Alternative Plant Protection Strategies Using Bacteria and Thyme to Improve Strawberry (cv. Elsanta) Yield and Quality
Abstract
1. Introduction
2. Results and Discussion
2.1. Plant Vegetative and Generative Development
2.2. Yield
2.3. Average Fruit Weight and Size
2.4. Strawberry Fruit Firmness
2.5. Soluble Solids
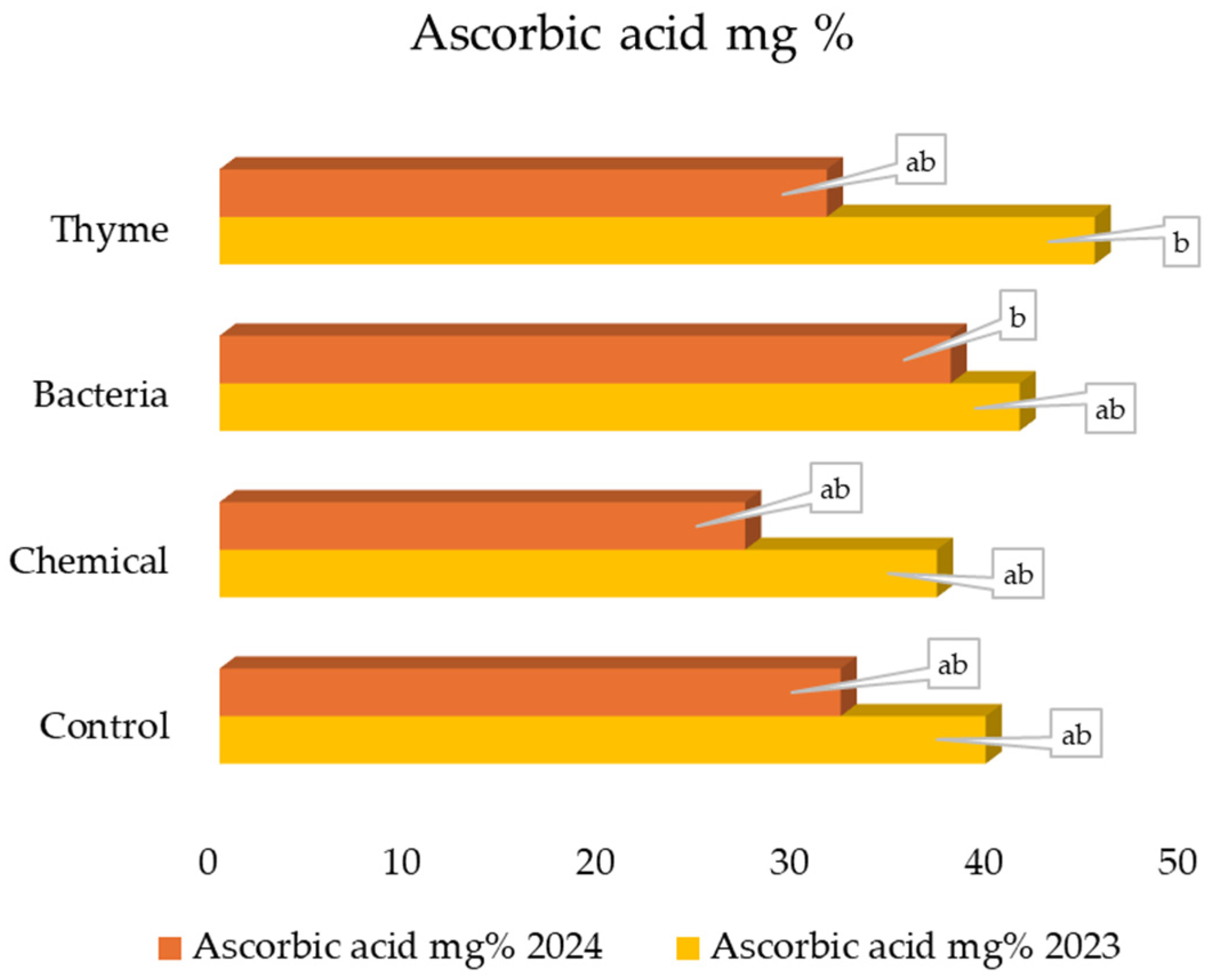
2.6. Content of Ascorbic Acid
3. Materials and Methods
3.1. Plant Material
3.2. Measurements
3.3. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oguntibeju, O.O.; Truter, E.J.; Esterhuyse, A.J. The Role of Fruit and Vegetable Consumption in Human Health and Disease Prevention. In Diabetes Mellitus—Insights and Perspectives; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef][Green Version]
- Madhavi, B.G.K.; Kim, N.E.; Basak, J.K.; Choi, G.M.; Kim, H.T. Comparative study of strawberry growth and fruit quality parameters in horizontal and vertical production systems. Hortic. Environ. Biotechnol. 2023, 64, 409–419. [Google Scholar] [CrossRef]
- Uddin, A.F.M.J.; Nasif, F.M.; Kaynat, B.; Maliha, M.; Rakibuzzaman, M. Organic Bio-stimulator Application on Growth and Yield of Strawberry. Int. J. Bus. Soc. Sci. Res. 2021, 9, 1–5. Available online: https://www.ijbssr.com/journal/details/organic-bio-stimulator-application-on-growth-and-yield-of-strawberry-14013420 (accessed on 25 April 2025).
- Elad, Y.; Vivier, M.; Fillinger, S. Botrytis, the Good, thew Bad and the Ugly. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–15. [Google Scholar]
- Elad, Y.; Williamson, B.; Ttudzynski, P.; Delen, N. Botrytis: Biology, Pathology and Control. Springer International Publishing: Cham, Switzerland, 2007; p. 403. [Google Scholar]
- Elad, Y.; Pertot, I.; Prado, A.M.C.; Stewart, A. Plant hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016a; pp. 413–486. [Google Scholar]
- Staats, M.; Baarlen, P.; Kan, J.A.L. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Mol. Biol. Evol. 2005, 22, 333–346. [Google Scholar] [CrossRef]
- Garrett, K.A.; Nita, M.; Wolf, E.D.; Esker, P.D.; Gomez-Montano, L.; Sparks, A.H. Chapter 21—Plant pathogens as indicators of climate change. In Climate Change: Observed Impacts on Planet Earth, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 325–338. [Google Scholar]
- Park, J.Y.; Kim, S.H.; Kim, N.H.; Lee, S.W.; Jeun, Y.C.; Hong, J.K. Differential inhibitory activities of four plant essential oils on in vitro growth of Fusarium oxysporum f. sp. fragariae causing Fusarium wilt in strawberry plants. Plant Pathol. J. 2017, 33, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguado, A.; Pastrana, A.M.; Santos, B.; Romero, F.; Sánchez, M.C.; Capote, N. The efficiency of natural products for the Control of Colletotrichum acutatum monitored by real-time PCR. Acta Hortic. 2014, 1049, 329–334. [Google Scholar] [CrossRef]
