Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Cucumber (Cucumis sativus)
Abstract
1. Introduction
2. Results
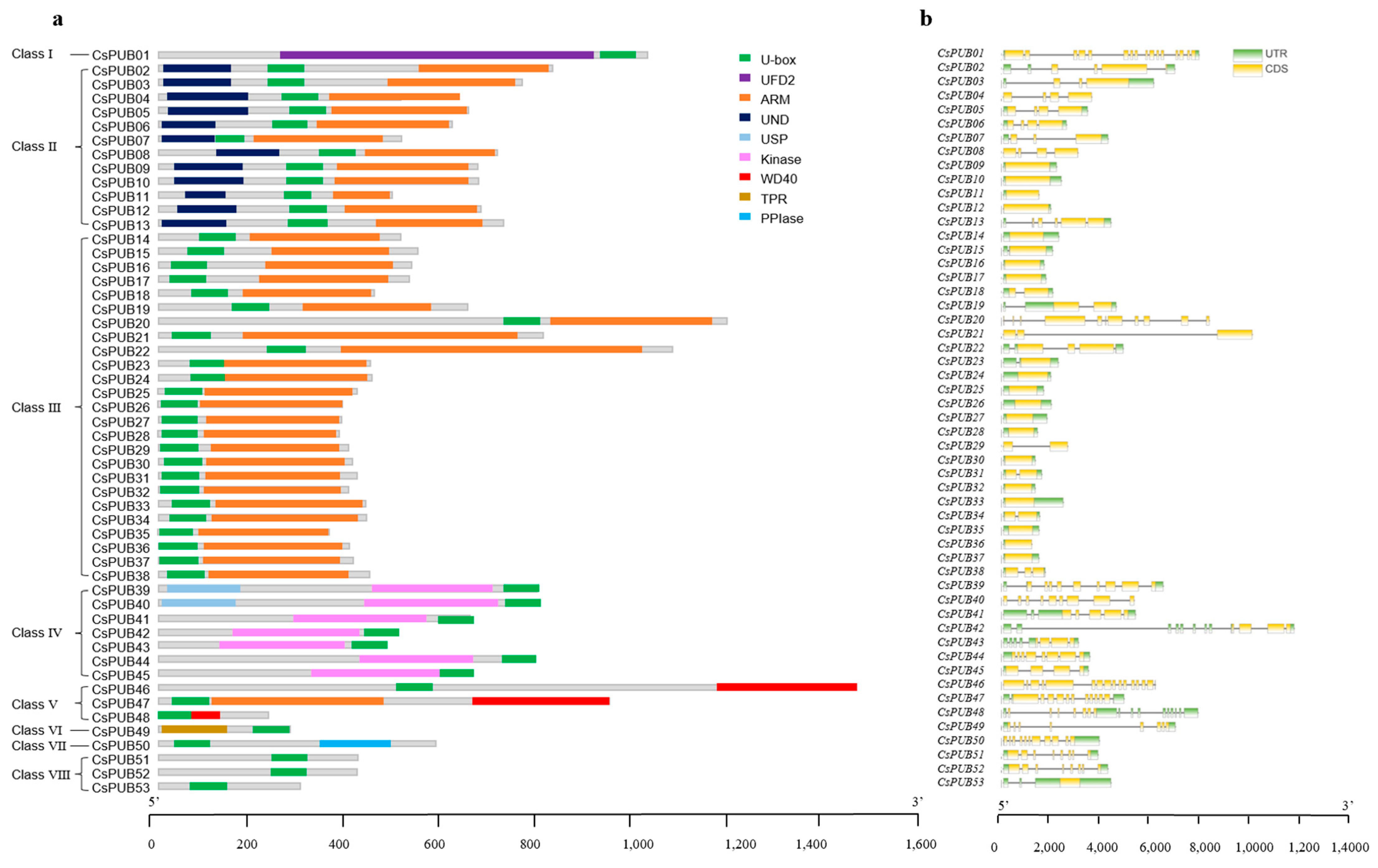
2.1. Genome-Wide Identification of U-Box Gene Family Members in Cucumber
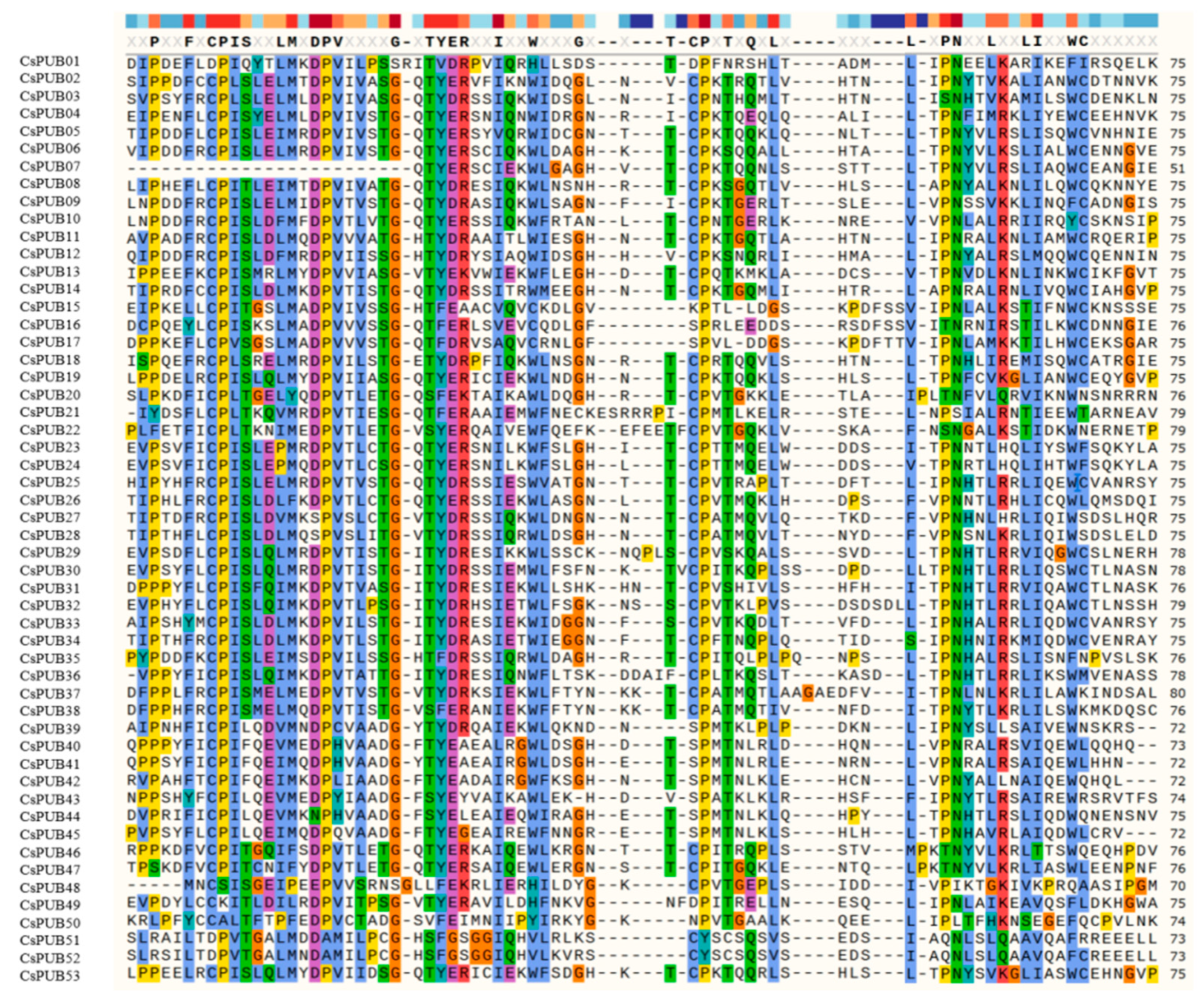
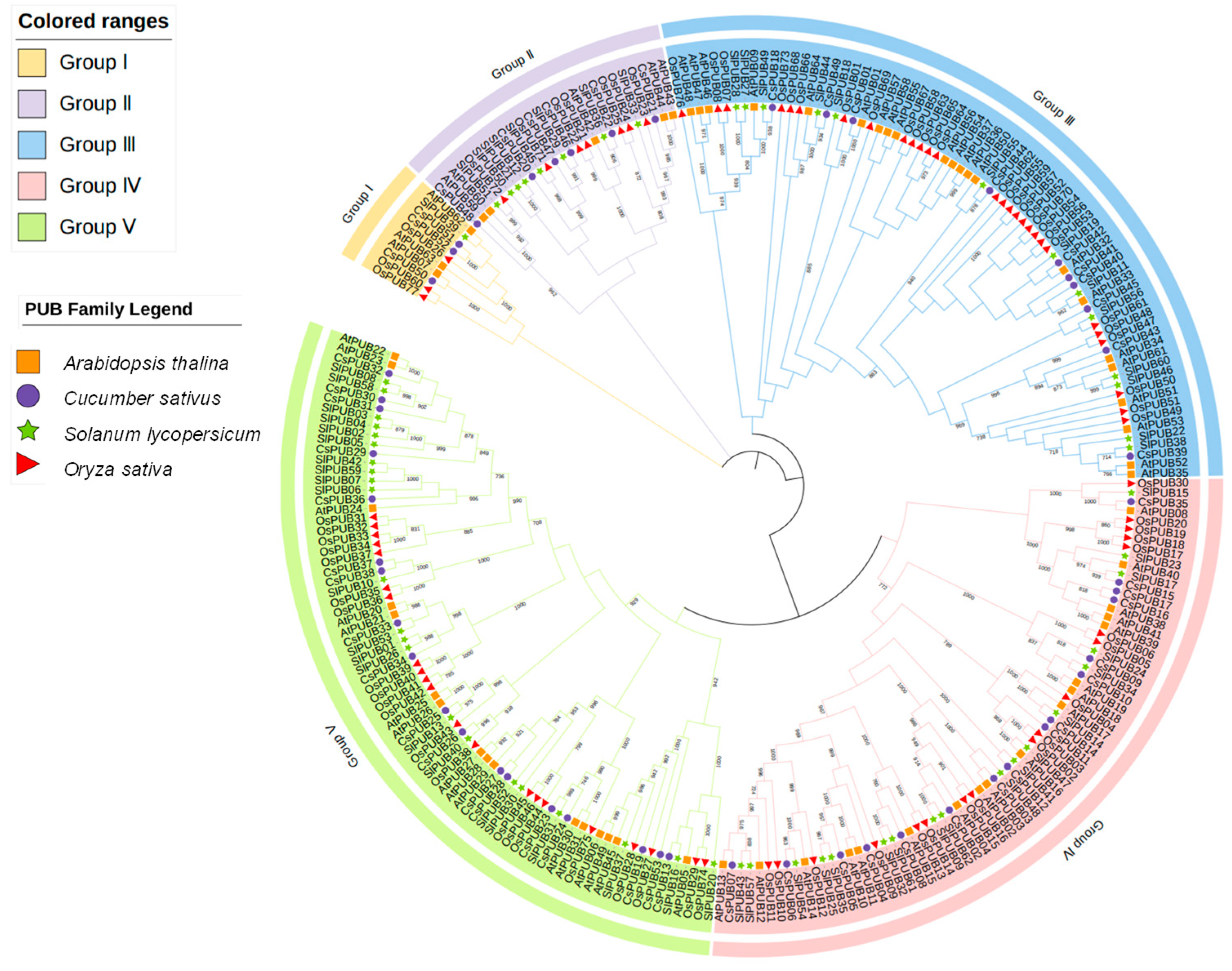
2.2. Evolutionary Analysis of CsPUB Members
2.3. Chromosome Localization and Collinearity Analysis of CsPUB Genes
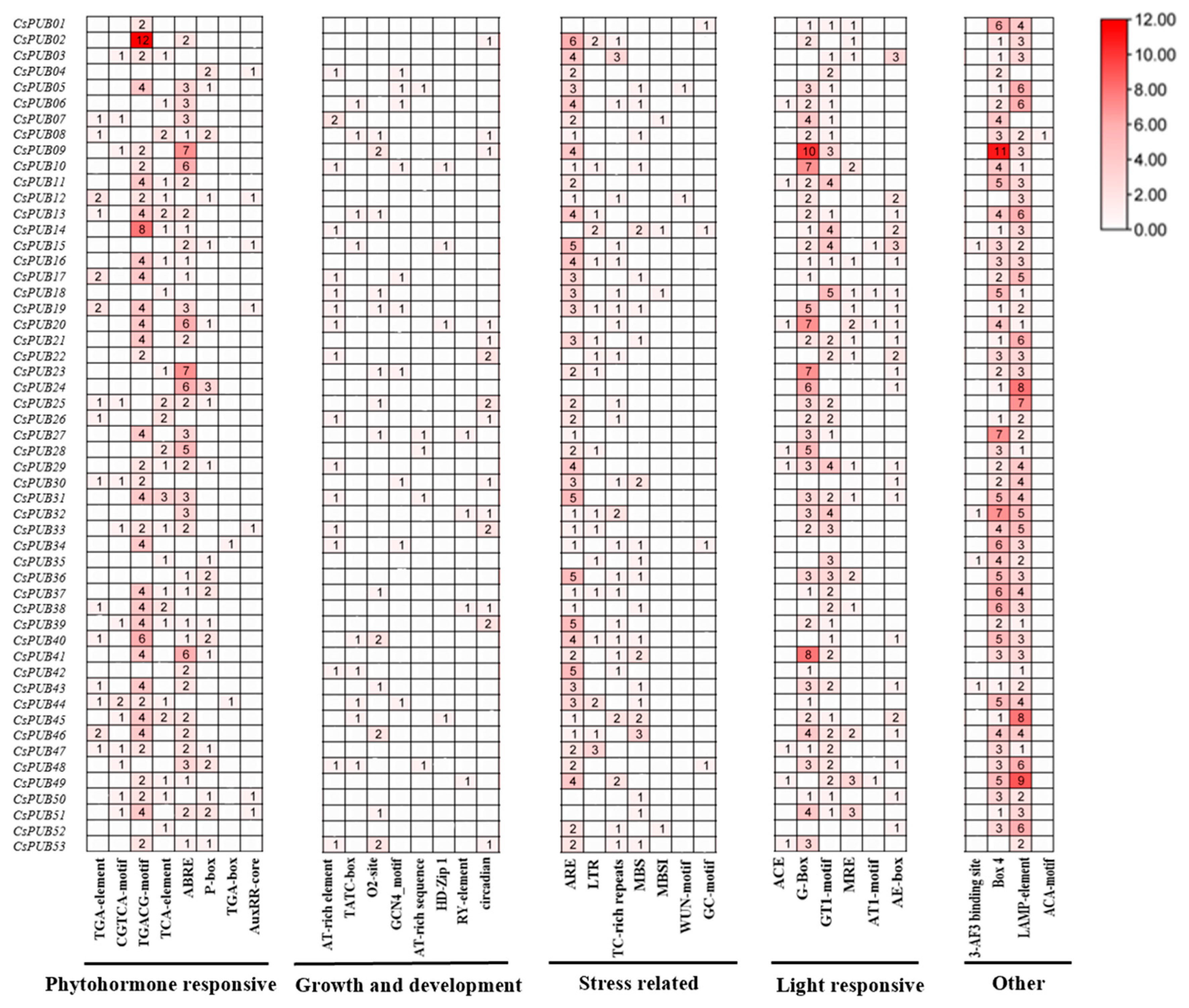
2.4. Cis-Acting Elements Analysis of the Promoter of CsPUBs
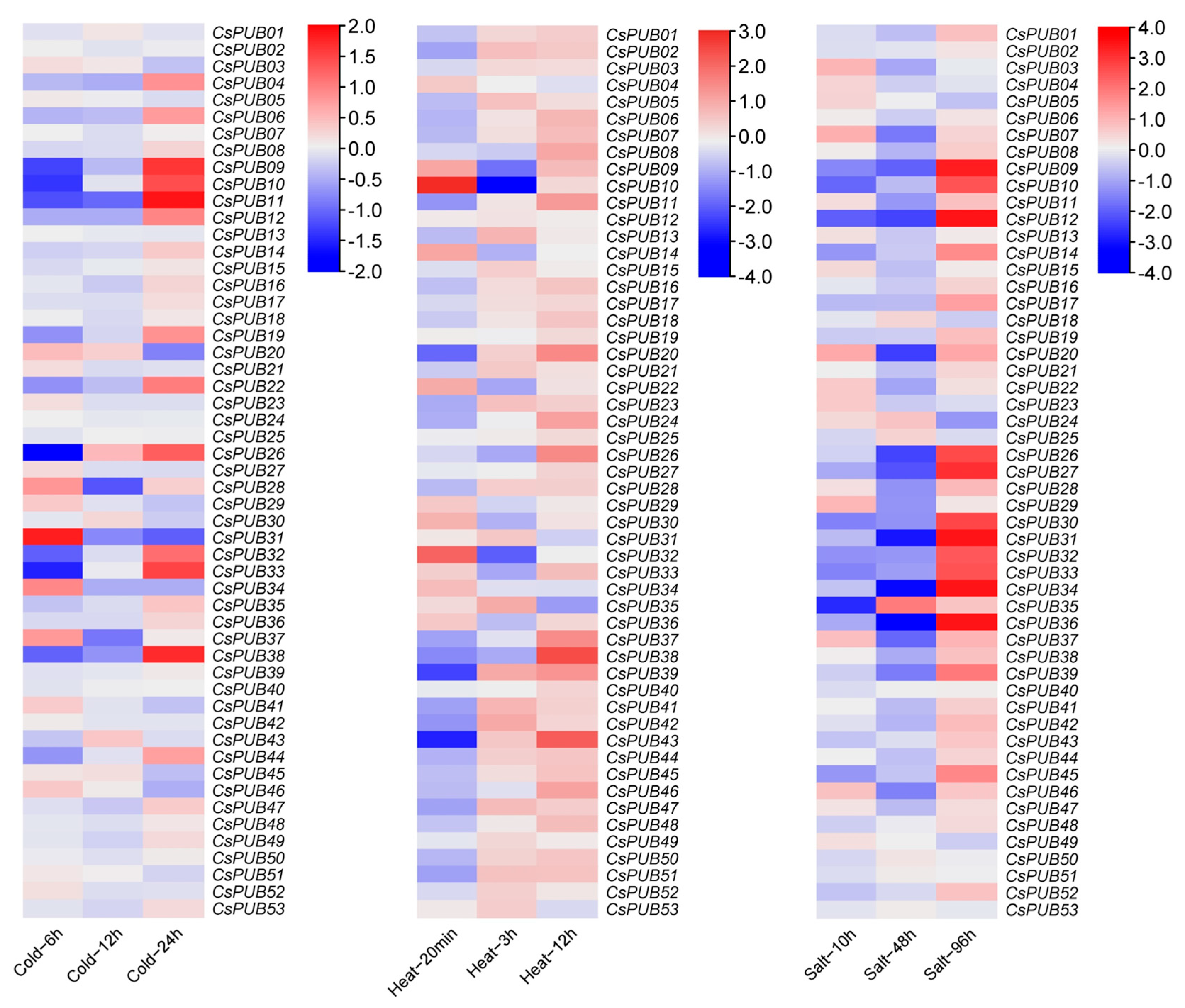
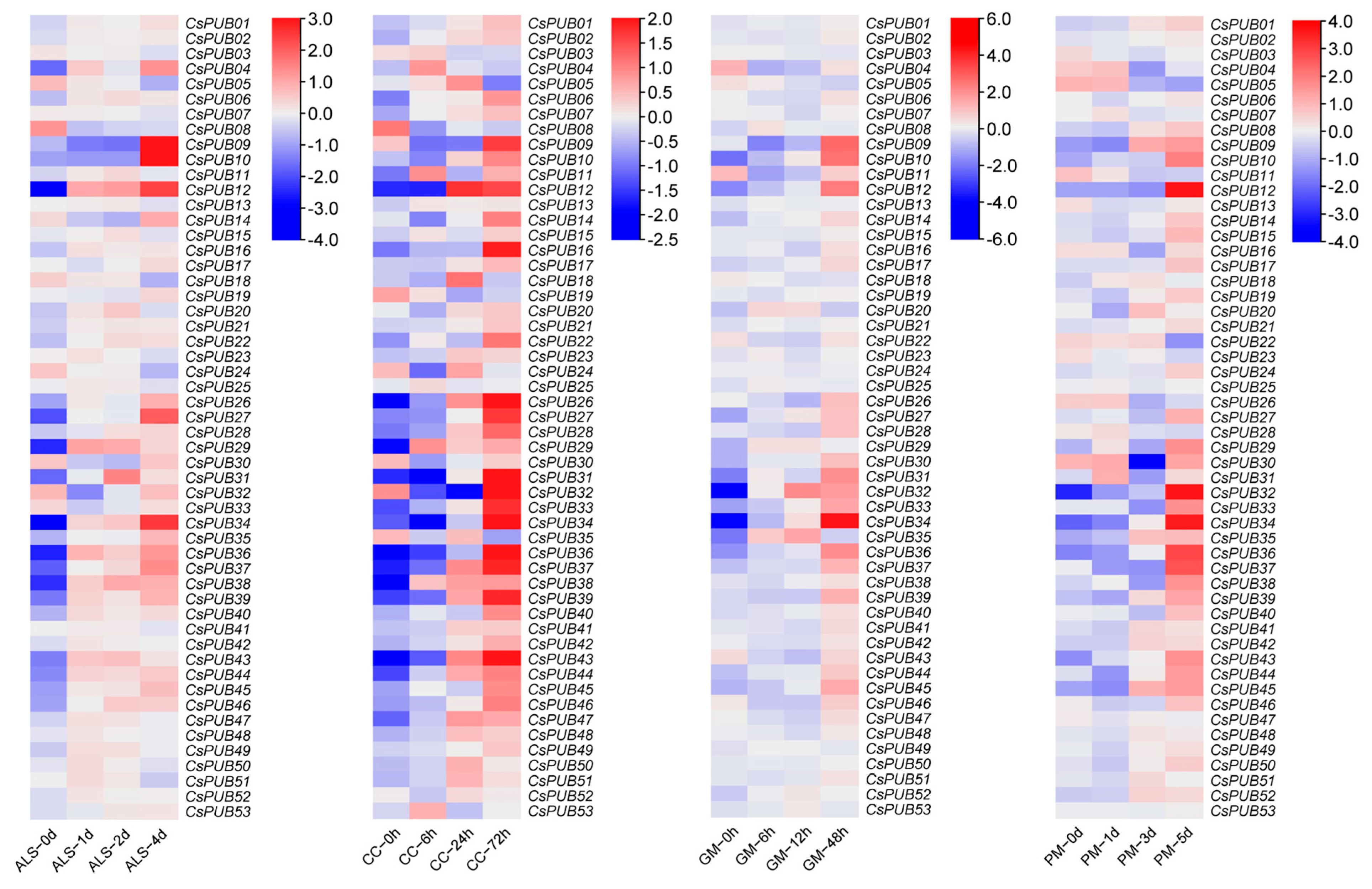
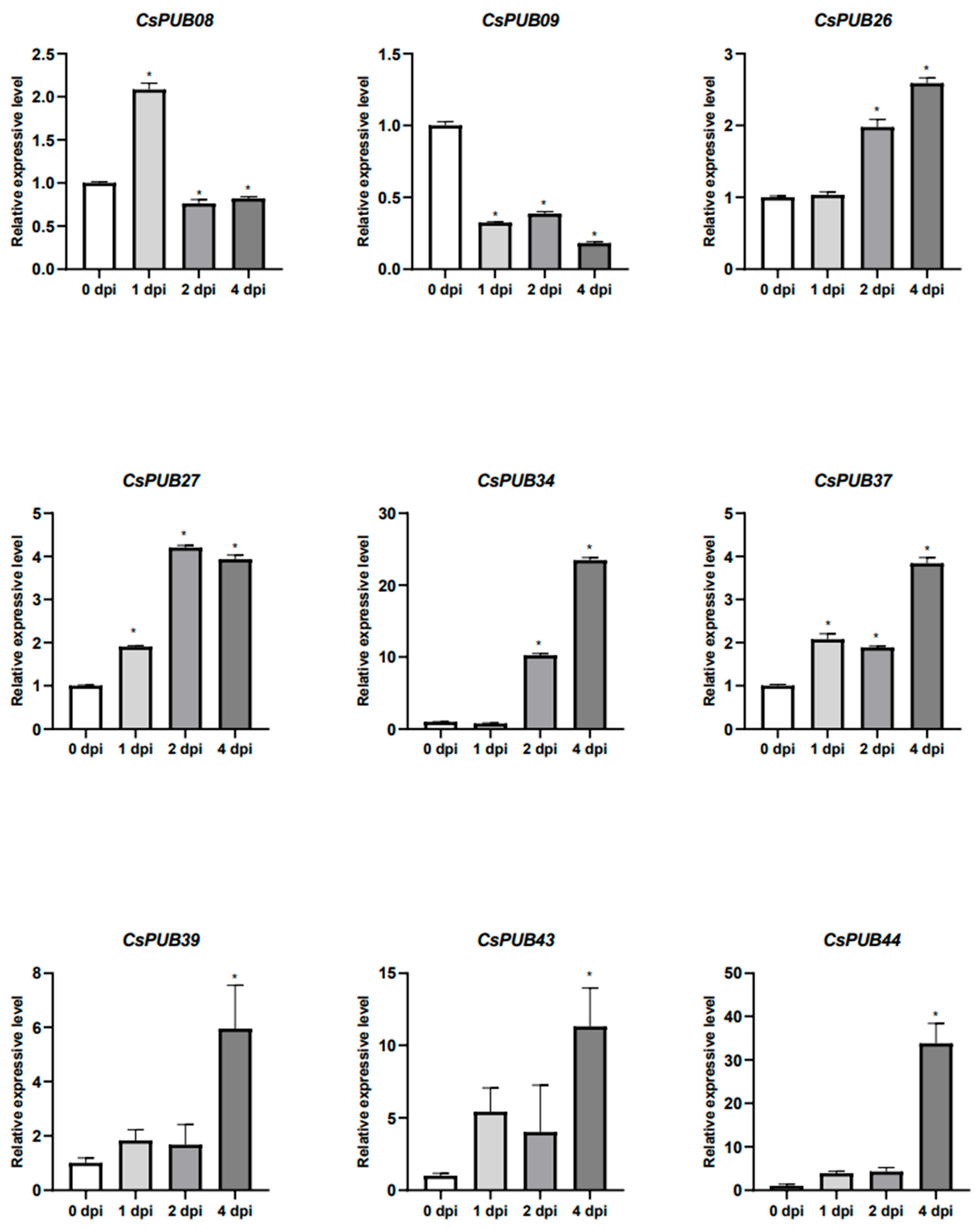
2.5. Expression Analysis of CsPUBs
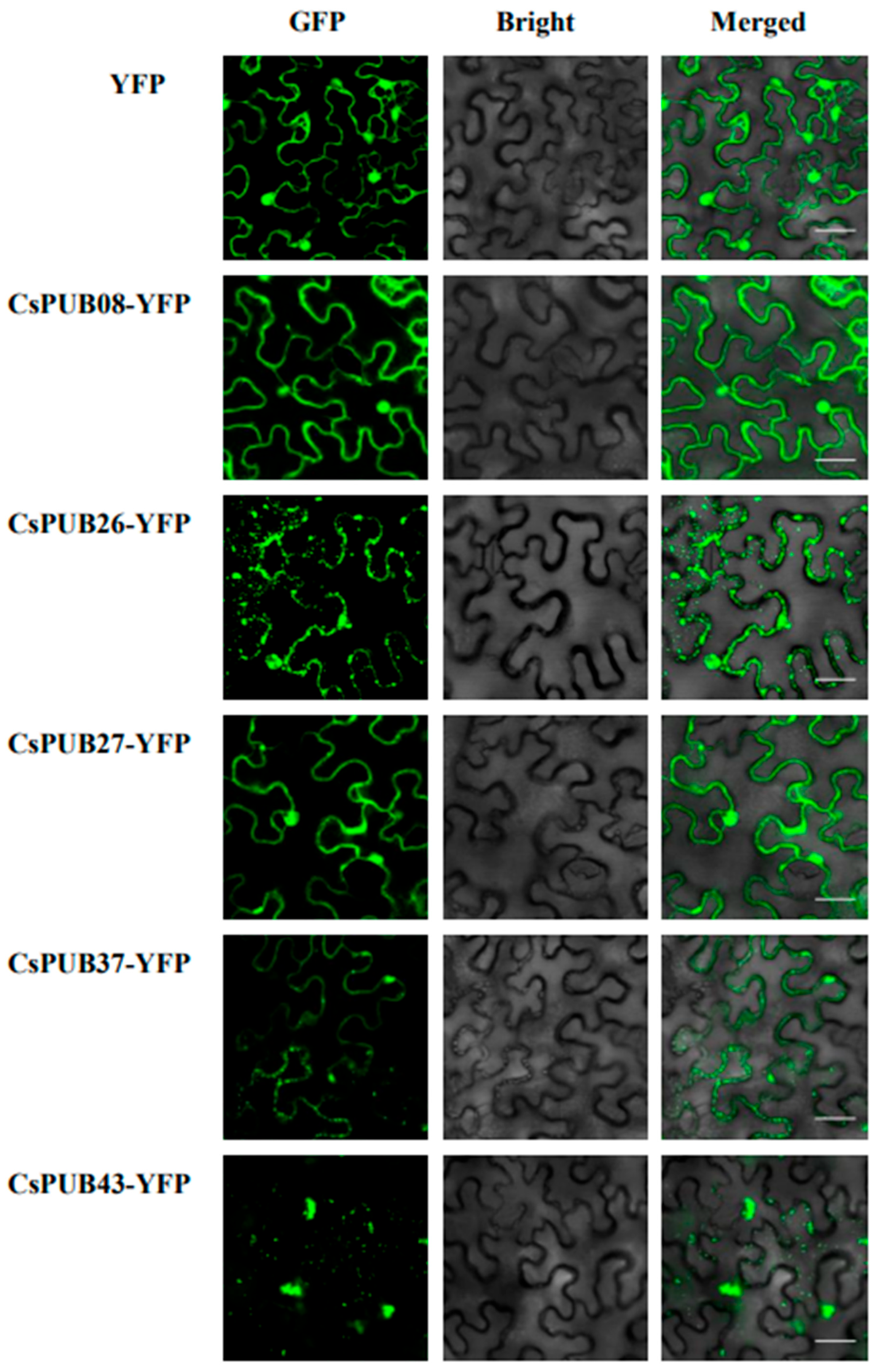
2.6. Subcellular Localization Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Identification of Plant U-Boxes and Construction of Evolutionary Tree and Protein Information Analysis
4.3. Chromosome Localization and Collinearity Analysis
4.4. Gene Structure, Conserved Domain, and Cis-Acting Element Analysis
4.5. Pathogen Treatment
4.6. Gene Expression Analysis
4.7. Subcellular Localization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vierstra, R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A. The ubiquitin-proteasome pathway: On protein death and cell life. EMBO J. 1998, 17, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L.; Hauksdóttir, H.; Troy, A.; Herschleb, J.; Kraft, E.; Callis, J. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 2005, 137, 13–30. [Google Scholar] [CrossRef]
- Hua, Z.; Vierstra, R.D. The cullin-RING ubiquitin-protein ligases. Annu. Rev. Plant Biol. 2011, 62, 299–334. [Google Scholar] [CrossRef]
- Chen, L.; Hellmann, H. Plant E3 ligases: Flexible enzymes in a sessile world. Mol. Plant 2013, 6, 1388–1404. [Google Scholar] [CrossRef]
- Aravind, L.; Koonin, E.V. The U box is a modified RING finger—A common domain in ubiquitination. Curr. Biol. 2000, 10, R132–R134. [Google Scholar] [CrossRef]
- Cyr, D.M.; Höhfeld, J.; Patterson, C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci. 2002, 27, 368–375. [Google Scholar] [CrossRef]
- Marín, I. Ancient origin of animal U-box ubiquitin ligases. BMC Evol. Biol. 2010, 10, 331. [Google Scholar] [CrossRef]
- Wiborg, J.; O’Shea, C.; Skriver, K. Biochemical function of typical and variant Arabidopsis thaliana U-box E3 ubiquitin-protein ligases. Biochem. J. 2008, 413, 447–457. [Google Scholar] [CrossRef]
