Olfactory Responses of Frankliniella occidentalis and Orius similis to Volatiles from Houttuynia cordata: Implications for Thrip Management
Abstract
1. Introduction
2. Results
2.1. Behavioral Responses of Frankliniella occidentalis to Plant Volatiles
2.2. Analysis of Houttuynia cordata Volatiles
2.3. Electroantennography Analyses
2.4. Behavioral Responses of Frankliniella occidentalis to Houttuynia cordata Volatiles in a Six-Arm Olfactometer
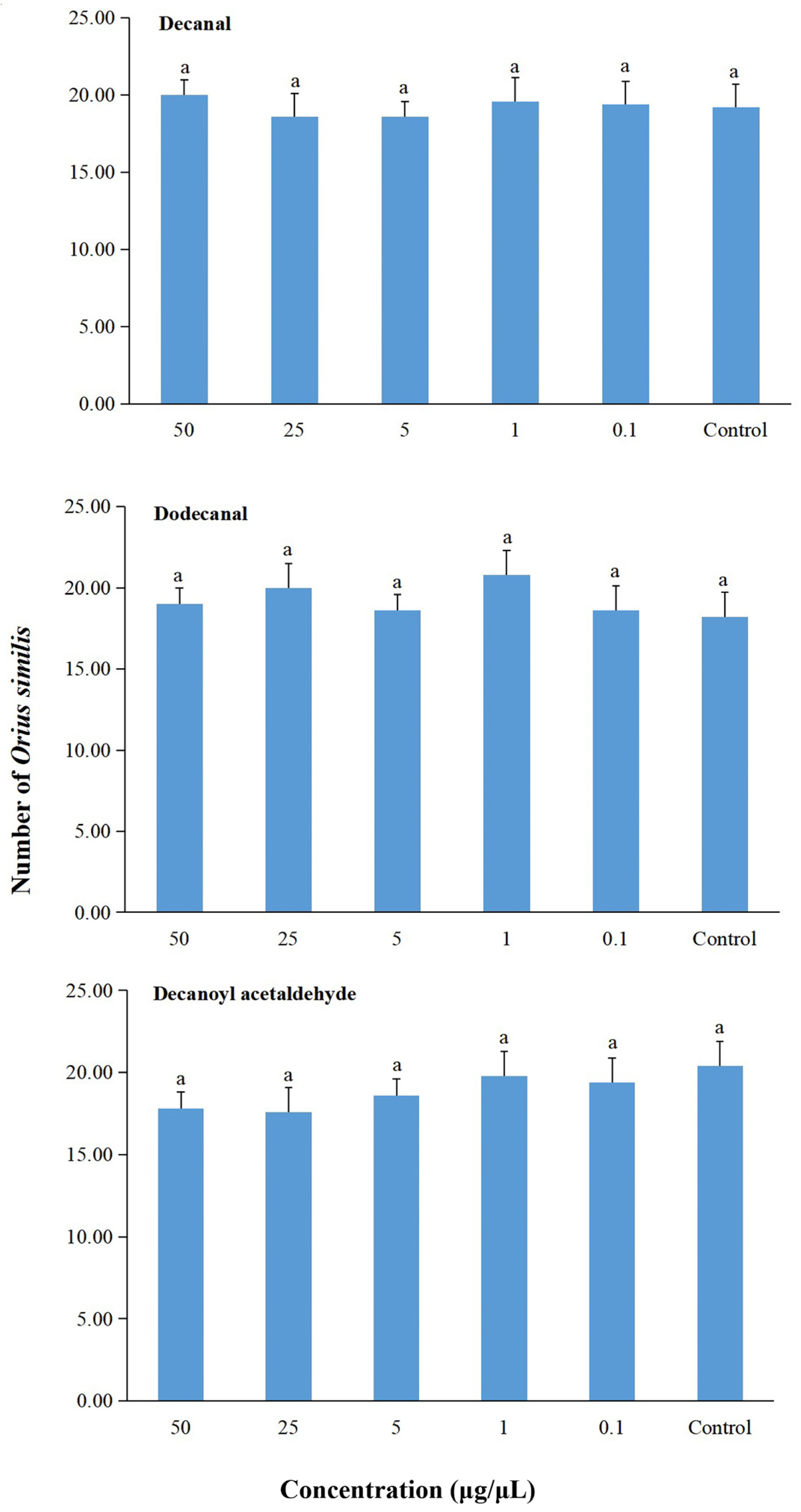
2.5. Behavioral Responses of Predator Orius similis to compounds of Houttuynia cordata Volatiles in a Six-Arm Olfactometer
3. Discussion
4. Materials and Methods
4.1. Insects and Plants
4.2. Behavioral Responses of Frankliniella occidentalis to Plant Volatiles in a Y-Tube Olfactometer
4.3. Gas Chromatography–Mass Spectrometry Analysis
4.4. Electroantennograms
4.5. Behavioral Responses of Frankliniella occidentalis to Houttuynia cordata Volatiles in a Six-Arm Olfactometer
4.6. Behavioral Responses of Orius similis to Houttuynia cordata Volatiles in a Six-Arm Olfactometer
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reitz, S.R. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): The making of a pest. Fla. Entomol. 2009, 92, 7–13. [Google Scholar] [CrossRef]
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. Tospovirus-thrips interactions. Annu. Rev. Phytopathol. 2005, 43, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T. Thrips as Crop Pests; CAB Int: Wallingford, UK, 1997. [Google Scholar]
- Reitz, S.R.; Gao, Y.L.; Kirk, W.D.J.; Hoddle, M.S.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef]
- Nyasani, J.O.; Meyhöfer, R.; Subramanian, S.; Poehling, H.M. Feeding and oviposition preference of Frankliniella occidentalis for crops and weeds in Kenyan French bean fields. J. Appl. Entomol. 2013, 137, 204–213. [Google Scholar] [CrossRef]
- Kirk, W.D.J.; Terry, L.I. The spread of the western fower thrips Frankliniella occidentalis (Pergande). Agric. For. Entomol. 2003, 5, 301–310. [Google Scholar] [CrossRef]
- Wu, S.Y.; Xing, Z.L.; Ma, T.T.; Xu, D.W.; Li, Y.Y.; Lei, Z.Z.; Gao, Y.L. Competitive interaction between Frankliniella occidentalis and locally present thrips species: A global review. J. Pest Sci. 2021, 94, 5–16. [Google Scholar] [CrossRef]
- Goldbach, R.; Peters, D. Possible causes of the emergence of tospovirus diseases. Semin. Virol. 1994, 5, 113–120. [Google Scholar] [CrossRef]
- Rotenberg, D.; Whitfield, A.E. Molecular interactions between tospoviruses and thrips vectors. Curr. Opin. Virol. 2018, 33, 191–197. [Google Scholar] [CrossRef]
