Characteristic Functional Genera (CFG) Mediate Nitrogen Priming Effect in the Microbiome of Saline–Alkaline Farmland
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Sites and Varieties
2.2. Experimental Design
2.3. Rhizosphere Soil Sampling
2.4. Determination of Soil Nutrient Content
2.5. High-Throughput Sequencing Analysis of Bacterial and Fungal Communities in Rhizosphere Soil
2.6. Data Processing and Analysis
3. Results
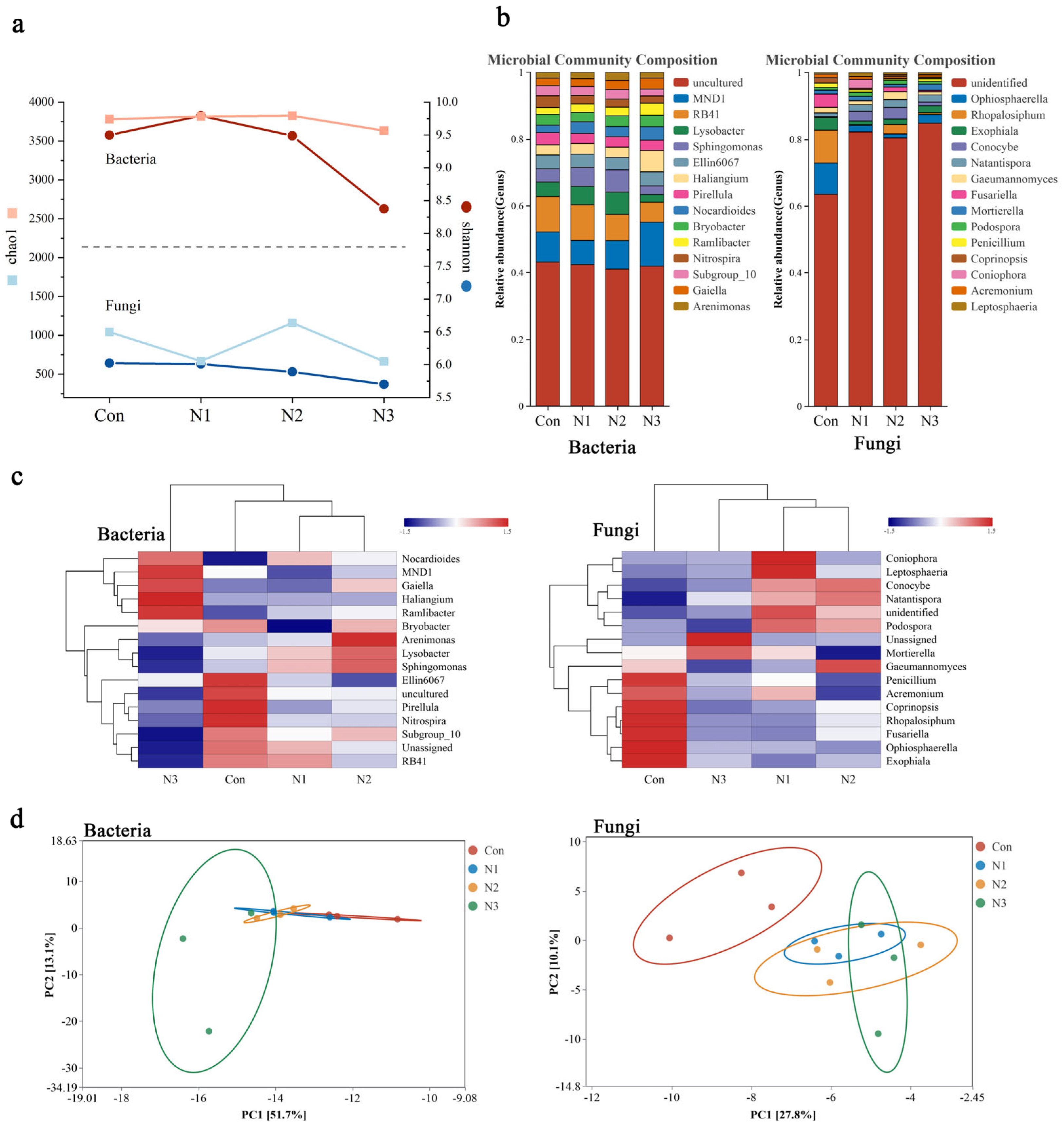
3.1. Effect of Nitrogen Priming on Microbial Dominant Flora Community
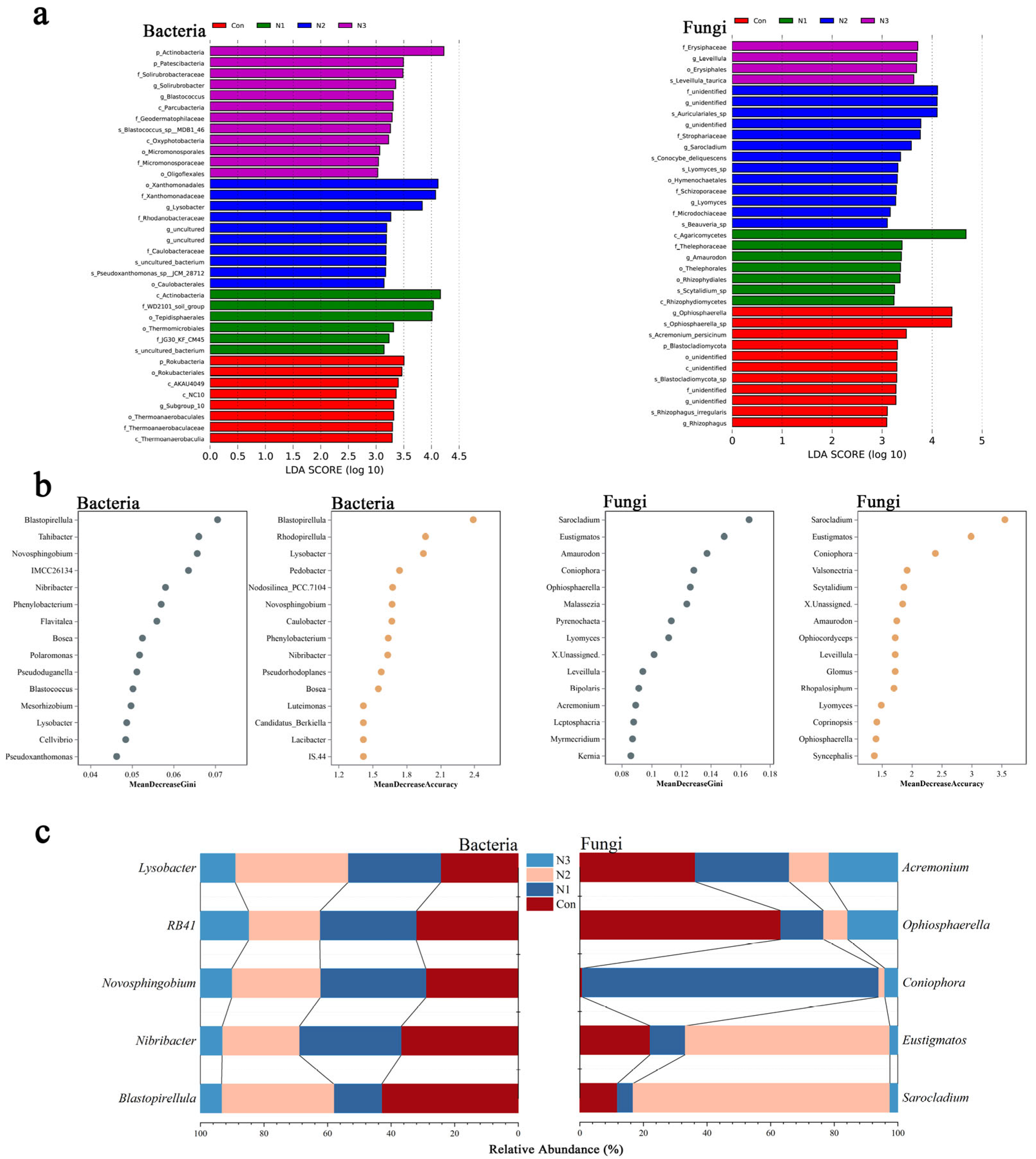
3.2. Screening of Characteristic Functional Bacteria Under Nitrogen Excitation Effect
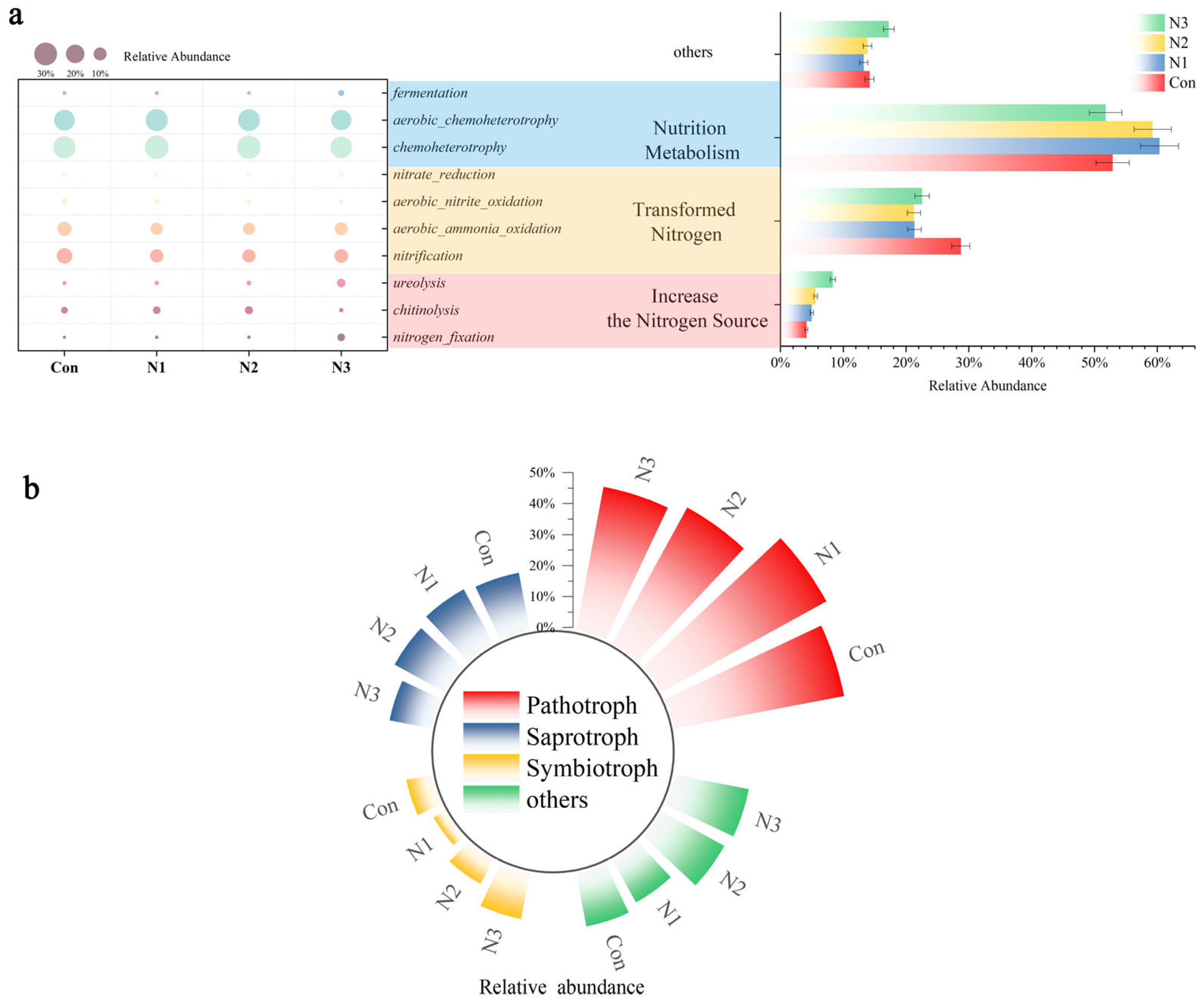
3.3. Effect of Nitrogen Priming on Microbial Function Prediction
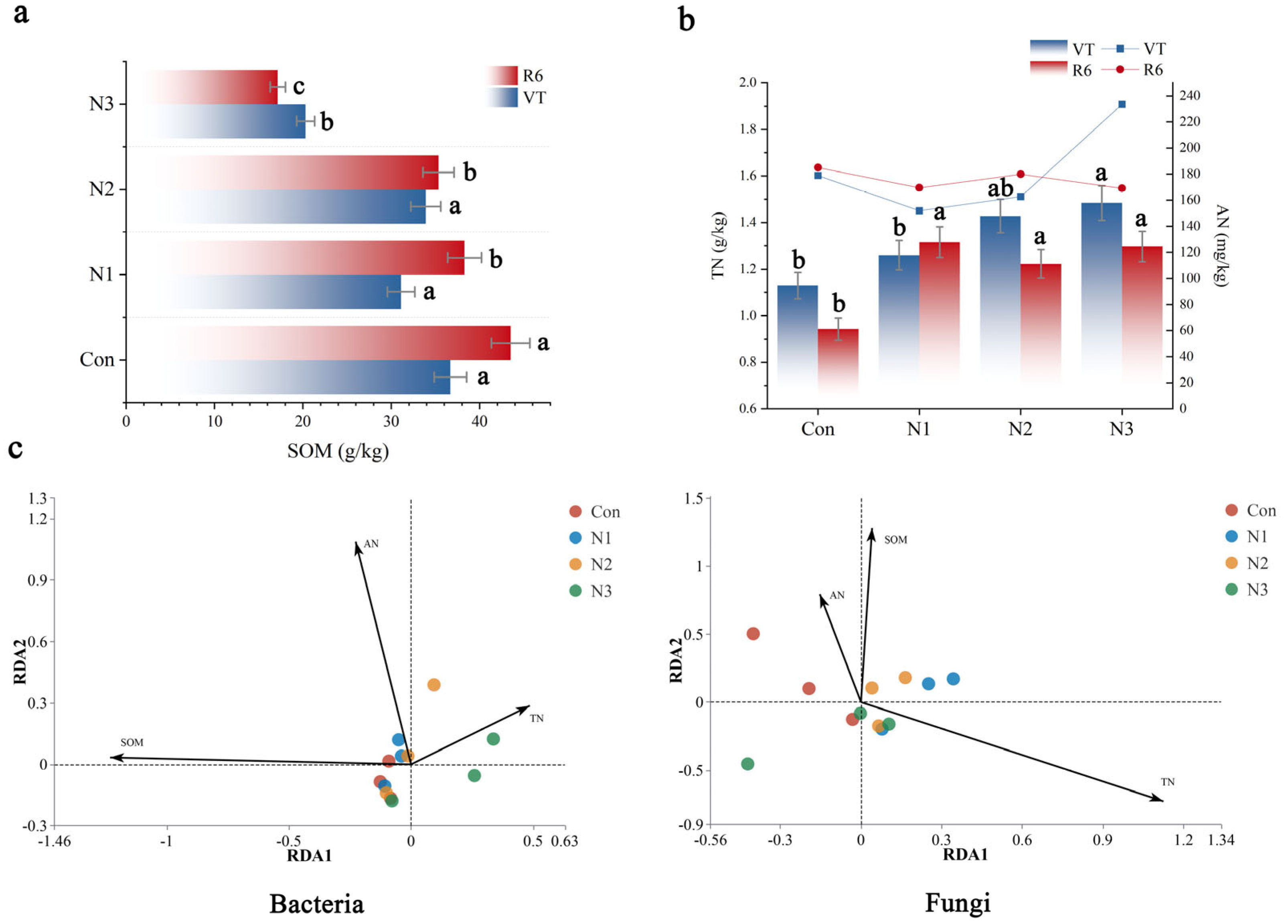
3.4. Effects of Nitrogen Priming on Soil Nitrogen and Organic Matter
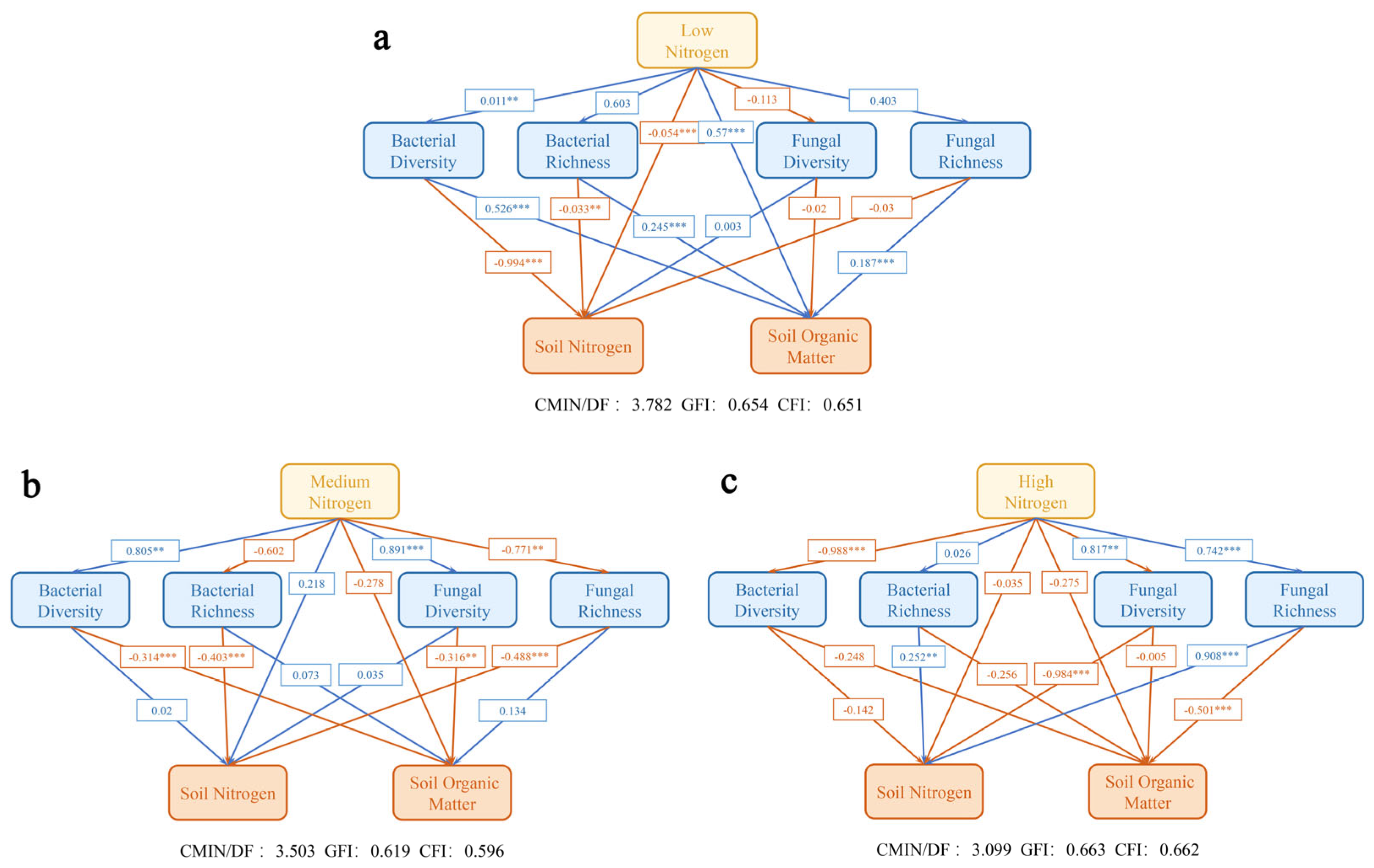
3.5. Path Analysis of Different Nitrogen Levels Under Nitrogen Excitation Effect
4. Discussion
4.1. Effects of Nitrogen Priming on Microbial Community Structure and Dominant Flora
4.2. Characteristics and Functions of Characteristic Functional Genera Under Nitrogen Priming Effect
4.3. Functional Analysis of Bacteria and Fungi Under Nitrogen Priming Effect
4.4. Complex Correlation Between Soil Fertility and Nitrogen and Analysis of Nitrogen Priming Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gu, S.; Jiang, R.; Zhang, X.; Hatano, R. Saline–Alkali Soil Reclamation Contributes to Soil Health Improvement in China. Agriculture 2024, 14, 1210. [Google Scholar] [CrossRef]
