Developmental and Temperature-Driven Variations in Metabolic Profile and Antioxidant Capacity of Broccoli (Brassica oleracea var. cymosa)
Abstract
1. Introduction
2. Results and Discussion
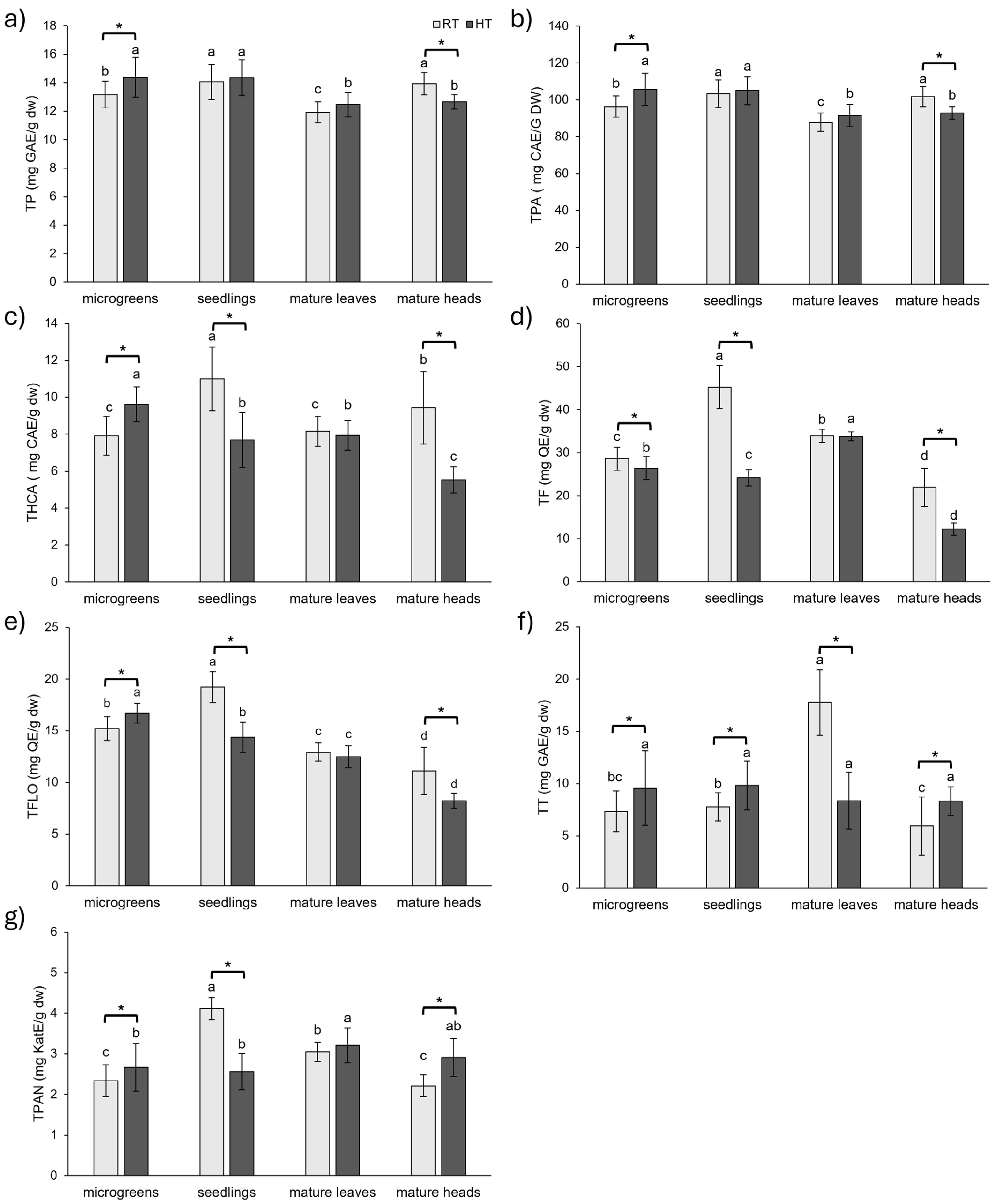
2.1. Polyphenolics in Broccoli Throughout Development and in Response to HT
2.1.1. Groups of Phenolic Compounds
2.1.2. Individual Phenolic Compounds
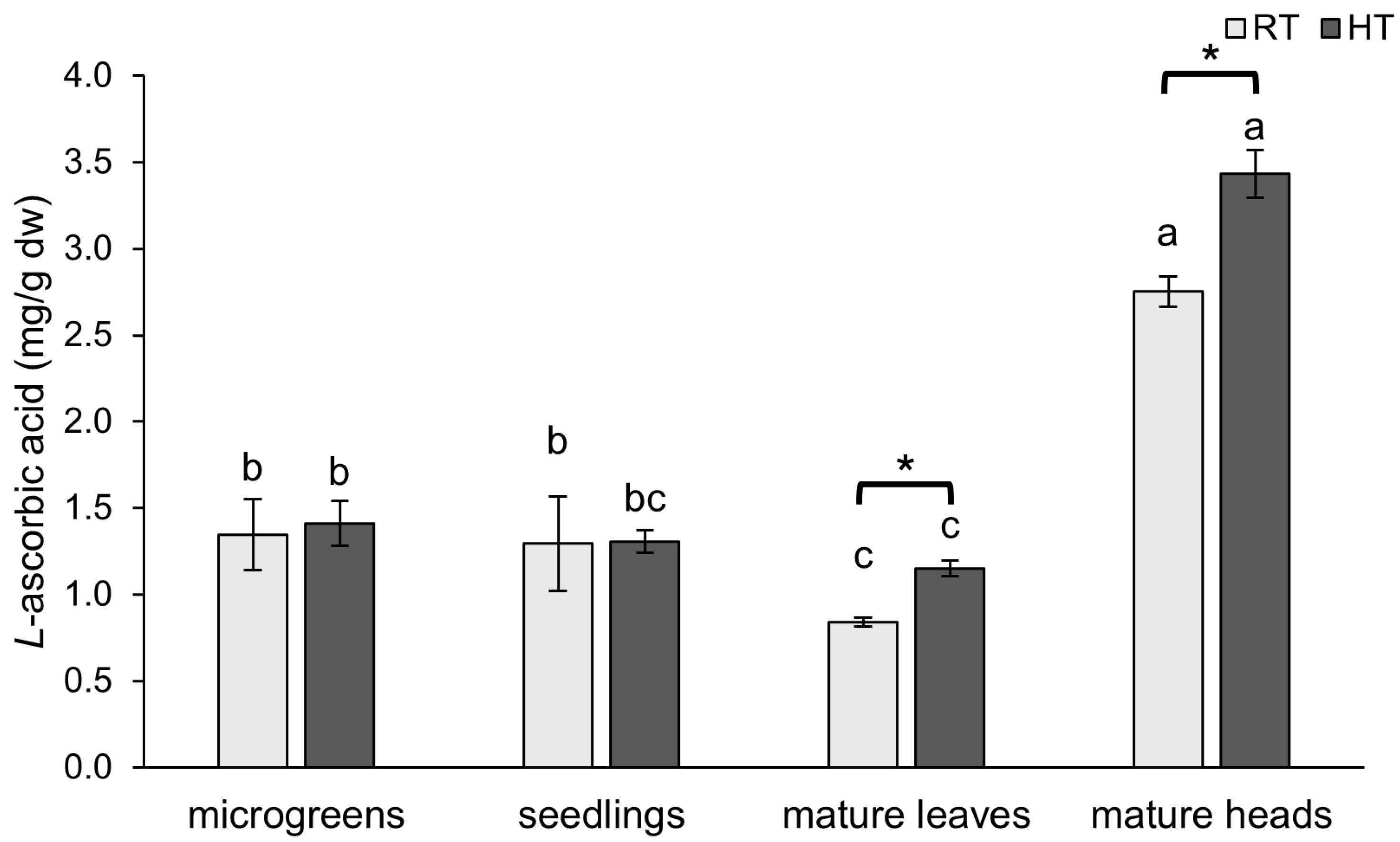
2.2. L-Ascorbic Acid
2.3. Soluble Sugars
2.4. Nitrogen-Containing Phytochemicals
2.5. Photosynthetic Pigments
2.6. Oxidative Stress Parameters
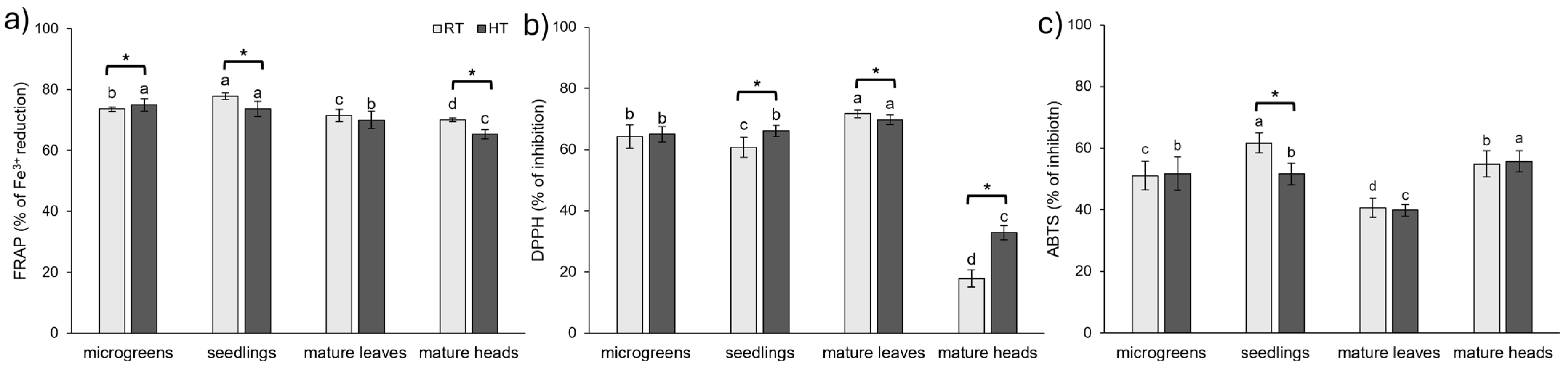
2.7. Antioxidant Capacity
2.8. Statistical Analysis
2.8.1. Two-Way Factorial ANOVA
2.8.2. Hierarchical Clustering
2.8.3. Principal Component Analysis
2.8.4. Pearson’s Correlation Coefficient
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Phytochemicals
3.3. Measurement of Different Groups of Polyphenolic Compounds
3.4. Measurement of Individual Polyphenolic Compounds and L-Ascorbic Acid
3.5. Measurement of Soluble Sugars
3.6. Measurement of Nitrogen-Containing Phytochemicals
3.7. Measurement of Photosynthetic Pigments
3.8. Measurement of Oxidative Stress Parameters
3.9. Measurement of Antioxidant Capacity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ANOVA | analysis of variance |
| BSAE | bovine serum albumin equivalent |
| CAE | caffeic acid equivalent |
| Car | carotenoid |
| Chl | chlorophyll |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| dw | dry weight |
| Fer ac | ferulic acid |
| FRAP | ferric ion-reducing antioxidant power |
| GAE | gallic acid equivalent |
| GLS | total intact glucosinolates |
| HC | hierarchical clustering |
| HT | high temperature |
| K | kaempferol |
| KatE | catechin equivalent |
| L-asc | L-ascorbic acid |
| PCA | principal component analysis |
| Porf | porphyrins |
| PROL | proline |
| PROT | soluble proteins |
| Q | quercetin |
| QE | quercetin equivalent |
| ROS | reactive oxygen species |
| RT | room temperature |
| Sin ac | sinapic acid |
| SINE | sinigrin equivalent |
| SS | soluble sugars |
| SucE | sucrose equivalent |
| TF | total flavonoids |
| TFLO | total flavonols |
| THCA | total hydroxycinnamic acids |
| TP | total phenolics |
| TPA | total phenolic acids |
| TPAN | total proanthocyanidins |
| TT | total tannins |
References
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef] [PubMed]
- World Meteorological Organization. WMO Global Annual to Decadal Climate Update; WMO: Geneva, Switzerland, 2024. [Google Scholar]
