Abstract
In this study, the functions of the photosynthetic machinery were evaluated using chlorophyll a fluorescence technique (PAM and JIP test) in pea plants (Pisum sativum L. cv Borec) and its LHC II oligomerization variants (mutants Costata 2/133 and Coeruleovireus 2/16). The oligomeric forms of LHCII increased in the following order: Costata 2/133 < Borec wt < Coeruleovireus 2/16. Data revealed that the mutant with higher LHCII oligomerization (Coeruleovireus 2/16) at low light intensity (LL, 150 µmol photons/m2·s) exhibited the following: (i) decreased energy dissipation and increased electron transport efficiency; (ii) higher reaction center density; (iii) increased amounts of the open reaction centers (qp) and their excitation efficiency (Φexc); and (iv) influenced the reoxidation of QA−, alleviating its interaction with plastoquinone. These effects enhanced photosynthetic performance related to PSII photochemistry (PIABS) and overall photosynthetic efficiency (PItotal). High light intensity (HL, 500 µmol photons/m2·s) caused a reduction in open reaction centers (qp), excitation efficiency (Φexc), photochemical energy conversion of PSII (ΦPSII), maximum efficiency of PSII photochemistry in light (Fv′/Fm′), and linear electron transport via PSII, with more pronounced effects observed in membranes with a lower degree of LHCII oligomerization (Costata 2/133). This study provides novel experimental evidence for the pivotal role of the LHCII structural organization in determining the efficiency of light-dependent reactions of photosynthesis.
1. Introduction
Photosynthesis, which converts light energy into chemical energy, is highly sensitive to climate changes [1]. The primary reactions of photosynthesis in higher plants occur within complexes embedded in the thylakoid membranes that constitute the photosynthetic apparatus [2]. Four multisubunit membrane–protein complexes participate in these processes: photosystem I (PSI), photosystem II (PSII), cytochrome b6f (cyt. b6f), and ATP synthase [3]. These complexes ensure optimal activity of the light-dependent reactions of photosynthesis under varying light conditions [4]. It is known that the PSII and PSI complexes are located in distinct domains of the thylakoid membrane: PSII and its light-harvesting complex (LHCII) reside in appressed grana stacks, while PSI and its light-harvesting complex (LHCI) are in non-appressed stroma thylakoid membranes [5].
Peripheral antenna systems of both PSII and PSI are composed of light-harvesting chlorophyll a/b proteins [6], where the chlorophylls a function as a primary electron donor of reaction centers of both photosystems [6]. The light-harvesting complex of PSII is crucial for photosynthesis in plants. It captures and transfers light energy to both the photosystems and consists of proteins and pigments like chlorophylls and carotenoids, embedded in thylakoid membranes [7,8]. The LHCII consists of six Lhcb proteins and can exist in various forms, such as monomers, dimers, trimers, and aggregates, which switch in response to changes in light intensity [5,9,10]. The trimeric forms, which consist of proteins Lhcb1, Lhcb2, and Lhcb3, are particularly effective in capturing and transferring light energy in PSII [10]. Trimeric forms of LHCII are bound with the PSII core by Lhcb4, Lhcb5, and Lhcb6 proteins. Phosphorylation influences LHCII’s organization within the thylakoid membrane, affecting energy distribution between photosystems. This dynamic organization is essential for optimizing light energy capture and ensuring efficient photosynthesis [5,10].
It is reported extensively that light intensity strongly affects the rate of the photosynthesis and the organization of LHCII, which is linked to changes in the levels of LHCII and the surface area of grana membranes [10,11]. In higher plants, low-light acclimation (shade plants) leads to an increase in the PSII antenna size and area of grana membranes [12,13], whereas sun plants exhibit a higher Chl a/b ratio, lower level of LHCII, and a lower degree of thylakoid stacking [1,14].
Chlorophyll a fluorescence is a fast, informative, and non-invasive method, widely used for the characterization of the photosynthesis of higher plants and gives information on the relationship between the structure and functions of the photosynthetic apparatus [15,16,17,18,19,20]. Pulse amplitude modulated (PAM) and chlorophyll a fluorescence induction are two measurement techniques widely used for characterization of the plant photosynthesis. A fast increase of the chlorophyll a fluorescence in dark-adapted leaves after applying light characterizes the primary photosynthetic processes. The energy trapping, electron transport, and dissipation of the energy in the antenna complexes depend on the structure of the photosynthetic apparatus [21].
Pigment mutants of higher plants are convenient models for studying the relationship between the structural organization of the complexes of the photosynthetic apparatus and their functions [22]. The study of pea chlorophyll mutant Chlorotica revealed that the reduction in the chlorophyll and carotenoid content corresponds with low photosynthetic activity [22]. It has been shown that the reduction of the chlorophyll content in mutants of maize and tomato leads to a decrease in the open PSII reaction centers (qp) and electron transport rate (ETR) [23,24]. The study of high pigment mutants in tomatoes (Solanum lycopersicum L.) demonstrates that they were characterized by higher net CO2 assimilation [25] and rate of photosynthesis [26]. Research on pea (Pisum sativum L.) mutants has shown that alterations in the amount and organization of LHCII modify the electric properties of thylakoid membranes, significantly impact energy distribution between both photosystems and affect the oxygen evolution [9,27].
The objects of the study in the present work are seedlings of Pisum sativum L. cv Borec (wt) and its mutants (Costata 2/133 and Coeruleovireus 2/16). In previous investigations, the thylakoid membranes of these plants were characterized (Table 1). It has been shown that the oligomeric forms of LHCII increased in the following order: Costata 2/133 < Borec wt < Coeruleovireus 2/16. The aim of this study is to assess how the degree of oligomerization of LHCII influences the light-dependent reactions of the photosynthetic apparatus. We used chlorophyll a fluorescence induction curves (OJIP curves) and pulse amplitude modulated (PAM) chlorophyll a fluorescence. The results give new information about the role of LHCII organization in the functional efficiency of the photosynthetic apparatus.

Table 1.
Characteristics of the chlorophyll content and ratio of chlorophyll protein complexes in thylakoid membranes isolated from wild type pea (Pisum sativum L. cv. Borec) and its mutants (Costata 2/133 and Coeruleovireus 2/16). The determination was made by nondenaturating SDS–PAGE. LHCIIo and LHCIIm are the oligomeric and monomeric forms of the major LHCII, respectively [9,27].
2. Results
2.1. Chlorophyll a Fluorescence Induction
Chlorophyll a fluorescence induction gives information on the functions of the photosynthetic apparatus in studied pea plants. The fluorescence curve follows a polyphasic increase, denoted as OJIP [28,29], and gives information about PSII functions and the efficiency of the electron transport chain [30]. The curves of the chlorophyll a fluorescence induction for Borec wt and its mutants Costata 2/133 and Coeruleovireus 2/16 are given in Figure 1. Selected parameters of the chlorophyll a fluorescence induction (JIP test) were calculated, which provide a comprehensive overview of the performance and efficiency of the photosynthetic apparatus, encompassing aspects of light energy dissipation (DIo/RC), the stability of the OEC (Wk), and electron transport efficiency φE, ψE, φPo.
