Phenolic Profile, Fatty Acid Composition, and Antioxidant Activity of Italian Riesling Grape Pomace from Two Transylvanian Microclimates
Abstract
1. Introduction
2. Results
2.1. Total Polyphenol Content
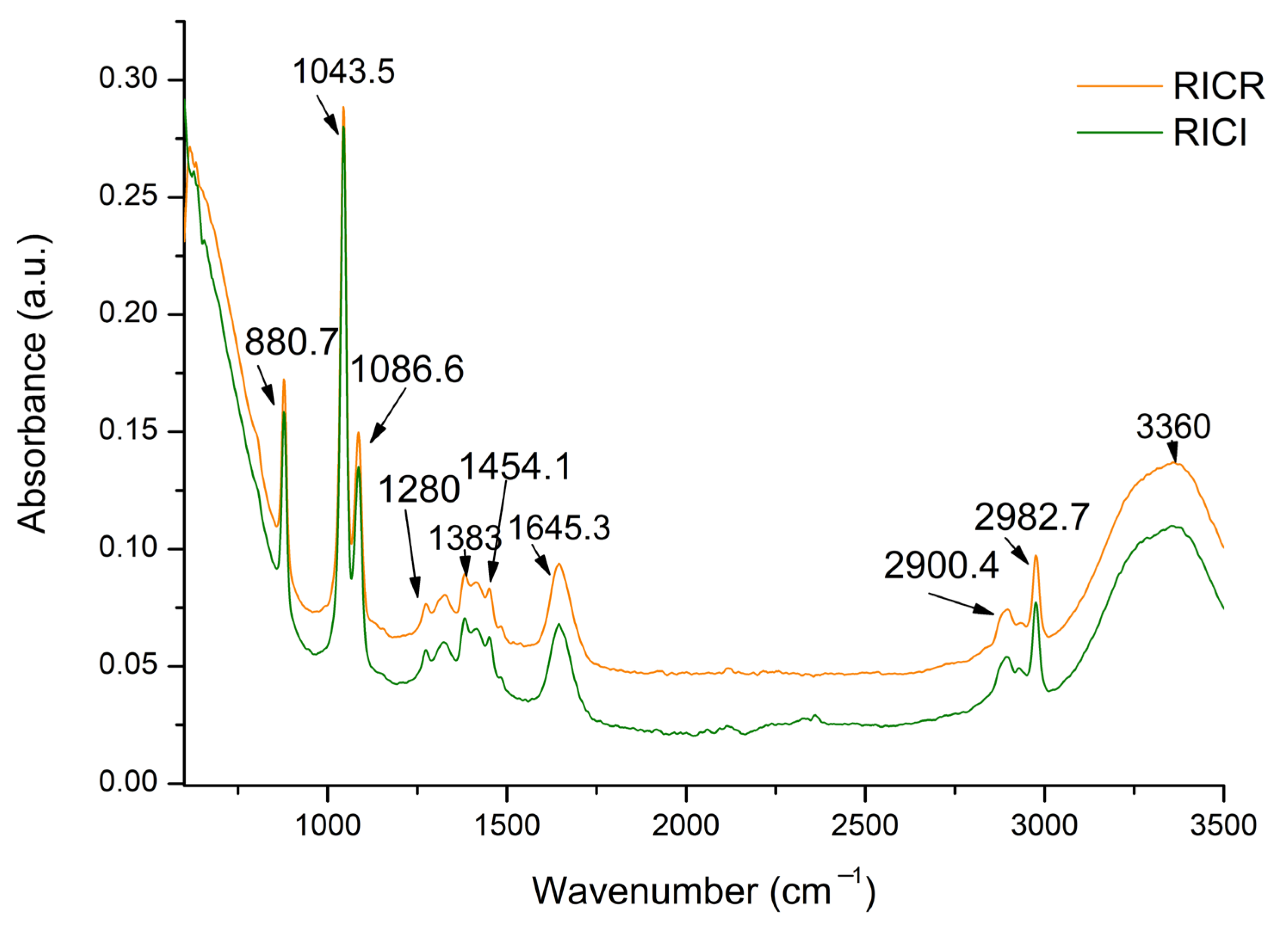
2.2. FTIR Analysis of Grape Pomace Extracts
2.3. HPLC-DAD-ESI-MS Analysis of Grape Pomace Extract Polyphenols
2.4. GC-FID Analysis of Fatty Acids
2.5. Antioxidant Activity
2.5.1. Antiradical Potential
2.5.2. Electron Transfer Assays
2.5.3. Metal Ion Chelation Assays
3. Discussion
4. Materials and Methods
4.1. Chemicals
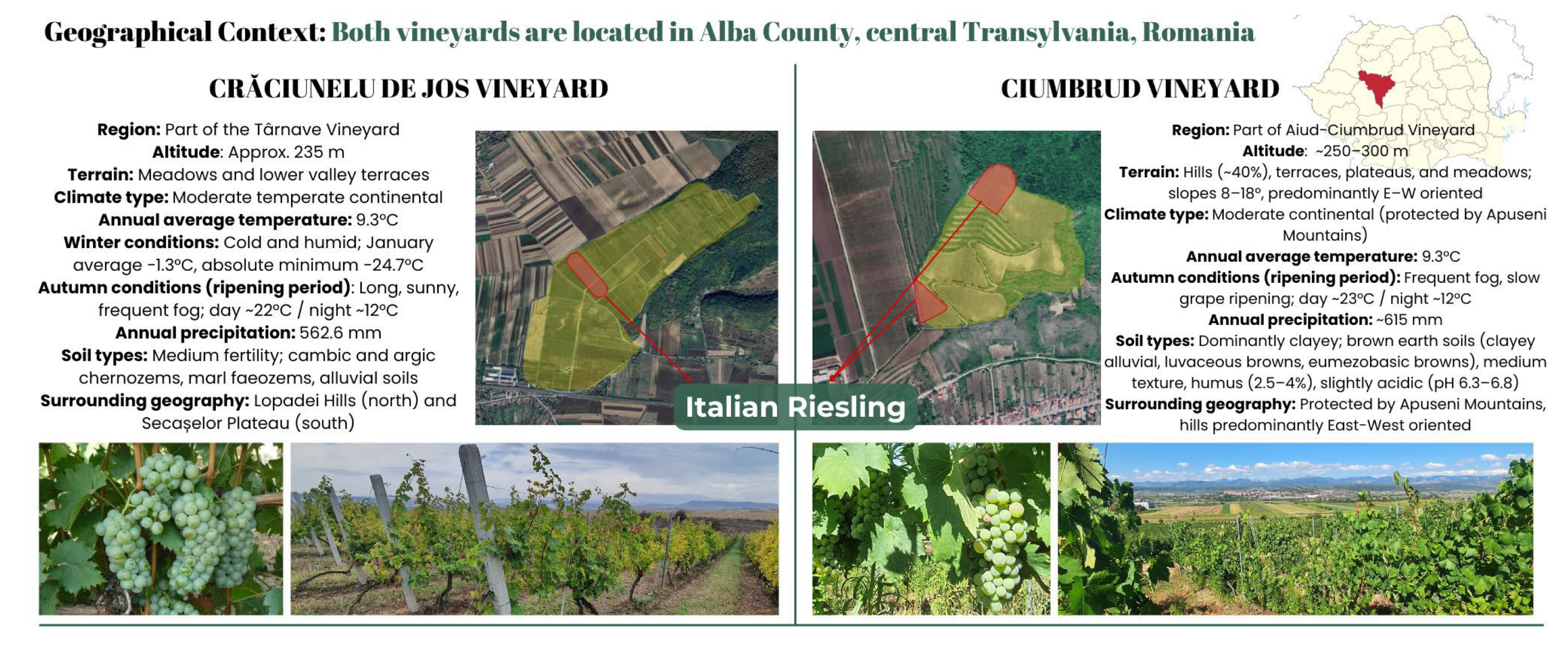
4.2. Grape Pomace Generation and Conditioning
4.3. Grape Pomace Polyphenol Extraction
4.4. Total Polyphenol Content (TPC) of Grape Pomace Polyphenol Extracts
4.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.6. Liquid Chromatography–Diode Array Detection–Electrospray Ionisation Mass Spectrometry (HPLC-DAD-ESI MS) Analysis
4.7. Gas Chromatography with Flame Ionisation Detector (GC-FID) Analysis of Fatty Acids
4.8. Antioxidant Activity
4.8.1. Antiradical Assays
Assessment of Relative DPPH Radical-Scavenging Ability
ABTS Cation Radical-Scavenging Capacity Measurement (ABTS)
4.8.2. Electron Transfer Assays
Measurement of Cupric Reducing Antioxidant Capacity (CUPRAC)
Measurement of Ferric Reducing Antioxidant Potential (FRAP)
Measurement of Reducing Power (RP)
4.8.3. Ferrous and Cupric Chelation Assays
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GP | grape pomace |