- Kirk, W.D.J.; de Kogel, W.J.; Koschier, E.H.; Teulon, D.A.J. Semiochemicals for Thrips and Their Use in Pest Management. Annu. Rev. Entomol. 2021, 66, 101–119. [Google Scholar] [CrossRef]
- Gao, Y.L.; Lei, Z.R.; Reitz, S.R. Western fower thrips resistance to insecticides: Detection, mechanisms and management strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Götte, E.; Rybak, M. Pest control of the western flower thrips Frankliniella occidentalis (Pergande) with proven insecticide resistance in cut flowers in greenhouses. Gesunde Pflanze 2011, 62, 117–123. [Google Scholar] [CrossRef]
- Li, D.G.; Shang, X.Y.; Reitz, S.R.; Nauen, R.; Lei, Z.R.; Lee, S.H.; Gao, Y.L. Field resistance to spinosad in western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae). J. Integr. Agric. 2016, 15, 2803–2808. [Google Scholar] [CrossRef]
- Wang, Z.H.; Gong, Y.J.; Jin, G.H.; Li, B.Y.; Chen, J.C. Field-evolved resistance to insecticides in the invasive western flower thrips Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) in China. Pest Manag. Sci. 2016, 72, 1440–1444. [Google Scholar] [CrossRef]
- Mavridis, K.; Ilias, A.; Papapostolou, K.M.; Varikou, K.; Michaelidou, K.; Tsagkarakou, A.; Vontas, J. Molecular diagnostics for monitoring insecticide resistance in the western flower thrips Frankliniella occidentalis. Pest Manag. Sci. 2023, 79, 1615–1622. [Google Scholar] [CrossRef]
- Broughton, S.; Cousins, D.A.; Rahman, T. Evaluation of semiochemicals for their potential application in mass trapping of Frankliniella occidentalis (Pergande) in roses. Crop Prot. 2015, 67, 130–135. [Google Scholar] [CrossRef]
- Beck, J.J.; Torto, B.; Vannette, R.L. Eavesdropping on plant-insectmicrobe chemical communications in agricultural ecology: A virtual issue on semiochemicals. J. Agric. Food Chem. 2017, 65, 5101–5103. [Google Scholar] [CrossRef]
- Renwick, A. Phytochemical modifcation of taste: An insect model. In Biologically Active Natural Products: Agrochemicals, 1st ed.; Cutler, H., Cutler, S., Eds.; Taylor & Francis: Atlanta, GA, USA, 1999; p. 320. [Google Scholar]
- Petroski, R.J.; Stanley, D.W. Natural compounds for pest and weed control. J. Agric. Food Chem. 2009, 57, 8171–8179. [Google Scholar] [CrossRef]
- Bhuyan, H.; Das, S.R.C. Evaluation of anthelmintic activity of Houttuynia cordata. J. Fundam. Pharm. Res. 2013, 1, 23–26. [Google Scholar]
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb: A review of phytochemistry and pharmacology and quality control. Chin. Med. 2013, 4, 101–123. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharmacogn. Rev. 2014, 8, 22–35. [Google Scholar] [PubMed]