- Luo, Q.; Li, Y.; Wang, W.; Fei, X.; Deng, X. Correction: Genome-Wide Survey and Expression Analysis of Chlamydomonas reinhardtii U-box E3 Ubiquitin Ligases (CrPUBs) Reveal a Functional Lipid Metabolism Module. PLoS ONE 2015, 10, e0142996. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.R.; Park, C.H.; Venu, R.C.; Gough, J.; Wang, G.L. Classification, expression pattern, and E3 ligase activity assay of rice U-box-containing proteins. Mol. Plant 2008, 1, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.Y.; Cho, S.K.; Hong, Y.; Kim, J.; Kim, J.H.; Kim, G.M.; Chen, Y.J.; Knoch, E.; Møller, B.L.; Kim, W.T.; et al. Classification of barley U-box E3 ligases and their expression patterns in response to drought and pathogen stresses. BMC Genom. 2019, 20, 326. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Taganna, J. Genome-wide analysis of the U-box E3 ubiquitin ligase enzyme gene family in tomato. Sci. Rep. 2020, 10, 9581. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Cong, Y.; Wang, T.; Zhong, X.; Yang, S.; Li, Y.; Gai, J. Genome-Wide Identification of Soybean U-Box E3 Ubiquitin Ligases and Roles of GmPUB8 in Negative Regulation of Drought Stress Response in Arabidopsis. Plant Cell Physiol. 2016, 57, 1189–1209. [Google Scholar] [CrossRef]
- Trujillo, M. News from the PUB: Plant U-box type E3 ubiquitin ligases. J. Exp. Bot. 2018, 69, 371–384. [Google Scholar] [CrossRef]
- Trenner, J.; Monaghan, J.; Saeed, B.; Quint, M.; Shabek, N.; Trujillo, M. Evolution and Functions of Plant U-Box Proteins: From Protein Quality Control to Signaling. Annu. Rev. Plant Biol. 2022, 73, 93–121. [Google Scholar] [CrossRef]
- Abhinandan, K.; Hickerson, N.M.N.; Lan, X.; Samuel, M.A. Disabling of ARC1 through CRISPR-Cas9 leads to a complete breakdown of self-incompatibility responses in Brassica napus. Plant Commun. 2023, 4, 100504. [Google Scholar] [CrossRef]
- Kinoshita, A.; ten Hove, C.A.; Tabata, R.; Yamada, M.; Shimizu, N.; Ishida, T.; Yamaguchi, K.; Shigenobu, S.; Takebayashi, Y.; Iuchi, S.; et al. A plant U-box protein, PUB4, regulates asymmetric cell division and cell proliferation in the root meristem. Development 2015, 142, 444–453. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Yu, B.; Yin, Z.; Xia, Y. EXTRA-LARGE G PROTEINs Interact with E3 Ligases PUB4 and PUB2 and Function in Cytokinin and Developmental Processes. Plant Physiol. 