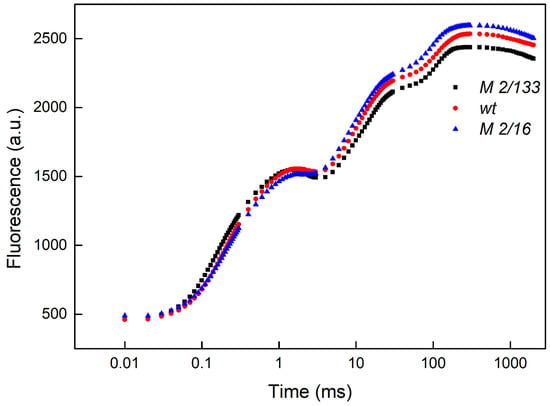
Figure 1.
The representative OJIP chlorophyll a fluorescence curves in Borec wild type (wt) and its mutants Costata 2/133 (M 2/133) and Coeruleovireus 2/16 (M 2/16). (for measurement details see Section 4).
The comparison of the JIP parameters revealed differences between Borec wt and its mutants (Figure 2 and Figure 3). The data showed that the light absorption per reaction center (ABS/RC) or functional PSII antenna size, was slightly smaller (by about 10%) in wt and mutant 2/16 than in the mutant 2/133, which reflects a bigger PSII antenna size in mutant 2/133 than both wt and mutant 2/16 (Figure 2). The plants with bigger oligomerization of LHCII (wt and mutant 2/16) have higher values of the parameter RC/ABS, φEo, φRo, and ψEo i.e., a higher number of the active reaction center per PSII antenna chlorophyll and better movement of the electron into electron transport chain beyond QA− (Figure 2). Dissipated energy (DIo/RC) decreased in membranes of wt (by 11%) and mutant 2/16 (by 14%) in comparison to the mutant 2/133. Experimental results also revealed a decrease in ABS/RC, Wk, N, and Vj parameters in wt and mutant 2/16 in comparison to mutant 2/133 (Figure 2). The parameter φP was similar in all studied plants, i.e., the maximum quantum yield of primary photochemistry was not affected by the degree of the LHCII oligomerization (Figure 2).
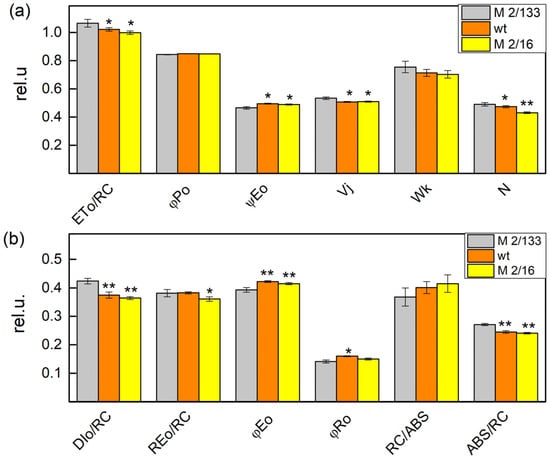
Figure 2.
Selected JIP parameters in Borec wild type and its mutants Costata 2/133 (M 2/133) and Coeruleovireus 2/16 (M 2/16). The parameters include the following: ETo/RC—electron transport flux further QA− per reaction center; φPo—maximum quantum yield of primary photochemistry; ψEo—the efficiency of electron transport beyond QA; Wk—the stability of the OEC; Vj—the fraction of closed reaction centers at the J-step in the chlorophyll a fluorescence curve; ABS/RC (×101)—absorption per reaction center; RC/ABS—the ratio of active reaction centers per absorbed light; DIo/RC—light energy dissipation; φEo—the quantum yield of electron transport beyond QA; φRo—the quantum yield of energy dissipation in the form of heat and fluorescence at the reaction center level; REo/RC—electron flux reducing end electron acceptors at the PSI acceptor side per reaction center; and N (×102)—maximum turnover of QA reducing until Fm was reached. Significant differences between the plant with the lowest degree of LHCII oligomerization (M 2/133) and plants with a higher degree of oligomerization (wt and M 2/16) were determined by Student’s t tests and are indicated by asterisks at p values less than 0.05 (*), 0.01 (**). (a) parameters: ETo/RC, φPo; ψEo, Vj, Wk, N; (b) parameters: Dio/RC, φEo, φRo, RC/ABS, ABS/RC.
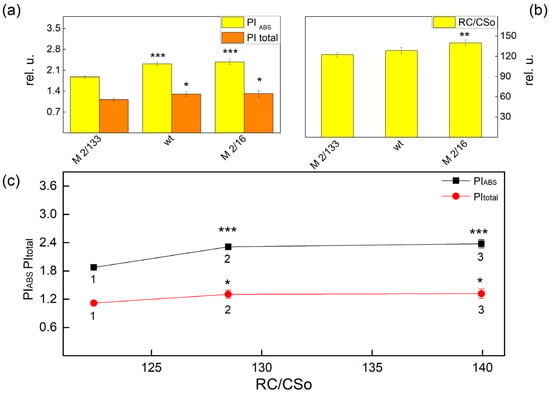
Figure 3.
Performance indices PIABS and PItotal (a), the amount of active PSII per excited cross-section reaction center (RC/CSo), (b) in Borec wild type and its mutants Costata 2/133 and Coeruleovireus 2/16. The relationship between the amount of active PSII per excited cross-section reaction center (RC/CSo) on the performance indices PIABS and PItotal. (c) 1, 2, 3 correspond with Costata 2/133, Borec wt, and Coeruleovireus 2/16, respectively. Significant differences between the plant with the lowest degree of LHCII oligomerization (M 2/133) and plants with a higher degree of oligomerization (wt and M 2/16) were determined by Student’s t test and are indicated by asterisks at p values less than 0.05 (*), 0.01 (**), and 0.001 (***).
Experimental results also revealed that the changes in the functional PSII antenna size influenced the performance indices (PIABS and PItotal) (Figure 2a). The increase of the ABS/RC corresponded with a decrease of both indices, i.e., ABS/RC increased while PIABS and PItotal decreased in the plants with a smaller degree of oligomerization (Figure 3a). At the same time the density of the reaction centers (RC/CSo, QA-reducing reaction centers) was also bigger in the wt and mutant 2/16 (Figure 3b). The correlation between the PIABS and PItotal and the amount of active PSII per excited cross-section reaction center (RC/CSo) are shown in Figure 3c.
A comparison of the changes in the studied parameters in the plant with the lowest degree of oligomerization (mutant 2/133) with those in the plants with a higher degree of oligomerization (wt and mutant 2/16) is presented in Figure 4.
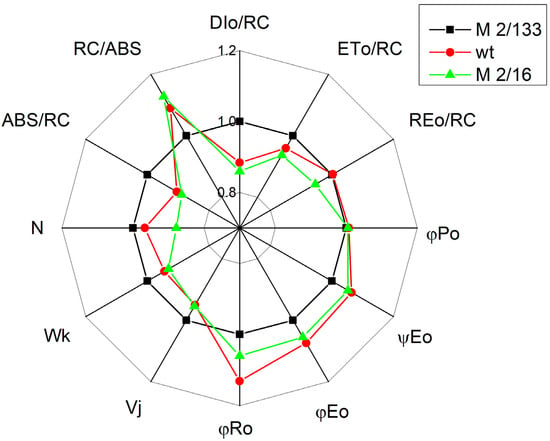
Figure 4.
