Validation of Management Zones, Variability, and Spatial Distribution of the Physiological Quality of Soybean Seeds
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection of Soil and Vegetation Attributes for the Definition of Management Zones
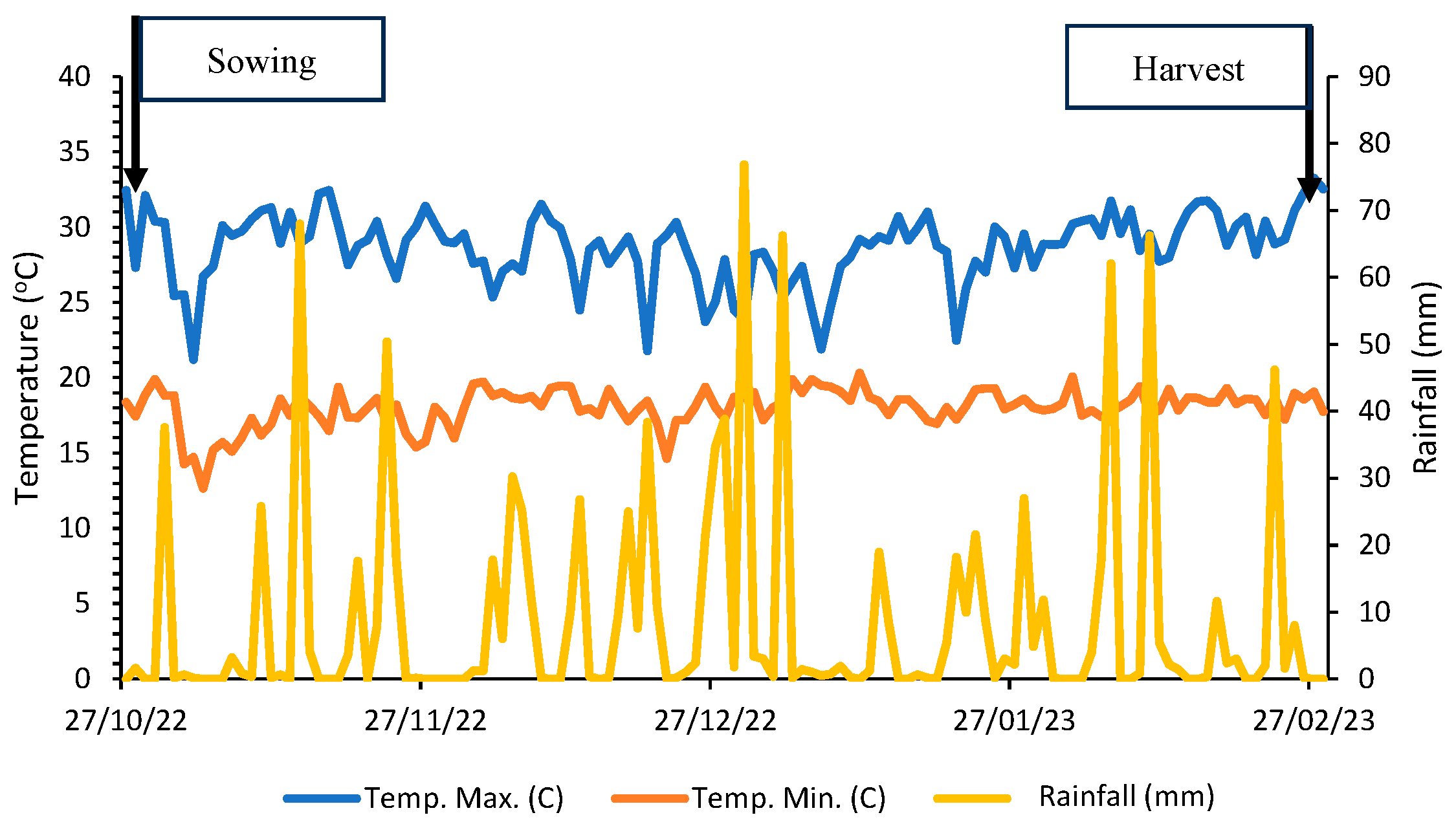
2.2. Crop Installation, Plot Marking, and Harvest
