Wheat Yield, N Use Efficiency, Soil Properties, and Soil Bacterial Community as Affected by Long-Term Straw Incorporation and Manure Under Wheat–Summer Maize Cropping System in Southern Shanxi Province, China
Abstract
1. Introduction
2. Results
2.1. Wheat Grain Yield and N Use Efficiency
2.2. Soil Chemical Properties
2.3. OTU Richness and Alpha-Diversity
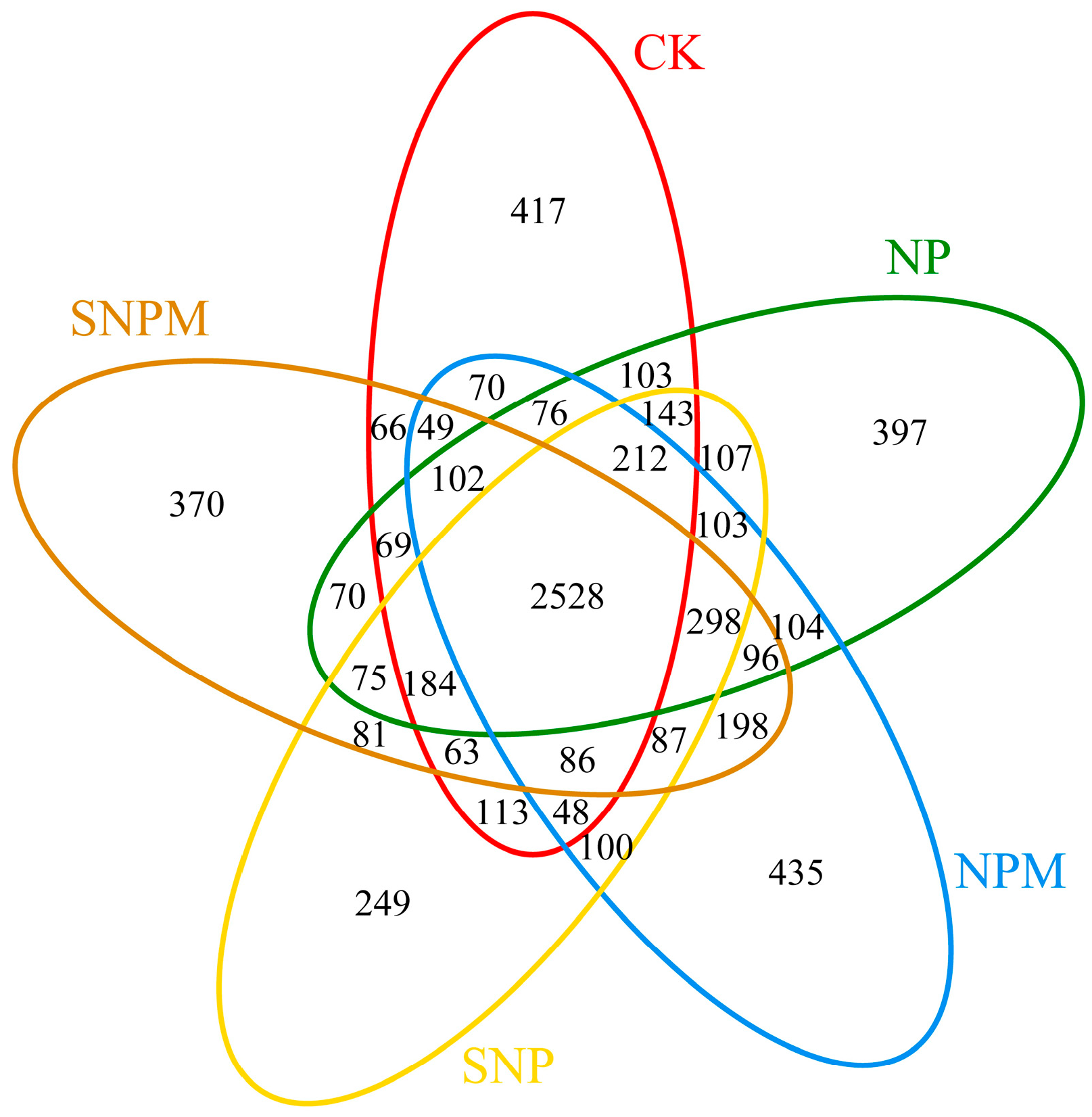
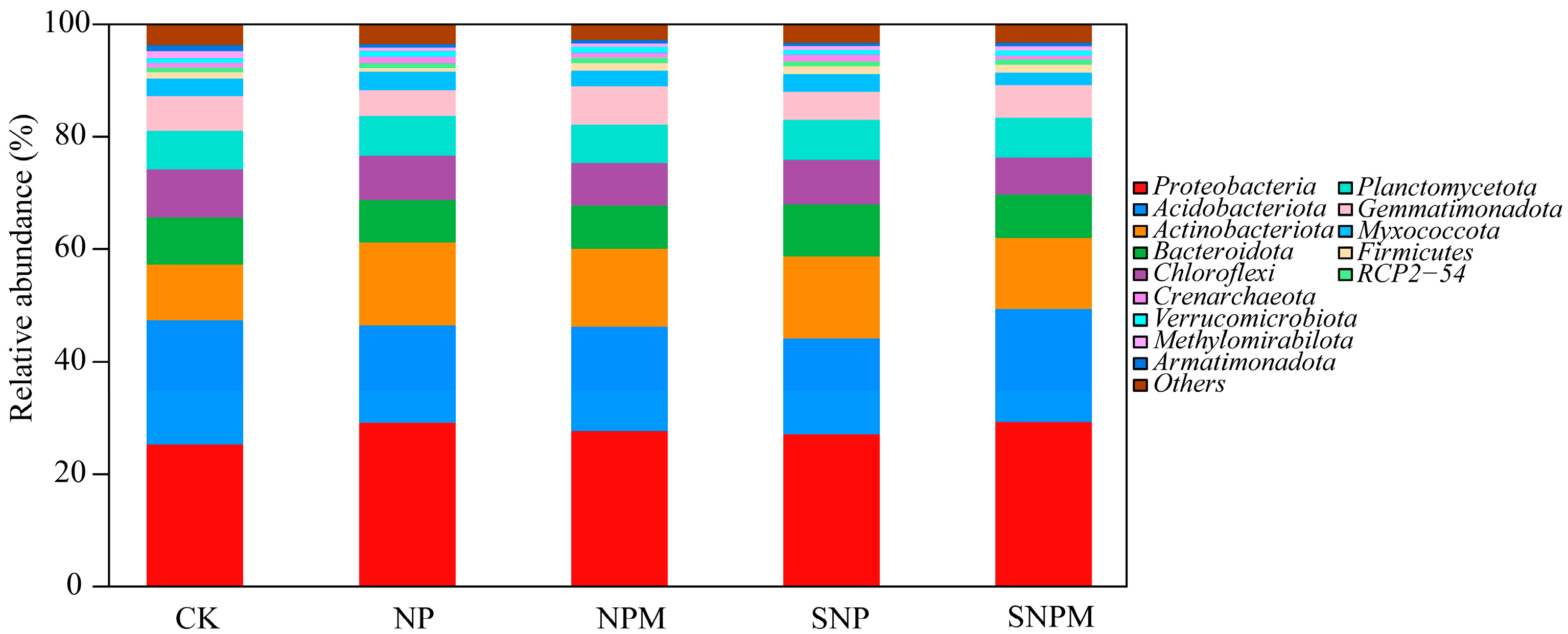