Radar plot representing various photosynthetic parameters in Borec wild type and its mutants Costata 2/133 and Coeruleovireus 2/16. The parameters are the same as in Figure 2. The parameters are normalized to the plant with the lowest degree of LHCII oligomerization (Costata 2/133).
Significant differences between studied plants were found in the performance indices (PIABS and PItotal) (Figure 3a). The parameter PIABS was higher in wt (by 24%) and mutant 2/16 (by 27%) than in mutant 2/133. The comparison of the PItotal of studied plants revealed that the values of this parameter were higher by 22% in the plants with a bigger oligomerization of LHII (wt and mutant 2/16) than its value in the mutant 2/133. The index PIABS is determined by the following: the number of active reaction centers per PSII antenna chlorophyll, γRC/(1 − γRC), the partial performance of primary photochemistry φPo/(1 − φPo), and the performance of thermal reactions of the intersystem electron carries ψ(Eo)/(1 − ψEo) [31,32,33]. Higher values of PIABS in wt and mutant 2/16 were a result of high values of the component, characterized by non-light-dependent reaction [ψEo/(1 − ψ(Eo)] and the number of active reaction centers per PSII antenna chlorophyll [γRC/(1 − γRC)] (Table 2). These parameters determined the higher values of PItotal in wt and mutant 2/16 than the values of this parameter in mutant 2/133, because the parameter [δREo/(1 – δREo)] has similar values in Borec wt and its mutants i.e., differences in the efficiency in the electron transfer from QB to PSI acceptors were not registered in studied plants.

Table 2.
Components of the performance indices PIABS and PItotal in Borec wild type and its mutants Costata 2/133 and Coeruleovireus 2/16. The parameters are in relative units. Significant differences between the plant with the lowest degree of LHCII oligomerization (mutant 2/133) and plants with a higher degree of oligomerization (wt and mutant 2/16) were determined by Student’s t tests and are indicated by asterisks at p values less than 0.05 (*).
2.2. PAM Chlorophyll a Fluorescence
The PAM chlorophyll a fluorescence signals revealed differences in studied parameters in pea plants (Borec wild type and its mutants Costata 2/133 and Coeruleovireus 2/16) (Figure 5, Figure 6 and Figure 7). The data also showed an influence of actinic light during the following measurements: a low light (LL, 150 μmol photons/m2·s actinic light) or a high light (HL, 500 μmol photons/m2·s actinic light). The maximum efficiency of PSII photochemistry (Fv/Fm) was higher by 3% at LL and 6% at HL in mutant 2/16 and wild type compared to mutant 2/133 (Figure 5). The ratio of the intensity of chlorophyll a fluorescence caused by photochemical processes to the intensity of the chlorophyll a fluorescence not excitonically bound to the reaction centers of PSII (Fv/Fo) was bigger in wt and mutant 2/16 (LL by 17–22%, HL by 33%) in comparison to the mutant 2/133.
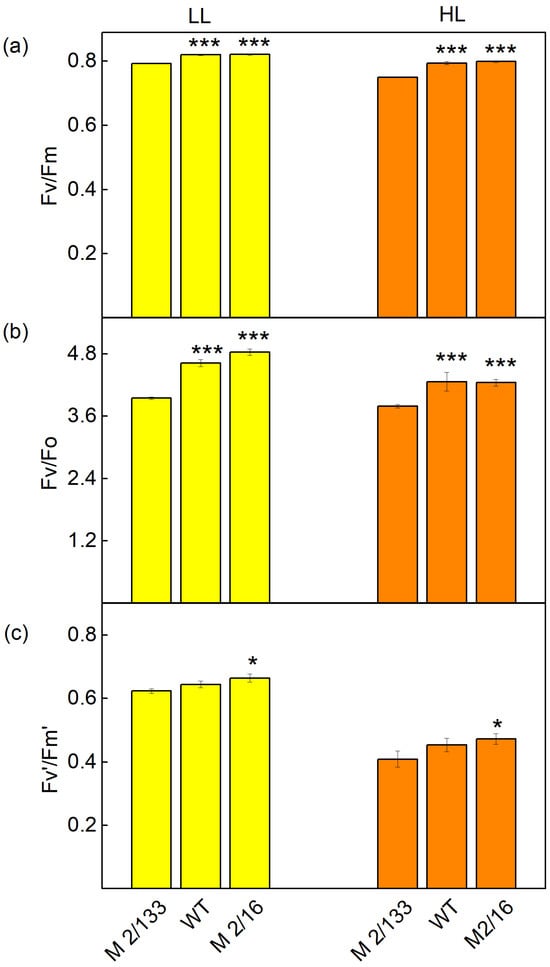
Figure 5.
The parameters of PAM chlorophyll a fluorescence in Borec wild type (wt) and its mutants Costata 2/133 (M 2/133) and Coeruleovireus 2/16 (M 2/16) at low light (LL, 150 μmol photons/m2·s actinic light) and high light (HL, 500 μmol photons/m2·s actinic light: (a) the maximal quantum yield) in dark-adapted state (Fv/Fm); (b) the intensity of chlorophyll a fluorescence caused by photochemical processes to intensity of the chlorophyll a fluorescence not excitonically bound to the reaction centers of PSII (Fv/Fo); (c) the effective quantum yield of PSII photochemistry (Fv′/Fm′). Significant differences between the plant with the lowest degree of LHCII oligomerization (M 2/133) and plants with a higher degree of oligomerization (wt and M 2/16) were determined by Student’s t tests and are indicated by asterisks at p values less than 0.05 (*) and 0.001 (***).
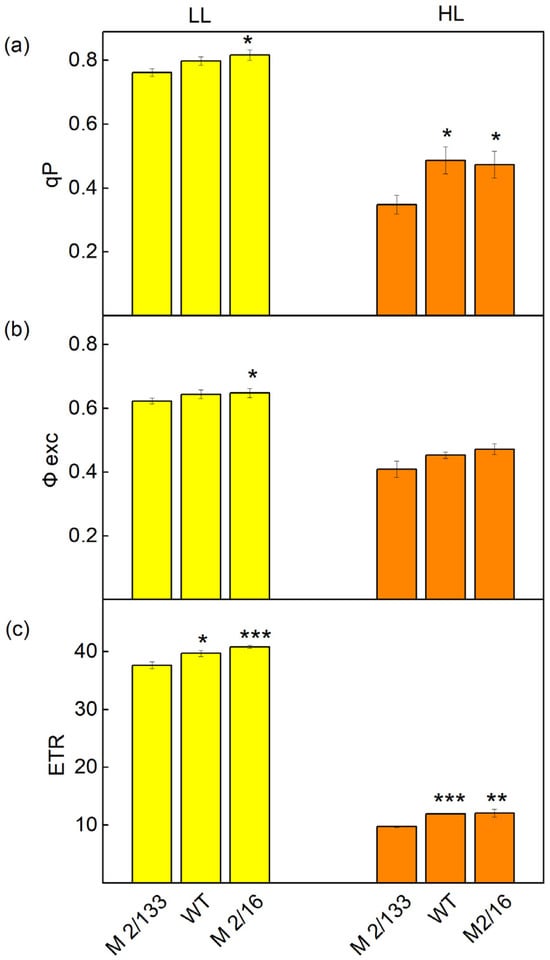
Figure 6.