2.3. Soybean Seed Quality Variability
2.4. Definition of Management Zones (MZs)
2.5. Statistical Analysis
3. Results and Discussion
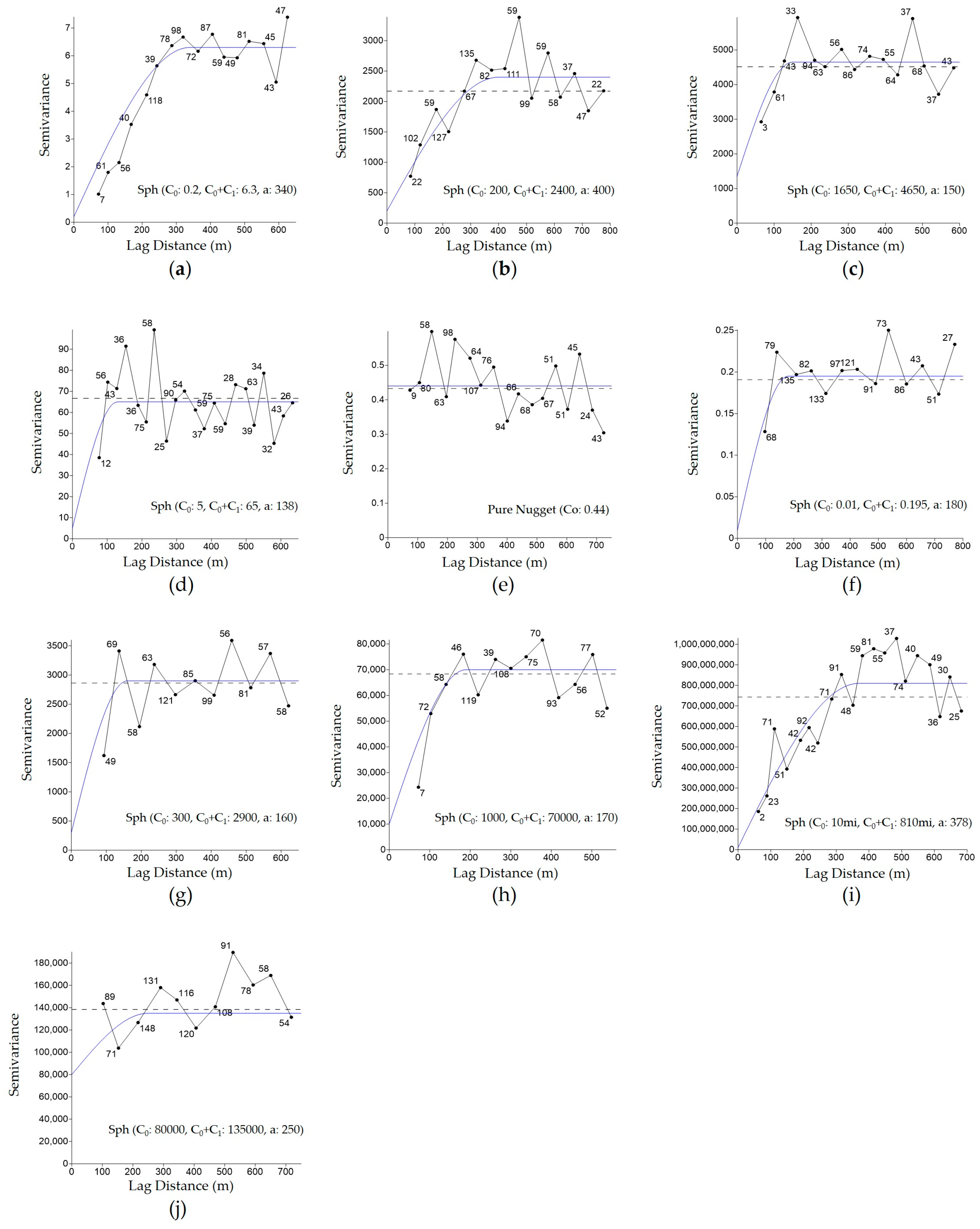
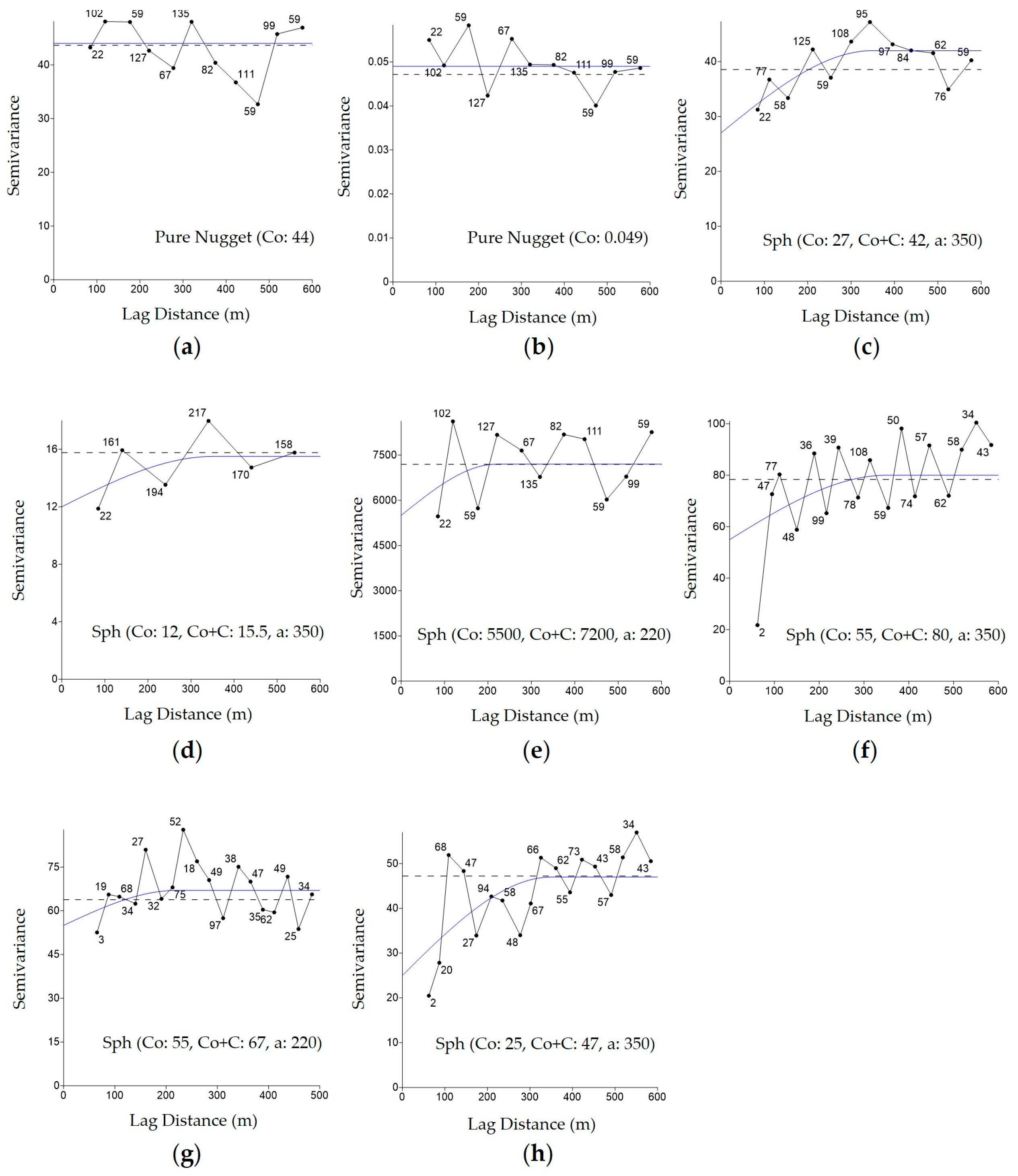
3.1. Creation of Semivariograms and Kriging Interpolation of Soil and Vegetation Attributes for the Development of Management Zones