| RICI | GP from Ciumbrud Italian Riesling grapes |
| RICR | GP from Crăciunelu de Jos Italian Riesling grapes |
| FTIR | Fourier transform infrared spectroscopy |
| HPLC-DAD-ESI MS | liquid chromatography–diode array detection–electrospray ionisation mass spectrometry |
| GC-FID | gas chromatography with flame ionisation detector |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical-scavenging capacity |
| ABTS | 2,2′-azinobis-(3-ethylbenzthiazolin-6-sulfonic acid) radical-scavenging capacity |
| CUPRAC | cupric reducing antioxidant capacity |
| FRAP | ferric reducing antioxidant potential |
| RP | reducing power |
| TI | thrombogenicity index |
| AI | atherogenicity index |
| H/H | ratio between hypo- and hypercholesterolemic fatty acids |
| COX | calculated oxidizability |
| TPC | total polyphenol content |
| GAE | gallic acid equivalent |
| TE | Trolox equivalent |
| EE | EDTA equivalent |
| SFA | saturated fatty acid |
| UFA | unsaturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
References
- Sweet, N. Riesling Selections. In Grape Program Newsletter; Foundation Plant Services, University of California, Davis: Davis, CA, USA, 2009; pp. 23–37. [Google Scholar]
- Calò, A.; Scienza, A.; Costacurta, A. Vitigni d’Italia; Calderini Edagricole: Bologna, Italy, 2001; ISBN 8820643677. [Google Scholar]
- Röckel, M. Vitis International Variety Catalogue. Available online: https://www.vivc.de/ (accessed on 19 March 2025).
