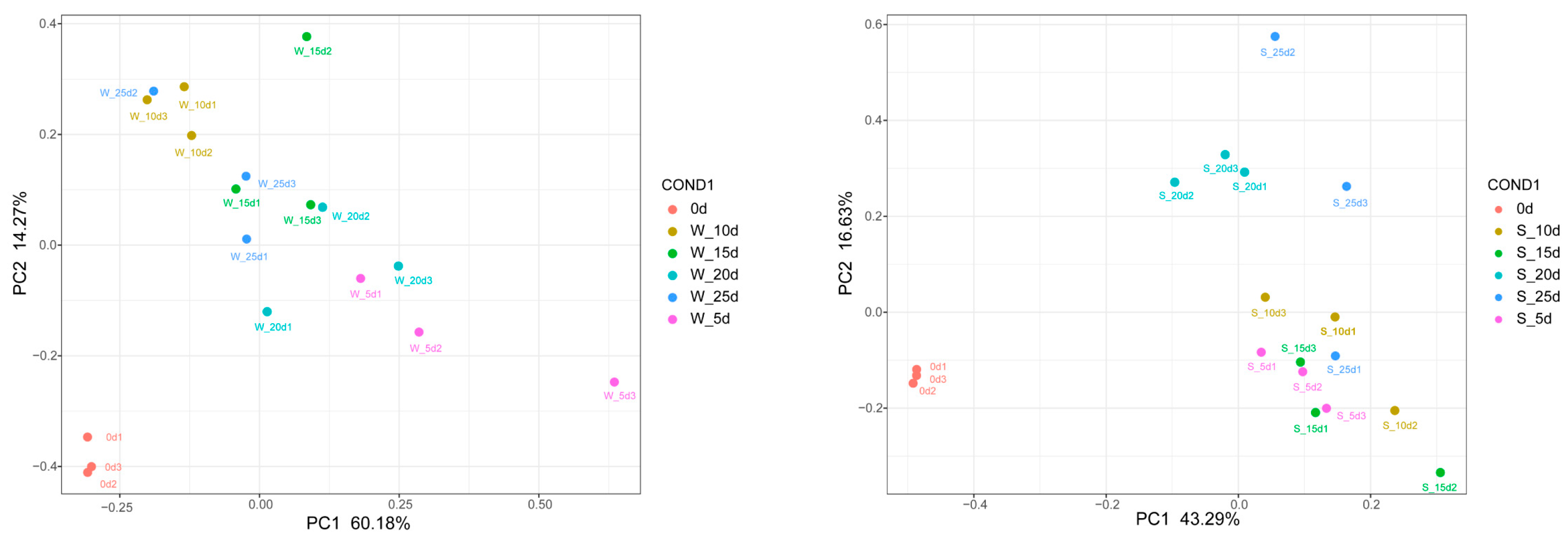
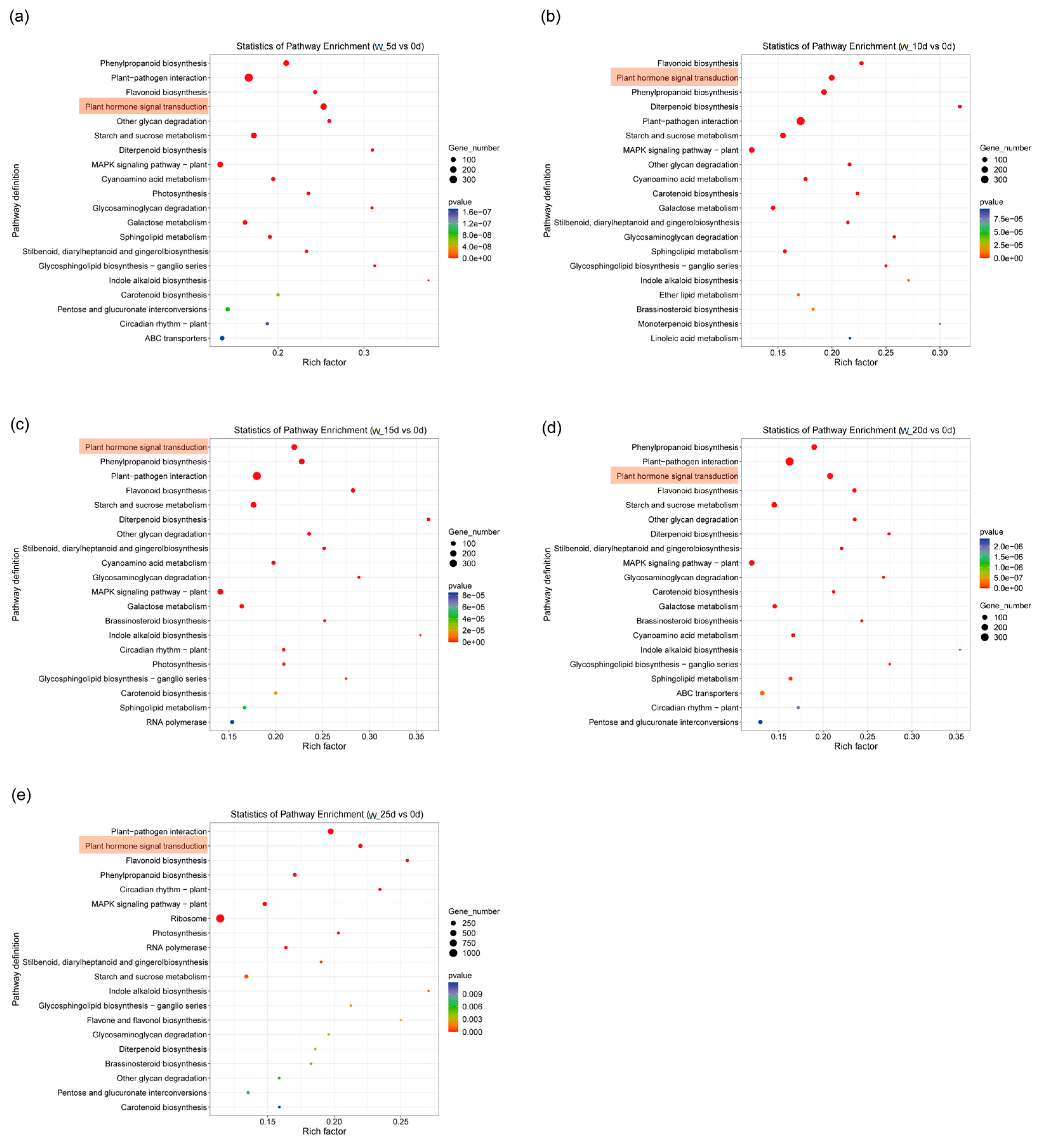
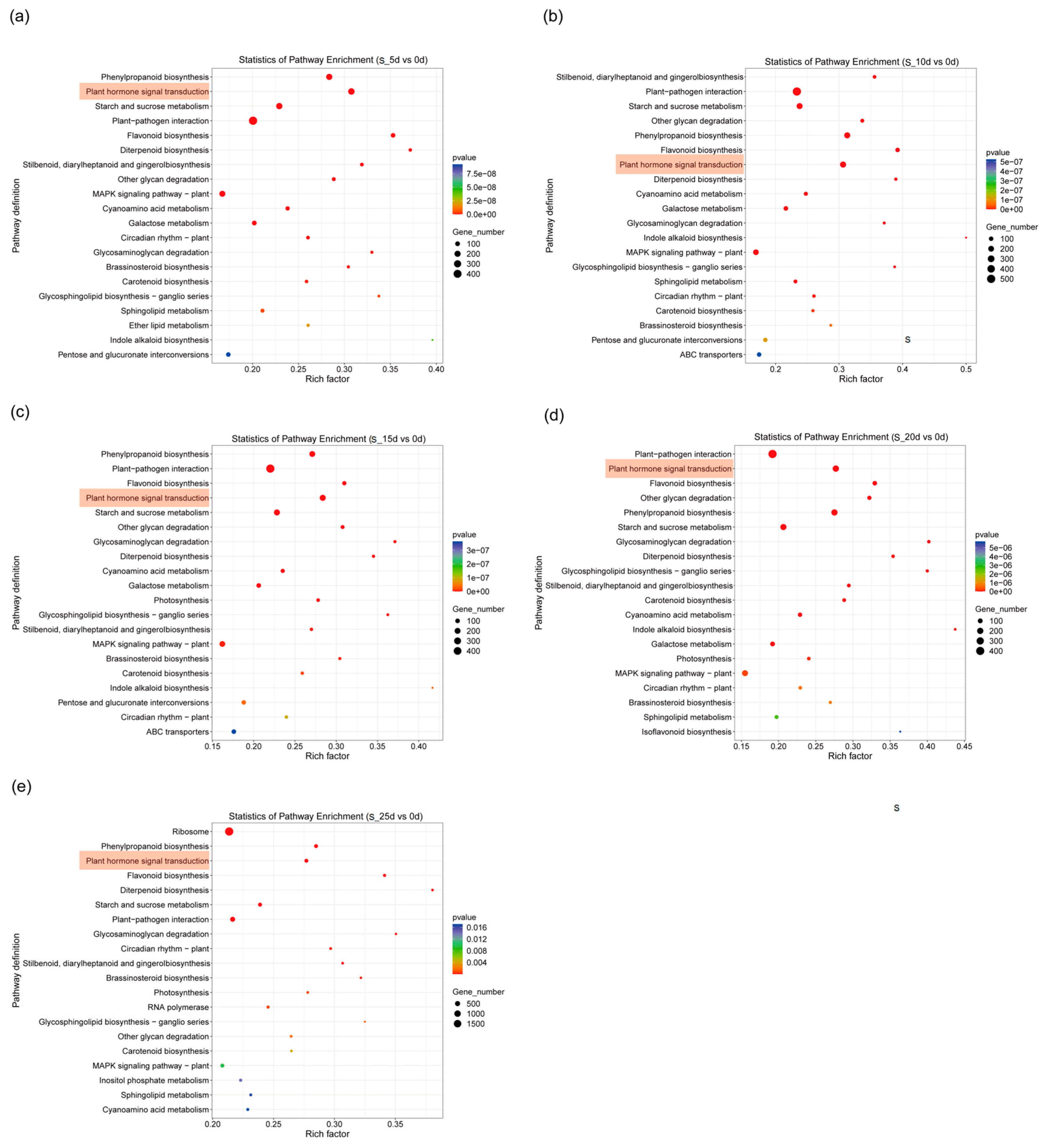

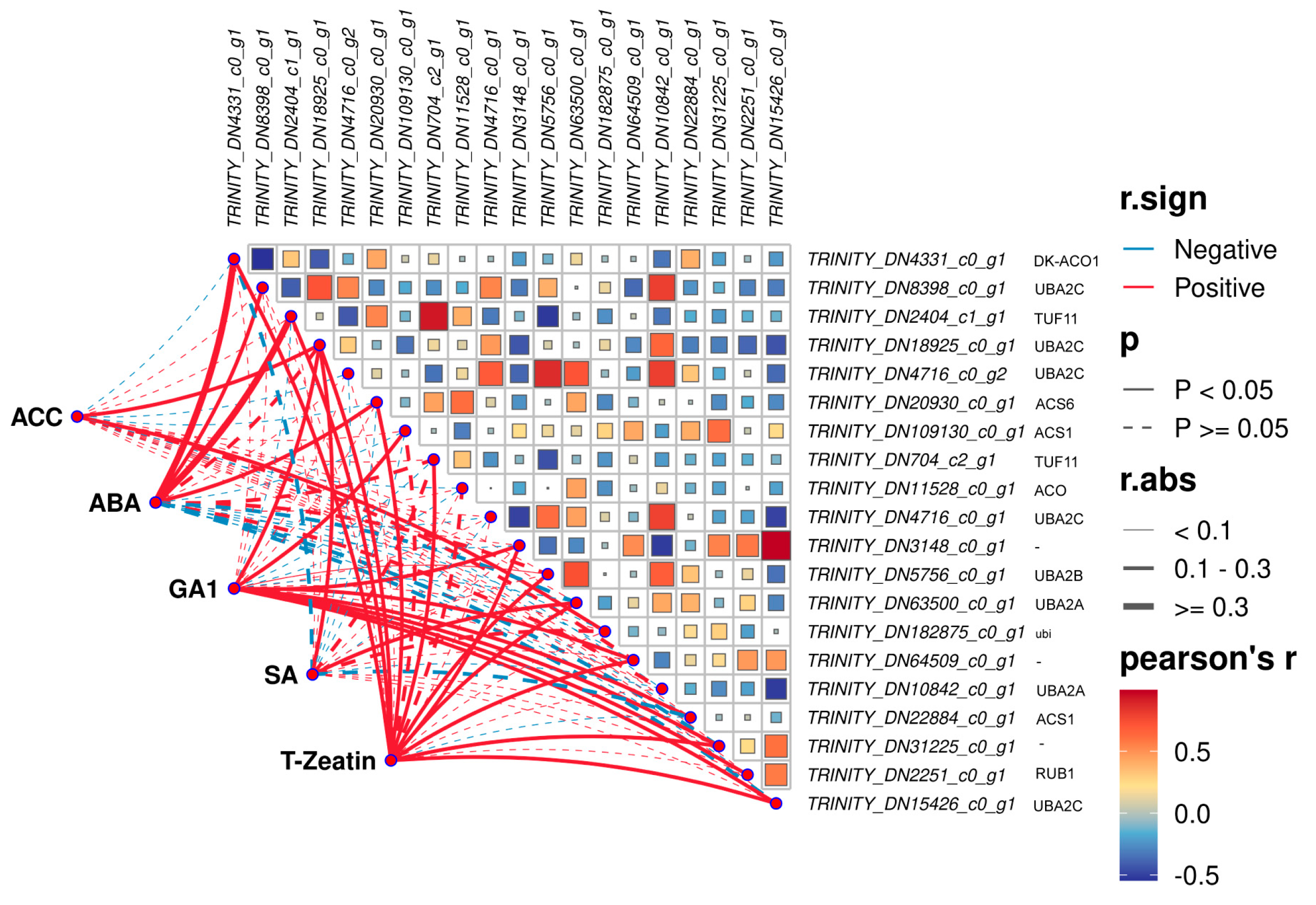

| Group | Total | Up | Down |
|---|---|---|---|
| Z_5 d vs. 0 d | 190 | 72 | 118 |
| Z_10 d vs. 0 d | 150 | 66 | 84 |
| Z_15 d vs. 0 d | 165 | 83 | 82 |
| Z_20 d vs. 0 d | 156 | 68 | 88 |
| Z_25 d vs. 0 d | 165 | 78 | 87 |
| L_5 d vs. 0 d | 231 | 94 | 137 |
| L_10 d vs. 0 d | 230 | 84 | 146 |
| L_15 d vs. 0 d | 213 | 75 | 138 |
| L_20 d vs. 0 d | 208 | 88 | 120 |
| L_25 d vs. 0 d | 208 | 82 | 126 |
| Gene Id | Annotation | Name | |
|---|---|---|---|
| Common DEGs | TRINITY_DN4331_c0_g1 | ACC oxidase 5 [Actinidia deliciosa] | DK-ACO1 |
| TRINITY_DN8398_c0_g1 | hypothetical protein HYC85_014601 [Camellia sinensis] | UBA2C | |
| TRINITY_DN2404_c1_g1 | ubiquitin-40S ribosomal protein S27a isoform X3 [Elaeis guineensis] | TUF11 | |
| TRINITY_DN18925_c0_g1 | UBP1-associated protein like [Actinidia chinensis var. chinensis] | UBA2C | |
| TRINITY_DN4716_c0_g2 | UBP1-associated protein 2C-like [Diospyros lotus] | UBA2C | |