- Klingelhöfer, D.; Braun, M.; Brüggmann, D.; Groneberg, D.A. Heatwaves: Does global research reflect the growing threat in the light of climate change? Global Health 2023, 19, 56. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.E.; McElrone, A.J.; Ostoja, S.M.; Forrestel, E.J. Extreme heat effects on perennial crops and strategies for sustaining future production. Plant Sci. 2020, 295, 110397. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Tewari, S.; Mishra, A. Flooding stress in plants and approaches to overcome. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 355–366. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and postharvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Toro, M.-T.; Fustos-Toribio, R.; Ortiz, J.; Becerra, J.; Zapata, N.; López-Belchí, M.D. Antioxidant responses and phytochemical accumulation in Raphanus species sprouts through elicitors and predictive models under high temperature stress. Antioxidants 2024, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.A.S.; Heaney, R.K.; Portas, C.A.M.; Fenwick, G.R. Changes in glucosinolate concentrations in Brassica crops (B. oleracea and B. napus) throughout growing seasons. J. Sci. Food Agric. 1996, 71, 237–244. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Šola, I.; Vujčić Bok, V.; Dujmović, M.; Rusak, G. Developmentally-related changes in phenolic and L-ascorbic acid content and antioxidant capacity of Chinese cabbage sprouts. J. Food Sci. Technol. 2019, 57, 702–712. [Google Scholar] [CrossRef]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- De la Fuente, B.; López-García, G.; Mañez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the bioaccessibility of antioxidant bioactive compounds and minerals of four genotypes of Brassicaceae microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef]
- Pereira, F.M.V.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J. Agric. Food. Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef]
- Lin, H.; Sun, J.; Hu, Z.; Cheng, C.; Lin, S.; Zou, H.; Yan, X. Variation in glucosinolate accumulation among different sprout and seedling stages of broccoli (Brassica oleracea var. italica). Plants 2022, 11, 1563. [Google Scholar] [CrossRef]