- Mouden, S.; Sarmiento, K.F.; Klinkhamer, P.G.L.; Leiss, K.A. Integrated pest management in western flower thrips: Past, present and future. Pest Manag. Sci. 2017, 73, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhi, J.R.; Cong, C.L.; Margolies, D.C. Olfactory cues used in host selection by Frankliniella occidentalis (Thysanoptera: Thripidae) in relation to host suitability. J. Insect Behav. 2014, 27, 41–56. [Google Scholar] [CrossRef]
- Cao, Y.; Zhi, J.R.; Zhang, R.Z.; Li, C.; Liu, Y.; Lv, Z.Y.; Gao, Y.L. Different population performances of Frankliniella occidentalis and Thrips hawaiiensis on flowers of two horticultural plants. J. Pest Sci. 2018, 1, 79–91. [Google Scholar] [CrossRef]
- Avellaneda1, J.; Díaz1, M.; Coy-Barrera, E.; Rodríguez1, D.; Osorio, C. Rose volatile compounds allow the design of new control strategies for the western flower thrips (Frankliniella occidentalis). J. Pest Sci. 2021, 94, 129–142. [Google Scholar] [CrossRef]
- Li, W.D.; Zhang, P.J.; Zhang, J.M.; Zhang, Z.J.; Huang, F.; Bei, Y.W.; Lin, W.C.; Lu, Y.B. An evaluation of Frankliniella occidentalis (Thysanoptera: Thripidae) and Frankliniella intonsa (Thysanoptera: Thripidae) performance on different plant leaves based on life history characteristics. J. Insect Sci. 2015, 15, 4. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Dyer, L.A.; Philbin, C.S.; Ochsenrider, K.M.; Richards, L.A.; Massad, T.J.; Smilanich, A.M.; Forister, M.L.; Parchman, T.L.; Galland, L.M.; Hurtado, P.J.; et al. Modern approaches to study plant–insect interactions in chemical ecology. Nat. Rev. Chem. 2018, 2, 50–64. [Google Scholar] [CrossRef]
- Terry, I.; Walter, G.H.; Moore, C.; Roemer, R.; Hull, C. Odor-mediated push-pull pollination in cycads. Science 2007, 318, 19–46. [Google Scholar] [CrossRef]
- Diabate, S.; Martin, T.; Murungi, L.; Fiaboe, K.; Subramanian, S.; Wesonga, J.; Delétré, E. Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019, 143, 855–866. [Google Scholar] [CrossRef]
- Tian, H.J.; Chen, Y.X.; Chen, Y.; Chen, X.Q.; Lin, S.; Zhang, J.; Yang, G.; Wei, H. A mixture of p-anisaldehyde and ethyl nicotinate elicits positive antennal and behavioral responses in Frankliniella occidentalis. Entomol. Exp. Appl. 2022, 170, 603–611. [Google Scholar] [CrossRef]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles functioning as host cues in a blend become non-host cues when presented alone to the black bean aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.J.; Galizia, C.G.; Stierle, J.; Dorn, S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exp. Biol. 2010, 213, 3388–3397. [Google Scholar] [CrossRef]
- Egger, B.; Spangl, B.; Koschier, E.H. Habituation in Frankliniella occidentalis to deterrent plant compounds and their blends. Entomol. Exp. Appl. 2014, 151, 231–238. [Google Scholar] [CrossRef]