The parameters of PAM chlorophyll a fluorescence in Borec wild type and its mutants Costata 2/133 and Coeruleovireus 2/16 at low light (LL, 150 μmol photons/m2·s actinic light) and high light (HL, 500 μmol photons/m2·s actinic light: (a) the photochemical quenching (qp); (b) excitation efficiency of open PSII center (Φexc); (c) the linear electron transport (ETR). Significant differences between the plant with the lowest degree of LHCII oligomerization (M 2/133) and plants with a higher degree of LHCII oligomerization (wt and M 2/16) were determined by Student’s t test and are indicated by asterisks at p values less than 0.05 (*), 0.01 (**), and 0.001 (***).
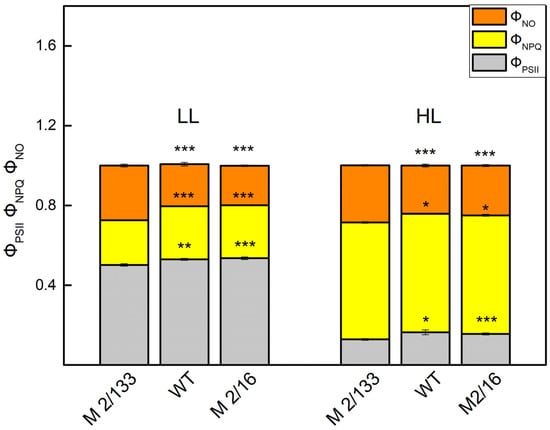
Figure 7.
The effective quantum yield of photochemical energy conversion of PSII (ΦPSII), and the regulated (ΦNPQ) and non-regulated (ΦNO) energy loss in Borec wild type (wt) and its mutants Costata 2/133 (M 2/133) and Coeruleovireus 2/16 (M 2/16) at low light (LL, 150 μmol photons/m2·s actinic light) and high light (HL, 500 μmol photons/m2·s actinic light). Significant differences between the plant with the lowest degree of LHCII oligomerization (M 2/133) and plants with a higher degree of oligomerization (wt and M 2/16) were determined by Student’s t test and are indicated by asterisks at p values less than 0.05 (*), 0.01 (**), and 0.001 (***).
Differences in the organization of the photosynthetic apparatus also affect the following: the effective quantum yield of PSII photochemistry (Fv′/Fm′), photochemical quenching (qp), excitation efficiency of open PSII center (Φexc), and linear electron transport rate (ETR). At low light (LL), a slight increase in Fv′/Fm′ (by 3–6%), qp (by 5–7%), and Φexc (by 4%) were registered in wt and mutant 2/16 in comparison to the mutant 2/133 (Figure 5 and Figure 6). The differences between studied plants were bigger at HL. The photochemical quenching (qp) was higher from 36% to 40% and ETR from 20% to 21% in wt and mutant 2/16 than the values of these parameters in mutant 2/133. The parameters Φexc and Fv′/Fm′ were bigger by 11–13% in wt and mutant 2/16 than in mutant 2/133 (Figure 6).
The energy absorbed by PSII is the sum of yields of photochemical energy conversion in PSII (ΦPSII), regulated (ΦNPQ), and non-regulated energy losses (ΦNO) [34]. At LL, the parameter ΦPSII was higher (5–8%) in plants with higher degree of oligomerization of LHCII (wt and mutant 2/16) than in the mutant 2/133 (Figure 7). At the same time, ΦNO was smaller (by about 25%), while ΦNPQ was bigger (by about 20%) in leaves of wt and mutant 2/16 in comparison to those in the mutant 2/133 (Figure 7).
Non-photochemical quenching of chlorophyll a fluorescence (NPQ) is essential for plant photoprotection [35]. The components that NPQ involves are as follows: energy-dependent quenching (qE), state transition quenching (qT), and photoinhibitory quenching (qI). The values of the component qE were similar in wt and studied mutants except for mutant 2/16 at HL (Table 3). The state transition quenching (qT) is about 9–10 times higher at LL and about 2 times at HL in wt and mutant 2/16 compared to mutant 2/133. Data also revealed a higher value of the qI in plants, with a higher degree of LHCII oligomerization (wt and mutant 2/16), by 41–45% at LL and by 12–14% at HL (Table 3).

Table 3.
Components of the non-photochemical quenching of chlorophyll a fluorescence in Borec wild type and its mutants Costata 2/133 and Coeruleovireus 2/16. qE—energy-dependent quenching; qT—state transition quenching; and qI—photoinhibitory quenching. Significant differences between the plant with the lowest degree of LHCII oligomerization (mutant 2/133) and plants with a higher degree of oligomerization (wt and mutant 2/16) were determined by Student’s t test and are indicated by asterisks at p values less than 0.05 (*), 0.01 (**), and 0.001 (***).
We estimated the influence of the LHCII organization on the QA− reoxidation by measuring the dark reduction of the chlorophyll a fluorescence after single saturated light pulse. Two components of the fluorescence signal characterized two pathways of QA− reoxidation [36,37]. Component A1, with rate constant k1, characterizes the interaction of QA with a plastoquinone, while component A2, with rate constant k2, characterizes an interaction of QA with the oxygen-evolving complex. The constant k1 data revealed that it did not differ in wt and two mutants, while the constant k2 was higher in mutant 2/133 than in wt and mutant 2/16 (Table 4). At the same time, the ratio of two components (A1/A2) was smaller in mutant 2/133 in comparison to the wt and mutant 2/16.

Table 4.
Kinetic characteristics of the dark relaxation of chlorophyll fluorescence induced by a single saturating light pulse in Borec wild type and its mutants Costata 2/133 (mutant 2/133) and Coeruleovireus 2/16 (mutant 2/16): k1—constant of the fast component; k2—constant of the slow component; A1/A2—the ratio of the fast and slow components. Significant differences between the plant with the lowest degree of LHCII oligomerization (mutants 2/133) and plants with a higher degree of oligomerization (wt and mutants 2/16) were determined by Student’s t test and are indicated by asterisks at p values less than 0.05 (*) and 0.001 (***).
2.3. Principal Component Analysis (PCA)
The first two components (Figure 8 and Table S1) account for 99.98% of the variability in the data. The pea mutant with the lowest oligomerization of LHCII complexes (mutant 2/133), positioned in the first quadrant, exhibits a negative correlation/relationship with parameters related to the number of active reaction centers per PSII antenna chlorophyll (RC/ABS), the quantum yield of electron transport (φEo), and the regulated energy losses from PSII (ΦNPQ), which are located in the third quadrant. Simultaneously, a more distinct positive correlation is observed in the mutant 2/133 for processes associated with non-photochemical quenching per reaction center unit (DIo/RC), nonregulated energy losses of PSII (ΦNO), and the stability of the OEC (Wk), all of which are located on the upper side of the F1 axis. In contrast, the mutant 2/16 and wt, located in the second quadrant and characterized by higher oligomerization of the LHII than mutant 2/133, show significantly improved photochemical activity, as indicated by the parameters in the same quadrant (Fv′/Fm′, Φexc, qp, ΦPSII).