- Hong, J.K.; Yang, H.I.; Jung, H.; Yoon, D.J.; Sang, M.K.; Jeun, Y.C. Application of volatile antifungal plant essential oils for controlling pepper fruit anthracnose by Colletotrichum gloeosporioides. Plant Pathol. J. 2015, 31, 269–277. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. Application of Plant Extracts to Control Postharvest Gray Mold and Susceptibility of Apple Fruits to B. cinerea from Different Plant Hosts. Foods 2020, 9, 1430. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The extracts of cinnamon and clove as potential biofungicides against strawberry grey mould. Plants 2020, 9, 613. [Google Scholar] [CrossRef]
- Naeini, A.; Ziglari, T.; Shokri, H.; Khosravi, A.R. Assessment of growth inhibiting effect of some plant essential oils on different Fusarium isolates. J. Med. Mycol. 2010, 20, 174–178. [Google Scholar] [CrossRef]
- Lopez, N.L.; Gutiérrez-Grijalva, E.P.; Vazquez-Oliva, G.; Heredia, J.B. Essential oils of Oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Palfi, M.; Konjevoda, P.; Vrandečić, K. Antifungal activity of essential oils on mycelial growth of Fusarium oxysporum and Botrytis cinerea. Emir. J. Food Agric. 2019, 31, 544–554. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × Piperita L. (peppermint) essential oils. J. King Saud Univ.-Sci. 2017, 31, 528–533. [Google Scholar] [CrossRef]
- Santoro, K.; Maghenzani, M.; Chiabrando, V.; Bosio, P.; Gullino, M.L.; Spadaro, D.; Giacalone, G. Thyme and savory essential oil vapor treatments control brown rot and improve the storage quality of peaches and nectarines, but could favor gray mold. Foods 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, T.; Kim, C.; Rahemi, A. Role of natural volatiles and essential oils in extending shelf life and controlling postharvest microorganisms of small fruits. Microorganisms 2018, 6, 104. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Grahovac, M.S.; Stanković, J.M.; Cvetković, M.T.; Maširević, S.N. Essential oil composition of different coriander (Coriandrum sativum L.) accessions and their influence on mycelial growth of Colletotrichum spp. Acta Sci. Pol. Hortorum Cultus 2016, 15, 35–44. [Google Scholar]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M.; Kang, S.M.; Yun, B.W.; Lee, I.J. Biocontrol Potential of Bacillus amyloliquefaciens against Botrytis pelargonii and Alternaria alternata on Capsicum annuum. J. Fungi 2021, 7, 472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hrustić, J.; Medić, O.; Berić, T.; Mihajlović, M.; Milijašević-Marčić, S.; Stanković, S.; Pešić, B. Suppression of Monilinia Brown Rot by Bacillus spp. Strains. Agron. 2023, 13, 2839. [Google Scholar] [CrossRef]