- Koschier, E.H.; Nielsen, M.C.; Spangl, B.; Davidson, M.M.; Teulon, D.A.J. The effect of background plant odours on the behavioural responses of Frankliniella occidentalis to attractive or repellent compounds in a Y-tube olfactometer. Entomol. Exp. Appl. 2017, 163, 160–169. [Google Scholar] [CrossRef]
- Brødsgaard, H.F. The effect of anisaldehyde as a scent attractant for Frankliniella occidentalis (Thysanoptera: Thripidae) and the response mechanism involved. IOBC WPRS Bull. 1990, 13, 36–38. [Google Scholar]
- Frey, J.E.; Cortada, R.V.; Helbling, H. The potential of flower odours for use in population monitoring of western flower thrips Frankliniella occidentalis Perg. (Thysanoptera: Thripidae). Biocontrol Sci. Technol. 1994, 4, 177–186. [Google Scholar] [CrossRef]
- Koschier, E.H.; de Kogel, W.J.; Visser, J.H. Assessing the attractiveness of volatile plant compounds to western flower thrips Frankliniella occidentalis. J. Chem. Ecol. 2000, 26, 2643–2655. [Google Scholar] [CrossRef]
- Davidson, M.M.; Perry, N.B.; Larsen, L.; Green, V.C.; Butler, R.C.; Teulon, D.A.J. 4-pyridyl carbonyl compounds as thrips lures: Effectiveness for western flower thrips in Y-tube bioassays. J. Agric. Food Chem. 2008, 56, 6554–6561. [Google Scholar] [CrossRef]
- Davidson, M.M.; Butler, R.C.; Teulon, D.A.J. Pyridine compounds increase thrips (Thysanoptera: Thripidae) trap capture in an onion crop. J. Econ. Entomol. 2009, 102, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Chermenskaya, T.D.; Burov, V.N.; Maniar, S.P.; Pow, E.M.; Roditakis, N.; Selytskaya, O.G.; Shamshev, I.V.; Wadhams, L.J.; Woodcock, C.M. Behavioural responses of western flower thrips, Frankliniella occidentalis (Pergande), to volatiles from three aromatic plants. Insect Sci. Appl. 2001, 21, 67–72. [Google Scholar] [CrossRef]
- Koschier, E.H.; Hoffmann, D.; Riefler, J. Influence of salicylaldehyde and methyl salicylate on post-landing behaviour of Frankliniella occidentalis Pergande. J. Appl. Entomol. 2007, 131, 362–367. [Google Scholar] [CrossRef]
- Davidson, M.M.; Nielsen, M.C.; Butler, R.C.; Castañé, C.; Alomar, O.; Riudavets, J.; Teulon, D.A.J. Can semiochemicals attract both western flower thrips and their anthocorid predators? Entomol. Exp. Appl. 2015, 155, 54–63. [Google Scholar] [CrossRef]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Dethier, V.G.; Browne, B.L.; Smith, C.L. The designation of chemicals in terms of the responses they elicit from insects. J. Econ. Entomol. 1960, 53, 134–136. [Google Scholar] [CrossRef]