- Xing, J.; Li, X.; Li, Z.; Wang, X.; Hou, N.; Li, D. Remediation of Soda-Saline-Alkali Soil through Soil Amendments: Microbially Mediated Carbon and Nitrogen Cycles and Remediation Mechanisms. Sci. Total Environ. 2024, 924, 171641. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of Mechanisms and Quantification of Priming Effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere Size and Shape: Temporal Dynamics and Spatial Stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, B. Global Patterns and Associated Drivers of Priming Effect in Response to Nutrient Addition. Soil Biol. Biochem. 2021, 153, 108118. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming Effects: Interactions between Living and Dead Organic Matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Zheng, W.; Yin, C.; Fan, X.; Ye, M.; Gao, Z.; Wu, C.; Liang, Y. Linking Nitrogen- and Straw-Sensitive Indicator Species and Their Co-Occurrences to Priming Effect in Agricultural Soil Exposed to Long-Term Nitrogen Fertilization. Soil Biol. Biochem. 2023, 176, 108881. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Fox, R.H.; Rayner, J.H. Interactions between Fertilizer Nitrogen and Soil Nitrogen—The So-called ‘Priming’ Effect. J. Soil Sci. 1985, 36, 425–444. [Google Scholar] [CrossRef]
- He, P.; Wan, S.-Z.; Fang, X.-M.; Wang, F.-C.; Chen, F.-S. Exogenous Nutrients and Carbon Resource Change the Responses of Soil Organic Matter Decomposition and Nitrogen Immobilization to Nitrogen Deposition. Sci. Rep. 2016, 6, 23717. [Google Scholar] [CrossRef]