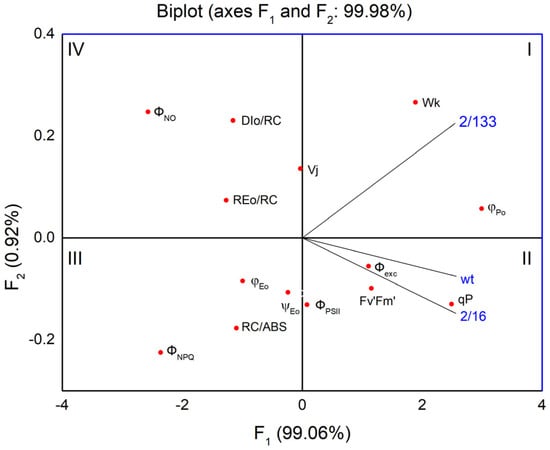
Figure 8.
Principal component analysis (PCA) shows variation among Borec wild type and its mutants Costata 2/133 and Coeruleovireus 2/16 (blue lines) in relation to selected parameters of chlorophyll a fluorescence (PAM and JIP test) shown as red dots.
3. Discussion
The regulatory role of LHCII in photosynthesis is well known [38,39,40]. The thylakoid membranes of studied pea plants are characterized by varying amounts and degrees of oligomerization of LHCII [27]. The ratio of oligomeric to monomeric forms of LHCII increases in the following order mutant 2/133 < wt < mutant 2/16 (Table 1). In this study, the functions of the photosynthetic apparatus are characterized in detail. A deeper understanding of the role of LHCII in primary photochemistry may help to understand the mechanisms of photosynthetic efficiency.
Variation of the chlorophyll content influences the absorbed light energy per active center (ABS/RC) (Figure 3 and Figure 4). The parameter ABS/RC was smaller in the membranes with a higher ratio of LHCIIo/LHCIIm, which reflects a smaller apparent antenna size in the membrane with a bigger degree of LHCII oligomerization. It has been shown that the ratio LHCII/PSII is higher in the plant (mutant 2/133) than wt and mutant 2/16 (Table 1), which suggests the synthesis of additional LHCII proteins [41]. The authors suggest that these additional synthesis proteins belong to the population of the LHCII molecules non-attached to PSII. This observation could explain the smaller apparent antenna size in the membrane with a bigger degree of LHCII oligomerization. At the same time, an increase in the number of active reaction centers per PSII antenna chlorophyll (RC/ABS) in the membrane with a bigger amount of the LHCIIo was registered. The data also showed a slight increase in the density of the reaction center (RC/CSo) in thylakoid membranes with the biggest amount of LHCIIo (mutant 2/16).
The experimental results revealed a variation in the parameters Wk and Vj, which could result from differences in the donor and acceptor side of PSII, respectively. The differences in parameter Wk in studied plants revealed an influence on the function of the OEC (Figure 2 and Figure 4) and correspond with changes in the kinetic parameters of the oxygen evolution, as a result of the modification of the Mn clusters and an influence on the oxygen-evolving reactions [27]. The influence of the donor side of the PSII complex could be connected by changes in the conformation of the OEC and/or of the proteins surrounding the complex and from variations of the surface electric parameters of the membrane [9]. The differences in parameter Fv/Fo in wt and mutants, which connected with the efficiency of the OEC [42,43,44] also revealed some differences in the donor side of PSII (Figure 2 and Figure 4).
Changes in the LHCII oligomerization are associated with modification not only on the donor side but also on the acceptor side of the PSII complex. The parameter Vj characterized the fraction of QA in the reduced state [45] was also influenced (Figure 2 and Figure 4). The current study showed that LHCII oligomerization influences the pathways of QA− reoxidation (Table 4). The constant characterizing QA− reoxidation through plastoquinone (k1) was similar in Borec wt and its mutants, but the constant k2, characterizing the interaction with OEC decreased in the membranes with a higher degree of LHCII oligomerization. At the same time, the ratio A1/A2 increased in wt and mutant 2/16, i.e., the electron flow from QA to plastoquinone is facilitated. The higher value of the Vj and smaller of the ψEo in mutant 2/133 compared with wt and mutant 2/16 (Figure 2) suggests decreased electron transfer beyond QA in this mutant, representing smaller electron movement through the electron transport chain.
At the same time, plants with a smaller amount of LHCIIo are characterized by increased dissipated energy flux per reaction center (DIo/RC) (Figure 2 and Figure 4). In addition, the data also revealed that the regulated energy losses (ΦNPQ) were bigger than the non-regulated energy losses (ΦNO) in all studied plants at HL actinic light (Figure 7). At the same time, ΦNO was bigger in mutant 2/133 in comparison to the plants with bigger LHCII oligomerization (wt and mutant 2/16). One of the reasons for high ΦNO at a smaller degree of oligomerization of LHCII could be due to changes in the organization of the OEC in comparison to the plants with higher oligomerization of LHCII. The differences in the organization of the OEC are connected with variations in the ratio of active PSIIα to PSIIβ centers as well as an influence on the So-S1 state distribution in darkness [9]. Furthermore, it was found that the modification of OEC is associated with high rates of non-radiative charge recombination between P680+ and QA [46]. The non-regulated energy losses (ΦNO) reflect energy quenching processes occurring within the PSII reaction center with QA in a reduced state [47]. Moreover, the reduction of QA has been suggested to be a major requirement for an efficient PSII reaction center-derived quenching [48]. The experimental data revealed that QA is more reduced in the plant with a smaller degree of the LHCII oligomerization (mutant 2/133) (Figure 6) and ΦNO values are significantly higher (Figure 7).
The components of non-photochemical quenching give more information about the dissipative mechanism in the thylakoid membrane (qE, qT, and qI) (Table 3). The data revealed that the parameter qT was higher in wt and mutant 2/16 in comparison to mutant 2/133. It is well known that state transition quenching (qT) is a process that redistributes excitation energy between two photosystems and it is very important for the protection of the photosynthetic apparatus [49,50]. The component qE was similar in all studied plants, but strongly increased at HL in all studied plants, as the increase is more pronounced in mutant 2/16 (Table 3), which supports a previous statement that qE protects PSII against short-term high light and fluctuations in light intensities [51]. It could be suggested/concluded that plants with higher LHCII oligomerization have better protection against high light.
The different organization of the LHCII influenced the amount of the open reaction center (qp) and their excitation efficiency (Φexc), as well as the electron transport rate (ETR) (Figure 6). These parameters had smaller values in the photosynthetic apparatus with a decreased amount of LHCIIo, i.e., in mutant 2/133 characterized by decreased pigment content and a smaller degree of oligomerization of LHCII. It could be concluded that differences in the organization of the LHCII-PSII complex, which corresponds with variation in the OEC and its functions [9], as well as an influence on the acceptor side (Vj) determined the differences in qp and (Φexc) in studied plants. The reduction of the parameters qp and ETR was also shown in maize mutants with reduced chlorophyll content [23]. A decrease of the parameter qp was also registered at the yellow left mutant of tomato (YLM) [24]. In addition, this study showed that the reduction of open centers in studied plants with decreased chlorophyll content and a small amount of LHCIIo was associated with a decrease in their efficiency (Figure 5). Moreover, at HL, the parameters qp, Φexc, and ETR decreased but these parameters were higher in the membrane with a bigger degree of oligomerization of LHCII (wt and mutant 2/16) (Figure 6). The different responses to changes in abiotic factors were observed in the yellow left mutant of tomatoes (YLM). The study of this mutant revealed higher sensitivity to low temperatures [24].
