Differential Impact of SiO2 Foliar Application on Lettuce Response to Temperature, Salinity, and Drought Stress
Abstract
1. Introduction
2. Results
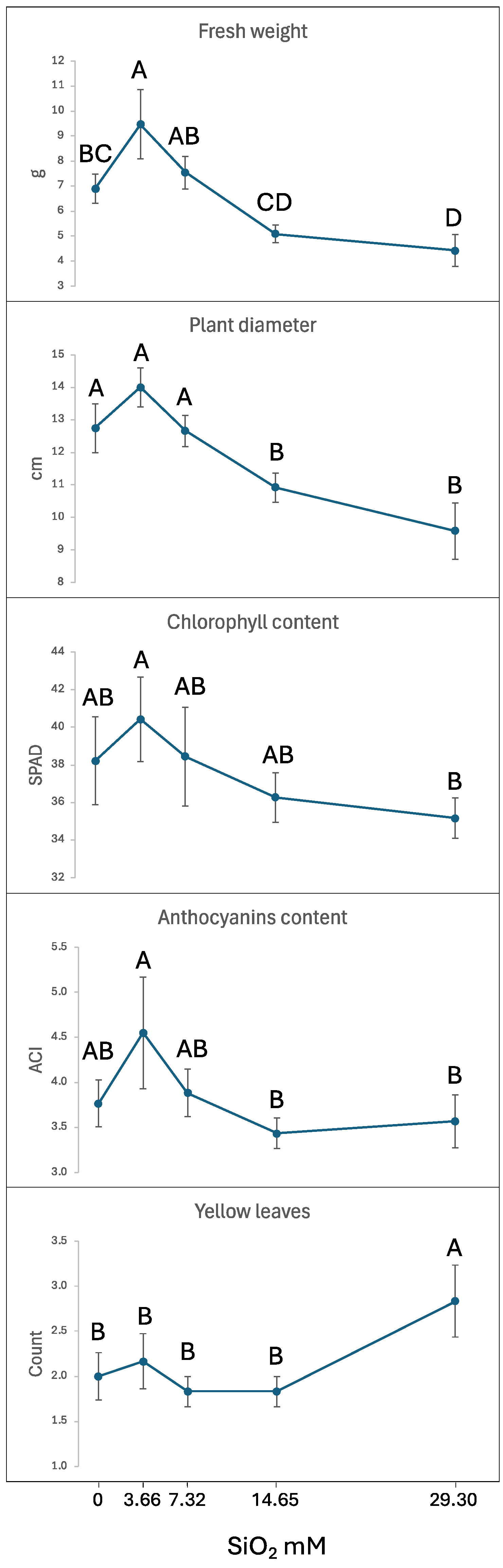
2.1. Effect of SiO2 Application on Plant Phenotypic Traits
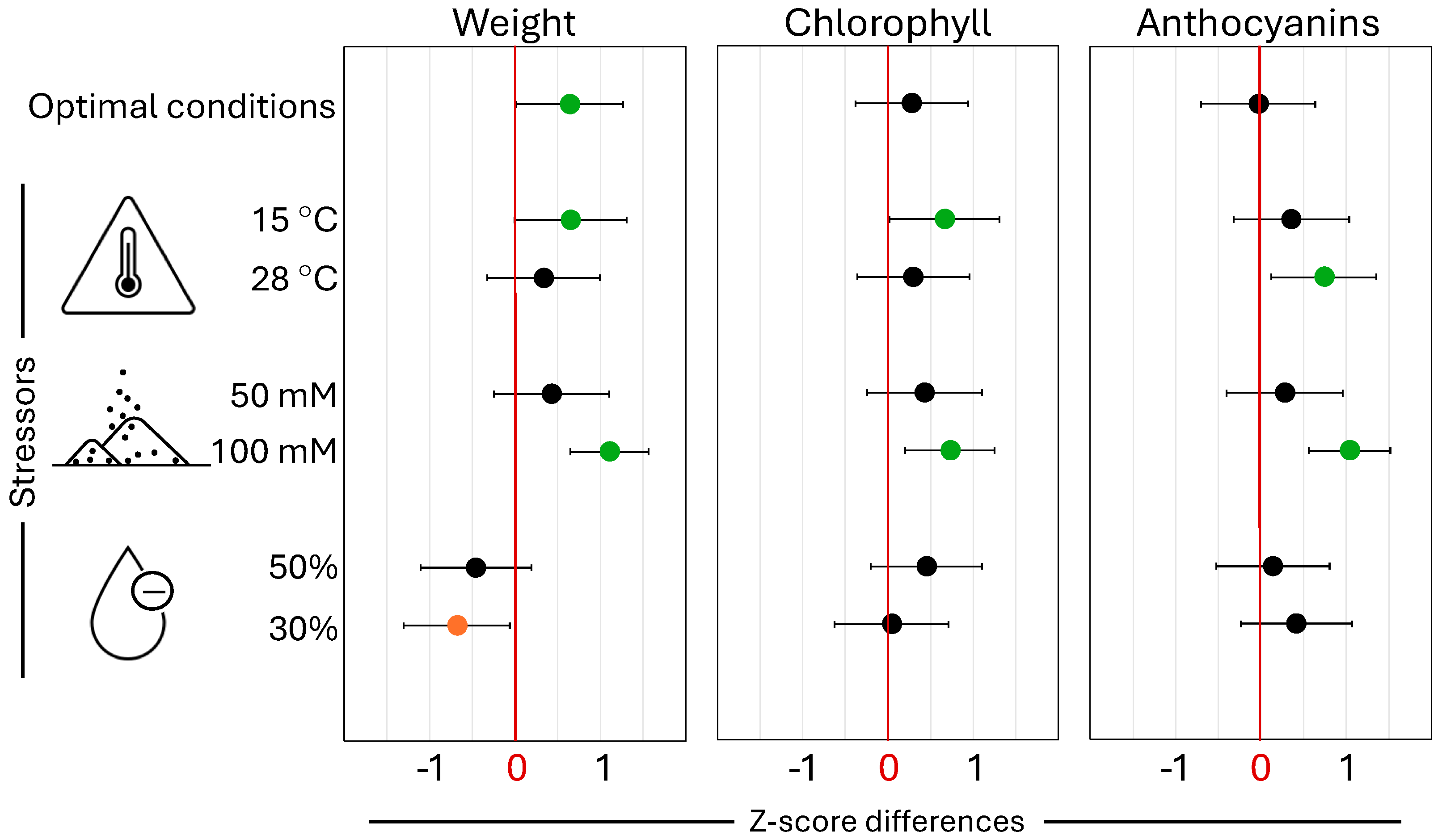
2.2. Effect of Increasing SiO2 Concentrations to Alleviate Salinity Stress
2.3. Effect of SiO2 Application on Mineral Composition
3. Discussion
3.1. Silicon Uptake and Translocation Mechanisms
3.1.1. Active vs. Passive Transport Systems
3.1.2. Transport and Distribution Mechanisms
3.1.3. Benefits for Plant Performance
3.2. Silicon Dynamics in Lettuce: A Non-Accumulator Species
3.2.1. Foliar Application: An Alternative Approach
3.2.2. Limited Mobility and Localized Effects
3.3. Effects of Silicon on Lettuce Cultivation and Physiology
3.3.1. Growth and Yield Enhancements
3.3.2. Stress Mitigation Capabilities
3.3.3. Photosynthesis and Post-Harvest Quality
3.4. Study Design and Rationale
3.4.1. Silicon-Mediated Response to Temperature
3.4.2. Silicon-Mediated Drought Stress Response
3.4.3. Silicon-Mediated Response to Salinity Stress
3.4.4. Mineral Elements Composition
4. Materials and Methods
4.1. Plant Material and Experimental Setup
4.2. Growth Conditions
4.3. Silicon Treatment and Experimental Design
- Optimal conditions (no stress): 20 °C; daily watering with distilled water to maintain 75% SWC.