- Heizer, M. Riesling Italian vs Riesling de Rhin; Wines of Romania: Bucharest, Romania, 2022; Available online: https://winesofromania.com/en/riesling-italian-vs-riesling-de-rhin/ (accessed on 19 March 2025).
- Oslobeanu, M.; Macici, M.; Georgescu, M.; Stoian, V. Zonarea Soiurilor de Vita de vie ın România; Editura Ceres: Bucharest, Romania, 1991. [Google Scholar]
- Balla, G.; Moldován, C.; Lunka, T.A.; Szekely-Varga, Z.; Kentelky, E. Effect of Different Phytotechnological Factors on the Qualitative and Quantitative Parameters of the Italian Riesling Grape Variety. Curr. Trends Nat. Sci. 2023, 12, 134–140. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupić, D.; Preiner, D.; Ašperger, D.; Kontić, J.K. Recovery of Flavonoids from Grape Skins by Enzyme-Assisted Extraction. Sep. Sci. Technol. 2016, 51, 255–268. [Google Scholar] [CrossRef]
- Voşloban, C.M.; Tomoiagă, L.; Iliescu, M.; Puşcă, G.; Chedea, V.S. Grape Pomace Yields from Transylvania Cultivated Grapes. ISB_INMA_TEH Agric. Mech. Eng. 2020, 61, 121–129. [Google Scholar]
- Tomoiagă, L.; Iliescu, M.L.; Răcoare, H.S.; Botea, V.; Sîrbu, A.D.; Puşcă, G.; Chedea, V.S. Grape Pomance Generation from Grape Cultivars Cultivated in Târnave Vineyards in the Framework of the Climate Change. Rom. J. Hortic. 2020, 1, 81–88. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Towards Comprehensive Utilization of Winemaking Residues: Characterization of Grape Skins from Red Grape Pomaces of Variety Touriga Nacional. Ind. Crops Prod. 2013, 43, 25–32. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable Wineries through Waste Valorisation: A Review of Grape Marc Utilisation for Value-Added Products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Blasi, F.; Trovarelli, V.; Mangiapelo, L.; Ianni, F.; Cossignani, L. Grape Pomace for Feed Enrichment to Improve the Quality of Animal-Based Foods. Foods 2024, 13, 3541. [Google Scholar] [CrossRef]
- Oancea, A.-G.; Saracila, M.; Vlaicu, A.; Varzaru, I.; Untea, A.; Dragomir, C. Nutritional Characterization of White Grape Pomace: Potential Feed Additive in Ruminants’ Nutrition. Sci. Pap. Anim. Sci. Biotechnol. 2024, 57, 21. [Google Scholar]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red Grape Pomace Rich in Polyphenols Diet Increases the Antioxidant Status in Key Organs—Kidneys, Liver, and Spleen of Piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef]
- Horodincu, L.; Proca, A.C.; Șlencu, B.G.; Trifan, A.; Pavel, G.; Solcan, G.; Solcan, C. Modulating Effects of Grape Pomace on the Intestinal Antioxidative and Inflammatory Status in Fattening Pigs. Agriculture 2025, 15, 740. [Google Scholar] [CrossRef]
- Siller-Sánchez, A.; Luna-Sánchez, K.A.; Israel Bautista-Hernández, M.; Chávez-González, L. Use of Grape Pomace from the Wine Industry for the Extraction of Valuable Compounds with Potential Use in the Food Industry. Curr. Food Sci. Technol. Rep. 2024, 2, 7–16. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stanišić, N.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Živković, V.; Jakovljević, V.; Mašković, P.Z.; Mašković, J. Valorizing Grape Pomace: A Review of Applications, Nutritional Benefits, and Potential in Functional Food Development. Foods 2024, 13, 4169. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.D.C.; Madureira, J.; Margaça, F.M.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Tsiapali, O.I.; Ayfantopoulou, E.; Tzourouni, A.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Unveiling the Utilization of Grape and Winery By-Products in Cosmetics with Health Promoting Properties. Appl. Sci. 2025, 15, 1007. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols From Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Xiong, Z.; Liao, Y.; Zhang, Z.; Wan, Z.; Liang, S.; Guo, J. Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies. Biomolecules 2025, 15, 670. [Google Scholar] [CrossRef]
- Chedea, V.S.; Macovei, Ș.O.; Bocsan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Perra, M.; Cuena-Lombraña, A.; Bacchetta, G.; Manca, M.L.; Manconi, M.; Maroun, R.G.; Muntoni, A.; Tuberoso, C.I.G.; Gil, K.A.; De Gioannis, G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability 2022, 14, 10690. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.L.; Jourdes, M.; Teissedre, P.L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Rahimi, M.; Babaei, A. An Overview on the Antioxidant, Anti-Inflammatory, Antimicrobial and Anti-Cancer Activity of Grape Extract. Biomed. Res. Clin. Pract. 2018, 3, 1–4. [Google Scholar] [CrossRef]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.E.; Răschip, I.E.; Nadăș, G.; Gheldiu, A.M.; Tuchiluș, C.; Rotaru, L. Antioxidant and Antimicrobial Effects of Grape Pomace Extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, Antiviral, and Antifungal Properties of Wines and Winery Byproducts in Relation to Their Flavonoid Content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Chirilă, F.; Ogola, H.J.O.; Pelmuş, R.Ş.; Călin, L.G.; Socaciu, C. Antioxidant/Prooxidant and Antibacterial/Probacterial Effects of a Grape Seed Extract in Complex with Lipoxygenase. Biomed. Res. Int. 2014, 2014, 313684. [Google Scholar] [CrossRef]
