The R3-MYB Transcription Factor DcMYB56 Regulates Anthocyanin Accumulation by Activating the Expression of Anthocyanin Biosynthesis-Related Genes in Dendrobium candidum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Anthocyanin Composition and Contents
2.3. Quantitative RT-PCR (qRT-PCR)
2.4. Gene Cloning and Amino Acid Sequence Analysis
2.5. Subcellular Localization Prediction
2.6. Yeast One-Hybrid Assay
2.7. Transcriptional Factor Activity Assay
2.8. Establishment of Transgenic A. thaliana Plants
2.9. Statistical Analysis
3. Results
3.1. Cyanidin Derivatives of Anthocyanins Related to the Red Color of D. candidum Stems
3.2. Isolation and Analysis of DcMYB56
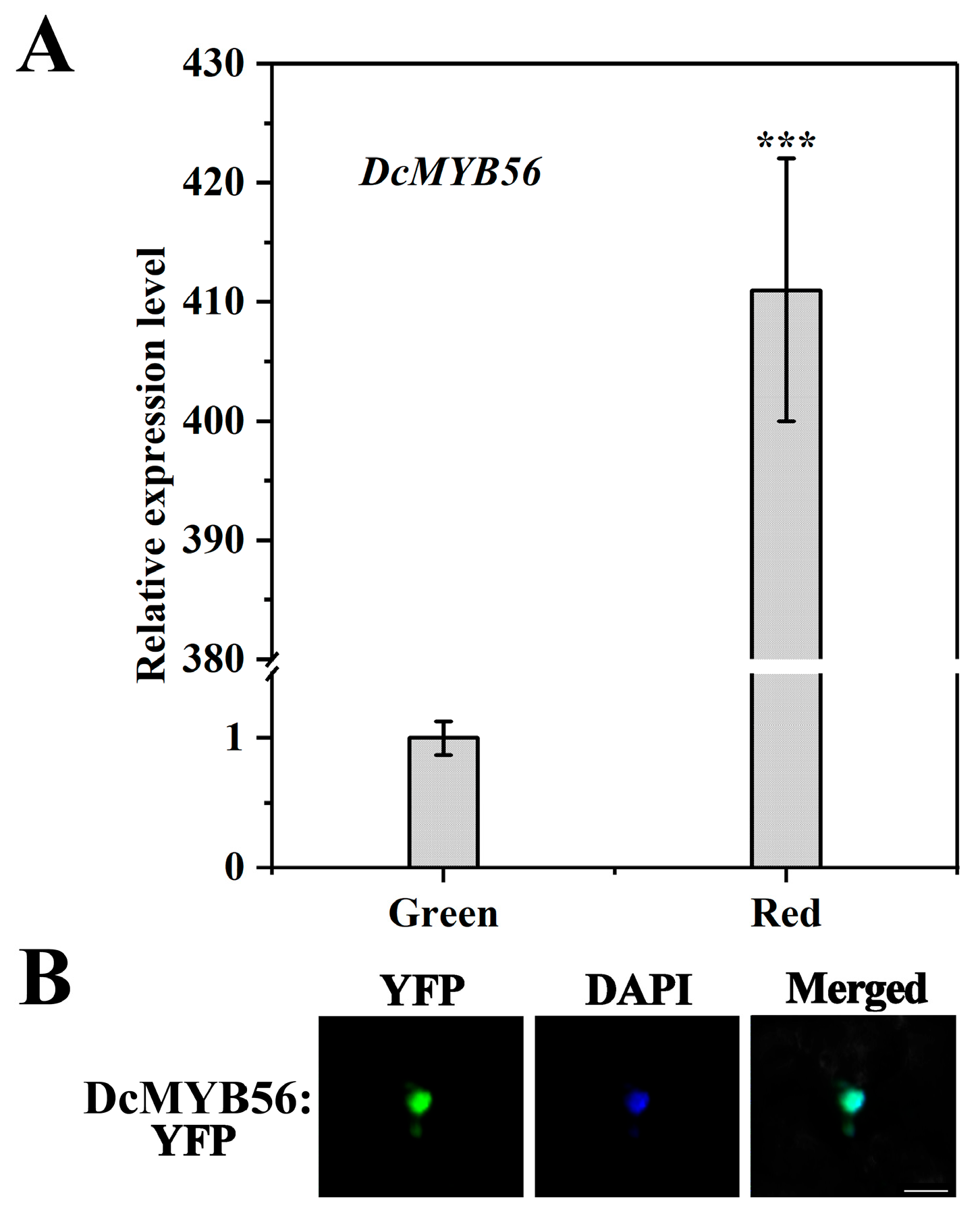
3.3. The Expression and Nuclear Localization of DcMYB56 in Red and Green Stems of D. candidum
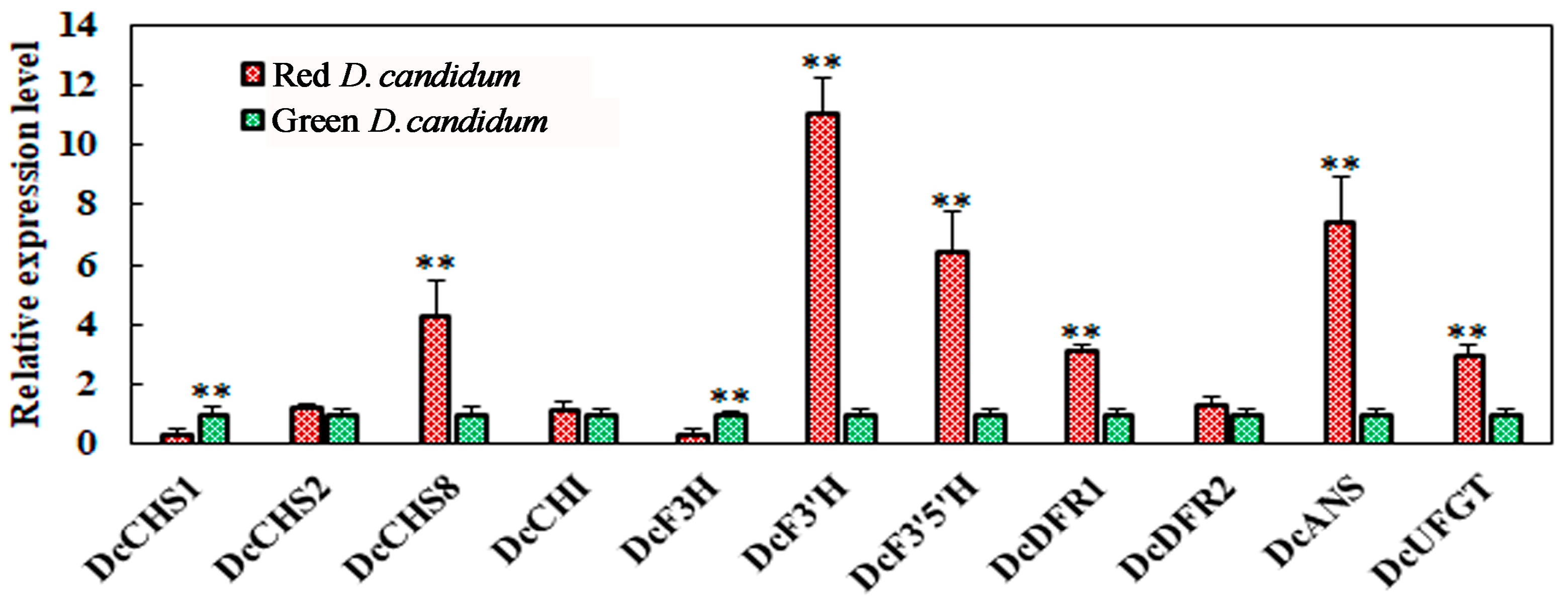
3.4. Expression of Key Anthocyanin Biosynthesis-Related Genes
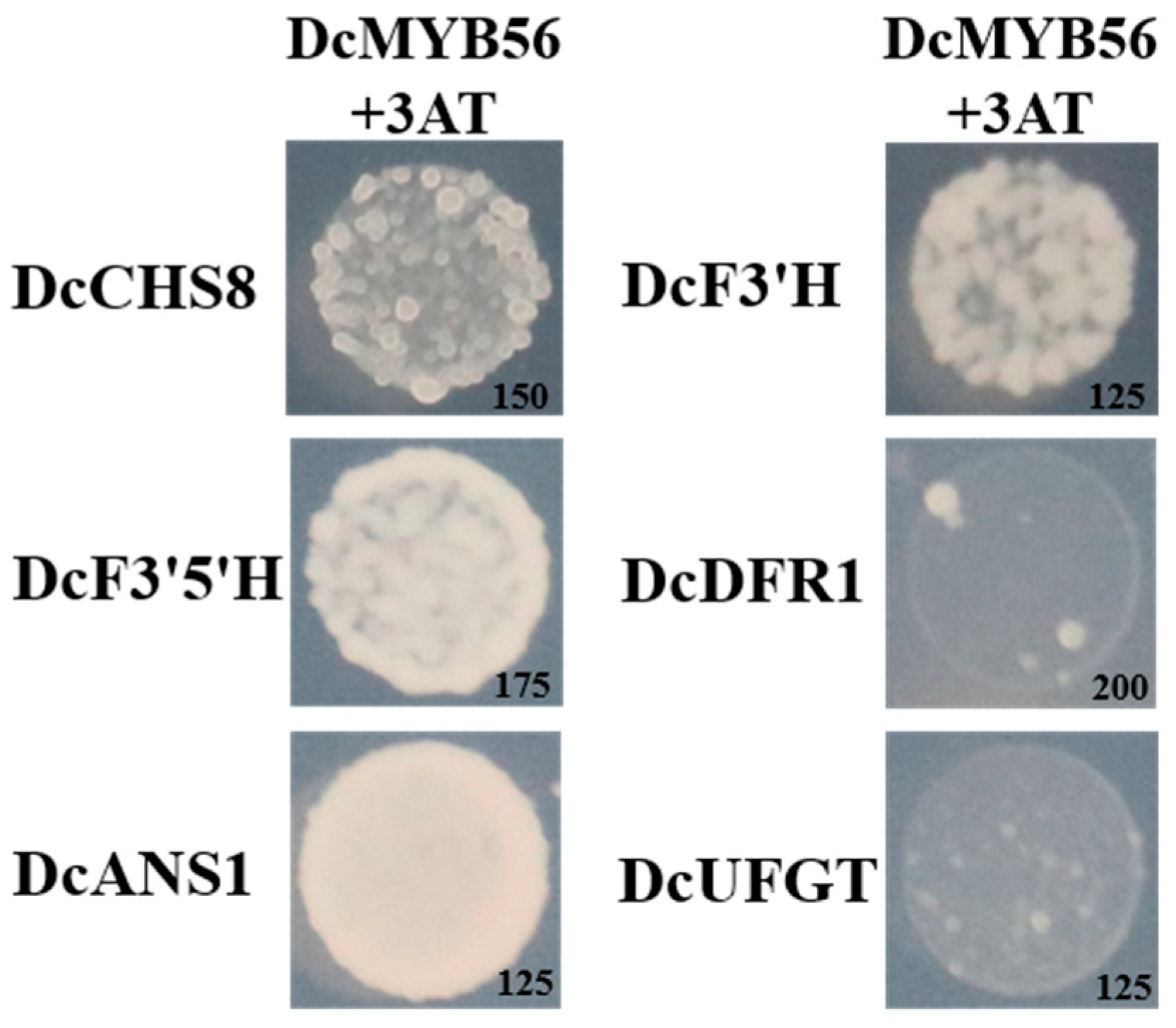
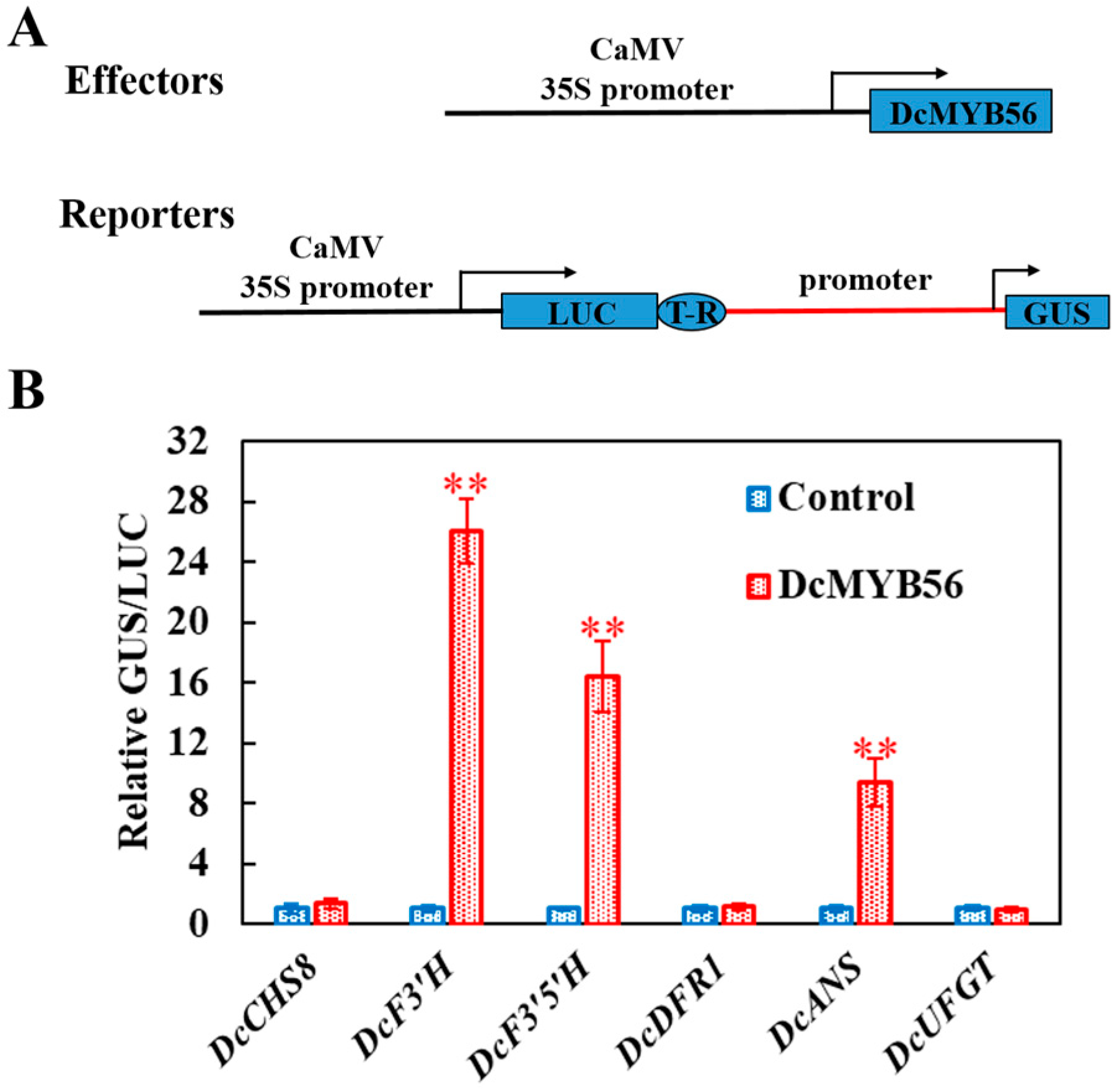
3.5. DcMYB56 Regulates the Expression of Anthocyanin Biosynthesis-Related Genes
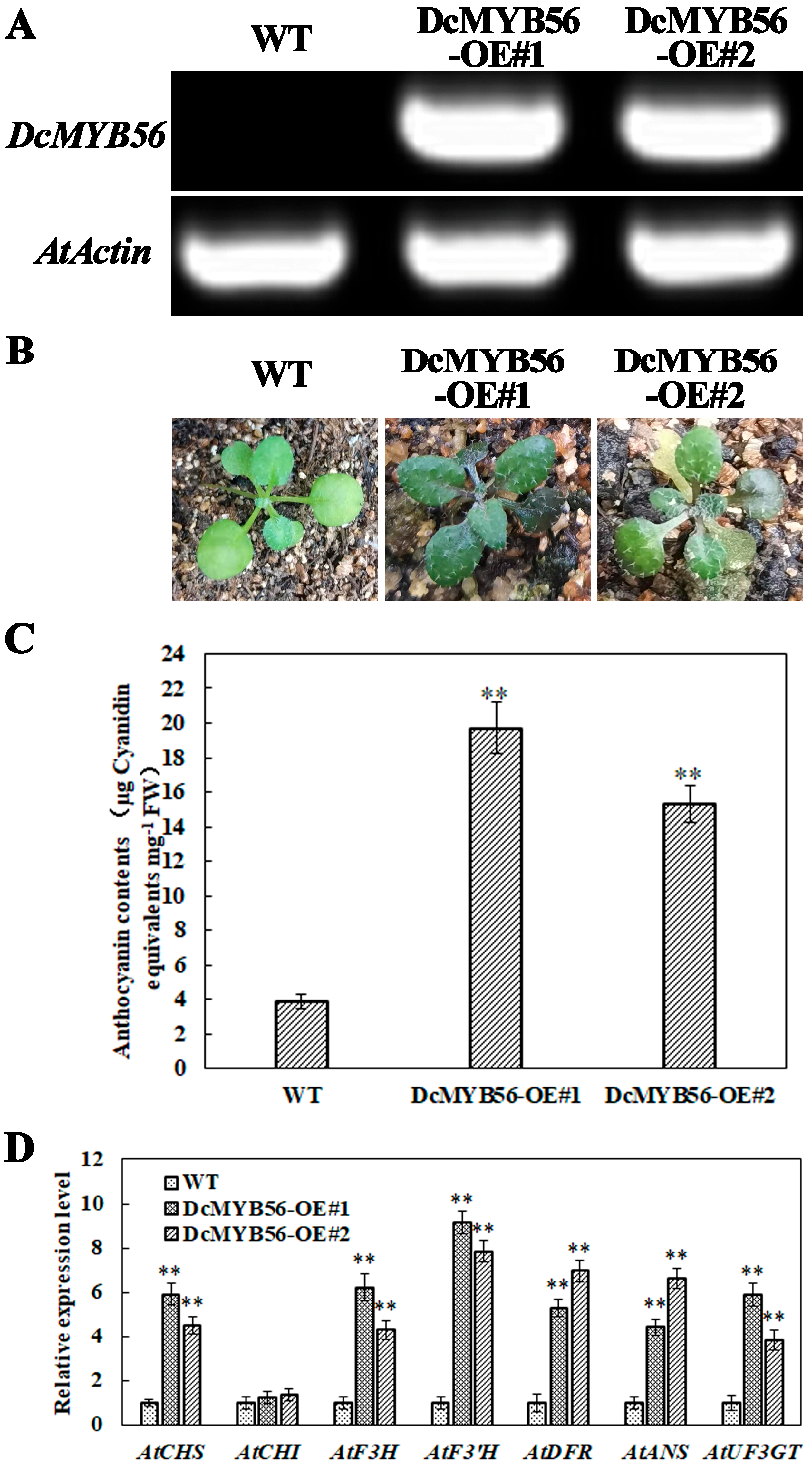
3.6. DcMYB56 Overexpression Increases the Anthocyanin Content in A. thaliana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ng, T.; Liu, J.; Wong, J.; Ye, X.; Wing Sze, S.C.; Tong, Y.; Zhang, K. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biot. 2012, 93, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.L.; Tang, Z.H.; Zhang, X.F.; Zhong, Y.H.; Yao, S.Z.; Wang, L.S.; Lin, C.; Luo, X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, M.; Fang, Z.; Chen, L.; Wu, J.; Zhang, Q. In vitro antiproliferative effect of a water-soluble Laminaria japonica polysaccharide on human melanoma cell line A375. Food Chem. Toxicol. 2013, 58, 56–60. [Google Scholar] [CrossRef]