- Suboptimal temperature: 15 °C; daily watering to maintain 75% SWC.
- Supraoptimal temperature: 28 °C; daily watering to maintain 75% SWC.
- Moderate salinity stress: 20 °C; watering with 50 mM NaCl solution.
- Severe salinity stress: 20 °C; watering with 100 mM NaCl solution.
- Moderate drought stress: 20 °C; watering every other day to maintain 50% SWC (with fluctuations as described above).
- Severe drought stress: 20 °C; watering every other day to maintain 30% SWC (with fluctuations as described above).
4.4. SiO2 Concentration and Salinity Stress
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simko, I.; Hayes, R.J.; Mou, B.; McCreight, J.D. Lettuce and Spinach. In Yield Gains in Major US Field Crops (CSSA Special Publications); Smith, S., Diers, B., Specht, J., Carver, B., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Crop Science Society of America, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 2014; pp. 53–86. [Google Scholar] [CrossRef]
- Davis, W.V.; Weber, C.; Wakefield, H.; Wechsler, S. Vegetables and Pulses Outlook: April 2025. VGS-375. Economic Research Service Situation and Outlook Report, United States Department of Agriculture. 2025. Available online: https://ers.usda.gov/sites/default/files/_laserfiche/outlooks/111478/VGS-375.pdf?v=51427 (accessed on 27 April 2025).
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Simko, I. Spatio-temporal dynamics of lettuce metabolome: A framework for targeted nutritional quality improvement. Plants 2024, 13, 3316. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]
- Peng, H.; Simko, I. Extending lettuce shelf life through integrated technologies. Curr. Opin. Biotechnol. 2023, 81, 102951. [Google Scholar] [CrossRef] [PubMed]
- Pathak, T.B.; Maskey, M.L.; Dahlberg, J.A.; Kearns, F.; Bali, K.M.; Zaccaria, D. Climate change trends and impacts on California agriculture: A detailed review. Agronomy 2018, 8, 25. [Google Scholar] [CrossRef]
- Rosental, L.; Still, D.W.; You, Y.; Hayes, R.J.; Simko, I. Mapping and identification of genetic loci affecting earliness of bolting and flowering in lettuce. Theor. Appl. Genet. 2021, 134, 3319–3337. [Google Scholar] [CrossRef]
- Simko, I.; Hayes, R.J.; Furbank, R.T. Non-destructive phenotyping of lettuce plants in early stages of development with optical sensors. Front. Plant Sci. 2016, 7, 1985. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How do plants respond to combined drought and salinity stress?—A systematic review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Behmann, J.; Steinrücken, J.; Plümer, L. Detection of early plant stress responses in hyperspectral images. ISPRS J. Photogramm. Remote Sens. 2014, 93, 98–111. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Gil Nam, H. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- de Souza Lemos Neto, H.; de Almeida Guimarães, M.; Sampaio, I.M.G.; de Araújo Hendges, A.R.A.; de Oliveira, A.B.; Filho, S.M. Silicon (Si) reduces the effects of salt stress on germination and initial growth of lettuce (Lactuca sativa L.). Aust. J. Crop Sci. 2018, 12, 1410–1418. [Google Scholar] [CrossRef]
- Eraslan, F.; Inal, A.; Savasturk, O.; Gunes, A. Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity. Sci. Hortic. 2007, 114, 5–10. [Google Scholar] [CrossRef]