Performance indices reveal the photosynthetic performance related to PSII photochemistry (PIABS) and the overall photosynthetic efficiency (PItotal) [21]. Data showed that both indices were bigger in wt and mutant 2/16, i.e., in the membranes with a bigger density of the reaction centers (RC/CS) and LHCII oligomerization (Figure 2). The differences of both performance indices were determined from the performance of the thermal reactions of the intersystem electron carries ψ(Eo)/(1 − ψEo) and the number of active reaction centers per PSII antenna chlorophyll, γRC/(1 − γRC) (Table 2), i.e., at the same time, differences in the efficiency in the electron transfer from QB to PSI acceptors were not registered in studied plants. Data revealed that the better efficiency of the function of the light-dependent reactions of the photosynthetic apparatus is connected with better photosynthetic performance of the PSII photochemistry.
4. Materials and Methods
4.1. Plant Material
Plants from wild type peas (Pisum sativum L. Borec) and two pea mutants (Costata 2/133 and Coeruleovireus 2/16) were grown in pots containing ½ Hoagland solution (pH 6.5). The characteristics of the plants from the previous study are given in Table 1. The composition of the nutrient medium is as follows: 2.5 mM KNO3, 2.5 mM Ca(NO3)2, 1 mM MgSO4, 0.5 mM NH4NO3, 0.5 mM K2HPO4, 23 µM H3BO3, 4.5 µM MnCl2, 0.4 µM ZnSO4, 0.2 µM CuSO4, 0.25 µM Na2MoO4, and 20 µM Fe-EDTA (pH 6.0). The solutions were changed every 3 days. Each pot contained 10 seedlings. Plants were maintained under growth under controlled conditions of 12 h light/dark photoperiod; 19 °C/23 °C night/day is the temperature; 150 µmol photons/m2·s is the light intensity; and 65% humidity. After two weeks (14 days) of growing, the leaves of the seedlings were collected, measured, and analyzed. Fully developed leaves were used for analysis. Three independent experiments were conducted. Four plants per experiment were made for each explored plant type.
4.2. Fast Chlorophyll a Fluorescence Kinetics
Fast chlorophyll fluorescence induction kinetics were measured using the Handy PEA fluorimeter (Hansatech Instruments, King’s Lynn, UK). Prior to measurement, samples were dark-adapted for 20 min using specialized leaf clips to allow full relaxation of photosynthetic components. The fluorimeter’s high-intensity saturating light pulse (up to 3000 µmol photon/m2·s) enabled precise quantification of fluorescence dynamics, capturing rapid changes in photosystem activity. Illumination was provided by an array of three light-emitting diodes. The applied light pulse lasted for 1 s, ensuring optimal excitation conditions. The recorded fluorescence data were subsequently utilized for the computation of JIP-test parameters, enabling a detailed assessment of photosynthetic performance. Key parameters analyzed included the following [28]:
Mo = TRo/RC − ETo/RC is the initial slope;
ABS/RC = Mo·(1/Vj)·(1/ΦPo) is the absorption per reaction center;
RC/CSo = (ABS/CS)/(ABS/RC) the number of active PSII reaction centers per excited cross-section;
RC/ABS = 1/ABS/RC is the ratio of active reaction centers per absorbed light;
ψEo = 1 − Vj is the efficiency that a trapped exciton can move an electron further than QA− into the electron transport chain;
DIo/RC = (ABS/RC) − (TR0/RC) is the light energy dissipation;
Wk − F300 μs/F2 ms represents the ratio between the specific rises in the fluorescence curve during the J phase and the K phase;
φPo = Fv/Fm is the quantum yield of primary photochemistry; the maximum quantum yield of primary photochemistry, representing the efficiency of energy conversion in PS II;
Vj = (F2ms − Fo)/(Fm − Fo) indicates the fraction of closed reaction centers at the J-step in the chlorophyll a fluorescence curve;
φRo = Fv/Fm (1 − Vj) is the quantum yield of energy dissipation in the form of heat and fluorescence at the reaction center level;
φEo = Fv/Fm (1 − VI)—the quantum yield of electron transport beyond QA (the primary quinone electron acceptor);
REo/RC = M0 (1/VJ) (1 − VI)—the rate of electron transport per reaction center, indicating the efficiency of electron flow through the photosynthetic system;
ETo/RC = M0 (1/VJ) (1 − VJ)—the electron transport rate per reaction center, reflecting the overall electron transport activity;
N = Sm·M0·(1/VJ) turn-over number QA− the relative size of the plastoquinone pool, which is involved in electron transport within the photosynthetic apparatus.
The performance indices PIABS (energy conversion from exciton to the reduction in intersystem electron acceptors) and PItotal (energy conversion from exciton to the reduction in PSI end acceptors) were also determined. The equations for their calculation are as follows [52]:
PIABS = RC/ABS × φPo/(1 − φPo) × ψEo/(1 − ψEo);
PItotal = PIABS × δREo/(1 − δREo).
Measurements were processed using the PEA+ software 1.13 for detailed OJIP analysis.
4.3. PAM Chlorophyll a Fluorescence Measurements
PAM chlorophyll fluorescence measurements at ambient temperature were carried out on dark-adapted leaf discs (1 cm in diameter) using a PAM fluorometer (model 101/103, Heinz Walz GmbH, Effeltrich, Germany) as in Stefanov et al. [53]. The minimum fluorescence level (Fo) was recorded after 20 min of dark adaptation by applying a weakly modulated measuring light (0.02 µmol photons/m2·s). The maximum fluorescence levels in the dark-adapted (Fm) and light-adapted (Fm′) states were determined using a 0.8 s saturating pulse at 3000 µmol photons/m2·s. Two actinic light (AL) intensities were applied: 150 µmol photons/m2·s (low light, LL) and 500 µmol photons/m2·s (high light, HL). The steady-state fluorescence level (F0′) was assessed 5–6 min after the onset of actinic illumination. Selected chlorophyll a fluorescence parameters were calculated as follows: the maximum quantum efficiency of PSII in the dark-adapted state (Fv/Fm = (Fm − F0)/Fm), the ratio of quantum yields of photochemical and concurrent non-photochemical processes in PSII, Fv/Fo = (Fm − Fo)/Fo the effective quantum yield of energy conversion in PSII (ΦPSII = (Fm′ − Fs)/Fm′), the photochemical quenching coefficient (qp = (Fm′ − Fs)/Fv′), the linear electron transport rate, ETR = ΦPSII × 150 × 0.5 × 0.84; the non-regulated (ΦNO = Fs/Fm) and regulated (ΦNPQ = Fs/Fm′ − Fs/Fm) energy loss in PSII; the effective quantum yield of PSII photochemistry, Fv′/Fm′ = (Fm′ − Fo′)/Fm′ [54,55]. The excitation efficiency of open PSII reaction centers (Φexc = ΦPSII/qp) was calculated using the Genty et al. [56] approach.