- Wang, S.; Tian, H.; Liu, J.; Pan, S. Pattern and Change of Soil Organic Carbon Storage in China: 1960s–1980s. Tellus B 2003, 55, 416–427. [Google Scholar] [CrossRef]
- Dorich, R.A.; Nelson, D.W. Evaluation of Manual Cadmium Reduction Methods for Determination of Nitrate in Potassium Chloride Extracts of Soils. Soil Sci. Soc. Am. J. 1984, 48, 72–75. [Google Scholar] [CrossRef]
- Ren, B.; Wang, W.; Shen, L.; Yang, W.; Yang, Y.; Jin, J.; Geng, C. Nitrogen Fertilization Rate Affects Communities of Ammonia-Oxidizing Archaea and Bacteria in Paddy Soils across Different Climatic Zones of China. Sci. Total Environ. 2023, 902, 166089. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, C.; Peacock, C.L.; Sørensen, S.J.; Redmile-Gordon, M.A.; Xiao, K.-Q.; Gao, C.; Liu, J.; Huang, Q.; Li, Z.; et al. Cooperative Microbial Interactions Drive Spatial Segregation in Porous Environments. Nat. Commun. 2023, 14, 4226. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil Microbial Community Composition Closely Associates with Specific Enzyme Activities and Soil Carbon Chemistry in a Long-Term Nitrogen Fertilized Grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Sun, X.; Wang, Y.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Wang, G. Distinct Influence of Conventional and Biodegradable Microplastics on Microbe-Driving Nitrogen Cycling Processes in Soils and Plastispheres as Evaluated by Metagenomic Analysis. J. Hazard. Mater. 2023, 451, 131097. [Google Scholar] [CrossRef]