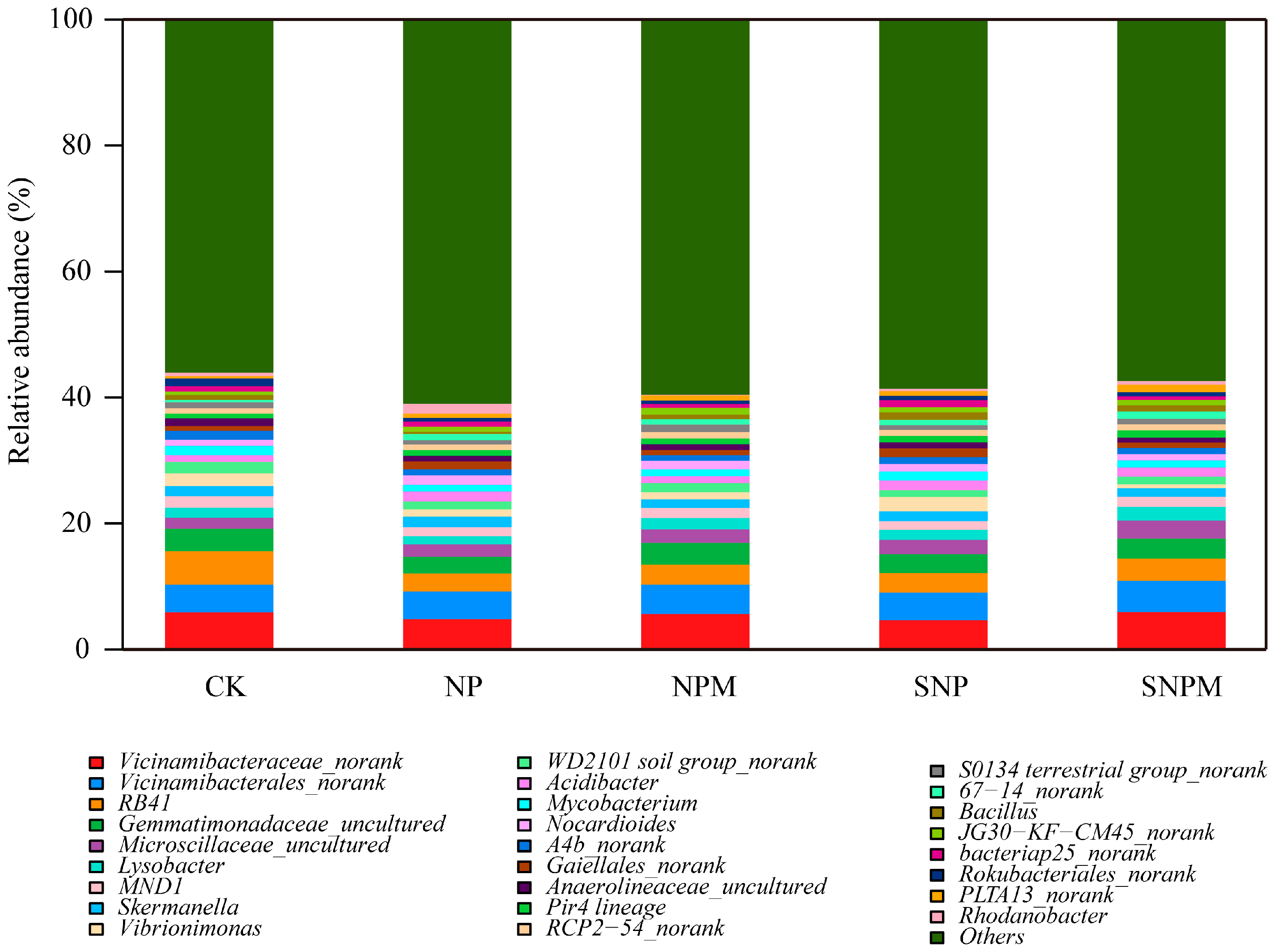
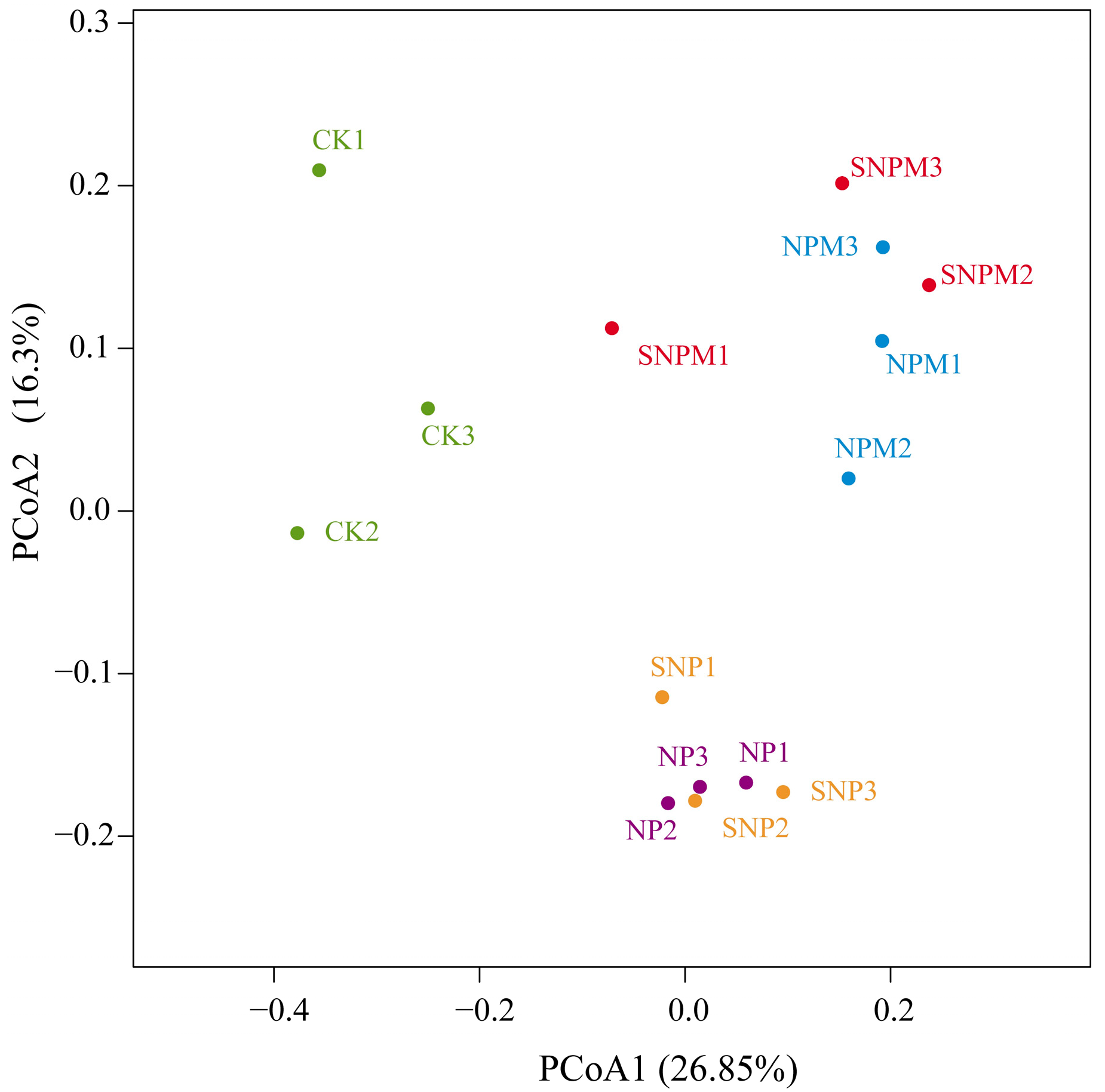
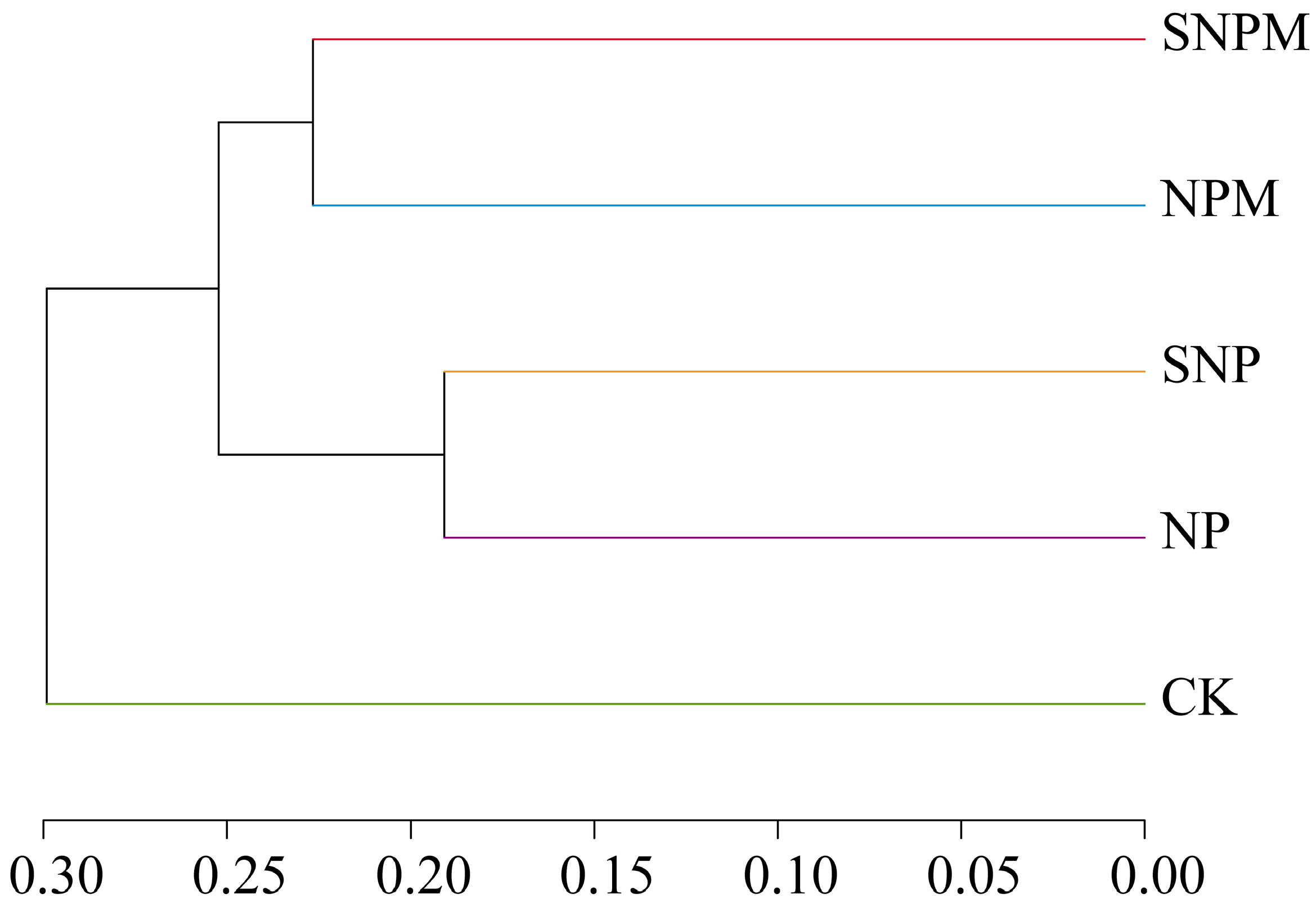