- Chedea, V.; Braicu, C.; Chirilǎ, F.; Ober, C.; Socaciu, C. Antibacterial Action of an Aqueous Grape Seed Polyphenolic Extract. Afr. J. Biotechnol. 2011, 10, 6276–6280. [Google Scholar]
- Carmona-Jiménez, Y.; Igartuburu, J.M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Fatty Acid and Tocopherol Composition of Pomace and Seed Oil from Five Grape Varieties Southern Spain. Molecules 2022, 27, 6980. [Google Scholar] [CrossRef]
- Bran, E.P.; Nicuţă, D.; Grosu, L.; Patriciu, O.-I.; Alexa, I.-C. Investigation Regarding the Potential Application of Grape Pomace Extracts on in Vitro Plant Growth and Development. Ovidius Univ. Ann. Chem. 2022, 33, 135–142. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes Versicolor: Medicinal Mushroom with Important Health Benefits. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The Lignicolous Fungus Trametes versicolor (L.) Lloyd (1920): A Promising Natural Source of Antiradical and AChE Inhibitory Agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Levai, A.M.; Bocsan, L.C.; Buzoianu, A.D. Pot Aloe Vera Gel—A Natural Source of Antioxidants. Not. Bot. Horti Agrobot. Cluj Napoca 2022, 50, 12732. [Google Scholar] [CrossRef]
- Ping, Y.; Zhang, J.; Xing, T.; Chen, G.; Tao, R.; Choo, K.H. Green Synthesis of Silver Nanoparticles Using Grape Seed Extract and Their Application for Reductive Catalysis of Direct Orange 26. J. Ind. Eng. Chem. 2018, 58, 74–79. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.; Rodriguez, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A.K. Physicochemical Analysis of Apple and Grape Pomaces. Bioresources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of Aloe Vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Wang, T.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Characterization of Wastes and Coproducts from the Coffee Industry for Composite Material Production. Bioresources 2016, 11, 7637–7653. [Google Scholar] [CrossRef]
- Lim, Z.X.; Cheong, K.Y. Effects of Drying Temperature and Ethanol Concentration on Bipolar Switching Characteristics of Natural Aloe Vera-Based Memory Devices. Phys. Chem. Chem. Phys. 2015, 17, 26833–26853. [Google Scholar] [CrossRef]
- Bele, A.A.; Khale, A. Comparison of Constituents in Aloe Vera Gel Collected in Different Seasons by Chromatography and Spectroscopy Techniques. World J. Pharm. Res. 2016, 5, 1028–1040. [Google Scholar]
- Xu, W.; Reddy, N.; Yang, Y. Extraction, Characterization and Potential Applications of Cellulose in Corn Kernels and Distillers’ Dried Grains with Solubles (DDGS). Carbohydr. Polym. 2009, 76, 521–527. [Google Scholar] [CrossRef]
- Ray, A.; Ghosh, S. Chemometrics for Functional Group Distribution, and UV Absorption Potential of Aloe vera L. Gel at Different Growth Periods. Mater. Today Proc. 2018, 5, 22245–22253. [Google Scholar] [CrossRef]
- Pastorova, I.; Botto, R.E.; Arisz, P.W.; Boon, J.J. Cellulose Char Structure: A Combined Analytical Py-GC-MS, FTIR, and NMR Study. Carbohydr. Res. 1994, 262, 27–47. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Jones, M.B. Plant Microclimate. Tech. Bioprod. Photosynth. 1985, 26–40. [Google Scholar] [CrossRef]
- Fraga, H. Viticulture and Winemaking under Climate Change. Agronomy 2019, 9, 783. [Google Scholar] [CrossRef]
- Mărculeț, I.; Mărculeţ, C. Crăciunelu de Jos (Alba County)—690 Years of Official Attestation. In Omul și Universul; Edit. Bibliostar: Râmnicu Vâlcea, Romania, 2015; pp. 120–125. [Google Scholar]
- Savu, S.; Tomoiaga, L.L.; Chedea, V.S. Ecological Microclimate Influence on Grapevine Phomopsis Viticola Attack Frequency in Aiud-Ciumbrud Vineyards. Bull. Hortic. 2020, 77, 1843–5394. [Google Scholar] [CrossRef]
- Muntean, M.D.; Tomoiagă, L.L.; Răcoare, H.S.; Sîrbu, A.D.; Giurcă, I.S.; Chedea, V.S.; Teușdea, A.C.; Comșa, M. Millerandage—One of the Grapevine Cultivation Challenges in the Climate Change Context. Horticulturae 2025, 11, 165. [Google Scholar] [CrossRef]
- ONVPV CAIET DE SARCINI. Pentru Producerea şi Comercializarea Vinului cu Denumire de Origine Controlată „TÂRNAVE”. Available online: https://www.onvpv.ro/sites/default/files/caiet_de_sarcini_doc_tarnave_modif_cf_cererii_1154_22.05.2017_no_track_changes_0.pdf (accessed on 7 April 2025).