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric characteristics, polyphenols and ascorbic acid variation in Brassica oleracea L. novel foods: Sprouts, microgreens and baby leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Du, Y.Y.; Tan, W.K.; Zou, L.; Lei, J.J.; Ong, C.N. New insights into the phenolic constituents and their relationships with antioxidant capacity during the growth of a commonly consumed Asian vegetable, Brassica rapa var. parachinensis (choy sum). Food Chem. Adv. 2022, 1, 100038. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food. Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Dietary factors, hormesis and health. Ageing Res. Rev. 2008, 7, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, L.; Picchi, V.; Tribulato, A.; Cavallaro, C.; Lo Scalzo, R.; Branca, F. The effect of the germination temperature on the phytochemical content of broccoli and rocket sprouts. Int. J. Food Sci. Nutr. 2016, 68, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Soengas, P.; Rodríguez, V.M.; Velasco, P.; Cartea, M.E. Effect of temperature stress on antioxidant defenses in Brassica oleracea. ACS Omega 2018, 3, 5237–5243. [Google Scholar] [CrossRef]
- Kisiel, A.; Krzemińska, A.; Cembrowska-Lech, D.; Miller, T. Data science and plant metabolomics. Metabolites 2023, 13, 454. [Google Scholar] [CrossRef]
- Bashir, K.; Todaka, D.; Sako, K.; Ueda, M.; Aziz, F.; Seki, M. Chemical application improves stress resilience in plants. Plant Mol. Biol. 2025, 115, 47. [Google Scholar] [CrossRef]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology—Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; Intech Open Ltd.: London, UK, 2022. [Google Scholar]
- Šola, I.; Stić, P.; Rusak, G. Effect of flooding and drought on the content of phenolics, sugars, photosynthetic pigments and vitamin C, and antioxidant potential of young Chinese cabbage. Eur. Food Res. Technol. 2021, 247, 1913–1920. [Google Scholar] [CrossRef]
- Šola, I.; Gmižić, D.; Miškec, K.; Ludwig-Müller, J. Impact of water stress on metabolic intermediates and regulators in broccoli sprouts, and cellular defense potential of their extracts. Int. J. Mol. Sci. 2025, 26, 632. [Google Scholar] [CrossRef]
- Šola, I.; Gmižić, D. Structural variations of broccoli polyphenolics and their antioxidant capacity as a function of growing temperature. Plants 2025, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Alhaithloul, H.A.S.; Galal, F.H.; Seufi, A.M. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L.). PeerJ 2021, 9, e11193. [Google Scholar] [CrossRef] [PubMed]
- López-Cervantes, J.; Tirado-Noriega, L.G.; Sánchez-Machado, D.I.; Campas-Baypoli, O.N.; Cantú-Soto, E.U.; Núñez-Gastélum, J.A. Biochemical composition of broccoli seeds and sprouts at different stages of seedling development. Int. J. Food Sci. Technol. 2013, 48, 2267–2275. [Google Scholar] [CrossRef]