- Olimi, E.; Kusstatscher, P.; Wicaksono, W.A.; Abdelfattah, A.; Cernava, T.; Berg, G. Insights into the microbiome assembly during different growth stages and storage of strawberry plants. Environ. Microbiome 2022, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of plant diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 102048. [Google Scholar] [CrossRef]
- Sessitsch, A.; Mitter, B. 21st century agriculture: Integration of plant microbiomes for improved crop production and food security. Microb. Biotechnol. 2015, 8, 32–33. [Google Scholar] [CrossRef]
- Yahya, G.; Ebada, A.; Khalaf, E.M.; Mansour, B.; Nouh, N.A.; Mosbah, R.A.; Saber, S.; Moustafa, M.; Negm, S.; El-Sokkary, M.M.A.; et al. Soil-Associated Bacillus Species: A Reservoir of Bioactive Compounds with Potential Therapeutic Activity against Human Pathogens. Microorganisms 2021, 9, 1131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dėnė, L.; Laužikė, K.; Juškevičienė, D.; Valiuškaitė, A.; Karklelienė, R. Initial Physiological, Biochemical and Elemental Garlic (Allium sativum L.) Clove Responses to T. vulgaris and S. aromaticum Extract Application. Horticulturae 2024, 10, 99. [Google Scholar]
- Dėnė, L.; Laužikė, K.; Rasiukevičiūtė, N.; Chrapačienė, S.; Brazaitytė, A.; Viršilė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Sutulienė, R.; Samuolienė, G.; et al. Defense response of strawberry plants against Botrytis cinerea influenced by coriander extract and essential oil. Front. Plant Sci. 2023, 13, 1098048. [Google Scholar] [CrossRef] [PubMed]
- Chrapačienė, S.; Rasiukevičiūtė, N.; Valiuškaitė, A. Control of seed-borne fungi by selected essential oils. Horticulturae 2022, 8, 220. [Google Scholar] [CrossRef]
- Hammock, H.A. The Impact of Blue and Red LED Lighting on Biomass Accumulation, Flavor Volatile Production, and Nutrient Uptake in Hydroponically Grown Genovese Basil. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2018. [Google Scholar]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef]
- Shala, A.Y.; Hameed, R.S.A.E.; Abd-ELbary, T.S. Essential Oils on Strawberry Growth and Fruit Quality. J. Sustain. Agric. Sci. 2024, 50, 13–23. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Pertot, I.; Zasso, R.; Amsalem, L.; Baldessari, M.; Angeli, G.; Elad, Y. Integrating biocontrol agents in strawberry powdery mildew control strategies in high tunnel growing systems. Crop Prot. 2008, 27, 622–631. [Google Scholar] [CrossRef]