The kinetic constants (k1 and k2) and the amplitude ratio (A1/A2) of the decay of variable chlorophyll a fluorescence relaxation after excitation by a saturating light pulse in dark-adapted leaves were also assessed [21,36,37,57].
The components of non-photochemical quenching: qE—the energy-dependent quenching component, qT—the state-transition quenching component, and qI—the photoinhibitory quenching component were determined as described in [58].
4.4. Principal Component Analysis (PCA)
Principal Component Analysis (PCA), a complex statistical technique, was applied to diminish a broad set of measured parameters into the most essential ones [59]. PCA was further employed to assess the influence of different LHCII oligomerization features of Pisum sativum L. Borec (wt) and two pea mutants (Costata 2/133 and Coeruleovireus 2/16) on JIP and PAM-derived fluorescence parameters. To determine the variability in response to structural changes/modifications in the LHCII complex, a clustering algorithm was utilized [60]. Multivariate statistical analysis using PCA was carried out, and a graphical illustration was created using Origin 9—software for data processing (OriginLab Corporation, Northampton, MA, USA). Meanwhile, the assumption of homoscedasticity is satisfied by the obtained data. Statistical significance between means was assessed using Student’s t-test. Differences were considered significant for p values < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
5. Conclusions
This study highlights the critical regulatory role of LHCII oligomerization in photosynthetic efficiency. The influence of the LHCII oligomerization on the light reaction of photosynthesis is summarized in Figure 9. The current study, for the first time, revealed that variations in LHCII organization significantly influence parameters such as energy absorption and electron transport efficiency. Increased LHCII oligomerization correlates with higher reaction center density and improved photosynthetic performance indices (PIABS and PItotal), which could be a result of an influence on the reoxidation of the QA−. Conversely, lower LHCII oligomerization is linked to increased energy dissipation and reduced electron transport efficiency. These findings suggest that LHCII’s structural organization plays a pivotal role in optimizing photosynthetic function under different environmental conditions. Insights into these mechanisms deepen our understanding of photosynthetic efficiency and its adaptation to abiotic factors. The conclusions regarding the role of LHCII oligomerization in the efficiency of the photosynthetic apparatus could be used to assess the possibility of evaluating the stability of new plant varieties in various environmental conditions.
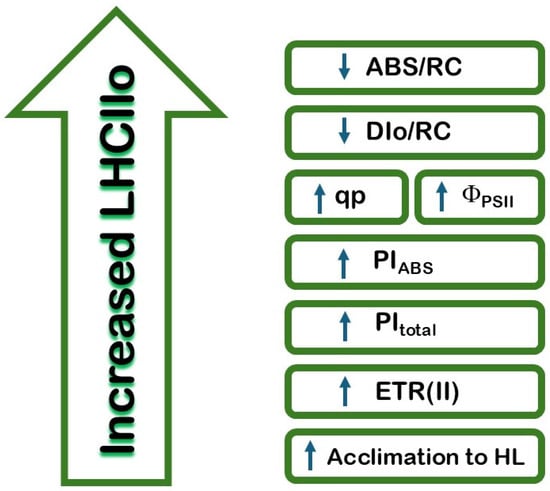
Figure 9.
Diagram of the main effects of LHCII oligomerization on the efficiency of the photosynthetic apparatus.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14121846/s1, Table S1. Contributions of variables (loadings) for the principal component analysis model shown in Figure 7.
Author Contributions
Conceptualization, A.N.M. and E.L.A.; methodology, G.D.R., M.A.S., A.N.M. and E.L.A.; software, G.D.R. and M.A.S.; validation, A.N.M. and E.L.A.; formal analysis, E.L.A.; investigation, G.D.R., M.A.S., A.N.M. and E.L.A.; writing—original draft preparation, E.L.A.; writing—review and editing, G.D.R., M.A.S., A.N.M. and E.L.A.; visualization, G.D.R. and M.A.S.; supervision, A.N.M. and E.L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Rochaix, J.-D. Assembly of the photosynthetic apparatus. Plant Physiol. 2011, 155, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Nevo, R.; Charuvi, D.; Tsabari, O.; Reich, Z. Composition, architecture and dynamics of the photosynthetic apparatus in higher plants. Plant J. 2012, 70, 157–176. [Google Scholar] [CrossRef]
- Perez-Boerema, A.; Engel, B.D.; Wietrzynski, W. Evolution of thylakoid structural diversity. Annu. Rev. Cell Dev. Biol. 2024, 40, 169–193. [Google Scholar] [CrossRef]
- Rantala, M.; Rantala, S.; Aro, E.-M. Composition, phosphorylation and dynamic organization of photosynthetic protein complexes in plant thylakoid membrane. Photochem. Photobiol. Sci. 2020, 19, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Lokstein, H.; Renger, G.; Götze, J. Photosynthetic light-harvesting (antenna) complexes—Structures and functions. Molecules 2021, 26, 3378. [Google Scholar] [CrossRef]
- Staehelin, L.A.; van der Staay, G.W.M. Structure, Composition, Functional Organization and Dynamic Properties of Thylakoid Membranes. In Oxygenic Photosynthesis: The Light Reactions; Springer: Dordrecht, The Netherlands, 1996; pp. 11–30. [Google Scholar] [CrossRef]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 12–39. [Google Scholar] [CrossRef]
- Apostolova, E.L.; Dobrikova, A.G.; Ivanova, P.I.; Petkanchin, I.B.; Taneva, S.G. Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J. Photochem. Photobiol. B Biol. 2006, 83, 114–122. [Google Scholar] [CrossRef]
- Janik-Zabrotowicz, E.; Gruszecki, W.I. LHCII—A protein like a “Swiss Army knife” with many mechanisms and functions. Photosynthetica 2023, 61, 405–416. [Google Scholar] [CrossRef]
- Apostolova, E.L.; Misra, A.N. Alterations in Structural Organization Affect the Functional Ability of Photosynthetic Apparatus. In Handbook of Plant and Crop Physiology, 3rd ed.; Pessarakli, M., Ed.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2014; pp. 103–120. ISBN 9781466553293. [Google Scholar]
- Grinzato, A.; Albanese, P.; Marotta, R.; Swuec, P.; Saracco, G.; Bolognesi, M.; Zanotti, G.; Pagliano, C. High-light versus low-light: Effects on paired photosystem ii supercomplex structural rearrangement in pea plants. Int. J. Mol. Sci. 2020, 21, 8643. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Sayre, R.T.; Lee, C.H. Identification of the optimal light harvesting antenna size for high-light stress mitigation in plants. Front. Plant Sci. 2020, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Guo, H.; Baskin, C.C.; Xiong, W.; Yang, C.; Li, Z.; Song, H.; Wang, T.; Yin, J.; Wu, X.; et al. Effect of light intensity on morphology, photosynthesis and carbon metabolism of alfalfa (Medicago sativa). Plants 2022, 11, 1688. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications, 1st ed.; Kalaji, M.H., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781315153605. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Mishra, A.N. Chlorophyll Fluorescence: A Practical Approach to Study Ecophysiology of Green Plants. In Advances in Plant Ecophysiology Techniques; Sánchez-Moreiras, A., Reigosa, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 77–97. [Google Scholar]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the photosynthetic apparatus functions by chlorophyll fluorescence and P700 absorbance in C3 and C4 plants under physiological conditions and under salt stress. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar] [CrossRef]