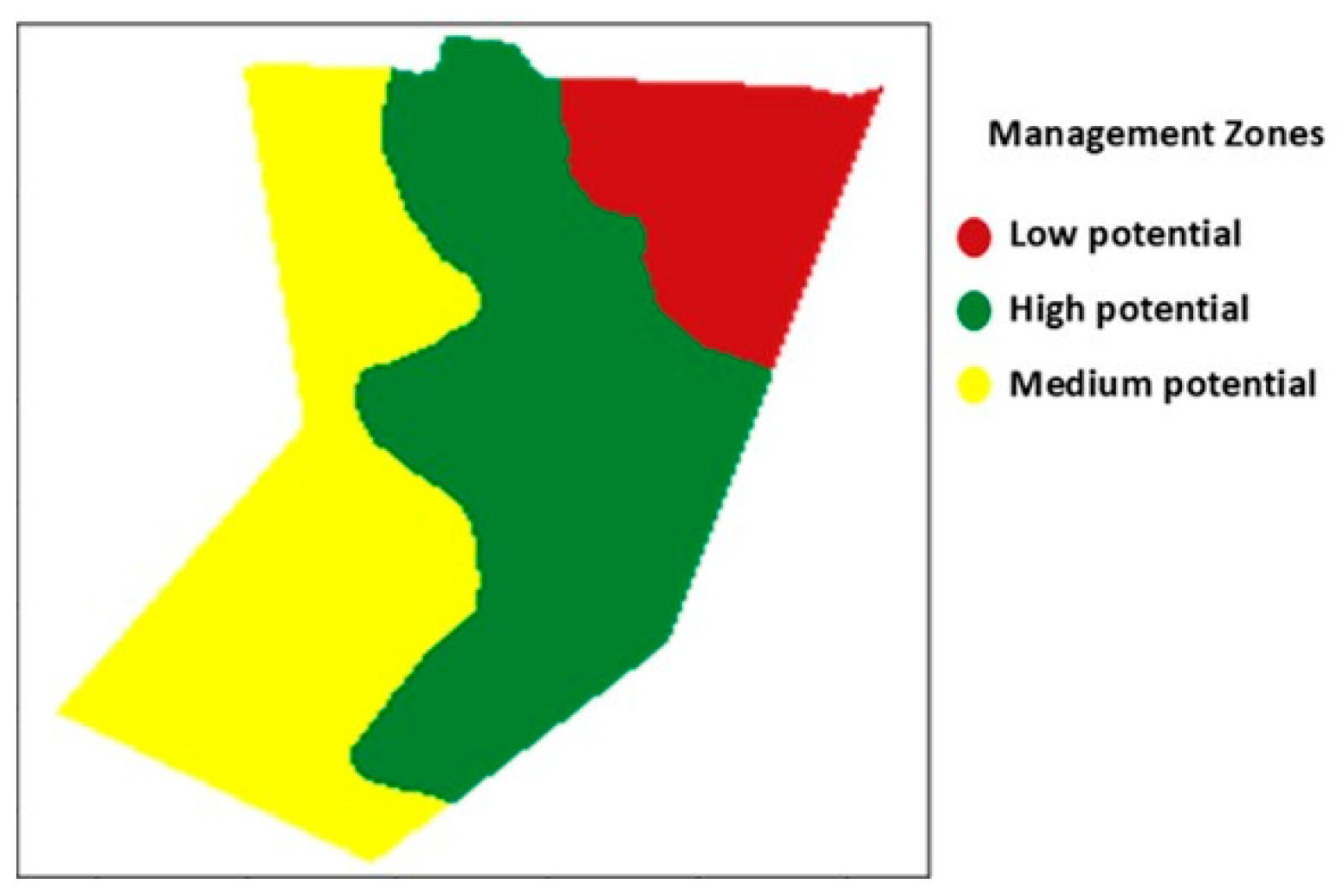
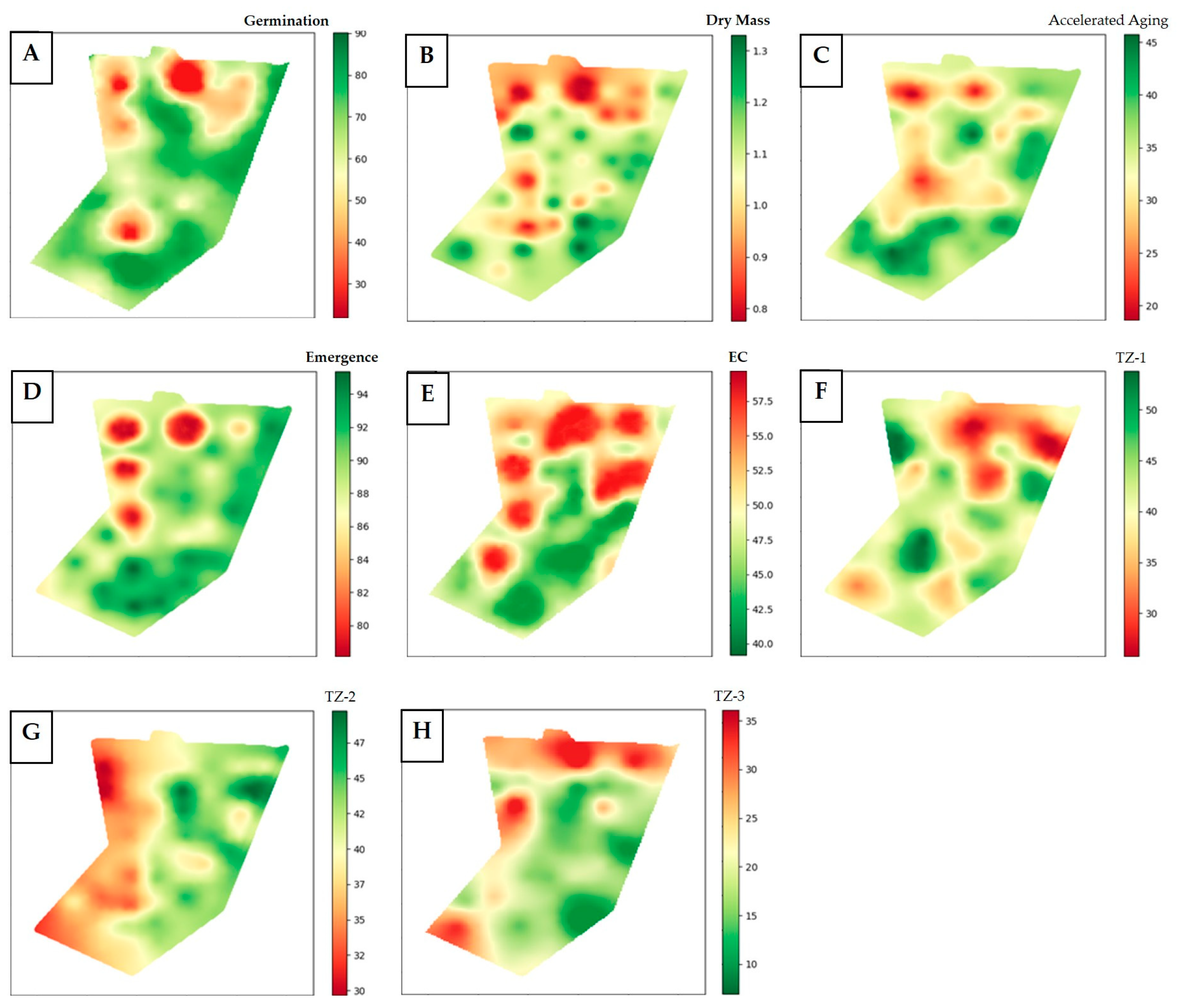
3.2. Definition of Management Zones in Soybean Seed Production Field
3.3. Variability in Soybean Seed Quality According to Management Zones (MZ)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World 2023; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; 316p, ISBN 978-92-5-137226-5. Available online: https://www.fao.org/documents/card/en/c/cc3017en (accessed on 12 September 2024).
- Companhia Nacional de Abastecimento. Acompanhamento de Safra Brasileira: Grãos, Décimo Segundo Levantamento, Março 2023; Conab: Brasília, Brazil, 2023. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 20 October 2024).