- Chong, C.; Wei, Y.; Fan, T.; Zhang, W.; Zhang, G.; Liu, Q.; Liu, H.; Wang, J.; Zhang, L. Bio-control bacillus strain TS02 for Control of strawberry powdery mildew and its molecular identification. Acta Hortic. 2014, 1049, 607–611. [Google Scholar] [CrossRef]
- Es-Soufi, R.; Tahiri, H.; Azaroual, L.; El Oualkadi, A.; Martin, P.; Badoc, A.; Lamarti, A. Biocontrol potential of Bacillus amyloliquefaciens Bc2 and Trichoderma harzianum TR against strawberry anthracnose under laboratory and field conditions. Agric. Sci. 2020, 11, 260–277. [Google Scholar]
- Sangiorgio, D.; Cellini, A.; Spinelli, F.; Donati, I. Promoting Strawberry (Fragaria × ananassa) Stress Resistance, Growth, and Yield Using Native Bacterial Biostimulants. Agronomy 2023, 13, 529. [Google Scholar] [CrossRef]
- Hong, S.; Kim, T.Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of Fungal Diseases and Fruit Yield Improvement of Strawberry Using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Xie, Z.; Liu, Y.; You, Y.; Zhang, X.; Sun, Z.; Li, W.; Li, Y.; et al. The impact of the postharvest environment on the viability and virulence of decay fungi. Crit. Rev. Food Sci. Nutr. 2017, 58, 1681–1687. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against botrytis cinerea causing postharvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Islam, M.T.; Hashidoko, Y.; Deora, A.; Ito, T.; Tahara, S. Suppression of damping-of disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne peronosporomycetes. Appl. Environ. Microbiol. 2005, 71, 3786–3796. [Google Scholar] [CrossRef]
- Singh, L.; Sadawarti, R.K.; Singh, S.K.; Shaifali; Mirza, A.A. Efficacy of Plant Growth Regulators for the Modulation in the Productivity of Strawberries (Fragaria × ananassa Duchesne). J. Plant Growth Regul. 2025, 44, 1072–1086. [Google Scholar] [CrossRef]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on postharvest shelf life of strawberries. LWT-Food. Sci. Technol. 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Ariza, M.T.; Miranda, L.; Gómez-Mora, J.A.; Medina, J.J.; Lozano, D.; Gavilán, P.; Soria, C.; Martínez-Ferri, E. Yield and Fruit Quality of Strawberry Cultivars under Different Irrigation Regimes. Agronomy. 2021, 11, 261. [Google Scholar] [CrossRef]