- Mølmann, J.A.B.; Steindal, A.L.H.; Bengtsson, G.B.; Seljåsen, R.; Lea, P.; Skaret, J.; Johansen, T.J. Effects of temperature and photoperiod on sensory quality and contents of glucosinolates, flavonols and vitamin C in broccoli florets. Food Chem. 2015, 172, 47–55. [Google Scholar] [CrossRef]
- Gmižić, D.; Pinterić, M.; Lazarus, M.; Šola, I. High growing temperature changes nutritional value of broccoli (Brassica oleracea L. convar. botrytis (L.) Alef. var. cymosa Duch.) seedlings. Foods 2023, 12, 582. [Google Scholar] [CrossRef]
- Šola, I.; Gmižić, D.; Pinterić, M.; Tot, A.; Ludwig-Müller, J. Adjustments of the phytochemical profile of broccoli to low and high growing temperatures: Implications for the bioactivity of its extracts. Int. J. Mol. Sci. 2024, 25, 3677. [Google Scholar] [CrossRef]
- Arikan, B.; Yildiztugay, E.; Ozfidan-Konakci, C. Protective role of quercetin and kaempferol against oxidative damage and photosynthesis inhibition in wheat chloroplasts under arsenic stress. Physiol. Plant 2023, 175, e13964. [Google Scholar] [CrossRef]
- Syed, R.U.; Moni, S.S.; Break, M.K.B.; Khojali, W.M.A.; Jafar, M.; Alshammari, M.D.; Abdelsalam, K.; Taymour, S.; Alreshidi, K.S.M.; Elhassan Taha, M.M.; et al. Broccoli: A multi-faceted vegetable for health: An in-depth review of its nutritional atributes, antimicrobial abilities, and anti-inflammatory properties. Antibiotics 2023, 12, 1157. [Google Scholar] [CrossRef]
- Hernández, V.; Hellín, P.; Fenoll, J.; Molina, M.V.; Garrido, I.; Flores, P. Impact of high temperature stress on ascorbic acid concentration in tomato. Acta Hortic. 2018, 1194, 985–990. [Google Scholar] [CrossRef]
- Alayafi, A.A.M. Exogenous ascorbic acid induces systemic heat stress tolerance in tomato seedlings: Transcriptional regulation mechanism. Environ. Sci. Pollut. Res. 2019, 27, 19186–19199. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Tsukatani, T.; Murayama, K.; Kurata, Y.; Takeda, E.; Otsuka, T.; Takai, M.; Miyazaki, Y.; Tachibana, H.; Yamada, K. Determination of vitamin C, S-methylmethionine and polyphenol contents, and functional activities of different parts of broccoli (Brassica oleracea var. italica). Nippon Shokuhin Kagaku Kogaku Kaishi 2015, 62, 242–249. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Impact of temperature on phenolic and osmolyte contents in in vitro cultures and micropropagated plants of two mediterranean plant species, Lavandula viridis and Thymus lotocephalus. Plants 2022, 11, 3516. [Google Scholar] [CrossRef]