- Liang, C.; Liang, Y.; Liu, H.; Zhu, D.; Hou, S.; Wu, Y.; Huang, S.; Lai, X. Effect of Dendrobium officinale on D-galactose-induced aging mice. Chin. J. Integr. Med. 2017. [Google Scholar] [CrossRef]
- Lin, G.; Luo, D.; Liu, J.; Wu, X.; Chen, J.; Huang, Q.; Su, L.; Zeng, L.; Wang, H.; Su, Z. Hepatoprotective effect of polysaccharides isolated from Dendrobium officinale against acetaminophen-induced liver injury in mice via regulation of the Nrf2-Keap1 signaling pathway. Oxid. Med. Cell. Longev. 2018, 2018, 6962439. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.; Wang, D.; Wang, D.; Wen, C.; Han, B.; Ouyang, Z. Characterization and anti-tumor activity of a polysaccharide isolated from Dendrobium officinale grown in the Huoshan County. Chin. Med. 2018, 13, 47. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, Z.; Du, B.; Xing, S.; Huang, S.; Li, Y.; Lei, Z.; Li, D.; Chen, H.; Huang, Y. Structure identification of viceninII extracted from Dendrobium officinale and the reversal of TGF-β1-induced epithelial–mesenchymal transition in lung adenocarcinoma cells through TGF-β/Smad and PI3K/Akt/mTOR signaling pathways. Molecules 2019, 24, 144. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Davies, K.M.; Albert, N.W.; Schwinn, K.E. From landing lights to mimicry: The molecular regulation of flower colouration and mechanisms for pigmentation patterning. Funct. Plant Biol. 2012, 39, 619–638. [Google Scholar] [CrossRef]
- Ogata, J.; Kanno, Y.; Itoh, Y.; Tsugawa, H.; Suzuki, M. Anthocyanin biosynthesis in roses. Nature 2005, 435, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Springob, K.; Nakajima, J.-I.; Yamazaki, M.; Saito, K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 2003, 20, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Jeewani, D.C.; Hua, W.Z. Recent advances in anthocyanin biosynthesis in colored wheat. Res. J. Biotechnol. 2017, 12, 57–62. [Google Scholar]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef]