- Mohammadi, P.; Khoshgoftarmanesh, A.H. The effectiveness of synthetic zinc (Zn)-amino chelates in supplying Zn and alleviating salt-induced damages on hydroponically grown lettuce. Sci. Hortic. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Muñoz-Rueda, A.; Mena-Petite, A. Lettuce production and antioxidant capacity are differentially modified by salt stress and light intensity under ambient and elevated CO2. J. Plant Physiol. 2013, 170, 1517–1525. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Evaluation of lettuce genotypes for salinity tolerance. HortScience 2015, 50, 1441–1446. [Google Scholar] [CrossRef]
- Shilpha, J.; Manivannan, A.; Soundararajan, P.; Jeong, B.R. Heat stress mitigation by silicon nutrition in plants: A comprehensive overview. In Benefits of Silicon in the Nutrition of Plants; de Mello Prado, R., Ed.; Springer: Cham, Switzerland, 2023; pp. 329–346. [Google Scholar] [CrossRef]
- Thakral, V.; Bhat, J.A.; Kumar, N.; Myaka, B.; Sudhakaran, S.; Patil, G.; Sonah, H.; Shivaraj, S.; Deshmukh, R. Role of silicon under contrasting biotic and abiotic stress conditions provides benefits for climate smart cropping. Environ. Exp. Bot. 2021, 189, 104545. [Google Scholar] [CrossRef]
- de Souza Lemos Neto, H.; Guimarães, M.; Mesquita, R.O.; Gomes Sampaio, I.; de Araújo Hendges, A.R.A.; de Oliveira, A.B. Silicon potential as attenuator of salinity effects on growth and post-harvest quality of lettuce. J. Agric. Sci. 2018, 10, 455–463. [Google Scholar] [CrossRef][Green Version]
- Dhiman, P.; Rajora, N.; Bhardwaj, S.; Sudhakaran, S.S.; Kumar, A.; Raturi, G.; Chakraborty, K.; Gupta, O.P.; Devanna, B.; Tripathi, D.K. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol. Biochem. 2021, 162, 110–123. [Google Scholar] [CrossRef]
- Villa e Vila, V.; Marques, P.A.A.; Gomes, T.M.; Nunes, A.F.; Montenegro, V.G.; Wenneck, G.S.; Franco, L.B. Deficit irrigation with silicon application as strategy to increase yield, photosynthesis and water productivity in lettuce crops. Plants 2024, 13, 1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Hodson, M.; White, P.J.; Mead, A.; Broadley, M. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 17–39. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef]
- Faisal, S.; Callis, K.; Slot, M.; Kitajima, K. Transpiration-dependent passive silica accumulation in cucumber (Cucumis sativus) under varying soil silicon availability. Botany 2012, 90, 1058–1064. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Raven, J.A. The transport and function of silicon in plants. Biol. Rev. 1983, 58, 179–207. [Google Scholar] [CrossRef]
- Kumar, S.; Soukup, M.; Elbaum, R. Silicification in grasses: Variation between different cell types. Front. Plant Sci. 2017, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- McLarnon, E.; McQueen-Mason, S.; Lenk, I.; Hartley, S.E. Evidence for active uptake and deposition of Si-based defenses in tall fescue. Front. Plant Sci. 2017, 8, 1199. [Google Scholar] [CrossRef]
- Agarie, S.; Agata, W.; Kubota, F.; Kaufman, P.B. Physiological roles of silicon in photosynthesis and dry matter production in rice plants: I. Effects of silicon and shading treatments. Jpn. J. Crop Sci. 1992, 61, 200–206. [Google Scholar] [CrossRef]
- Cho, E.; Gurdon, C.; Zhao, R.; Peng, H.; Poulev, A.; Raskin, I.; Simko, I. Phytochemical and agronomic characterization of high-flavonoid lettuce lines grown under field conditions. Plants 2023, 12, 3467. [Google Scholar] [CrossRef]