- Šola, I.; Davosir, D.; Kokić, E.; Zekirovski, J. Effect of hot- and cold-water treatment on broccoli bioactive compounds, oxidative stress parameters and biological effects of their extracts. Plants 2023, 12, 1135. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Y.; Yue, L.; Chen, G.; Yuan, L.; Zhang, S.; Li, F.; Zhang, H.; Li, G.; Zhu, S.; et al. Heat stress response in Chinese cabbage (Brassica rapa L.) revealed by transcriptome and physiological analysis. PeerJ 2022, 10, e13427. [Google Scholar] [CrossRef]
- Sihag, P.; Kumar, U.; Sagwal, V.; Kapoor, P.; Singh, Y.; Mehla, S.; Balyan, P.; Mir, R.R.; Varshney, R.K.; Singh, K.P.; et al. Effect of terminal heat stress on osmolyte accumulation and gene expression during grain filling in bread wheat (Triticum aestivum L.). Plant Genome 2024, 17, e20307. [Google Scholar] [CrossRef]
- Kourani, M.; Anastasiadi, M.; Hammond, J.P.; Mohareb, F. Prolonged heat stress in Brassica napus during flowering negatively impacts yield and alters glucosinolate and sugars metabolism. Front. Plant Sci. 2025, 16, 1507338. [Google Scholar] [CrossRef]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112–115. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; Sun, L.; Wang, X.-J.; Sun, R.; An, Y.-Q.; An, B.-L.; Zhu, M.-X.; Zhao, C.-F.; Bai, J.-G. Ferulic acid pretreatment alleviates heat stress in blueberry seedlings by inducing antioxidant enzymes, proline, and soluble sugars. Biol. Plant 2018, 62, 534–542. [Google Scholar] [CrossRef]
- Cho, L.-H.; Pasriga, R.; Yoon, J.; Jeon, J.-S.; An, G. Roles of sugars in controlling flowering time. J. Plant. Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Camejo, D.; Torres, W. High temperature effect on tomato (Lycopersicon esculentum) pigment and protein content and cellular viability. Cultiv. Trop. 2001, 22, 13–17. [Google Scholar]
- Yang, L.Y.; Yang, S.L.; Li, J.Y.; Ma, J.H.; Pang, T.; Zou, C.M.; He, B.; Gong, M. Effects of different growth temperatures on growth, development, and plastid pigments metabolism of tobacco (Nicotiana tabacum L.). Plants Bot. Stud. 2018, 59, 5. [Google Scholar] [CrossRef]
- Renseigné, N.; Umar, S.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Cantliffe, D.J. Nitrate accumulation in spinach grown at different temperatures. J. Am. Soc. Hortic. Sci. 1972, 97, 674–676. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Hunt, J. Effect of nitrogen fertiliser on the nitrate contents of field vegetables grown in Britain. J. Sci. Food Agric. 1986, 37, 373–383. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Soengas, P.; Cartea, E.; Sotelo, T.; Velasco, P. Suitability of a European nuclear collection of Brassica oleracea L. landraces to grow at high temperatures. J. Agr. Crop. Sci. 2013, 200, 183–190. [Google Scholar] [CrossRef]