- Niu, Y.; Yang, X.; Hao, S.; Hei, Z.; Chen, B.; Hu, H.; Wan, S.; Chen, Y. Temperate Grassland Soil Nitrifiers Are More Sensitive to Nitrogen Addition than Simulated Warming. Appl. Soil Ecol. 2024, 195, 105214. [Google Scholar] [CrossRef]
- Mukhtar, H.; Hao, J.; Xu, G.; Bergmeyer, E.; Ulutas, M.; Yang, J.; Schachtman, D.P. Nitrogen Input Differentially Shapes the Rhizosphere Microbiome Diversity and Composition across Diverse Maize Lines. Biol. Fertil. Soils 2025, 61, 1–12. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil Microbial Diversity–Biomass Relationships Are Driven by Soil Carbon Content across Global Biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- He, C.; Li, K.; Li, J.; Fan, P.; Ruan, Y.; Jia, Z. Rice Straw Increases Microbial Nitrogen Fixation, Bacterial and nifH Genes Abundance with the Change of Land Use Types. Front. Microbiol. 2024, 14, 1283675. [Google Scholar] [CrossRef]
- Nicolás, C.; Martin-Bertelsen, T.; Floudas, D.; Bentzer, J.; Smits, M.; Johansson, T.; Troein, C.; Persson, P.; Tunlid, A. The Soil Organic Matter Decomposition Mechanisms in Ectomycorrhizal Fungi Are Tuned for Liberating Soil Organic Nitrogen. ISME J. 2019, 13, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Konishi, N.; Ma, J.F. Three Polarly Localized Ammonium Transporter 1 Members Are Cooperatively Responsible for Ammonium Uptake in Rice under Low Ammonium Condition. New Phytol. 2021, 232, 1778–1792. [Google Scholar] [CrossRef] [PubMed]