- Ladygin, V.G. Pigment composition and photosynthetic activity of pea chlorophyll mutants. Biol. Bull. Russ. Acad. Sci. 2003, 30, 370–377. [Google Scholar] [CrossRef]
- Zhong, X.M.; Sun, S.F.; Li, F.H.; Wang, J.; Shi, Z.S. Photosynthesis of a yellow-green mutant line in maize. Photosynthetica 2015, 53, 499–505. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, Y.; Xu, M.; Zhao, M.; Chen, H.; Lu, Y.; Pang, S.; Xu, W. Characterization of low-temperature sensitivity and chlorophyll fluorescence in yellow leaf mutants of tomato. Agronomy 2024, 14, 2382. [Google Scholar] [CrossRef]
- Pereira, A.M.; Martins, A.O.; Batista-Silva, W.; Condori-Apfata, J.A.; Nascimento, V.L.; Silva, V.F.; Oliveira, L.A.; Medeiros, D.B.; Martins, S.C.V.; Fernie, A.R.; et al. Elevated carbon assimilation and metabolic reprogramming in tomato high pigment mutants support the increased production of pigments. Plant Cell Rep. 2022, 41, 1907–1929. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Ahmed, F.; Wang, Z.; Harlina, P.W.; Nishawy, E.; Ayaad, M.; Manan, A.; Maher, M.; Ewas, M. Comparative analysis of two phytochrome mutants of tomato (Micro-Tom cv.) reveals specific physiological, biochemical, and molecular responses under chilling stress. J. Genet. Eng. Biotechnol. 2020, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.; Morgan, R.M.; Ivanov, A.G.; Apostolova, E.; Petkanchin, I.; Huner, N.P.A.; Taneva, S.G. Electric properties of thylakoid membranes from pea mutants with modified carotenoid and chlorophyll-protein complex composition. Photosynth. Res. 2000, 65, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll A Fluorescence; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Haldimann, P.; Strasser, R.J. Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (Pisum sativum L.). Photosynth. Res. 1999, 62, 67–83. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Bussotti, F.; Pollastrini, M.; Piekut, K.; Kowalik, W.; Wróbel, J.; Kalaji, H.M. Photosynthetic efficiency of Microcystis ssp. under salt stress. Environ. Exp. Bot. 2021, 186, 104459. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee; Bosa, K.; Kościelniak, J.; Zuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Giorio, P.; Sellami, M.H. Polyphasic OKJIP Chlorophyll a Fluorescence Transient in a Landrace and a Commercial Cultivar of Sweet Pepper (Capsicum annuum, L.) under Long-Term Salt Stress. Plants 2021, 10, 887. [Google Scholar] [CrossRef]
- Bussotti, F.; Desotgiu, R.; Pollastrini, M.; Cascio, C. The JIP test: A tool to screen the capacity of plant adaptation to climate change. Scand. J. For. Res. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Samson, G.; Carpentier, R. Nonphotosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress. The pool size of stromal reductants. Photochem. Photobiol. 2001, 74, 438. [Google Scholar] [CrossRef]
- Shirao, M.; Kuroki, S.; Kaneko, K.; Kinjo, Y.; Tsuyama, M.; Förster, B.; Takahashi, S.; Badger, M.R. Gymnosperms have increased capacity for electron leakage to oxygen (Mehler and PTOX reactions) in photosynthesis compared with angiosperms. Plant Cell Physiol. 2013, 54, 1152–1163. [Google Scholar] [CrossRef]
- Andrews, J.R.; Fryer, M.J.; Baker, N.R. Consequences of LHC II deficiency for photosynthetic regulation in chlorina mutants of barley. Photosynth. Res. 1995, 44, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. Protein Phosphorylation in Regulation of Photosynthesis. Biochim. Biophys. Acta Bioenerg. 1992, 1098, 275–335. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Rees, D.; Pascal, A.A.; Noctor, G.; Young, A.J. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll—Protein complex. FEBS Lett. 1991, 292, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kouřil, R.; Wientjes, E.; Bultema, J.B.; Croce, R.; Boekema, E.J. High-light vs. low-light: Effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 411–419. [Google Scholar] [CrossRef]
- Moustakas, M.; Bayçu, G.; Sperdouli, I.; Eroğlu, H.; Eleftheriou, E.P. Arbuscular mycorrhizal symbiosis enhances photosynthesis in the medicinal herb Salvia fruticosa by improving photosystem II photochemistry. Plants 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Govindachary, S.; Bukhov, N.G.; Joly, D.; Carpentier, R. Photosystem II inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and -tolerant plants. Physiol. Plant. 2004, 121, 322–333. [Google Scholar] [CrossRef]
- Mosadegh, H.; Trivellini, A.; Lucchesini, M.; Ferrante, A.; Maggini, R.; Vernieri, P.; Sodi, A.M. UV-B physiological changes under conditions of distress and eustress in sweet basil. Plants 2019, 8, 396. [Google Scholar] [CrossRef]
- Jiang, C.D.; Shi, L.; Gao, H.Y.; Schansker, G.; Toth, S.Z.; Strasser, R.J. Development of photosystems 2 and 1 during leaf growth in grapevine seedlings probed by chlorophyll a fluorescence transient and 820 nm transmission in vivo. Photosynthetica 2006, 44, 454–463. [Google Scholar] [CrossRef]
- Jursinic, P. Govindjee Effects of hydroxylamine and silicomolybdate on the decay in delayed light emission in the 6–100 μs range after a single 10 ns flash in pea thylakoids. Photosynth. Res. 1982, 3, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Heber, U.; Wiese, C.; Shuvalov, V.A. Energy dissipation in photosynthesis: Does the quenching of chlorophyll fluorescence originate from antenna complexes of photosystem II or from the reaction center? Planta 2001, 212, 749–758. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P. Dynamics of higher plant photosystem cross-section associated with state transitions. Photosynth. Res. 2009, 99, 173–183. [Google Scholar] [CrossRef]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 468–485. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Stefanov, M.A.; Rashkov, G.D.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Different sensitivity levels of the photosynthetic apparatus in Zea mays L. and Sorghum bicolor L. under salt stress. Plants 2021, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Guo, P. Chlorophyll Fluorescence: A Useful Tool in Barley Plant Breeding Programs. In Photochemistry Research Progress; Sánchez, A., Gutierrez, S.J., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 447–471. ISBN 9781604562323. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Rashkov, G.D.; Ivanova, K.; Markovska, Y.; Apostolova, E.L. Effects of salinity on the photosynthetic apparatus of two Paulownia lines. Plant Physiol. Biochem. 2016, 101, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, C.R.; Virzo De Santo, A.; D’Ambrosio, N. A revised energy partitioning approach to assess the yields of non-photochemical quenching components. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Jinhang, S. The Analysis and Research of Clustering Algorithm Based on PCA. In Proceedings of the 2017 13th IEEE International Conference on Electronic Measurement & Instruments (ICEMI), Yangzhou, China, 20–22 October 2017; pp. 361–365. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).