| TRINITY_DN20930_c0_g1 | hypothetical protein RHSIM_Rhsim07G0098100 [Rhododendron simsii] | ACS6 | |
| TRINITY_DN109130_c0_g1 | hypothetical protein HYC85_011085 [Camellia sinensis] | ACS1 | |
| TRINITY_DN704_c2_g1 | ubiquitin-40S ribosomal protein S27a isoform X3 [Elaeis guineensis] | TUF11 | |
| TRINITY_DN11528_c0_g1 | 1-aminocyclopropane-1-carboxylate oxidase-like [Actinidia eriantha] | ACO | |
| TRINITY_DN4716_c0_g1 | UBP1-associated protein 2C [Camellia lanceoleosa] | UBA2C | |
| TRINITY_DN3148_c0_g1 | bi-ubiquitin [Scenedesmus sp. NREL 46B-D3] | - | |
| TRINITY_DN5756_c0_g1 | UBP1-associated protein 2B [Camellia lanceoleosa] | UBA2B | |
| TRINITY_DN63500_c0_g1 | hypothetical protein F0562_016212 [Nyssa sinensis] | UBA2A | |
| TRINITY_DN182875_c0_g1 | UBQ10 [Scenedesmus sp. PABB004] | ubi | |
| TRINITY_DN64509_c0_g1 | polyubiquitin-like [Hordeum vulgare subsp. vulgare] | - | |
| Unique to waterlogging stress | TRINITY_DN10842_c0_g1 | UBP1-associated protein 2A-like [Camellia sinensis] | UBA2A |