2017, 173, 1235–1246. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Zhong, H.; Chen, S.; Wong, K.B.; Xia, Y. Arabidopsis PUB2 and PUB4 connect signaling components of pattern-triggered immunity. New Phytol. 2022, 233, 2249–2265. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Derkacheva, M.; Rufian, J.S.; Brillada, C.; Kowarschik, K.; Jiang, S.; Derbyshire, P.; Ma, M.; DeFalco, T.A.; Morcillo, R.J.L.; et al. The Arabidopsis E3 ubiquitin ligase PUB4 regulates BIK1 and is targeted by a bacterial type-III effector. EMBO J. 2022, 41, e107257. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Lin, W.; Gao, X.; Wu, S.; Cheng, C.; Avila, J.; Heese, A.; Devarenne, T.P.; He, P.; Shan, L. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 2011, 332, 1439–1442. [Google Scholar] [CrossRef]
- Wang, J.; Grubb, L.E.; Wang, J.; Liang, X.; Li, L.; Gao, C.; Ma, M.; Feng, F.; Li, M.; Li, L.; et al. A Regulatory Module Controlling Homeostasis of a Plant Immune Kinase. Mol. Cell 2018, 69, 493–504.e496. [Google Scholar] [CrossRef]
- Ma, A.; Zhang, D.; Wang, G.; Wang, K.; Li, Z.; Gao, Y.; Li, H.; Bian, C.; Cheng, J.; Han, Y.; et al. Verticillium dahliae effector VDAL protects MYB6 from degradation by interacting with PUB25 and PUB26 E3 ligases to enhance Verticillium wilt resistance. Plant Cell 2021, 33, 3675–3699. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Yu, C.; Shi, X.; Lan, W.; Gao, C.; Yang, J.; Dai, H.; Zhang, X.; Zhang, H.; et al. Release of a ubiquitin brake activates OsCERK1-triggered immunity in rice. Nature 2024, 629, 1158–1164. [Google Scholar] [CrossRef]
- Mou, B.; Zhao, G.; Wang, J.; Wang, S.; He, F.; Ning, Y.; Li, D.; Zheng, X.; Cui, F.; Xue, F.; et al. The OsCPK17-OsPUB12-OsRLCK176 module regulates immune homeostasis in rice. Plant Cell 2024, 36, 987–1006. [Google Scholar] [CrossRef]
- Hao, Z.; Tian, J.; Fang, H.; Fang, L.; Xu, X.; He, F.; Li, S.; Xie, W.; Du, Q.; You, X.; et al. A VQ-motif-containing protein fine-tunes rice immunity and growth by a hierarchical regulatory mechanism. Cell Rep. 2022, 40, 111235. [Google Scholar] [CrossRef]
- Ichimaru, K.; Yamaguchi, K.; Harada, K.; Nishio, Y.; Hori, M.; Ishikawa, K.; Inoue, H.; Shigeta, S.; Inoue, K.; Shimada, K.; et al. Cooperative regulation of PBI1 and MAPKs controls WRKY45 transcription factor in rice immunity. Nat. Commun. 2022, 13, 2397. [Google Scholar] [CrossRef]