- Bagateli, J.R.; Dörr, C.S.; Schuch, L.O.B.; Meneghello, G.E. Productive performance of soybean plants originated from seed lots with increasing vigor levels. J. Seed Sci. 2019, 41, 151–159. [Google Scholar] [CrossRef]
- Krzyzanowski, F.C.; França-Neto, J.d.B.; Henning, A.A. A Alta Qualidade da Semente de Soja: Fator Importante para a Produção da Cultura; Circular Técnica, 136; Embrapa Soja: Londrina, Brazil, 2018; 24p, Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/177391/1/CT136-online.pdf (accessed on 15 October 2024).
- Corrêa, M.F.; Gadotti, G.I.; Pinheiro, R.M.; Nadal, A.P.; Schuch, L.O.B.; Vergara, R.O. Análise de variabilidade espacial e temporal em um campo de produção de sementes de soja e trigo. Rev. Cereus 2021, 13, 182–194. [Google Scholar]
- Tey, Y.S.; Brindal, M. Factors influencing the adoption of precision agricultural technologies: A review for policy implications. Precis. Agric. 2012, 13, 713–730. [Google Scholar] [CrossRef]
- Catão, H.C.R.M.; Hurtado, S.M.C. Chemical treatment and storage of sorghum seeds produced in different management zones. J. Seed Sci. 2023, 45. [Google Scholar] [CrossRef]
- Corrêa, M.F.; Gadotti, G.I.; Pinheiro, R.d.M.; Nadal, A.P.; Vergara, R.d.O.; Navroski, R.; Schuch, L.O.B. Análise dos componentes do rendimento e da qualidade fisiológica de sementes de soja sob variabilidade espacial do solo. Divers. J. 2022, 7, 542–554. [Google Scholar] [CrossRef]
- Gazolla-Neto, A.; Vergara, R.O.; Gadotti, G.I.; Villela, F.A. Rastreabilidade e variabilidade espacial da qualidade fisiológica de sementes soja em campo de produção. Rev. Bras. Tecnol. Agropecu. 2017, 1, 65–73. Available online: https://revistas.fw.uri.br/rbdta/article/view/2317 (accessed on 20 December 2024).
- Köppen, W. Climatología: Con un Estudio de los Climas de la Tierra; Fondo de Cultura Económica: Mexico City, Mexico, 1948. [Google Scholar]
- QGIS Development Team QGIS Geographic Information System. Open Source Geospacial Found. Proj. 2018. Available online: https://qgis.org/ (accessed on 20 December 2024).
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; Secretaria de Defesa Agropecuária/Mapa/ACS: Brasília, Brazil, 2009; 399p. Available online: https://www.gov.br/agricultura/pt-br/assuntos/lfda/arquivos-publicacoes-laboratorio/regras-para-analise-de-sementes.pdf/view (accessed on 20 December 2024).
- Krzyzanowski, F.C.; França-Neto J de, B.; Gomes-Junior, F.G.; Nakagawa, J. Vigor de Sementes: Conceitos e Testes; Abrates: Londrina, Brazil, 2020; 601p, ISBN 978-65-992000-0-7. [Google Scholar]
- Marcos-Filho, J. Teste de envelhecimento acelerado. In Vigor de Sementes: Conceitos e Testes; Krzyzanowski, F.C., Vieira, R.D., de B. França-Neto, J., Marcos-Filho, J., Eds.; Abrates: Londrina, Brazil, 2020; pp. 185–237. ISBN 978-65-992000-0-7. [Google Scholar]
- Vieira, R.D.; Marcos-Filho, J. Teste de Condutividade Elétrica. Vigor de Sementes: Conceitos e Testes; Krzyzanowski, F.C., Vieira, R.D., de B. França-Neto, J., Marcos-Filho, J., Eds.; Abrates: Londrina, Brazil, 2020; pp. 334–375. ISBN 978-65-992000-0-7. [Google Scholar]
- França-Neto, J.B.; Krzyzanowski, F.C. Metodologia do Teste de Tetrazólio em Sementes de Soja; Documentos, 449; Embrapa CNPS: Londrina, Brazil, 2022; 111p. [Google Scholar]
- Vieira, S.R. Geoestatística em Estudos de Variabilidade Espacial do Solo. In Tópicos em Ciência do Solo; Novais, R.F., Alvarez, V.V.H., Schaeffer, C.E.G.R., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2000; pp. 1–54. [Google Scholar]
- Golden Software. Surfer Plotting Software Information Brochure; Versão 10.1.561 (32-bit); Golden Software: Golden, CO, USA, 2011. [Google Scholar]
- Gomes, F.P. Curso de Estatística Experimental, 15th ed.; Fealq: Piracicaba, Brazil, 2009; ISBN 9788571330559. [Google Scholar]
- Bertolani, F.C.; Vieira, S.R. Variabilidade espacial da taxa de infiltração de água e da espessura do horizonte A, em um Argissolo Vermelho-Amarelo, sob diferentes usos. Rev. Bras. Cienc. Solo 2001, 25, 987–995. [Google Scholar] [CrossRef]
- Souza, Z.M.d.; Júnior, J.M.; Pereira, G.T. Variabilidade espacial da estabilidade de agregados e matéria orgânica em solos de relevos diferentes. Pesqui. Agropecu. Bras. 2004, 39, 491–499. [Google Scholar] [CrossRef]
- Lemos Filho, L.C.A.; de Oliveira, E.L.; de Faria, M.A.; de Andrade, L.A.B. Spatial variability of soil density and organic matter content in area cultivated with sugar cane (Saccharum officinarum L.) Variação espacial da densidade do solo e matéria orgânica em área cultivada com cana-de-açúcar (Saccharum officinarum L.). Rev. Cienc. Agrônomica 2008, 39, 193–202. Available online: https://www.redalyc.org/articulo.oa?id=195317754002 (accessed on 20 December 2024).