- Gaudinier, A.; Rodriguez-Medina, J.; Zhang, L.; Olson, A.; Liseron-Monfils, C.; Bågman, A.-M.; Foret, J.; Abbitt, S.; Tang, M.; Li, B.; et al. Transcriptional Regulation of Nitrogen-Associated Metabolism and Growth. Nature 2018, 563, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Trzcińska, M.; Pawlik-Skowrońska, B. Differences in Zn and Pb Resistance of Two Ecotypes of the Microalga Eustigmatos Sp. Inhabiting Metal Loaded Calamine Mine Spoils. J. Appl. Phycol. 2013, 25, 277–284. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere Soil Properties, Microbial Community, and Enzyme Activities: Short-Term Responses to Partial Substitution of Chemical Fertilizer with Organic Manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Dalton, H.; Stirling, D.I.; Quayle, J.R.; Higgins, I.J.; Quayle, J.R.; Bull, A.T. Co-Metabolism. Philos. Trans. R. Soc. London. B Biol. Sci. 1997, 297, 481–496. [Google Scholar] [CrossRef]
- Tomioka, K.; Sekiguchi, H.; Nomiyama, K.; Kawaguchi, A.; Kawakami, A.; Masunaka, A.; Kobayashi, H.; Chiba, M.; Nagata, K.; Ishikawa, N. Early Dead Ripe of Bread Wheat and Durum Wheat Caused by Ophiosphaerella Korrae and Evaluation of the Fungal Influence on Barley, Rice, and Soybean. J. Gen. Plant Pathol. 2021, 87, 273–280. [Google Scholar] [CrossRef]
- Kaur, P.; Balomajumder, C. Bioremediation Process Optimization and Effective Reclamation of Mixed Carbamate-Contaminated Soil by Newly Isolated Acremonium sp. Chemosphere 2020, 249, 125982. [Google Scholar] [CrossRef]