- Cabrita, L.; Fossen, T.; Andersen, O.M. Colour and stability of the six common anthocyanidin 3-glucosides in aqueous solutions. Food Chem. 2000, 68, 101–107. [Google Scholar] [CrossRef]
- Onik, J.C.; Hu, X.; Lin, Q.; Wang, Z. Comparative transcriptomic profiling to understand pre- and post-ripening hormonal regulations and anthocyanin biosynthesis in early ripening apple fruit. Molecules 2018, 23, 1908. [Google Scholar] [CrossRef]
- Jin, J.; Li, Z.; Zhao, L.; Li, Z. Cloning and Analysis of RrF3’H in Rosa rugosa. Am. J. Mol. Biol. 2019, 10, 51. [Google Scholar]
- Toda, K.; Yang, D.; Yamanaka, N.; Watanabe, S.; Harada, K.; Takahashi, R. A single-base deletion in soybean flavonoid 3′-hydroxylase gene is associated with gray pubescence color. Plant Mol. Biol. 2002, 50, 187–196. [Google Scholar] [CrossRef]
- Zabala, G.; Vodkin, L. Cloning of the pleiotropic T locus in soybean and two recessive alleles that differentially affect structure and expression of the encoded flavonoid 3′ hydroxylase. Genetics 2003, 163, 295–309. [Google Scholar] [CrossRef]
- Shimada, Y.; Ohbayashi, M.; Nakano-Shimada, R.; Okinaka, Y.; Kiyokawa, S.; Kikuchi, Y. Genetic engineering of the anthocyanin biosynthetic pathway with flavonoid-3′, 5′-hydroxylase: Specific switching of the pathway in petunia. Plant Cell Rep. 2001, 20, 456–462. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Wang, J.-J.; Liu, J.; Jiang, J.; Sun, J.; Yan, P.; Sun, Y.; Wan, P.; Ye, W.; Fan, B. DcTT8, a bHLH transcription factor, regulates anthocyanin biosynthesis in Dendrobium candidum. Plant Physiol. Biochem. 2021, 162, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lou, Q.; Ma, J.; Su, B.; Gao, Z.; Liu, Y. Cloning and functional characterization of dihydroflavonol 4-reductase gene involved in anthocyanidin biosynthesis of grape hyacinth. Int. J. Mol. Sci. 2019, 20, 4743. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Y.; Wang, H.; Tian, Z.; Xin, S.; Zhu, P. The dihydroflavonol 4-reductase BoDFR1 drives anthocyanin accumulation in pink-leaved ornamental kale. Theor. Appl. Genet. 2021, 134, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Zhang, J.; Yang, B.; Yu, Y.; Liu, T.; Nie, B.; Song, B. Functional analysis of an anthocyanin synthase gene StANS in potato. Sci. Hortic. 2020, 272, 109569. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishimaru, M.; Ding, C.K.; Yakushiji, H.; Goto, N. Comparison of UDP-glucose: Flavonoid 3-O-glucosyltransferase (UFGT) gene sequences between white grapes (Vitis vinifera) and their sports with red skin. Plant Sci. 2001, 160, 543–550. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, H.; Zhang, Y.; Zhao, Y.; Zhang, Y.; Feng, X.; Lin, H. Diverse roles of MYB transcription factors in plants. J. Integr. Plant Biol. 2025, 67, 539–562. [Google Scholar]
- Ramsay, N.A.; Glover, B.J. MYB–bHLH–WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef]
- Qi, Y.; Gu, C.; Wang, X.; Gao, S.; Li, C.; Zhao, C.; Li, C.; Ma, C.; Zhang, Q. Identification of the Eutrema salsugineum EsMYB90 gene important for anthocyanin biosynthesis. BMC Plant Biol. 2020, 20, 186. [Google Scholar] [CrossRef]
- Xi, H.; He, Y.; Chen, H. Functional characterization of SmbHLH13 in anthocyanin biosynthesis and flowering in eggplant. Hortic. Plant J. 2021, 7, 73–80. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, H.; Jiang, X.; Wang, P.; Dai, X.; Chen, W.; Gao, L.; Xia, T. A WD40 repeat protein from Camellia sinensis regulates anthocyanin and proanthocyanidin accumulation through the formation of MYB–bHLH–WD40 ternary complexes. Int. J. Mol. Sci. 2018, 19, 1686. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Kim, J.H.; Lee, W.J.; Jin, J.Y.; Kim, J.; Hong, S.-W.; Lee, H. AtMyb56 regulates anthocyanin levels via the modulation of AtGPT2 expression in response to sucrose in Arabidopsis. Mol. Cells 2018, 41, 351–361. [Google Scholar]