- Chaves, L.H.G.; Farias, C.H.A. Variabilidade espacial de cobre e manganês em Argissolo sob cultivo de cana-de-açúcar. Rev. Ciência Agronômica 2009, 40, 211–218. Available online: https://www.redalyc.org/pdf/1953/195318233007.pdf (accessed on 15 December 2024).
- Oda-Souza, M.; Barbin, D.; Ribeiro Júnior, P.J.; Stape, J.L. Aplicação de métodos geoestatísticos para identificação de dependência espacial na análise de dados de um ensaio de espaçamento florestal em delineamento sistemático tipo leque. Rev. Árvore 2008, 32, 499–509. [Google Scholar] [CrossRef]
- Ceccon, G.; DA Silva, J.F.; Makino, P.A.; Neto, A.L. Consórcio Milho-Braquiária com Densidades Populacionais da Forrageira no Centro-Sul do Brasil. Rev. Bras. Milho Sorgo 2018, 17, 157–167. [Google Scholar] [CrossRef]
- Tahir, S.; Marschner, P. Clay Addition to Sandy Soil Reduces Nutrient Leaching—Effect of Clay Concentration and Ped Size. Commun. Soil. Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Melo, B.M.R.d.; Paglis, C.M.; de Oliveira, M.S.; Teixeira, M.B.R.; da Silva, J.S.M.; Lima, D.F.F. Zonas de manejo em função de propriedades de solo, relevo e produtividade da lavoura cafeeira. Rev. Agrogeoambiental 2017, 9, 49–59. [Google Scholar] [CrossRef][Green Version]
- Santos, F.S. Seleção de Variáveis e Definição de Zonas de Manejo para Agricultura de Precisão. Master’s Thesis, Curso de Engenharia Agrícola, Universidade Federal de Viçosa, Viçosa, Brazil, 2017. [Google Scholar]
- Umbelino, A.D.S.; De Oliveira, D.G.; Martins, M.P.D.O.; Dos Reis, E.F. Definições de zona de manejo para soja de alta produtividade. Rev. Ciências Agrárias 2018, 41, 674–682. [Google Scholar] [CrossRef]
- Vieira, S.R.; Filho, O.G.; Chiba, M.K.; Mellis, E.V.; Dechen, S.C.F.; De Maria, I.C. Variabilidade espacial dos teores foliares de nutrientes e da produtividade da soja em dois anos de cultivo em um latossolo vermelho. Rev. Bras. Cienc. Solo 2010, 34, 1503–1514. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa nº 45, de 17 de Setembro de 2013. Institui os Padrões Para a Produção e a Comercialização de Sementes. Diário Oficial da União: Seção 1, Brasília, DF, Ano 181, pp. 16–38, 18 Set. 2013. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/sementes-e-mudas/publicacoes-sementes-e-mudas/copy_of_INN45de17desetembrode2013.pdf (accessed on 29 November 2024).
- Peske, S.T.; Barros, A.C.S.A. Produção de Sementes. In Sementes: Fundamentos Científicos e Tecnológicos; Peske, S.T., Lucca, O.F., Barros, A.C.S.A., Eds.; UFPel: Pelotas, Brazil, 2012; Chapter 2; pp. 13–100. ISBN 85-7192-320-5. [Google Scholar]
- Delouche, J.C. Germinação, deterioração e vigor da semente. Seed News Pelotas 2002, 6, 24–31. Available online: https://seednews.com.br/artigos/2018-germinacao-deterioracao-e-vigor-da-semente-edicao-dezembro-2002 (accessed on 20 December 2024).