- Ishikawa, K.; Yamaguchi, K.; Sakamoto, K.; Yoshimura, S.; Inoue, K.; Tsuge, S.; Kojima, C.; Kawasaki, T. Bacterial effector modulation of host E3 ligase activity suppresses PAMP-triggered immunity in rice. Nat. Commun. 2014, 5, 5430. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Ning, Y.; Shirsekar, G.; Cai, Y.; Wang, X.; Dai, L.; Wang, Z.; Liu, W.; Wang, G.L. The U-Box E3 ligase SPL11/PUB13 is a convergence point of defense and flowering signaling in plants. Plant Physiol. 2012, 160, 28–37. [Google Scholar] [CrossRef]
- Tian, Y.; Li, K.; Li, T.; Gai, W.; Zhou, J.; Deng, X.W.; Xue, Y.; Deng, Y.; He, H.; Zhang, X. The near-complete genome assembly of pickling cucumber and its mutation library illuminate cucumber functional genomics and genetic improvement. Mol. Plant 2025, 18, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Santos-Rosa, M.J.; Shirasu, K. The U-box protein family in plants. Trends Plant Sci. 2001, 6, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yu, C.; Li, L.; Wang, M.; Yang, L.; Zhang, Y.; Zhang, Y.; Wang, J.; Li, C.; Reynolds, M.P.; et al. How Many Faces Does the Plant U-Box E3 Ligase Have? Int. J. Mol. Sci. 2022, 23, 2285. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Q.; Zhang, J.; Li, E.; Yang, X.; Zhang, Z. Genome-wide identification of the plant U-box (PUB) gene family and their global expression analysis in tomato (Solanum lycopersicum). Veg. Res. 2023, 3, 16. [Google Scholar] [CrossRef]
- Woodson, J.D.; Joens, M.S.; Sinson, A.B.; Gilkerson, J.; Salome, P.A.; Weigel, D.; Fitzpatrick, J.A.; Chory, J. Ubiquitin facilitates a quality-control pathway that removes damaged chloroplasts. Science 2015, 350, 450–454. [Google Scholar] [CrossRef]
- Stegmann, M.; Anderson, R.G.; Ichimura, K.; Pecenkova, T.; Reuter, P.; Zarsky, V.; McDowell, J.M.; Shirasu, K.; Trujillo, M. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 2012, 24, 4703–4716. [Google Scholar] [CrossRef]
- Seo, D.H.; Ahn, M.Y.; Park, K.Y.; Kim, E.Y.; Kim, W.T. The N-Terminal UND Motif of the Arabidopsis U-Box E3 Ligase PUB18 Is Critical for the Negative Regulation of ABA-Mediated Stomatal Movement and Determines Its Ubiquitination Specificity for Exocyst Subunit Exo70B1. Plant Cell 2016, 28, 2952–2973. [Google Scholar] [CrossRef]
- Furlan, G.; Nakagami, H.; Eschen-Lippold, L.; Jiang, X.; Majovsky, P.; Kowarschik, K.; Hoehenwarter, W.; Lee, J.; Trujillo, M. Changes in PUB22 Ubiquitination Modes Triggered by MITOGEN-ACTIVATED PROTEIN KINASE3 Dampen the Immune Response. Plant Cell 2017, 29, 726–745. [Google Scholar] [CrossRef]