- Renwick, J.A.A. Oviposition stimulants and deterrents. In CRC Handbook of Natural Pesticides, Attractants and Repellents; Morgan, E.D., Mandava, N.B., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 151–153. [Google Scholar]
- Yang, T.; Stoopen, G.; Thoen, M.; Wiegers, G.; Jongsma, M.A. Chrysanthemum expressing a linalool synthase gene “smells good”, but “tastes bad” to western flower thrips. Plant Biotechnol. J. 2013, 11, 875–882. [Google Scholar] [CrossRef]
- McKellar, R.C.; McGarvey, B.D.; Tsao, R.; Lu, X.W.; Knight, K.P. Application of the electronic nose to the classification of resistance to western flower thrips in chrysanthemums. J. Chem. Ecol. 2005, 31, 2439–2450. [Google Scholar] [CrossRef]
- Cao, Y.; Li, C.; Yang, H.; Li, J.; Li, S.; Wang, Y.W.; Gao, Y.L. Laboratory and field investigation on the orientation of Frankliniella occidentalis (Thysanoptera: Thripidae) to more suitable host plants driven by volatiles and component analysis of volatiles. Pest Manag. Sci. 2019, 75, 598–606. [Google Scholar] [CrossRef]
- Abdullah, Z.S.; Ficken, K.J.; Greenfield, B.P.J.; Butt, T.M. Innate responses to putative ancestral hosts: Is the attraction of western flower thrips to pine pollen a result of relict olfactory receptors? J. Chem. Ecol. 2014, 40, 534–540. [Google Scholar] [CrossRef]
- Cao, Y.; Benelli, G.; Germinara, G.S.; Maggi, F.; Zhang, Y.; Luo, S.L.; Yang, H.; Li, C. Innate positive chemotaxis to paeonal from highly attractive Chinese medicinal herbs in the cigarette beetle, Lasioderma serricorne. Sci. Rep. 2019, 9, 6995. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Chen, H.H.; Li, J.K.; Zhang, R.; Turlings, T.C.J.; Li, C. Volatiles released by Chinese liquorice roots mediate host location behaviour by neonate Porphyrophora sophorae, (Hemiptera: Margarodidae). Pest Manag. Sci. 2016, 72, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Davison, A.C.; Tamo, C. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 2010, 29, 45–55. [Google Scholar] [CrossRef]



| Number | Compound | Molecular Formula | Molecular Weight | Content (%) |
|---|---|---|---|---|
| 1 | 3-Hexenal | C6H10O | 98 | 0.61 |
| 2 | 3-Hexen-1-ol, (E)- | C6H12O | 100 | 2.15 |
| 3 | 2-Hexenal, (E)- | C6H10O | 98 | 0.79 |
| 4 | 1-Hexanol | C6H14O | 102 | 0.09 |
| 5 | Nonane | C9H20 | 128 | 0.31 |
| 6 | 2,4-Hexadienal, (E,E)- | C6H8O | 96 | 0.05 |
| 7 | α-Thujene | C10H16 | 136 | 0.04 |
| 8 | α-Pinene | C10H16 | 136 | 0.29 |
| 9 | Camphene | C10H16 | 136 | 0.05 |
| 10 | 4-Oxohex-2-enal | C6H8O2 | 112 | 0.08 |
| 11 | Sabinene | C10H16 | 136 | 1.57 |
| 12 | β-Pinene | C10H16 | 136 | 0.41 |
| 13 | β-Myrcene | C10H16 | 136 | 5.12 |
| 14 | Octanal | C8H16O | 128 | 0.06 |
| 15 | α-Terpinene | C10H16 | 136 | 0.07 |