- Couto, A.P.S.; Brzezinski, C.R.; Abati, J.; Colombo, R.C.; Henning, F.A.; Fonseca, I.C.d.B.; Zucareli, C.; Gdm, S. Electrical conductivity test in the evaluation of the physiological potential of treated and stored soybean seeds. Semin. Agrar. 2021, 42, 3135–3148. [Google Scholar] [CrossRef]
- Mattioni, N.M.; Schuch, L.O.B.; Villela, F.A. Variabilidade espacial da produtividade e da qualidade das sementes de soja em um campo de produção. Rev. Bras. Sement. 2011, 33, 608–615. [Google Scholar] [CrossRef]
- Vergara, R.O.; Gazolla-Neto, A.; Gadotti, G.I. Space distribution of soybean seed storage potential. Rev. Caatinga 2019, 32, 399–410. [Google Scholar] [CrossRef]
- Gazolla-Neto, A.; Fernandes, M.C.; Gomes, A.D.; Gadotti, G.I.; Villela, F.A. Distribuição espacial da qualidade fisiológica de sementes de soja em campo de produção. Rev. Caatinga 2015, 28, 119–127. [Google Scholar] [CrossRef][Green Version]
- Castellanos, C.I.S.; Gadotti, G.I.; Meneghello, G.E.; Cavalcante, J.A.; Pinheiro, R.d.M. Influence of spatial soil variability on productivity and physiological quality of soybean seeds. Rev. Agrogeoambiental 2022, 14, e20221660. [Google Scholar] [CrossRef]

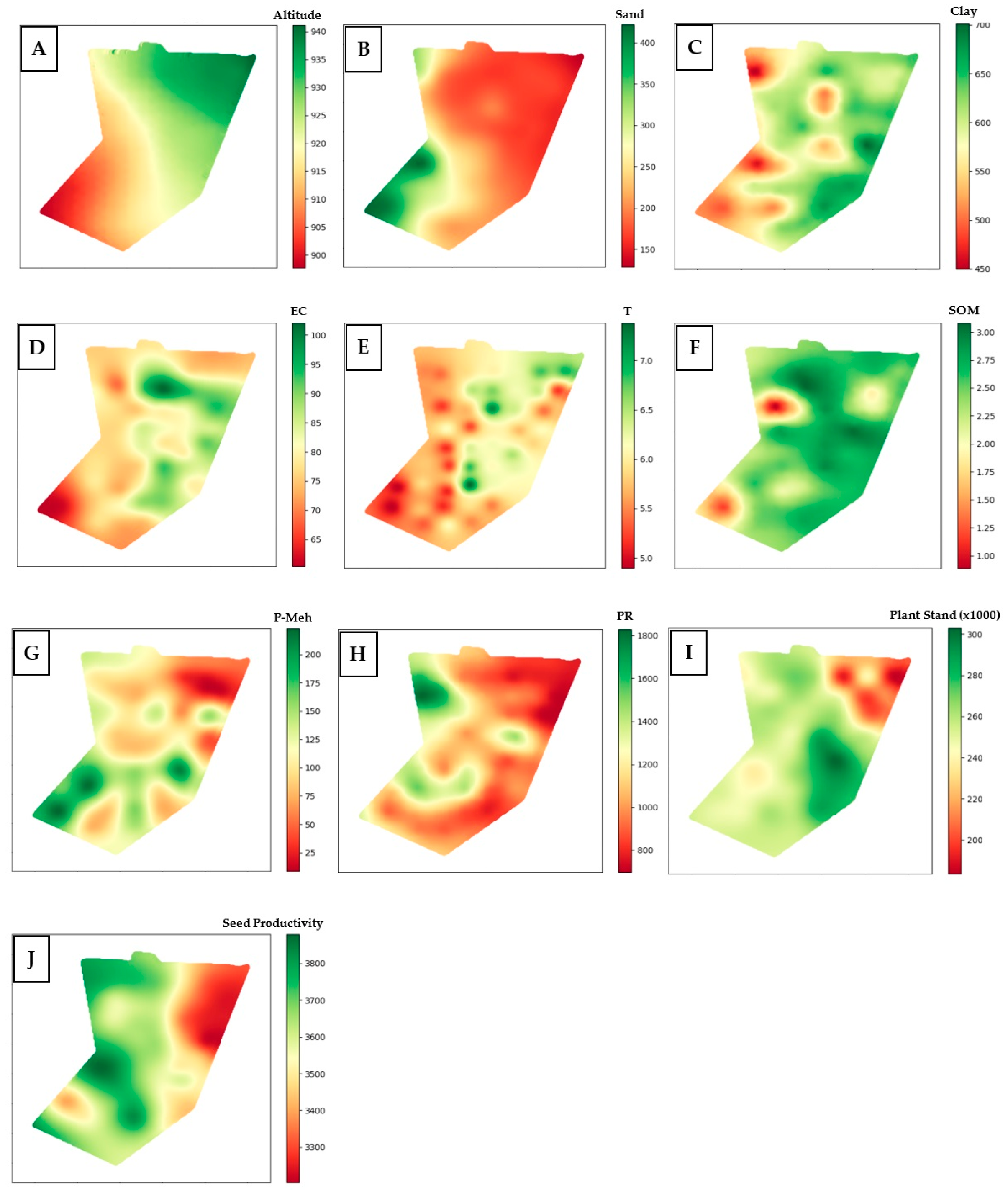
| Attributes 1 | Minimum | Maximum | Mean | Median | * C.V. (%) |
|---|---|---|---|---|---|
| Altitude (m) | 901.3 | 937.8 | 921.5 | 921.7 | 1.0 |
| Clay | 393.8 | 739.9 | 593.4 | 611.0 | 12.6 |
| EC | 60.2 | 106.9 | 81.2 | 79.0 | 11.5 |
| T | 4.8 | 7.9 | 5.9 | 6.0 | 12.4 |
| P-Mehlich (mg/dm3) | 5.3 | 255.6 | 109.3 | 93.2 | 58.2 |
| PR | 670.4 | 1921.1 | 1100.2 | 1024.8 | 28.8 |
| SOM | 0.7 | 3.1 | 2.5 | 2.6 | 18.6 |
| Initial plant stand (plants/m2) | 165,000.0 | 311,000.0 | 254,125.0 | 255,000.0 | 11.5 |
| Soybean seed productivity (kg/ha) | 2560.6 | 4500.0 | 3580.6 | 3604.6 | 11.0 |
| Attributes 1 | Model | Nugget (C0) | Partial Sill (C1) | Sill (C0 + C1) | Range (a) |
|---|---|---|---|---|---|
| Altitude | Spherical | 0.2 | 6.1 | 6.3 | 340 |
| Clay (20 cm) | Spherical | 1.350 | 3.300 | 4650 | 150 |
| Sand (20 cm) | Spherical | 200 | 2200 | 2400 | 400 |
| EC (20 cm) | Spherical | 5 | 60 | 65 | 138 |
| T (20 cm) | Pure Nugget | 0.44 | -- | -- | -- |
| P-Mehlich (20 cm) | Spherical | 300 | 2.600 | 2.900 | 160 |
| PR (20 cm) | Spherical | 1.000 | 60.000 | 70.000 | 190 |
| SOM (20 cm) | Spherical | 0.01 | 0.185 | 0.195 | 180 |
| Initial Plant Stand (plants/m2) | Spherical | 10,000,000 | 800,000,000 | 810,000,000 | 378 |