- Rao, S.-Q.; Chen, X.-Q.; Wang, K.-H.; Zhu, Z.-J.; Yang, J.; Zhu, B. Effect of short-term high temperature on the accumulation of glucosinolates in Brassica rapa. Plant Physiol. Biochem. 2021, 161, 222–233. [Google Scholar] [CrossRef]
- Cui, L.; Li, J.; Fan, Y.; Xu, S.; Zhang, Z. High temperature effects on photosynthesis, PSII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility. Bot. Stud. 2006, 47, 61–69. [Google Scholar]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Xiang, X.; Fu, J.; Wang, X.; Zhou, Y.; Xing, W. Biosynthesis and extraction of chlorophyll, carotenoids, anthocyanins, and betalaine in vivo and in vitro. Curr. Issues Mol. Biol. 2024, 46, 10662–10676. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, M.; Abdullah, S.N.A.; Namasivayam, P. The impact of heat atress on morpho-physiological response and expression of specific genes in the heat stress-responsive transcriptional regulatory network in Brassica oleracea. Plants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Masood, A.; Yusuf, M.; Fariduddin, Q.; Ahmad, A. Growth of Indian mustard (Brassica juncea L.) in response to salicylic acid under high-temperature stress. Braz. J. Plant Physiol. 2009, 21, 187–195. [Google Scholar] [CrossRef]
- Sharma, P.; Lakra, N.; Ahlawat, Y.; Zaid, A.; Abd-ElGawad, A.M.; Elansary, H.O.; Gupta, A. Putrescine mitigates high temperature effects by modulating morpho-physiological and biochemical attributes in Brassica juncea seedlings. Agronomy 2023, 13, 1879. [Google Scholar] [CrossRef]
- Awais Ghani, M.; Mehran Abbas, M.; Ali, B.; Ziaf, K.; Azam, M.; Anjum, R.; Iqbal, Q.; Nadeem, M.; Noor, A.; Jillani, U. Role of salicylic acid in heat stress tolerance in tri-genomic Brassica napus L. Bioagro 2020, 33, 13–20. [Google Scholar] [CrossRef]
- Hou, W.; Sun, A.H.; Chen, H.L.; Yang, F.S.; Pan, J.L.; Guan, M.Y. Effects of chilling and high temperatures on photosynthesis and chlorophyll fluorescence in leaves of watermelon seedlings. Biol. Plant 2016, 60, 148–154. [Google Scholar] [CrossRef]
- Kalia, P.; Sharma, S.R. Current researches in hybrid broccoli. J. New Seeds 2005, 6, 109–134. [Google Scholar] [CrossRef]