- Liao, X.; Tang, T.; Li, J.; Wang, J.; Neher, D.A.; Zhang, W.; Xiao, J.; Xiao, D.; Hu, P.; Wang, K.; et al. Nitrogen Fertilization Increases the Niche Breadth of Soil Nitrogen-Cycling Microbes and Stabilizes Their Co-Occurrence Network in a Karst Agroecosystem. Agric. Ecosyst. Environ. 2024, 374, 109177. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. Nutrient–Hormone Relations: Driving Root Plasticity in Plants. Mol. Plant 2022, 15, 86–103. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, I.; Cheng, X.; de Jager, V.; Expósito, R.G.; Watrous, J.; Patel, N.; Postma, J.; Dorrestein, P.C.; Kobayashi, D.; Raaijmakers, J.M. Comparative Genomics and Metabolic Profiling of the Genus Lysobacter. BMC Genom. 2015, 16, 991. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and Mechanisms of Responses by Soil Microbial Communities to Nitrogen Addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Peng, L.; Huang, J.; Huang, C.; Yang, H. Genetic Sequencing Provides Insights into Molecular and Genetic Mechanisms of Lysobacter Enzymogenes HYP18 Involved in Soil Organic Nitrogen and Phosphorus Mobilization and Plant Growth Promotion. Plant Soil 2023, 491, 525–542. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N Availability Determine the Priming Effect: Microbial N Mining and Stoichiometric Decomposition Theories. Glob. Change Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Han, Z.; Lu, Y.; Zhao, Y.; Wang, Y.; Han, Z.; Han, Y.; Zhang, J. Analysis of Relative Expression of Key Enzyme Genes and Enzyme Activity in Nitrogen Metabolic Pathway of Two Genotypes of Potato (Solanum tuberosum L.) under Different Nitrogen Supply Levels. Horticulturae 2022, 8, 769. [Google Scholar] [CrossRef]