- ONVPV CAIET DE SARCINI. Pentru Producerea şi Comercializarea Vinului cu Denumire de Origine Controlată “AIUD”. Available online: https://www.onvpv.ro/sites/default/files/pdfs/caiet_de_sarcini_doc_aiud_modificat_cf_cerere_modificare_1153_din_25072014_368ro.pdf (accessed on 7 April 2025).
- Vojáčkova, K.; Mlček, J.; Škrovankova, S.; Adamkova, A.; Adamek, M.; Orsavova, J.; Bučkova, M.; Fic, V.; Kouřimska, L.; Buran, M. Biologically Active Compounds Contained in Grape Pomace. Potravin. Slovak J. Food Sci. 2020, 14, 854–861. [Google Scholar] [CrossRef]
- Wittenauer, J.; Schweiggert-Weisz, U.; Carle, R. In Vitro-Study of Antioxidant Extracts from Garcinia Mangostana Pericarp and Riesling Grape Pomace-a Contribution to by-Products Valorization as Cosmetic Ingredients. J. Appl. Bot. Food Qual. 2016, 89, 249–257. [Google Scholar] [CrossRef]
- Winkler, A.; Weber, F.; Ringseis, R.; Eder, K.; Dusel, G. Determination of Polyphenol and Crude Nutrient Content and Nutrient Digestibility of Dried and Ensiled White and Red Grape Pomace Cultivars. Arch. Anim. Nutr. 2015, 69, 187–200. [Google Scholar] [CrossRef]
- Gaita, C.; Alexa, E.; Popescu, I.; Popescu, S.; Negrea, M.; Poiana, M.-A. Grape Pomace: A Potential Sustainable Resource for Natural Bioactive Compounds Recovery. J. Agroaliment. Process. Technol. 2017, 23, 141–147. [Google Scholar]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent Selection for Efficient Extraction of Bioactive Compounds from Grape Pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagă, L.L.; Ranga, F.; Muntean, M.-D.; Sîrbu, A.; Comșa, M.; Cruceru, J.; Răcoare, H.-S.; Pop, R.M. From Grape to Wine-Muscat Ottonel from Blaj-Târnave Vineyard Chemical and Sensory Analysis. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2023, 80, 25–36. [Google Scholar] [CrossRef]
- Borbalan, A.; Zorro, L.; Guillen, D.; Garcia-Barroso, C. Study of the Polyphenol Content of Red and White Grape Varieties by Liquid Chromatography–Mass Spectrometry and Its Relationship to Antioxidant Power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Cheng, G.; Zhou, S.H.; Liu, Y.; Yue, T.X.; Zhang, Z.W. Effect of Bearing Position on Phenolics Profiles in the Skins of Four Cultivars of Grapevine (Vitis vinifera L.). J. Hortic. Sci. Biotechnol. 2015, 90, 356–363. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and Quantification of Phenolic Compounds in Berry Skin, Pulp, and Seeds in 13 Grapevine Varieties Grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, R.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutié Rrez, I. Flavonol Profiles of Vitis vinifera White Grape Cultivars. J. Food Compos. Anal. 2010, 23, 699–705. [Google Scholar] [CrossRef]
- Kropek, I.E.; Štefan, M.B.; Rajkovača, K.; Petković, T.; Cvetnić, M.; Bolanča, T.; Vladimir-Knežević, S. Comparative Phenolic Profiles of Monovarietal Wines from Different Croatian Regions. Appl. Sci. 2023, 13, 3031. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Khoulati, A.; Kadda, S.; Bencheikh, N.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.E.; Lahmass, I.; Benabbes, R.; et al. Antioxidant Activity, Metal Chelating Ability and DNA Protective Effect of the Hydroethanolic Extracts of Crocus Sativus Stigmas, Tepals and Leaves. Antioxidants 2022, 11, 932. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Caprioli, G.; Abouelenein, D.; Mustafa, A.M.; Uba, A.I.; Ak, G.; Ozturk, R.B.; Zengin, G.; Yagi, S. Phenolic Profile, Antioxidant and Enzyme Inhibitory Activities of Leaves from Two Cassia and Two Senna Species. Molecules 2022, 27, 5590. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoǧlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Huyut, Z.; Beydemir, Ş.; Gülçin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent Effects on the Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants Measured by CUPRAC, ABTS/Persulphate and FRAP Methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant Evaluation Protocols: Food Quality or Health Effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Rock, P.A. The Standard Oxidation Potential of the Ferrocyanide-Ferricyanide Electrode at 25° and the Entropy of Ferrocyanide Ion. J. Phys. Chem. 1966, 70, 576–580. [Google Scholar] [CrossRef]