- Bahmani, R.; Razavi, F.; Mortazavi, S.N.; Gohari, G.; Juárez-Maldonado, A. Evaluation of Proline-Coated Chitosan Nanoparticles on Decay Control and Quality Preservation of Strawberry Fruit (cv. Camarosa) during Cold Storage. Horticulturae 2022, 8, 648. [Google Scholar]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Alam Khan, M.; Mohi-Ud-Din, M.; Miah, G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Mazeikiene, I.; Frercks, B.; Kurgonaite, M.; Rasiukeviciute, N.; Macioniene, I. Genomic insights into Vaccinium spp. endophytes B. halotolerans and B. velezensis and their antimicrobial potential. Int. J. Mol. Sci. 2025, under review. [Google Scholar]
- EPPO. Botryotinia fuckeliana on strawberries. EPPO Bull. 2015, 45, 333–335. [Google Scholar] [CrossRef]
- EPPO. Regulation of growth in strawberry. EPPO Bull. 2009, 39, 254–256. [Google Scholar] [CrossRef]
- Luksiene, Z.; Rasiukeviciute, N.; Zudyte, B.; Uselis, N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 203, 111656. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis. Vitamin C (ascorbic acid) in vitamin preparations and juices. In Official Analytical Chemists, 15th ed.; Helrich, K., Ed.; AOAC: Gaithersburg, MD, USA, 1990. [Google Scholar]

| Crowns | Leaves | Inflorescences | Flowers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2023 | |||||||||
| Control | 2.40 | ±0.31 | 13.35 | ±1.36 | 3.65 | ±0.51 | 25.40 | ±4.49 | a |
| Chemical | 2.55 | ±0.23 | 15.35 | ±1.52 | 3.95 | ±0.57 | 31.65 | ±5.96 | ab |
| Bacteria | 2.70 | ±0.21 | 14.20 | ±1.18 | 4.15 | ±0.50 | 33.30 | ±4.37 | b |
| Thyme | 2.45 | ±0.32 | 12.70 | ±1.35 | 3.85 | ±0.60 | 27.70 | ±3.84 | ab |
| 2024 | |||||||||
| Control | 4.25 | ±0.74 | 16.90 | ±2.34 | 5.90 | ±0.19 | 50.60 | ±2.57 | a |
| Chemical | 4.20 | ±0.90 | 18.20 | ±1.68 | 6.05 | ±0.64 | 57.90 | ±3.32 | ab |
| Bacteria | 4.55 | ±0.70 | 21.55 | ±2.43 | 6.90 | ±0.50 | 64.35 | ±4.97 | b |
| Thyme | 4.15 | ±0.64 | 17.50 | ±3.31 | 7.00 | ±0.93 | 58.50 | ±2.00 | ab |
| ns | ns | ns | |||||||
| Treatment | First Picking | Second Picking | Third Picking | Total | |||
|---|---|---|---|---|---|---|---|
| Healthy | Rotten | Healthy Rotten | Healthy | Rotten | |||
| 2023 | |||||||
| Control | 2525.00 ab | 0.00 ± 0.00 | 2875.00 ab | 9.74 ± 1.74 | 2430.00 ab | 0.00 ± 0.00 | 7839.74 |
| Chemical | 1723.00 a | 0.00 ± 0.00 | 2930.00 ab | 11.98 ± 3.00 | 2600.00 ab | 0.00 ± 0.00 | 7264.98 |
| Bacteria | 3395.00 bc | 0.00 ± 0.00 | 3305.00 bc | 13.57 ± 3.39 | 2870.00 b | 0.00 ± 0.00 | 9583.57 |
| Thyme | 2665.00 ab | 0.00 ± 0.00 | 2650.00 ab | 8.85 ± 2.21 | 2205.00 ab | 0.00 ± 0.00 | 7528.85 |
| 2024 | |||||||
| Control | 2056.00 a | 0.00 ± 0.00 | 5515.00 ab | 70.00 ± 2.00 | 7630.00 ab | 70.00 ± 1.00 | 14,634.14 |