| Soybean Seed Productivity (kg/ha) | Spherical | 80.000 | 55.000 | 135.000 | 250 |
| Physiological Attributes (n = 48) | Minimum | Maximum | Mean | Median | C.V. (%) |
|---|---|---|---|---|---|
| Germination (%) | 17 | 91 | 73 | 79 | 20.1 |
| Accelerated Aging (%) | 3 | 59 | 34 | 34 | 41.6 |
| Dry Mass (g) | 0.3 | 1.5 | 1.0 | 1.1 | 21.7 |
| Emergence (%) | 48 | 98 | 89 | 91 | 10.5 |
| E. Conductivity (μS cm−1 g−1) | 25.5 | 88.2 | 49.2 | 46.1 | 26.0 |
| Tetrazolium 1 (%) | 19 | 66 | 40 | 40 | 22.7 |
| Tetrazolium 2 (%) | 21 | 60 | 40 | 40 | 22.2 |
| Tetrazolium 3 (%) | 6 | 40 | 19 | 18 | 39.3 |
| Management Zones | G (%) | AA (%) | DM (g) | E (%) | EC (μS cm−1 g−1) | TZ1 (%) | TZ2 (%) | TZ3 (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low potential | 71 | B | 36 | a | 1.0 | a | 90 | a | 55.3 | a | 37 | b | 41 | a | 22 | a |
| Medium potential | 71 | B | 35 | a | 1.0 | a | 89 | a | 49.8 | ab | 45 | ab | 34 | b | 21 | a |
| High potential | 80 | A | 36 | a | 1.1 | a | 91 | a | 44.0 | b | 40 | b | 44 | a | 16 | b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Filho, M.A.; Santos, A.L.C.; Domingues, R.F.; Melazzo, G.M.; Pontes, B.S.; da Silva, R.J.; Hurtado, S.M.C.; Catão, H.C.R.M. Validation of Management Zones, Variability, and Spatial Distribution of the Physiological Quality of Soybean Seeds. Plants 2025, 14, 1856. https://doi.org/10.3390/plants14121856
de Oliveira Filho MA, Santos ALC, Domingues RF, Melazzo GM, Pontes BS, da Silva RJ, Hurtado SMC, Catão HCRM. Validation of Management Zones, Variability, and Spatial Distribution of the Physiological Quality of Soybean Seeds. Plants. 2025; 14(12):1856. https://doi.org/10.3390/plants14121856
Chicago/Turabian Stylede Oliveira Filho, Maurício Alves, Ana Laura Costa Santos, Ricardo Ferreira Domingues, Gabriela Mariano Melazzo, Brenda Santos Pontes, Rafael Jacinto da Silva, Sandro Manuel Carmelino Hurtado, and Hugo César Rodrigues Moreira Catão. 2025. "Validation of Management Zones, Variability, and Spatial Distribution of the Physiological Quality of Soybean Seeds" Plants 14, no. 12: 1856. https://doi.org/10.3390/plants14121856
APA Stylede Oliveira Filho, M. A., Santos, A. L. C., Domingues, R. F., Melazzo, G. M., Pontes, B. S., da Silva, R. J., Hurtado, S. M. C., & Catão, H. C. R. M. (2025). Validation of Management Zones, Variability, and Spatial Distribution of the Physiological Quality of Soybean Seeds. Plants, 14(12), 1856. https://doi.org/10.3390/plants14121856