- Byun, M.Y.; Cui, L.H.; Oh, T.K.; Jung, Y.J.; Lee, A.; Park, K.Y.; Kang, B.G.; Kim, W.T. Homologous U-box E3 Ubiquitin Ligases OsPUB2 and OsPUB3 Are Involved in the Positive Regulation of Low Temperature Stress Response in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 16. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Jin, X.; Sun, Y.; Wu, Y.; Wang, W.; Huang, J. Rice OsPUB16 modulates the ‘SAPK9-OsMADS23-OsAOC’ pathway to reduce plant water-deficit tolerance by repressing ABA and JA biosynthesis. PLoS Genet. 2022, 18, e1010520. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Liu, M.; Yan, Y.; Guo, X.; Cao, X.; Jiao, Y.; Zheng, J.; Ma, Y.; Xie, Y.; Li, H.; et al. The U-box E3 ubiquitin ligase PUB35 negatively regulates ABA signaling through AFP1-mediated degradation of ABI5. Plant Cell 2024, 36, 3277–3297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, C.; Li, N.; Ge, Y.; Wang, L.; Tang, Y.; Wang, Y.; Li, Y.; Zhang, C. Ubiquitin ligase VvPUB26 in grapevine promotes proanthocyanidin synthesis and resistance to powdery mildew. Plant Physiol. 2024, 195, 2891–2910. [Google Scholar] [CrossRef]
- Trujillo, M.; Ichimura, K.; Casais, C.; Shirasu, K. Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr. Biol. 2008, 18, 1396–1401. [Google Scholar] [CrossRef]
- Kim, M.S.; Le, V.T.; Jung, Y.J.; Kang, K.K.; Cho, Y.G. OsPUB9 Gene Edited by CRISPR/Cas9 Enhanced Resistance to Bacterial Leaf Blight in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2024, 25, 7145. [Google Scholar] [CrossRef]
- Wang, D.R.; Zhang, X.W.; Xu, R.R.; Wang, G.L.; You, C.X.; An, J.P. Apple U-box-type E3 ubiquitin ligase MdPUB23 reduces cold-stress tolerance by degrading the cold-stress regulatory protein MdICE1. Hortic. Res. 2022, 9, uhac171. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Song, C.P.; Gong, Z.; Yang, S.; Ding, Y. PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis. Plant Cell 2023, 35, 3585–3603. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, X.; Qu, C.; Jin, N.; Qin, T.; Jin, L.; Huang, J. OsPUB75-OsHDA716 mediates deactivation and degradation of OsbZIP46 to negatively regulate drought tolerance in rice. Plant Physiol. 2024, 197, kiae545. [Google Scholar] [CrossRef]