| Chemical | 4345.00 bc | 0.00 ± 0.00 | 5250.00 ab | 0.00 ± 0.00 | 7100.00 ab | 20.00 ± 0.10 | 16,695.02 |
| Bacteria | 3300.00 ab | 10.00 ± 0.10 | 5650.00 ab | 0.00 ± 0.00 | 8844.00 b | 30.00 ± 0.10 | 17,794.04 |
| Thyme | 4120.00 bc | 0.00 ± 0.0 | 6310.00 b | 70.00 ± 1.00 | 7710.00 ab | 170.00 ± 20.00 | 18,140.23 |
| Treatment | First Picking | Second Picking | Third Picking |
|---|---|---|---|
| 2023 | |||
| Control | 6.76 ± 0.71 | 6.73 ± 0.32 | 5.15 ± 0.53 a |
| Chemical | 7.39 ± 0.45 | 8.28 ± 0.37 | 7.14 ± 0.42 b |
| Bacteria | 8.19 ± 0.28 | 7.35 ± 0.28 | 6.60 ± 0.34 ab |
| Thyme | 7.05 ± 0.53 | 6.34 ± 1.01 | 5.99 ± 0.44 ab |
| ns | ns | ||
| 2024 | |||
| Control | 11.44 ± 0.72 | 9.78 ± 0.43 a | 6.36 ± 0.30 |
| Chemical | 12.97 ± 0.88 | 10.42 ± 0.69 a | 6.68 ± 0.30 |
| Bacteria | 13.93 ± 1.00 | 10.72 ± 1.07 a | 7.29 ± 0.52 |
| Thyme | 12.60 ± 1.18 | 15.53 ± 4.83 b | 6.40 ± 0.65 |
| ns | ns | ||
| Treatment | First Picking | Second Picking | Third Picking |
|---|---|---|---|
| 2023 | |||
| Control | 23.78 ± 1.58 a | 25.05 ± 0.59 | 24.05 ± 0.19 |
| Chemical | 25.68 ± 0.56 ab | 30.03 ± 1.88 | 24.75 ± 0.78 |
| Bacteria | 26.75 ± 0.72 b | 29.78 ± 1.13 | 24.35 ± 0.54 |
| Thyme | 25.30 ± 0.74 ab | 24.53 ± 2.32 | 23.30 ± 0.52 |
| ns | ns | ||
| 2024 | |||
| Control | 29.78 ± 1.18 | 28.55 ± 0.75 | 24.70 ± 0.83 |
| Chemical | 31.43 ± 1.43 | 30.18 ± 0.79 | 23.50 ± 0.47 |
| Bacteria | 33.53 ± 1.78 | 33.93 ± 3.09 | 24.75 ± 1.26 |
| Thyme | 30.65 ± 1.81 | 31.30 ± 0.94 | 24.55 ± 1.08 |
| ns | ns | ns | |
| Treatment | First Picking | Second Picking | Third Picking |
|---|---|---|---|
| 2023 | |||
| Control | 8.44 ± 0.71 | 11.98 ± 0.74 | 11.22 ± 0.27 ab |
| Chemical | 8.46 ± 0.57 | 12.07 ± 0.47 | 12.81 ± 0.45 ab |
| Bacteria | 9.84 ± 0.62 | 11.44 ± 1.00 | 11.36 ± 0.43 ab |
| Thyme | 8.34 ± 0.49 | 11.43 ± 0.74 | 10.92 ± 0.60 a |
| ns | ns | ||
| 2024 | |||
| Control | 10.49 ± 0.58 | 11.64 ± 0.56 | 15.31 ± 1.32 b |
| Chemical | 10.61 ± 1.62 | 12.14 ± 0.80 | 12.58 ± 0.51 a |
| Bacteria | 11.23 ± 0.21 | 10.59 ± 0.88 | 11.33 ± 0.55 a |
| Thyme | 10.29 ± 0.49 | 11.64 ± 0.62 | 11.22 ± 0.54 a |
| ns | ns | ||
| Treatment | First Picking | Second Picking | Third Picking |
|---|---|---|---|
| 2023 | |||
| Control | 9.40 ± 0.20 | 11.25 ± 0.24 | 11.03 ± 0.58 b |
| Chemical | 9.23 ± 0.13 | 10.78 ± 0.43 | 9.50 ± 0.44 a |
| Bacterial | 8.45 ± 0.39 | 10.78 ± 0.27 | 11.08 ± 0.41 b |
| Thyme | 9.40 ± 0.25 | 10.43 ± 0.40 | 11.40 ± 0.37 b |
| ns | ns | ||
| 2024 | |||
| Control | 6.69 ± 0.26 a | 8.11 ± 0.36 | 7.48 ± 0.46 |
| Chemical | 7.36 ± 0.57 ab | 6.75 ± 0.46 | 8.39 ± 0.67 |
| Bacterial | 7.99± 0.51 b | 7.05 ± 0.64 | 7.44 ± 0.95 |