- Burgess, J. Redox Potentials. In Ions in Solution; Elsevier: Amsterdam, The Netherlands, 1999; pp. 93–105. [Google Scholar]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Ferreira, R.; Lourenço, S.; Lopes, A.; Andrade, C.; Câmara, J.S.; Castilho, P.; Perestrelo, R. Evaluation of Fatty Acids Profile as a Useful Tool towards Valorization of By-Products of Agri-Food Industry. Foods 2021, 10, 2867. [Google Scholar] [CrossRef]
- Yehuda, S.; Rabinovitz, S.; Mostofsky, D.I. Mixture of Essential Fatty Acids Lowers Test Anxiety. Nutr. Neurosci. 2005, 8, 265–267. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The Effect of Palmitic Acid on Inflammatory Response in Macrophages: An Overview of Molecular Mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of Bioactive Compounds from Grape Pomace (Vitis vinifera and Vitis labrusca) by Spectrophotometric, Chromatographic and Spectral Analyses. J. Chromatogr. B 2015, 1007, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kolláthová, R.; Hanušovský, O.; Gálik, B.; Biro, D. Fatty Acid Profile Analysis of Grape By-Products from Slovakia and Austria. Acta Fytotech. Zootech. 2020, 23, 78–84. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Kovačević, D.B.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Russo, V.M.; Jacobs, J.L.; Hannah, M.C.; Moate, P.J.; Dunshea, F.R.; Leury, B.J. In Vitro Evaluation of the Methane Mitigation Potential of a Range of Grape Marc Products. Anim. Prod. Sci. 2017, 57, 1437–1444. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.F.E.; Ghafoor, K.; Banjanin, T.; Alqah, H.A. Chemical Composition, Bioactive Compounds, Mineral Contents, and Fatty Acid Composition of Pomace Powder of Different Grape Varieties. J. Food Process Preserv. 2020, 44, e14539. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Bondioli, P.; Folegatti, L.; Rovellini, P. Oils Rich in Alpha Linolenic Acid: Chemical Composition of Perilla (Perilla frutescens) Seed Oil. OCL J. 2020, 27, 67. [Google Scholar] [CrossRef]
- Sabir, A.; Unver, A.; Kara, Z. The Fatty Acid and Tocopherol Constituents of the Seed Oil Extracted from 21 Grape Varieties (Vitis spp.). J. Sci. Food Agric. 2012, 92, 1982–1987. [Google Scholar] [CrossRef]
- Gómez, M.E.; Igartuburu, J.M.; Pando, E.; Rodríguez Luis, F.; Mourente, G. Lipid Composition of Lees from Sherry Wine. J. Agric. Food Chem. 2004, 52, 4791–4794. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-Tocopherol) Levels in Plant Oils. Flavour. Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Sendra, E.; Carbonell-Barrachina, Á.; Legua, P.; Hernández, F. Fatty Acid Profile of Fruits (Pulp and Peel) and Cladodes (Young and Old) of Prickly Pear [Opuntia ficus-indica (L.) Mill.] from Six Spanish Cultivars. J. Food Compos. Anal. 2019, 84, 103294. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Chemical, Rheological and Nutritional Characteristics of Sesame and Olive Oils Blended with Linseed Oil. Adv. Pharm. Bull. 2018, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.T.; Islam, M.M.; Bostami, A.B.M.R.; Mun, H.S.; Kim, Y.J.; Yang, C.J. Meat Composition, Fatty Acid Profile and Oxidative Stability of Meat from Broilers Supplemented with Pomegranate (Punica granatum L.) by-Products. Food Chem. 2015, 188, 481–488. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Habeanu, M.; Lefter, N.; Gheorghe, A.; Nagy, A.; Marin, D.; Ropota, M. Effects of Dietary Flaxseed Oil on the Muscle Fatty Acid Composition in Mangalitsa Pigs in an Extensive Rearing System. S. Afr. J. Anim. Sci. 2014, 44, 240–244. [Google Scholar] [CrossRef]