- Cai, O.; Zhang, H.; Yang, L.; Wu, H.; Qin, M.; Yao, W.; Huang, F.; Li, L.; Lin, S. Integrated Transcriptome and Metabolome Analyses reveal bamboo culm color formation mechanisms involved in anthocyanin biosynthetic in Phyllostachys nigra. Int. J. Mol. Sci. 2024, 25, 1738. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Yang, Z.; Wang, S.; Wang, C.; Ma, W.; Zhang, J.; Zhang, A.; Zhang, J. Establishment of Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry Method for Determination of 18 Individual Anthocyanins in Wine. Food Sci. 2019, 40, 229–235. [Google Scholar]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Liang, X.; Zhou, X.; Yang, F.; Liu, J.; He, S.; Guo, Z. Alternative splicing of rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiol. 2016, 171, 1427–1442. [Google Scholar] [CrossRef]
- Jia, N.; Liu, J.; Sun, Y.; Tan, P.; Cao, H.; Xie, Y.; Wen, B.; Gu, T.; Liu, J.; Li, M. Citrus sinensis MYB transcription factors CsMYB330 and CsMYB308 regulate fruit juice sac lignification through fine-tuning expression of the Cs4CL1 gene. Plant Sci. 2018, 277, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Sun, Y.; Qian, M.; Yang, F.; Ni, J.; Tao, R.; Li, L.; Shu, Q.; Zhang, D.; Teng, Y. Transcriptome analysis of bagging-treated red Chinese sand pear peels reveals light-responsive pathway functions in anthocyanin accumulation. Sci. Rep. 2017, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ren, Y.; Huang, S.; Huang, S.; Yang, G. Floral colors of Phalaenopsis type dendrobium and their flavonoid composition. Acta Hortic. Sin. 2013, 40, 107. [Google Scholar]