| Unique to submergence | TRINITY_DN22884_c0_g1 | hypothetical protein C3L33_16073, partial [Rhododendron williamsianum] | ACS1 |
| TRINITY_DN31225_c0_g1 | hypothetical protein COHA_002734 [Chlorella ohadii] | - | |
| TRINITY_DN2251_c0_g1 | LOW QUALITY PROTEIN: polyubiquitin-A-like [Panicum hallii] | RUB1 | |
| TRINITY_DN15426_c0_g1 | hypothetical protein CBR_g19053 [Chara braunii] | UBA2C |
| Gene Id | Annotation | Name | |
|---|---|---|---|
| Common DEGs | TRINITY_DN2741_c0_g1 | hypothetical protein HYC85_002681 [Camellia sinensis] | MHZ4 |
| TRINITY_DN34233_c0_g1 | hypothetical protein LOK49_LG10G01334 [Camellia lanceoleosa] | - | |
| TRINITY_DN7803_c0_g1 | hypothetical protein HHK36_032021 [Tetracentron sinense] | ADH1 | |
| TRINITY_DN699_c0_g1 | hypothetical protein RHGRI_006931 [Rhododendron griersonianum] | ADH1 | |
| TRINITY_DN12950_c0_g1 | hypothetical protein F0562_010481 [Nyssa sinensis] | NCED1 | |
| TRINITY_DN9242_c0_g1 | Molybdenum cofactor sulfurase [Camellia lanceoleosa] | FLACCA | |
| TRINITY_DN25154_c0_g1 | hypothetical protein RHMOL_Rhmol04G0049500 [Rhododendron molle] | XERICO | |