- Pele, R.; Marc, G.; Stana, A.; Ionuț, I.; Nastasă, C.; Tiperciuc, B.; Oniga, I.; Pîrnău, A.; Vlase, L.; Oniga, O. Synthesis of New Phenolic Derivatives of Quinazolin-4(3H)-One as Potential Antioxidant Agents—In Vitro Evaluation and Quantum Studies. Molecules 2022, 27, 2599. [Google Scholar] [CrossRef]
- Marc, G.; Stana, A.; Franchini, A.H.; Vodnar, D.C.; Barta, G.; Tertiş, M.; Şanta, I.; Cristea, C.; Pîrnau, A.; Ciorîta, A.; et al. Phenolic Thiazoles with Antioxidant and Antiradical Activity. Synthesis, in Vitro Evaluation, Toxicity, Electrochemical Behavior, Quantum Studies and Antimicrobial Screening. Antioxidants 2021, 10, 1707. [Google Scholar] [CrossRef]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Marc, G.; Franchini, A.H.; Oniga, O.; Vlase, L.; Bogdan, M. Synthesis and Molecular Interaction Study of a Diphenolic Hidrazinyl-Thiazole Compound with Strong Antioxidant and Antiradical Activity with HSA. J. Mol. Struct. 2021, 1244, 131278. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Shiau, C.Y.; Chen, H.M.; Chiou, T.K.; Wu, H.-C.; Shiau, C.-Y.; Chen, H.-M.; Chiou, T.-K. Antioxidant Activities of Carnosine, Anserine, Some Free Amino Acids and Their Combination. J. Food Drug Anal. 2020, 11, 13. [Google Scholar] [CrossRef]
- Cesari, L.; Mutelet, F.; Canabady-Rochelle, L. Antioxidant Properties of Phenolic Surrogates of Lignin Depolymerisation. Ind. Crops Prod. 2019, 129, 480–487. [Google Scholar] [CrossRef]
| Tentative Identification | Concentration † (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Retention Time Rt (min) | UV λmax (nm) | [M + H]+ (m/z) | Compounds | Subclass | RICR | RICI |
| 3.29 | 270 | 138 | 2-Hydroxybenzoic acid | Hydroxybenzoic acid | 100.78 ± 4.81 a | 107.22 ± 3.03 a |
| 5.96 | 279 | 171 | Gallic acid | Hydroxybenzoic acid | 22.19 ± 0.33 a | 30.35 ± 0.34 b |
| Total phenolic acids | 122.98 ± 5.15 a | 137.57 ± 3.38 b | ||||
| 10.42 | 280 | 579, 291 | Procyanidin dimer B3 | Flavanol | 23.41 ± 0.41 a | 26.30 ± 0.29 b |
| 11.63 | 280 | 579, 291 | Procyanidin dimer B1 | Flavanol | 29.88 ± 0.40 a | 33.56 ± 0.44 b |
| 12.28 | 280 | 579, 291 | Procyanidin dimer B2 | Flavanol | 14.86 ± 0.37 a | 35.05 ± 0.40 b |
| 12.61 | 280 | 291 | Catechin | Flavanol | 107.32 ± 4.98 a | 63.25 ± 2.05 b |
| 13.95 | 280 | 291 | Epicatechin | Flavanol | 142.62 ± 5.85 a | 87.38 ± 3.19 b |
| 16.40 | 280 | 443 | Epicatechingallate | Flavanol | 26.58 ± 0.39 a | 20.17 ± 0.25 b |
| 22.91 | 280 | n.d | Catechin derivate | Flavanol | 328.68 ± 17.34 a | 280.58 ± 8.40 b |
| Total flavanols | 673.34 ± 29.72 a | 546.29 ± 15.03 b | ||||
| 16.23 | 355 | 465 | Quercetin-glucoside | Flavonol | 9.09 ± 0.43 a | 12.34 ± 0.23 b |
| 17.33 | 253, 350 | 449 | Kaempferol-glucoside | Flavonol | 1.47 ± 0.01 a | 1.52 ± 0.00 b |
| Total flavonols | 10.55 ± 0.44 a | 13.85 ± 0.24 b | ||||
| Sample/Fatty Acid (g FAME/100 g Total FAME) | RICR | RICI |
|---|---|---|
| Saturated Fatty Acids (SFAs) | ||
| Caprylic (C 8:0) | 0.53 ± 0.04 a | 0.03 ± 0.02 b |
| Capric (C 10:0) | 0.09 ± 0.01 a | 0.06 ± 0.01 a |
| Lauric (C12:0) | 0.02 ± 0.00 a | 0.03 ± 0.02 a |
| Myristic (C 14:0) | 0.22 ± 0.02 a | 0.22 ± 0.05 a |
| Pentadecanoic (C15:0) | 0.06 ± 0.02 a | 0.04 ± 0.01 a |
| Palmitic (C16:0) | 11.19 ± 0.05 a | 11.16 ± 0.00 a |
| Heptadecanoic (C17:0) | 0.04 ± 0.01 a | 0.09 ± 0.01 a |
| Stearic (C18:0) | 4.19 ± 0.00 a | 4.32 ± 0.04 a |
| Arachidic (C 20:0) | 0.34 ± 0.03 a | 0.07 ± 0.02 b |
| Lignoceric (C 24:0) | 0.18 ± 0.00 a | 0.19 ± 0.03 a |
| Total SFAs | 16.86 ± 0.07 a | 16.23 ± 0.13 b |