- Hosoki, T.; Hamada, M.; Kando, T.; Moriwaki, R.; Inaba, K. Comparative study of anthocyanins in tree peony flowers. J. Jpn. Soc. Hortic. Sci. 1991, 60, 395–403. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hashimoto, F.; Shimizu, K.; Sakata, Y. A new acylated anthocyanin from the red flowers of Camellia hongkongensis and characterization of anthocyanins in the section Camellia species. J. Integr. Plant Biol. 2009, 51, 545–552. [Google Scholar] [CrossRef]
- Yu, Z.; Liao, Y.; Teixeira da Silva, J.A.; Yang, Z.; Duan, J. Differential accumulation of anthocyanins in Dendrobium officinale stems with red and green peels. Int. J. Mol. Sci. 2018, 19, 2857. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Yue, Q.; Xu, L.; Xiang, G.; Yu, X.; Yao, Y. Characterization of gene expression profile, phenolic composition, and antioxidant capacity in red-fleshed grape berries and their wines. J. Agric. Food Chem. 2018, 66, 7190–7199. [Google Scholar] [CrossRef]
- Jia, N.; Wang, J.; Wang, Y.; Ye, W.; Liu, J.; Jiang, J.; Sun, J.; Yan, P.; Wang, P.; Wang, F.; et al. The Light-Induced WD40-Repeat Transcription Factor DcTTG1 Regulates Anthocyanin Biosynthesis in Dendrobium candidum. Front. Plant Sci. 2021, 12, 633333. [Google Scholar] [CrossRef]
- Lipsick, J.S. One billion years of Myb. Oncogene 1996, 13, 223–235. [Google Scholar]
- Borevitz, J.O.; Xia, Y.; Blount, J.; Dixon, R.A.; Lamb, C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 2000, 12, 2383–2393. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Huang, H.; Ren, Q.; Wu, D.; Chang, C.; Peng, W.; Liu, Y.; Peng, J.; Xie, D. The jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell 2011, 23, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, Z.; Zhang, Y.; Li, Y.; Zhou, S.; Chen, G. A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica Oleracea var. acephala f. tricolor). Plant Cell Rep. 2012, 31, 281–289. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Sun, Q.; Zhang, T.; Liu, W.; Wang, N.; Chen, X. MdMYB114 regulates anthocyanin biosynthesis and functions downstream of MdbZIP4-like in apple fruit. J. Plant Physiol. 2021, 257, 153353. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, S.; Yang, Q.; Gao, H.; Sheng, O.; Bi, F.; Li, C.; Dong, T.; Yi, G.; He, W. MaMYB4, an R2R3-MYB repressor transcription factor, negatively regulates the biosynthesis of anthocyanin in banana. Front. Plant Sci. 2021, 11, 600704. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Cheng, Y.; Liu, H.; Wang, Q.; Wang, Q.; Wang, C.; Su, R.; Chen, F.; Wang, H.; Du, L. Differential anthocyanin accumulation in radish taproot: Importance of RsMYB1 gene structure. Plant Cell Rep. 2020, 39, 217–226. [Google Scholar] [CrossRef]
- Xu, H.; Wang, N.; Liu, J.; Qu, C.; Wang, Y.; Jiang, S.; Lu, N.; Wang, D.; Zhang, Z.; Chen, X. The molecular mechanism underlying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes. Plant Mol. Biol. 2017, 94, 149–165. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Cheng, Y.; Wang, J.; Wang, X.; Liu, R.; Han, J. Functional identification of PsMYB57 involved in anthocyanin regulation of tree peony. BMC Genet. 2020, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, S.; Ichihi, A.; Tanaka, Y.; Fujishige, S.; Koeda, S.; Shimizu, K. The R2R3-MYB transcription factor MiMYB1 regulates light dependent red coloration of ‘Irwin’mango fruit skin. Sci. Hortic. 2020, 272, 109567. [Google Scholar] [CrossRef]