- Simko, I.; Zhao, R. Phenotypic characterization, plant growth and development, genome methylation, and mineral elements composition of neotetraploid lettuce (Lactuca sativa L.). Front. Plant Sci. 2023, 14, 1296660. [Google Scholar] [CrossRef]
- Peng, H.; Zhao, R.; Smith, R.; Simko, I. Phenotypic and genetic analyses of yellow spot malady in lettuce. Sci. Hortic. 2022, 305, 111389. [Google Scholar] [CrossRef]
- de Andrade, F.A.; Junior, O.A.; Perini, L.J.; de Jesus Andrade, C.G.T.; Miglioranza, É. Yield, nutritional state and silicon accumulation in lettuce cultivars fertilized with calcium silicate. Agron. Sci. Biotechnol. 2016, 2, 29. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, S.; Hussain, S.; Kataria, S.; Hajihashemi, S.; Kumari, P.; Yang, X.; Brestic, M. Does silicon really matter for the photosynthetic machinery in plants…? Plant Physiol. Biochem. 2021, 169, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Maqsood, M.A.; Rehman Hu Mahboob, W.; Sarwar, N.; Hafeez, O.B.A.; Hussain, S.; Ercisli, S.; Akhtar, M.; Aziz, T. Silicon nutrition in plants under water-deficit conditions: Overview and prospects. Water 2023, 15, 739. [Google Scholar] [CrossRef]
- Frantz, J.M.; Khandekar, S.; Leisner, S. Silicon differentially influences copper toxicity response in silicon-accumulator and non-accumulator species. J. Am. Soc. Hortic. Sci. 2011, 136, 329–338. [Google Scholar] [CrossRef]
- Hoffmann, J.; Berni, R.; Hausman, J.-F.; Guerriero, G. A review on the beneficial role of silicon against salinity in non-accumulator crops: Tomato as a model. Biomolecules 2020, 10, 1284. [Google Scholar] [CrossRef] [PubMed]
- Tebow, J.B.; Houston, L.L.; Dickson, R.W. Silicon foliar spray and substrate drench effects on plant growth, morphology, and resistance to wilting with container-grown edible species. Horticulturae 2021, 7, 263. [Google Scholar] [CrossRef]
- Puppe, D.; Sommer, M. Experiments, uptake mechanisms, and functioning of silicon foliar fertilization—A review focusing on maize, rice, and wheat. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 152, pp. 1–49. [Google Scholar] [CrossRef]
- Cooke, J.; Carey, J.C. Stress alters the role of silicon in controlling plant water movement. Funct. Ecol. 2023, 37, 2985–2999. [Google Scholar] [CrossRef]
- Pilon, C.; Soratto, R.P.; Moreno, L.A. Effects of soil and foliar application of soluble silicon on mineral nutrition, gas exchange, and growth of potato plants. Crop Sci. 2013, 53, 1605–1614. [Google Scholar] [CrossRef]
- Jang, S.-W.; Sadiq, N.B.; Hamayun, M.; Jung, J.; Lee, T.; Yang, J.-S.; Lee, B.; Kim, H.-Y. Silicon foliage spraying improves growth characteristics, morphological traits, and root quality of Panax ginseng C.A.Mey. Ind. Crops Prod. 2020, 156, 112848. [Google Scholar] [CrossRef]
- Hussain, S.; Shuxian, L.; Mumtaz, M.; Shafiq, I.; Iqbal, N.; Brestic, M.; Shoaib, M.; Sisi, Q.; Li, W.; Mei, X.; et al. Foliar application of silicon improves stem strength under low light stress by regulating lignin biosynthesis genes in soybean (Glycine max (L.) Merr.). J. Hazard. Mater. 2021, 401, 123256. [Google Scholar] [CrossRef]
- Frasetya, B.; Subandi, M.; Sofiani, I. (Eds) The Effect of Silica Source Concentration to Improve Growth of lactuca sativa L. on Floating Hydroponic System; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar] [CrossRef]
- de Souza Alonso, T.A.; da Silva, D.L.; de Mello Prado, R.; Soares, P.L.M.; Tenesaca, L.F.L.; Ferreira, R.J. Silicon promotes the control of Meloidogyne incognita in lettuce by increasing ascorbic acid and phenolic compounds. J. Pest Sci. 2022, 95, 1453–1466. [Google Scholar] [CrossRef]