| Unsaturated Fatty Acids (UFAs) | ||
| Monounsaturated Fatty Acids (MUFAs) | ||
| Pentadecanoic (C15:1) | 0.05 ± 0.01 a | 0.08 ± 0.01 a |
| Palmitoleic (C16:1) | 0.87 ± 0.03 a | 0.76 ± 0.04 a |
| Oleic cis (C18:1) | 16.55 ± 0.04 a | 18.13 ± 0.02 b |
| Total MUFAs | 17.47 ± 0.00 a | 18.96 ± 0.05 b |
| Polyunsaturated Fatty Acids (PUFAs) | ||
| Polyunsaturated Fatty Acid Omega 3 (PUFA ω-3) | ||
| Linolenic (C18:3n3) | 0.85 ± 0.01 a | 1.26 ± 0.01 b |
| Octadecatetraenoic (C18:4n3) | 0.36 ± 0.02 a | 0.69 ± 0.02 b |
| Eicosatrienoic (C20(3n3)) | 0.07 ± 0.00 a | 0.07 ± 0.01 a |
| Eicosapentaenoic (C 20:5n3) | 0.10 ± 0.00 a | 0.12 ± 0.02 a |
| Total PUFA ω-3 | 1.39 ± 0.03 a | 2.13 ± 0.03 b |
| Polyunsaturated Fatty Acid Omega 6 (PUFA ω-6) | ||
| Linoleic cis (C 18:2n6) | 63.73 ± 0.03 a | 61.18 ± 0.06 b |
| Eicosadienoic (C20(2n6)) | 0.12 ± 0.00 a | 0.00 ± 0.00 b |
| Eicosatrienoic (C20(3n6)) | 0.05 ± 0.01 a | 0.07 ± 0.01 a |
| Arachidonic (C20(4n6)) | 0.08 ± 0.00 a | 0.27 ± 0.01 b |
| Docosadienoic (C 22:2n6) | 0.11 ± 0.01 a | 0.36 ± 0.01 b |
| Total PUFA ω-6 | 64.08 ± 0.04 a | 61.87 ± 0.02 b |
| Total PUFA | 65.47 ± 0.02 a | 64.00 ± 0.05 b |
| Total UFA (MUFA+PUFA) | 82.94 ± 0.02 a | 82.96 ± 0.10 a |
| Other fatty acids | 0.20 ± 0.09 a | 0.81 ± 0.04 b |
| SFA/UFA | 0.20 ± 0.00 a | 0.20 ± 0.00 a |
| PUFA/MUFA | 3.75 ± 0.00 a | 3.38 ± 0.01 b |
| ω-6/ω-3 | 46.26 ± 0.89 a | 29.07 ± 0.42 b |
| UFA/SFA | 4.92 ± 0.02 a | 5.11 ± 0.05 a |
| PUFA/SFA | 3.88 ± 0.01 a | 3.94 ± 0.04 a |
| COX | 6.91±0.00 a | 6.75 ± 0.01 b |
| AI | 0.15 ± 0.00 a | 0.15 ± 0.00 a |
| TI | 0.35 ± 0.00 a | 0.34 ± 0.00 b |
| H/H | 7.12 ± 0.04 a | 7.08 ± 0.04 a |
| % | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Sample/ Antioxidant Activity | DPPH | ABTS | CUPRAC | FRAP | RP | Ferrous Ion Chelation | Cupric Ion Chelation |
|---|---|---|---|---|---|---|---|
| µM TE/mL Extract | mg GAE/mL Extract | mg GAE/mL Extract | mg GAE/mL Extract | mg GAE/mL Extract | µM EE/mL Extract | µm EE/mL Extract | |
| RICR | 81.16 ± 0.9 a | 3.23 ± 0.06 a | 0.89 ± 0.01 a | 1.54 ± 0.05 a | 1.14 ± 0.04 a | 0.23 ± 0.00 a | 0.41 ± 0.02 a |
| RICI | 88.61 ± 0.79 b | 3.00 ± 0.03 b | 1.08 ± 0.02 b | 1.81 ± 0.02 b | 1.11 ± 0.03 a | 0.22 ± 0.01 a | 0.34 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chedea, V.S.; Tomoiagă, L.L.; Ropota, M.; Marc, G.; Ranga, F.; Comșa, M.; Muntean, M.D.; Sîrbu, A.D.; Giurca, I.S.; Răcoare, H.S.; et al. Phenolic Profile, Fatty Acid Composition, and Antioxidant Activity of Italian Riesling Grape Pomace from Two Transylvanian Microclimates. Plants 2025, 14, 1809. https://doi.org/10.3390/plants14121809
Chedea VS, Tomoiagă LL, Ropota M, Marc G, Ranga F, Comșa M, Muntean MD, Sîrbu AD, Giurca IS, Răcoare HS, et al. Phenolic Profile, Fatty Acid Composition, and Antioxidant Activity of Italian Riesling Grape Pomace from Two Transylvanian Microclimates. Plants. 2025; 14(12):1809. https://doi.org/10.3390/plants14121809
Chicago/Turabian StyleChedea, Veronica Sanda, Liliana Lucia Tomoiagă, Mariana Ropota, Gabriel Marc, Floricuta Ranga, Maria Comșa, Maria Doinița Muntean, Alexandra Doina Sîrbu, Ioana Sorina Giurca, Horia Silviu Răcoare, and et al. 2025. "Phenolic Profile, Fatty Acid Composition, and Antioxidant Activity of Italian Riesling Grape Pomace from Two Transylvanian Microclimates" Plants 14, no. 12: 1809. https://doi.org/10.3390/plants14121809
APA StyleChedea, V. S., Tomoiagă, L. L., Ropota, M., Marc, G., Ranga, F., Comșa, M., Muntean, M. D., Sîrbu, A. D., Giurca, I. S., Răcoare, H. S., Bocsan, C. I., Buzoianu, A. D., Kisher, H., & Pop, R. M. (2025). Phenolic Profile, Fatty Acid Composition, and Antioxidant Activity of Italian Riesling Grape Pomace from Two Transylvanian Microclimates. Plants, 14(12), 1809. https://doi.org/10.3390/plants14121809