- Anwar, M.; Wang, G.; Wu, J.; Waheed, S.; Allan, A.C.; Zeng, L. Ectopic overexpression of a novel R2R3-MYB, NtMYB2 from Chinese narcissus represses anthocyanin biosynthesis in tobacco. Molecules 2018, 23, 781. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, Y.; Zhou, C.; Li, D.; Gao, X.; Zhang, S.; Chen, M. Genome-wide identification of direct targets of the TTG1–bHLH–MYB complex in regulating trichome formation and flavonoid accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 5014. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Xie, D. Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent Pat. Biotechnol. 2014, 8, 47–60. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, G.; Chen, L.; Zhang, H.; Cui, Y.; Luo, P. A feedback loop comprising RhMYB114 and RhMYB16 regulates anthocyanin accumulation and tissue acidification in Rosa hybrida. Hortic. Plant J. 2024, 679. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, N.; Ye, W.; Jiang, J.; Wang, P.; Liu, J. The R3-MYB Transcription Factor DcMYB56 Regulates Anthocyanin Accumulation by Activating the Expression of Anthocyanin Biosynthesis-Related Genes in Dendrobium candidum. Plants 2025, 14, 1805. https://doi.org/10.3390/plants14121805
Jia N, Ye W, Jiang J, Wang P, Liu J. The R3-MYB Transcription Factor DcMYB56 Regulates Anthocyanin Accumulation by Activating the Expression of Anthocyanin Biosynthesis-Related Genes in Dendrobium candidum. Plants. 2025; 14(12):1805. https://doi.org/10.3390/plants14121805
Chicago/Turabian StyleJia, Ning, Wei Ye, Jinlan Jiang, Peiyu Wang, and Jiqin Liu. 2025. "The R3-MYB Transcription Factor DcMYB56 Regulates Anthocyanin Accumulation by Activating the Expression of Anthocyanin Biosynthesis-Related Genes in Dendrobium candidum" Plants 14, no. 12: 1805. https://doi.org/10.3390/plants14121805
APA StyleJia, N., Ye, W., Jiang, J., Wang, P., & Liu, J. (2025). The R3-MYB Transcription Factor DcMYB56 Regulates Anthocyanin Accumulation by Activating the Expression of Anthocyanin Biosynthesis-Related Genes in Dendrobium candidum. Plants, 14(12), 1805. https://doi.org/10.3390/plants14121805