| 16 | Limonene | C10H16 | 136 | 0.11 |
| 17 | (Z)-β-Ocimene | C10H16 | 136 | 0.06 |
| 18 | (E)-β-Ocimene | C10H16 | 136 | 1.69 |
| 19 | γ-Terpinene | C10H16 | 136 | 0.11 |
| 20 | Undecane | C11H24 | 156 | 0.06 |
| 21 | Nonanal | C9H18O | 142 | 3.50 |
| 22 | 1-Nonanol | C9H20O | 144 | 1.32 |
| 23 | Decanal | C10H20O | 156 | 47.21 |
| 24 | 1-Decanol | C10H22O | 158 | 11.02 |
| 25 | l-Bornyl acetate | C12H20O2 | 196 | 0.12 |
| 26 | 2-Undecanone | C11H22O | 170 | 1.65 |
| 27 | Undecanal | C11H22O | 170 | 3.48 |
| 28 | n-Decanoic acid | C10H20O2 | 172 | 0.12 |
| 29 | Geranyl acetate | C12H20O2 | 196 | 0.78 |
| 30 | Dodecanal | C12H24O | 184 | 7.13 |
| 31 | Decanoyl acetaldehyde | C12H22O2 | 198 | 3.76 |
| 32 | (E)-Caryophyllene | C15H24 | 204 | 0.86 |
| 33 | α-Humulene | C15H24 | 204 | 0.08 |
| 34 | (E)-β-Farnesene | C15H24 | 204 | 0.67 |
| 35 | Bicyclogermacrene | C15H24 | 204 | 0.49 |
| 36 | (E, E)-α-Farnesene | C15H24 | 204 | 0.15 |
| 37 | Tetradecanal | C14H28O | 212 | 0.47 |
| 38 | Hexadecanal | C16H32O | 240 | 0.07 |
| Parameter | Source | df | MS | F | p |
|---|---|---|---|---|---|
| Relative EAG value (mv) | Compound | 4 | 0.613 | 221.11 | <0.001 |
| Concentration | 4 | 0.046 | 16.56 | <0.001 | |
| Compound × Concentration | 16 | 0.093 | 33.41 | <0.001 | |
| Error | 80 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; Lin, S.; Jiang, F.; Zhang, C.; Yuan, R.; Huang, S.; Wang, L.; Cao, Y.; Maggi, F.; Germinara, G.S. Olfactory Responses of Frankliniella occidentalis and Orius similis to Volatiles from Houttuynia cordata: Implications for Thrip Management. Plants 2025, 14, 1855. https://doi.org/10.3390/plants14121855
Zeng G, Lin S, Jiang F, Zhang C, Yuan R, Huang S, Wang L, Cao Y, Maggi F, Germinara GS. Olfactory Responses of Frankliniella occidentalis and Orius similis to Volatiles from Houttuynia cordata: Implications for Thrip Management. Plants. 2025; 14(12):1855. https://doi.org/10.3390/plants14121855
Chicago/Turabian StyleZeng, Guang, Shuo Lin, Feiyu Jiang, Changrong Zhang, Rongrong Yuan, Shuai Huang, Lijuan Wang, Yu Cao, Filippo Maggi, and Giacinto Salvatore Germinara. 2025. "Olfactory Responses of Frankliniella occidentalis and Orius similis to Volatiles from Houttuynia cordata: Implications for Thrip Management" Plants 14, no. 12: 1855. https://doi.org/10.3390/plants14121855
APA StyleZeng, G., Lin, S., Jiang, F., Zhang, C., Yuan, R., Huang, S., Wang, L., Cao, Y., Maggi, F., & Germinara, G. S. (2025). Olfactory Responses of Frankliniella occidentalis and Orius similis to Volatiles from Houttuynia cordata: Implications for Thrip Management. Plants, 14(12), 1855. https://doi.org/10.3390/plants14121855