- Kushwaha, A.; Das, A.; Dave, R.; Bhattacharya, B.K. A non-destructive estimation of chlorophyll-a and -b over different crops using airborne imaging spectroscopy observations. Adv. Space Res. 2024, 73, 1290–1303. [Google Scholar] [CrossRef]
- Fang, Z.; Bouwkamp, J.C.; Solomos, T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar]
- Chauhan, J.; Srivastava, J.P.; Singhal, R.K.; Soufan, W.; Dadarwal, B.K.; Mishra, U.N.; Anuragi, H.; Rahman, M.A.; Sakran, M.I.; Brestic, M.; et al. Alterations of oxidative stress indicators, antioxidant enzymes, soluble sugars, and amino acids in mustard [Brassica juncea (L.) Czern and Coss.] in response to varying sowing time, and field temperature. Front. Plant Sci. 2022, 13, 875009. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant defense system in plants: Reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Ho, C.-H.; Yang, M.-H.; Lin, H.-L. Effects of cultivation temperature on phenolic compound content and antioxidant capacity in the leaves and stems of the vegetable Gynura bicolor (Roxb. ex Willd.) DC. HortScience 2024, 59, 1828–1832. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Meghanathan, N. Assortativity analysis of real-world network graphs based on centrality metrics. Comput. Inf. Sci. 2016, 9, 7–25. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Czigle, S.; Filep, R.; Balažová, E.; Szentgyörgyi, H.; Balázs, V.L.; Kocsis, M.; Purger, D.; Papp, N.; Farkas, Á. Antioxidant capacity determination of Hungarian-, Slovak-, and Polish-origin goldenrod honeys. Plants 2022, 11, 792. [Google Scholar] [CrossRef]
- Howard, L.R.; Clear, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Sangeetha, R.; Vedasree, N. In vitro α-amylase inhibitory activity of the leaves of Thespesia populnea. ISRN Pharmacol. 2012, 2012, 515634. [Google Scholar] [CrossRef] [PubMed]
- Weidner, S.; Karolak, M.; Karamać, M.; Kosínska, A.; Amarowicz, R. Phenolic compounds and properties of antioxidants in grapevine roots (Vitis vinifera L.) under drought stress followed by recovery. Acta Soc. Bot. Pol. 2009, 78, 97–103. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.; Hawkesford, M.; De Kok, L.J. The significance of glucosinolates for sulfur storage in Brassicaceae seedlings. Front. Plant Sci. 2014, 5, 704. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Ljubej, V.; Karalija, E.; Salopek-Sondi, B.; Šamec, D.; Hanaka, A.; Jaroszuk, J.; Majewska, M.; Francini, A. Effects of short-term exposure to low temperatures on proline, pigments, and phytochemicals level in kale (Brassica oleracea var. acephala). Horticulturae 2021, 7, 341. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef]