- Li, Y.; Cui, J.; Kang, J.; Zhao, W.; Yang, K.; Fu, J. Trichoderma Rhizosphere Soil Improvement: Regulation of Nitrogen Fertilizer in Saline–Alkali Soil in Semi-Arid Region and Its Effect on the Microbial Community Structure of Maize Roots. Agronomy 2024, 14, 2340. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Liu, X.; Lu, Y.; Wang, Y. Saline-Alkali Soil Applied with Vermicompost and Humic Acid Fertilizer Improved Macroaggregate Microstructure to Enhance Salt Leaching and Inhibit Nitrogen Losses. Appl. Soil Ecol. 2020, 156, 103705. [Google Scholar] [CrossRef]
- Zhou, B.; Jia, R.; Chen, X.; Yang, L.; Duan, M.; Xiao, F.; Liang, C.; Zhou, D.; Li, W.; Liu, C. Impact of Bacteria-Nitrogen Coupling on Cotton Growth and Nitrogen Utilization under Different Salt Stress. Agric. Water Manag. 2023, 280, 108221. [Google Scholar] [CrossRef]
- Qu, W.; Han, G.; Eller, F.; Xie, B.; Wang, J.; Wu, H.; Li, J.; Zhao, M. Nitrogen Input in Different Chemical Forms and Levels Stimulates Soil Organic Carbon Decomposition in a Coastal Wetland. CATENA 2020, 194, 104672. [Google Scholar] [CrossRef]
- Dong, L.; Yao, X.; Zhang, H.; Deng, Y.; Hu, T.; Baquerizo, M.D.; Wang, W. Microbial Diversity Is Especially Important for Supporting Soil Function in Low Nitrogen Ecosystems. Soil Biol. Biochem. 2024, 194, 109442. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
| Treatments Coded | Treatments |
|---|---|