- de Souza Lemos Neto, H.; de Almeida Guimaraes, M.; Sampaio, I.M.G.; da Silva Rabelo, J.; dos Santos Viana, C.; Mesquita, R.O. Can silicon (Si) influence growth, physiology and postharvest quality of lettuce? Aust. J. Crop Sci. 2020, 14, 71–77. [Google Scholar] [CrossRef]
- de Souza Lemos Neto, H.; de Almeida Guimarães, M.; Mesquita, R.O.; Sousa Freitas, W.E.; de Oliveira, A.B.; da Silva Dias, N.; Gomes-Filho, E. Silicon supplementation induces physiological and biochemical changes that assist lettuce salinity tolerance. Silicon 2021, 13, 4075–4089. [Google Scholar] [CrossRef]
- Helms, K.M.; Dickson, R.W.; Bertucci, M.B.; Rojas, A.A.; Gibson, K.E. Metal micronutrient and silicon concentration effects on growth and susceptibility to pythium root rot forhydroponic lettuce (Lactuca sativa). Horticulturae 2023, 9, 670. [Google Scholar] [CrossRef]
- Tazekand, F.M.; Ghasemi, K.; Roosta, H. Effect of silicon on the quantity and quality of Batavia lettuce in soilless culture conditions. J. Soil Plant Interact. 2022, 13, 64–77. [Google Scholar] [CrossRef]
- Abdalla, K.A.; Youssef, S.M.S.; Ibrahim, M.F.; Salama, Y.A.; Metwally, A.A. Impacts of cobalt, selenium and silicon biofortification on the growth, productivity and nutritional value of lettuce. Egypt. J. Hortic. 2024, 51, 71–86. [Google Scholar] [CrossRef]
- Çelik, Y. Effects of different irrigation levels and varying doses of silicon applications on yield and some physiological parameters in lettuce cultivation. Acta Sci. Pol. Hortorum Cultus 2023, 22, 3–12. [Google Scholar] [CrossRef]
- Alkahtani, M.; Hafez, Y.; Attia, K.; Al-Ateeq, T.; Ali, M.A.M.; Hasanuzzaman, M.; Abdelaal, K. Bacillus thuringiensis and silicon modulate antioxidant metabolism and improve the physiological traits to confer salt tolerance in lettuce. Plants 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Santiago, L.; Navarro-León, E.; López-Moreno, F.J.; Arjó, G.; González, L.M.; Ruiz, J.M.; Blasco, B. The application of the silicon-based biostimulant Codasil® offset water deficit of lettuce plants. Sci. Hortic. 2021, 285, 110177. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Ding, X.; Liao, X.; Wang, R. Spraying silicon and/or cerium sols favorably mediated enhancement of Cd/Pb tolerance in lettuce grown in combined Cd/Pb contaminated soil. Procedia Environ. Sci. 2013, 18, 68–77. [Google Scholar] [CrossRef][Green Version]
- Pereira, A.S.; Bortolin, G.S.; Dorneles, A.O.S.; Meneghello, G.E.; do Amarante, L.; Mauch, C.R. Silicon seed priming attenuates cadmium toxicity in lettuce seedlings. Environ. Sci. Pollut. Res. 2021, 28, 21101–21109. [Google Scholar] [CrossRef]
- de Cássia Alves, R.; dos Santos Zucco, M.F.; Oliveira, K.R.; Checchio, M.V.; Franco, C.A.; Körösi, K.; Gratão, P.L. Seed priming with silicon improves plant resistance to downy mildew (Bremia lactucae) in lettuce seedlings by intensifying antioxidant defense systems. Silicon 2022, 14, 12721–12731. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Cogliati, E.E.; Gullino, M.L. Silicon and increased electrical conductivity reduce downy mildew of soilless grown lettuce. Eur. J. Plant Pathol. 2012, 132, 123–132. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Sario, S.; Mendes, R.J.; Correia, C.V.; Moutinho-Pereira, J.; Correia, C.M.; Santos, C. Silicon titanium oxide nanoparticles can stimulate plant growth and the photosynthetic pigments on lettuce crop. Agriculture 2020, 66, 148–160. [Google Scholar] [CrossRef]