- Poljuha, D.; Šola, I.; Bilić, J.; Dudaš, S.; Bilušić, T.; Markić, J.; Rusak, G. Phenolic composition, antioxidant capacity, energy content and gastrointestinal stability of Croatian wild edible plants. Eur. Food Res. Technol. 2015, 241, 573–585. [Google Scholar] [CrossRef]







| Developmental Stage | Temperature | Stage × Temperature | |
|---|---|---|---|
| TP | 0.31 *** | 0.01 | 0.13 * |
| TPA | 0.12 *** | 0.00 | 0.20 *** |
| THCA | 0.21 *** | 0.30 *** | 0.49 *** |
| TF | 0.82 *** | 0.64 *** | 0.70 *** |
| TFLO | 0.81 *** | 0.28 *** | 0.52 *** |
| TT | 0.07 * | 0.17 *** | 0.00 |
| TPAN | 0.47 *** | 0.01 | 0.57 *** |
| K | 0.95 *** | 0.80 *** | 0.76 *** |
| Q | 0.79 *** | 0.20 * | 0.39 ** |
| Sin ac | 0.80 *** | 0.07 | 0.10 |
| Fer ac | 0.95 *** | 0.05 | 0.62 *** |
| L-asc | 0.96 *** | 0.43 ** | 0.41 ** |
| SS | 0.64 *** | 0.73 *** | 0.56 *** |
| PROT | 0.80 *** | 0.02 | 0.50 *** |
| NO3− | 0.88 *** | 0.13 * | 0.67 *** |
| GLS | 0.75 *** | 0.08 * | 0.16 * |
| Chl a | 0.94 *** | 0.20 *** | 0.08 * |
| Chl b | 0.92 *** | 0.39 *** | 0.24 *** |
| Car | 0.94 *** | 0.09 * | 0.68 *** |
| Porf | 0.93 *** | 0.39 *** | 0.20 *** |
| Chl | 0.93 *** | 0.30 *** | 0.05 |
| Chl + Car | 0.93 *** | 0.26 *** | 0.04 |
| Chl a/b | 0.91 *** | 0.76 *** | 0.80 *** |
| Chl/Car | 0.79 *** | 0.56 *** | 0.60 *** |
| PROL | 0.96 *** | 0.92 *** | 0.86 *** |
| H2O2 | 0.67 *** | 0.33 *** | 0.47 *** |
| ABTS | 0.66 *** | 0.07 * | 0.29 *** |
| DPPH | 0.97 *** | 0.58 *** | 0.45 *** |
| FRAP | 0.74 *** | 0.27 *** | 0.35 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gmižić, D.; Šola, I. Developmental and Temperature-Driven Variations in Metabolic Profile and Antioxidant Capacity of Broccoli (Brassica oleracea var. cymosa). Plants 2025, 14, 1825. https://doi.org/10.3390/plants14121825
Gmižić D, Šola I. Developmental and Temperature-Driven Variations in Metabolic Profile and Antioxidant Capacity of Broccoli (Brassica oleracea var. cymosa). Plants. 2025; 14(12):1825. https://doi.org/10.3390/plants14121825
Chicago/Turabian StyleGmižić, Daria, and Ivana Šola. 2025. "Developmental and Temperature-Driven Variations in Metabolic Profile and Antioxidant Capacity of Broccoli (Brassica oleracea var. cymosa)" Plants 14, no. 12: 1825. https://doi.org/10.3390/plants14121825
APA StyleGmižić, D., & Šola, I. (2025). Developmental and Temperature-Driven Variations in Metabolic Profile and Antioxidant Capacity of Broccoli (Brassica oleracea var. cymosa). Plants, 14(12), 1825. https://doi.org/10.3390/plants14121825