- Fan, W.; Liao, X.; Tan, Y.; Wang, X.; Schroeder, J.I.; Li, Z. Arabidopsis PLANT U-BOX44 down-regulates osmotic stress signaling by mediating Ca2+-DEPENDENT PROTEIN KINASE4 degradation. Plant Cell 2023, 35, 3870–3888. [Google Scholar] [CrossRef]
- Saini, L.K.; Sharma, M.; Ravi, B.; Ghosh, S.; Pahuja, S.; Singh, N.; Pandey, G.K. Overexpression of ARM repeat/U-box containing E3 ligase, PUB2 positively regulates growth and oxidative stress response in Arabidopsis. Biochem. J. 2023, 480, 555–571. [Google Scholar] [CrossRef]
- Monaghan, J.; Xu, F.; Gao, M.; Zhao, Q.; Palma, K.; Long, C.; Chen, S.; Zhang, Y.; Li, X. Two Prp19-like U-box proteins in the MOS4-associated complex play redundant roles in plant innate immunity. PLoS Pathog. 2009, 5, e1000526. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Ryu, M.Y.; Song, C.; Kwak, J.M.; Kim, W.T. Arabidopsis PUB22 and PUB23 are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell 2008, 20, 1899–1914. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, Y.; Huang, L.; Du, F.; Zhao, X.; Li, Z.; Wang, W.; Fu, B. A U-box E3 ubiquitin ligase OsPUB67 is positively involved in drought tolerance in rice. Plant Mol. Biol. 2020, 102, 89–107. [Google Scholar] [CrossRef]
- Deb, S.; Sankaranarayanan, S.; Wewala, G.; Widdup, E.; Samuel, M.A. The S-Domain Receptor Kinase Arabidopsis Receptor Kinase2 and the U Box/Armadillo Repeat-Containing E3 Ubiquitin Ligase9 Module Mediates Lateral Root Development under Phosphate Starvation in Arabidopsis. Plant Physiol. 2014, 165, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]


| Gene ID | Gene Name | AA | MW | pI | Chr | Location | SD |
|---|---|---|---|---|---|---|---|
| CsaV4_3G004078 | CsPUB01 | 1043 | 117,952.08 | 5.4 | 3 | 38,288,116–38,297,099 | + |
| CsaV4_2G001478 | CsPUB02 | 1142 | 131,432.04 | 6.37 | 2 | 14,584,660–14,591,279 | − |
| CsaV4_7G001821 | CsPUB03 | 776 | 84,567.83 | 6.1 | 7 | 23,414,401–23,421,303 | − |
| CsaV4_5G002287 | CsPUB04 | 642 | 17,463.87 | 4.95 | 5 | 28,595,090–28,597,391 | + |
| CsaV4_5G001121 | CsPUB05 | 661 | 72,164.67 | 6.32 | 5 | 10,256,146–10,260,002 | − |
| CsaV4_5G003054 | CsPUB06 | 189 | 20,192.19 | 6.34 | 5 | 33,278,778–33,279,989 | − |
| CsaV4_1G002812 | CsPUB07 | 519 | 56,015.17 | 6.65 | 1 | 29,784,570–29,789,371 | − |
| CsaV4_6G001024 | CsPUB08 | 292 | 31,777.82 | 6.01 | 6 | 8,320,818–8,324,444 | − |
| CsaV4_1G000903 | CsPUB09 | 681 | 75,410.55 | 8.32 | 1 | 6,312,230–6,314,661 | − |