| Control N1 N2 N3 | In different fertilization periods, the same disturbance treatment was applied to the soil, but no nitrogen fertilizer was applied. Basal fertilizer: 20 kg N ha−1; seventh leaf stage: 40 kg N ha−1; tasseling stage: 20 kg N ha−1. Total amount of nitrogen fertilizer: 60 kg N ha−1. Basal fertilizer: 45 kg N ha−1; seventh leaf stage: 90 kg N ha−1; tasseling stage: 45 kg N ha−1. Total amount of nitrogen fertilizer: 180 kg N ha−1. Basal fertilizer: 75 kg N ha−1; seventh leaf stage: 150 kg N ha−1; tasseling stage: 75 kg N ha−1. Total amount of nitrogen fertilizer: 300 kg N ha−1. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiao, Y.; Zhao, W.; Kang, J.; Yang, K.; Fu, J. Characteristic Functional Genera (CFG) Mediate Nitrogen Priming Effect in the Microbiome of Saline–Alkaline Farmland. Plants 2025, 14, 1806. https://doi.org/10.3390/plants14121806
Li Y, Xiao Y, Zhao W, Kang J, Yang K, Fu J. Characteristic Functional Genera (CFG) Mediate Nitrogen Priming Effect in the Microbiome of Saline–Alkaline Farmland. Plants. 2025; 14(12):1806. https://doi.org/10.3390/plants14121806
Chicago/Turabian StyleLi, Yicong, Yao Xiao, Wei Zhao, Jiarui Kang, Kejun Yang, and Jian Fu. 2025. "Characteristic Functional Genera (CFG) Mediate Nitrogen Priming Effect in the Microbiome of Saline–Alkaline Farmland" Plants 14, no. 12: 1806. https://doi.org/10.3390/plants14121806
APA StyleLi, Y., Xiao, Y., Zhao, W., Kang, J., Yang, K., & Fu, J. (2025). Characteristic Functional Genera (CFG) Mediate Nitrogen Priming Effect in the Microbiome of Saline–Alkaline Farmland. Plants, 14(12), 1806. https://doi.org/10.3390/plants14121806