| TRINITY_DN39455_c1_g2 | secoisolariciresinol dehydrogenase-like [Camellia sinensis] | ADH1 | |
| TRINITY_DN3317_c0_g1 | momilactone A synthase-like [Camellia sinensis] | Os04g0179200 | |
| Unique to waterlogging stress | TRINITY_DN34480_c0_g1 | hypothetical protein LOK49_LG07G02934 [Camellia lanceoleosa] | NCED2 |
| Unique to submergence | TRINITY_DN7095_c0_g1 | carotenoid 9,10(9′,10′)-cleavage dioxygenase 1-like [Camellia sinensis] | NCED5 |
| ACC | ABA, GA1, SA, T-Zeatin |
|---|---|
| 1. Add ddH2O, pre-cooled to 4 °C, and extract at 4 °C for 2 h; 2. Centrifuge 10,000× g at 4 °C for 5 min, take the supernatant, extract the precipitate once again, combine the supernatants, pass through MCX pre-loading, and elute with 8 mL of water; 3. Pass through a 0.22 µm filter membrane and place in a 4 °C refrigerator for on-machine testing. | 1. Extract at 4 °C overnight, centrifuge at 12,000× g for 5 min, and take the supernatant; 2. Add five times the volume of acetonitrile solution to the precipitate again, extract twice, and combine to obtain the supernatant; 