- Galati, V.C.; Magalhães Marques, K.; Ascari Morgado, C.M.; Corrêa Muniz, A.C.; Cecílio Filho, A.B.; Mattiuz, B.-H. Silicon in the turgidity maintenance of American lettuce. Afr. J. Agric. Res. 2015, 10, 4699–4705. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Nazaralian, S.; Majd, A.; Irian, S.; Najafi, F.; Ghahremaninejad, F.; Landberg, T.; Greger, M. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017, 115, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, A.L.; Imran, M.; Asaf, S.; Kim, Y.-H.; Bilal, S.; Numan, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Silicon-induced thermotolerance in Solanum lycopersicum L. via activation of antioxidant system, heat shock proteins, and endogenous phytohormones. BMC Plant Biol. 2020, 20, 248. [Google Scholar] [CrossRef]
- Sonobe, K.; Hattori, T.; An, P.; Tsuji, W.; Eneji, E.; Tanaka, K.; Inanaga, S. Diurnal variations in photosynthesis, stomatal conductance and leaf water relation in sorghum grown with or without silicon under water stress. J. Plant Nutr. 2009, 32, 433–442. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Ruppenthal, V.; Zoz, T.; Steiner, F.; do Carmo, L.M.; Castagnara, D.D. Silicon does not alleviate the adverse effects of drought stress in soybean plants. Semin. Ciências Agrárias 2016, 37, 3941–3954. [Google Scholar] [CrossRef][Green Version]
- Maillard, A.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.-C.; Hosseini, S.A. Silicon transcriptionally regulates sulfur and ABA metabolism and delays leaf senescence in barley under combined sulfur deficiency and osmotic stress. Environ. Exp. Bot. 2018, 155, 394–410. [Google Scholar] [CrossRef]
- Vandegeer, R.K.; Zhao, C.; Cibils-Stewart, X.; Wuhrer, R.; Hall, C.R.; Hartley, S.E.; Tissue, D.T.; Johnson, S.N. Silicon deposition on guard cells increases stomatal sensitivity as mediated by K+ efflux and consequently reduces stomatal conductance. Physiol. Plant. 2021, 171, 358–370. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. The effect of silicon on osmotic and drought stress tolerance in wheat landraces. Plants 2021, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef]
- White, P.J. The pathways of calcium movement to the xylem. J. Exp. Bot. 2001, 52, 891–899. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Demidchik, V.; Nichols, C.; Davies, J.M. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1564, 299–309. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron homeostasis in plants–a brief overview. Metallomics 2017, 9, 813–823. [Google Scholar] [CrossRef]
- Thomine, S.; Vert, G. Iron transport in plants: Better be safe than sorry. Curr. Opin. Plant Biol. 2013, 16, 322–327. [Google Scholar] [CrossRef]
- Ródenas, R.; García-Legaz, M.F.; López-Gómez, E.; Martínez, V.; Rubio, F.; Ángeles Botella, M. NO3−, PO43− and SO42− deprivation reduced LKT1-mediated low-affinity K+ uptake and SKOR-mediated K+ translocation in tomato and Arabidopsis plants. Physiol. Plant. 2017, 160, 410–424. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, S.-M.; Kim, B.-K.; Yoon, I.-S.; Kwon, T.-R. Identification of rice accessions associated with K+/Na+ ratio and salt tolerance based on physiological and molecular responses. Rice Sci. 2017, 24, 360–364. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z.; Maurović, N.; Kondres, N.; Filipović, V.; Savić, R.; Blagojević, B.; Tanaskovik, V.; Gergichevich, C.M.; Romić, D. Growth and element uptake by salt-sensitive crops under combined NaCl and Cd stresses. Plants 2021, 10, 1202. [Google Scholar] [CrossRef]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The effect of NaCl stress on the response of lettuce (Lactuca sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- Assaha, D.V.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Pang, Z.; Peng, H.; Lin, S.; Liang, Y. Theory and application of a Si-based defense barrier for plants: Implications for soil-plant-atmosphere system health. Crit. Rev. Environ. Sci. Technol. 2024, 54, 722–746. [Google Scholar] [CrossRef]



| Element | Symbol | 0 mM SiO2 | 3.66 mM SiO2 | 7.32 mM SiO2 | 14.65 mM SiO2 | 29.30 mM SiO2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aluminum | Al | 32.2 | - | 32.4 | - | 39.3 | - | 24.0 | - | 33.6 | - |
| Barium | Ba | 2.54 | - | 2.42 | - | 2.44 | - | 2.68 | - | 2.51 | - |
| Boron | B | 13.2 | - | 13.2 | - | 13.0 | - | 12.5 | - | 13.7 | - |
| Cadmium | Cd | 0.204 | AB | 0.188 | B | 0.233 | A | 0.230 | AB | 0.120 | C |
| Calcium | Ca | 5639 | - | 5245 | - | 5313 | - | 6042 | - | 5627 | - |
| Chloride | Cl− | 31,054 | C | 36,989 | A | 32,610 | BC | 31,993 | BC | 33,393 | B |
| Copper | Cu | 2.73 | C | 3.38 | A | 3.02 | B | 3.06 | B | 2.66 | C |
| Iron | Fe | 36.0 | AB | 38.9 | AB | 36.9 | AB | 33.3 | B | 42.0 | A |
| Lithium | Li | 0.511 | - | 0.586 | - | 0.560 | - | 0.547 | - | 0.630 | - |
| Magnesium | Mg | 2036 | B | 2482 | A | 2057 | B | 2298 | AB | 2254 | AB |
| Manganese | Mn | 87.8 | AB | 88.4 | AB | 92.8 | A | 94.2 | A | 79.1 | B |
| Molybdenum | Mo | 0.337 | - | 0.188 | - | 0.257 | - | 0.187 | - | 0.214 | - |
| Nitrogen | N | 1.37 | AB | 1.62 | A | 1.51 | AB | 1.14 | B | 1.44 | AB |
| Phosphorus | P | 3215 | AB | 3240 | A | 2996 | BC | 2813 | C | 3284 | A |
| Potassium | K | 16,015 | C | 20,406 | A | 16,733 | BC | 15,423 | C | 17,864 | B |
| Silicon | Si | 58.6 | D | 69.3 | CD | 75.0 | BC | 84.8 | B | 103.6 | A |
| Sodium | Na | 14,464 | B | 16,185 | A | 14,687 | B | 13,781 | C | 15,885 | A |
| Strontium | Sr | 18.3 | - | 16.4 | - | 16.8 | - | 18.7 | - | 17.4 | - |
| Sulfur | S | 777 | B | 980 | A | 797 | B | 757 | B | 953 | A |
| Tin | Sn | 1.55 | A | 0.98 | B | 1.15 | AB | 1.07 | AB | 0.84 | B |
| Titanium | Ti | 0.607 | - | 0.574 | - | 0.602 | - | 0.419 | - | 0.602 | - |
| Zinc | Zn | 31.2 | AB | 32.0 | AB | 31.6 | AB | 28.6 | B | 35.3 | A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simko, I.; Zhao, R.; Peng, H. Differential Impact of SiO2 Foliar Application on Lettuce Response to Temperature, Salinity, and Drought Stress. Plants 2025, 14, 1845. https://doi.org/10.3390/plants14121845
Simko I, Zhao R, Peng H. Differential Impact of SiO2 Foliar Application on Lettuce Response to Temperature, Salinity, and Drought Stress. Plants. 2025; 14(12):1845. https://doi.org/10.3390/plants14121845
Chicago/Turabian StyleSimko, Ivan, Rebecca Zhao, and Hui Peng. 2025. "Differential Impact of SiO2 Foliar Application on Lettuce Response to Temperature, Salinity, and Drought Stress" Plants 14, no. 12: 1845. https://doi.org/10.3390/plants14121845
APA StyleSimko, I., Zhao, R., & Peng, H. (2025). Differential Impact of SiO2 Foliar Application on Lettuce Response to Temperature, Salinity, and Drought Stress. Plants, 14(12), 1845. https://doi.org/10.3390/plants14121845