| CsaV4_5G002605 | CsPUB10 | 683 | 76,794.22 | 7.42 | 5 | 30,687,010–30,689,661 | − |
| CsaV4_4G002233 | CsPUB11 | 498 | 55,784.36 | 6.3 | 4 | 37,600,005–37,601,623 | + |
| CsaV4_6G000201 | CsPUB12 | 688 | 75,882.62 | 7.85 | 6 | 1,364,117–1,366,286 | + |
| CsaV4_4G000578 | CsPUB13 | 738 | 82,591.93 | 6.04 | 4 | 6,139,853–6,144,787 | + |
| CsaV4_1G002691 | CsPUB14 | 517 | 55,681.77 | 6.81 | 1 | 28,557,228–28,559,764 | − |
| CsaV4_5G003208 | CsPUB15 | 554 | 60,418.4 | 7.56 | 5 | 34,474,333–34,476,587 | − |
| CsaV4_1G000891 | CsPUB16 | 540 | 59,407.43 | 6.63 | 1 | 6,255,763–6,257,621 | + |
| CsaV4_1G000189 | CsPUB17 | 452 | 49,441.99 | 5.65 | 1 | 2,219,249–2,221,155 | + |
| CsaV4_3G002876 | CsPUB18 | 291 | 32,302.81 | 8.79 | 3 | 30,441,628–30,442,788 | − |
| CsaV4_2G002560 | CsPUB19 | 659 | 72,594.83 | 5.16 | 2 | 43,412,255–43,417,433 | − |
| CsaV4_1G003549 | CsPUB20 | 1213 | 137,319.25 | 6.23 | 1 | 35,703,460–35,712,913 | + |
| CsaV4_6G002143 | CsPUB21 | 495 | 53,838.87 | 5.11 | 6 | 24,740,406–24,742,324 | − |
| CsaV4_5G002792 | CsPUB22 | 1014 | 113,306.44 | 5.35 | 5 | 31,741,490–31,746,985 | + |
| CsaV4_4G003100 | CsPUB23 | 453 | 49,087.09 | 5.83 | 4 | 46,390,827–46,393,341 | + |
| CsaV4_2G002621 | CsPUB24 | 455 | 49,490.86 | 6.67 | 2 | 43,808,284–43,810,452 | − |
| CsaV4_3G001913 | CsPUB25 | 425 | 46,364.36 | 5.97 | 3 | 14,930,324–14,932,165 | − |
| CsaV4_2G001668 | CsPUB26 | 399 | 44,849.97 | 5.85 | 2 | 16,182,318–16,184,507 | − |
| CsaV4_6G003001 | CsPUB27 | 404 | 44,791.15 | 8.2 | 6 | 31,428,505–31,430,499 | − |
| CsaV4_2G001635 | CsPUB28 | 387 | 43,290.96 | 7.91 | 2 | 15,924,847–15,926,401 | − |
| CsaV4_1G003701 | CsPUB29 | 265 | 29,234.28 | 6.14 | 1 | 37,471,774–37,474,930 | − |
| CsaV4_6G002670 | CsPUB30 | 414 | 45,873.46 | 8.67 | 6 | 29,000,714–29,002,167 | − |
| CsaV4_4G001209 | CsPUB31 | 424 | 46,727.27 | 8.96 | 4 | 12,441,210–12,442,959 | − |
| CsaV4_5G001204 | CsPUB34 | 444 | 49,923.81 | 8.17 | 5 | 11,491,336–11,492,993 | + |
| CsaV4_3G003735 | CsPUB32 | 406 | 44,468.67 | 9.08 | 3 | 36,008,291–36,009,734 | − |
| CsaV4_3G002201 | CsPUB33 | 442 | 49,149.68 | 8.4 | 3 | 18,505,395–18,508,135 | − |
| CsaV4_6G003794 | CsPUB35 | 365 | 39,439.79 | 8.5 | 6 | 35,988,799–35,990,428 | − |
| CsaV4_1G003699 | CsPUB36 | 408 | 45,751.58 | 9.04 | 1 | 37,393,795–37,395,087 | + |
| CsaV4_3G001063 | CsPUB37 | 415 | 46,407.31 | 6.91 | 3 | 8,949,370–8,950,992 | − |