| Thyme | 7.53 ± 0.52 ab | 6.94 ± 0.60 | 6.68 ± 0.27 |
| ns | ns | ||
| Treatment | Composition | Dose |
|---|---|---|
| Control | None | None, not treated |
| Chemical | Boscalid 267 g l−1 + pyraclostrobin 37 g L−1 | 1.8 kg ha−1 |
| Ciprodinil 375 g l−1 + fludioksonil 250 g L−1 | 1.0 kg ha−1 | |
| Bacteria * | B. halotolerans Bil-LT1_1 B. halotolerans Bil-LT1_2 B. velezensis Cran-LT_1_8 B. velezensis Ling-NOR_4_15 | 3.3 × 10−6 |
| Thyme | Thymus vulgaris | 600 µL/L |
| Essential Oils | Thymus vulgaris | |
|---|---|---|
| Component | PA 1 (%) | RT 2 |
| α-thujene | 0.54 | 5.293 |
| α-pinene | 0.83 | 5.449 |
| Camphene | 0.37 | 5.800 |
| β-pinene | 0.22 | 6.501 |
| 1-octen-3-ol | 1.57 | 6.677 |
| Myrcene | 1.79 | 6.895 |
| 3-octanol | 0.20 | 7.119 |
| α-phellandrene | 0.25 | 7.241 |
| α-terpinene | 1.59 | 7.572 |
| p-cymene | 12.37 | 7.865 |
| Limonene | 0.28 | 7.935 |
| Eucalyptol | 1.72 | 7.984 |
| γ-terpinene | 8.39 | 8.798 |
| cis-sabinene hydrate | 1.04 | 9.036 |
| Linalool | 4.26 | 10.010 |
| Camphor | 0.46 | 11.442 |
| Borneol | 1.24 | 11.913 |
| Ε-caryophyllene | 1.16 | 19.000 |
| Terpinen-4-ol | 1.39 | 12.233 |
| α-terpineol | 0.31 | 12.900 |
| Thymol methyl ether | 0.52 | 13.842 |
| Thymol | 52.22 | 16.045 |
| Carvacyl methy ether | 0.58 | 14.110 |
| Carvacrol | 3.38 | 16.162 |
| Caryophyllene oxide | 0.68 | 23.179 |
| Other 3 | 2.64 | |
| Total Identified | 100.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasiukevičiūtė, N.; Morkeliūnė, A.; Mažeikienė, I.; Lanauskas, J.; Valiuškaitė, A. Alternative Plant Protection Strategies Using Bacteria and Thyme to Improve Strawberry (cv. Elsanta) Yield and Quality. Plants 2025, 14, 1827. https://doi.org/10.3390/plants14121827
Rasiukevičiūtė N, Morkeliūnė A, Mažeikienė I, Lanauskas J, Valiuškaitė A. Alternative Plant Protection Strategies Using Bacteria and Thyme to Improve Strawberry (cv. Elsanta) Yield and Quality. Plants. 2025; 14(12):1827. https://doi.org/10.3390/plants14121827
Chicago/Turabian StyleRasiukevičiūtė, Neringa, Armina Morkeliūnė, Ingrida Mažeikienė, Juozas Lanauskas, and Alma Valiuškaitė. 2025. "Alternative Plant Protection Strategies Using Bacteria and Thyme to Improve Strawberry (cv. Elsanta) Yield and Quality" Plants 14, no. 12: 1827. https://doi.org/10.3390/plants14121827
APA StyleRasiukevičiūtė, N., Morkeliūnė, A., Mažeikienė, I., Lanauskas, J., & Valiuškaitė, A. (2025). Alternative Plant Protection Strategies Using Bacteria and Thyme to Improve Strawberry (cv. Elsanta) Yield and Quality. Plants, 14(12), 1827. https://doi.org/10.3390/plants14121827