3. Pass through a C18 solid–phase extraction column, shake vigorously for 30 s, centrifuge at 10,000× g for 5 min, and take the supernatant; 4. Vacuum centrifugal concentration until dry, resolubilize with 200 μL methanol, pass through a 0.22 μm filter membrane, and place in a −20 °C refrigerator for on-machine testing. |
| Condition | Name | ACC | ABA, GA1, SA, T-Zeatin |
|---|---|---|---|
| liquid chromatography conditions | Chromatographic column | Poroshell 120 SB-C18 Reversed phase column (2.1 × 150, 2.7 um) | |
| Column temperature | 35 °C | 30 °C | |
| Mobile phase | A:B = acetonitrile: (water/0.1% formic acid) = 3:7 | A:B = (methanol/0.1% formic acid): (water/0.1% formic acid) | |
| Elution gradient | Isometric gradient elution | Table S4 | |
| Injection volume | 2 µL | ||
| mass spectrometry parameters | Ionization mode | ESI positive ion mode | ESI positive and negative ion modes are monitored separately |
| Scanning type | MRM | ||
| curtain gas | 15 psi | ||
| Spray voltage | +4500 v | +4500 v, −4500 v | |
| Atomized gas pressure | 65 psi | ||
| Auxiliary gas pressure | 70 psi | ||
| Atomization temperature | 350 °C | 400 °C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Dong, J.; Zhang, G.; Zhu, Q.; Yu, F. Root Ethylene and Abscisic Acid Responses to Flooding Stress in Styrax japonicus: A Transcriptomic Perspective. Plants 2025, 14, 1870. https://doi.org/10.3390/plants14121870
Han C, Dong J, Zhang G, Zhu Q, Yu F. Root Ethylene and Abscisic Acid Responses to Flooding Stress in Styrax japonicus: A Transcriptomic Perspective. Plants. 2025; 14(12):1870. https://doi.org/10.3390/plants14121870
Chicago/Turabian StyleHan, Chao, Jinghan Dong, Gaoyuan Zhang, Qinglin Zhu, and Fangyuan Yu. 2025. "Root Ethylene and Abscisic Acid Responses to Flooding Stress in Styrax japonicus: A Transcriptomic Perspective" Plants 14, no. 12: 1870. https://doi.org/10.3390/plants14121870
APA StyleHan, C., Dong, J., Zhang, G., Zhu, Q., & Yu, F. (2025). Root Ethylene and Abscisic Acid Responses to Flooding Stress in Styrax japonicus: A Transcriptomic Perspective. Plants, 14(12), 1870. https://doi.org/10.3390/plants14121870