| CsaV4_6G000661 | CsPUB38 | 451 | 50,629.08 | 8.33 | 6 | 5,445,539–5,447,447 | − |
| CsaV4_5G000484 | CsPUB39 | 808 | 90,431.07 | 8.09 | 5 | 3,491,174–3,498,511 | + |
| CsaV4_6G000061 | CsPUB40 | 813 | 91,533.06 | 5.66 | 6 | 406,116–412,114 | + |
| CsaV4_3G000934 | CsPUB41 | 669 | 75,495.52 | 5.72 | 3 | 8,154,010–8,160,068 | − |
| CsaV4_3G001789 | CsPUB42 | 510 | 58,056.97 | 6.07 | 3 | 13,700,345–13,713,702 | + |
| CsaV4_2G000042 | CsPUB43 | 489 | 55,675.74 | 5.98 | 2 | 941,836–945,270 | − |
| CsaV4_6G003328 | CsPUB44 | 806 | 91,648.28 | 5.72 | 6 | 33,673,401–33,677,361 | + |
| CsaV4_6G001517 | CsPUB45 | 671 | 75,819.23 | 5.67 | 6 | 12,689,616–12,693,513 | − |
| CsaV4_3G004576 | CsPUB46 | 1489 | 165,762.75 | 5.84 | 3 | 42,249,414–42,256,392 | − |
| CsaV4_1G003910 | CsPUB47 | 962 | 106,853.78 | 5.24 | 1 | 40,158,873–40,164,408 | − |
| CsaV4_3G001224 | CsPUB48 | 235 | 26,027.79 | 6.72 | 3 | 9,905,953–9,914,891 | − |
| CsaV4_3G002875 | CsPUB49 | 281 | 32,050.38 | 5.41 | 3 | 30,429,405–30,437,301 | + |
| CsaV4_6G003237 | CsPUB50 | 592 | 65,020.26 | 8.16 | 6 | 33,109,149–33,113,551 | − |
| CsaV4_5G000913 | CsPUB51 | 425 | 47,216.88 | 5.04 | 5 | 7,225,532–7,229,873 | − |
| CsaV4_3G004442 | CsPUB52 | 308 | 33,595.38 | 4.43 | 3 | 41,242,520–41,247,309 | − |
| CsaV4_5G002475 | CsPUB53 | 303 | 33,708.96 | 4.77 | 5 | 29,913,450–29,918,387 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhao, T.; Song, H.; Sha, S.; Ma, J.; Zhang, R.; Kong, W.; Yang, S.; Liu, J.; Wang, Y. Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Cucumber (Cucumis sativus). Plants 2025, 14, 1801. https://doi.org/10.3390/plants14121801
Chen Q, Zhao T, Song H, Sha S, Ma J, Zhang R, Kong W, Yang S, Liu J, Wang Y. Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Cucumber (Cucumis sativus). Plants. 2025; 14(12):1801. https://doi.org/10.3390/plants14121801
Chicago/Turabian StyleChen, Quanqing, Tian Zhao, Hao Song, Siyuan Sha, Jun Ma, Ruihan Zhang, Weiwen Kong, Shuying Yang, Jinglan Liu, and Yiping Wang. 2025. "Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Cucumber (Cucumis sativus)" Plants 14, no. 12: 1801. https://doi.org/10.3390/plants14121801
APA StyleChen, Q., Zhao, T., Song, H., Sha, S., Ma, J., Zhang, R., Kong, W., Yang, S., Liu, J., & Wang, Y. (2025). Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Cucumber (Cucumis sativus). Plants, 14(12), 1801. https://doi.org/10.3390/plants14121801