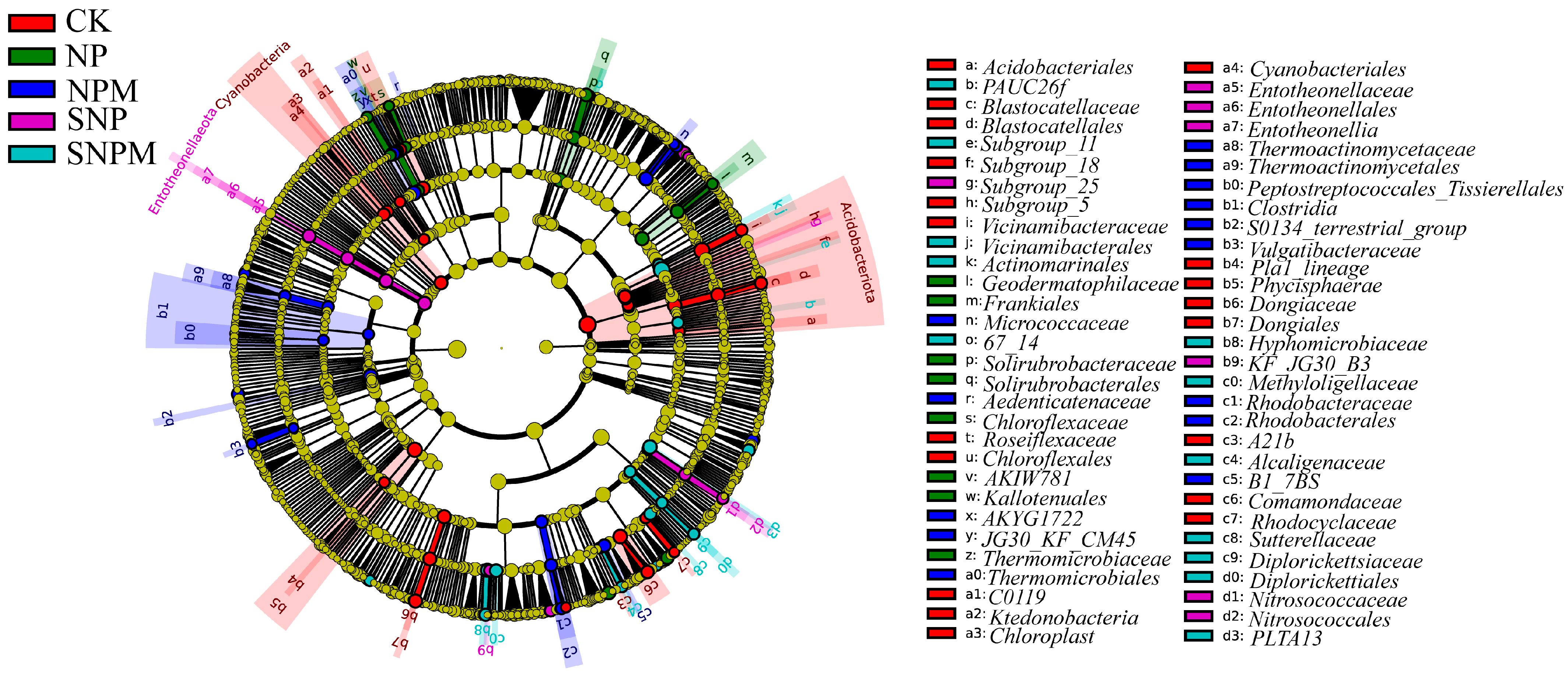
2.4. Composition and Structure of Bacteria
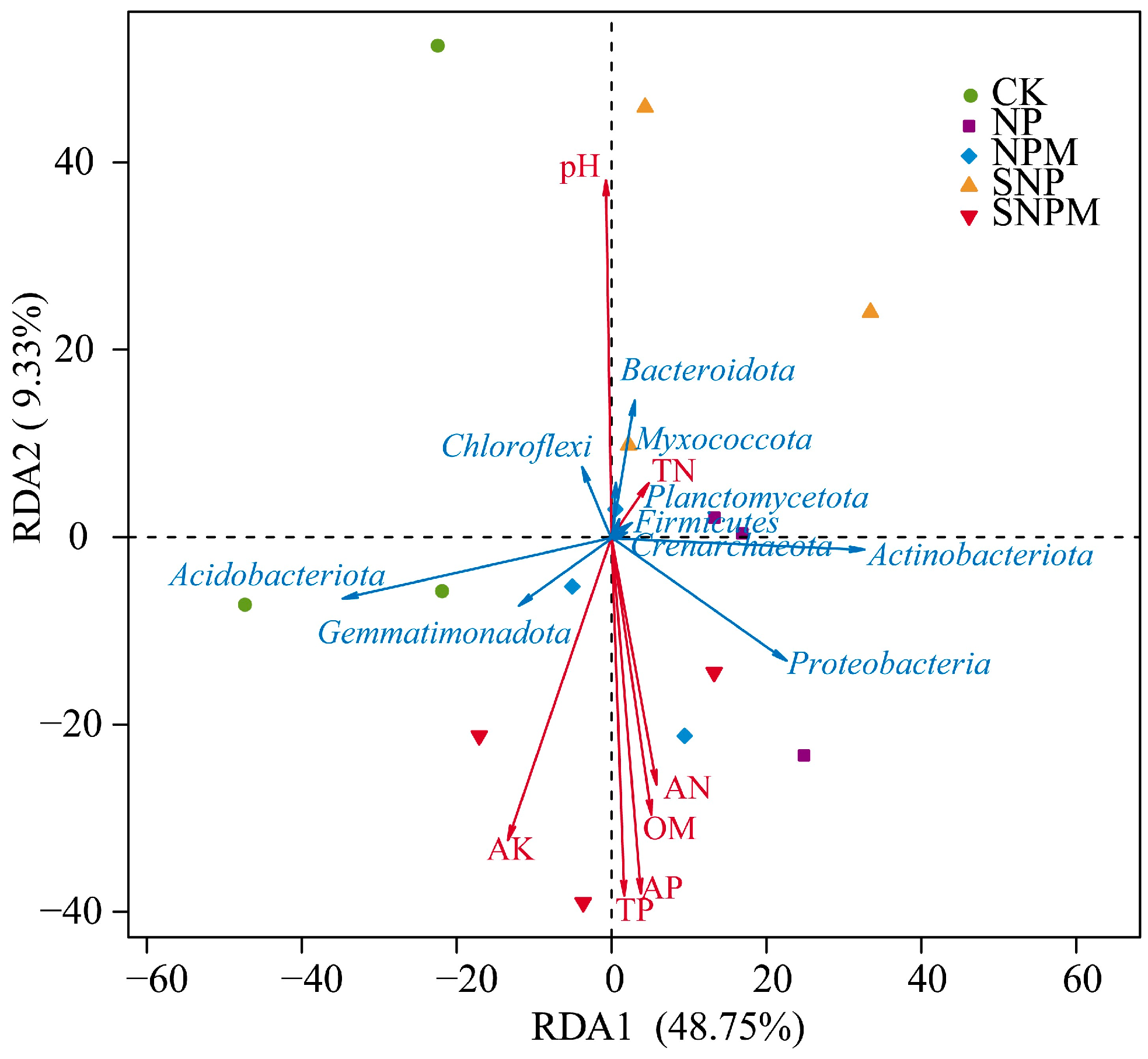
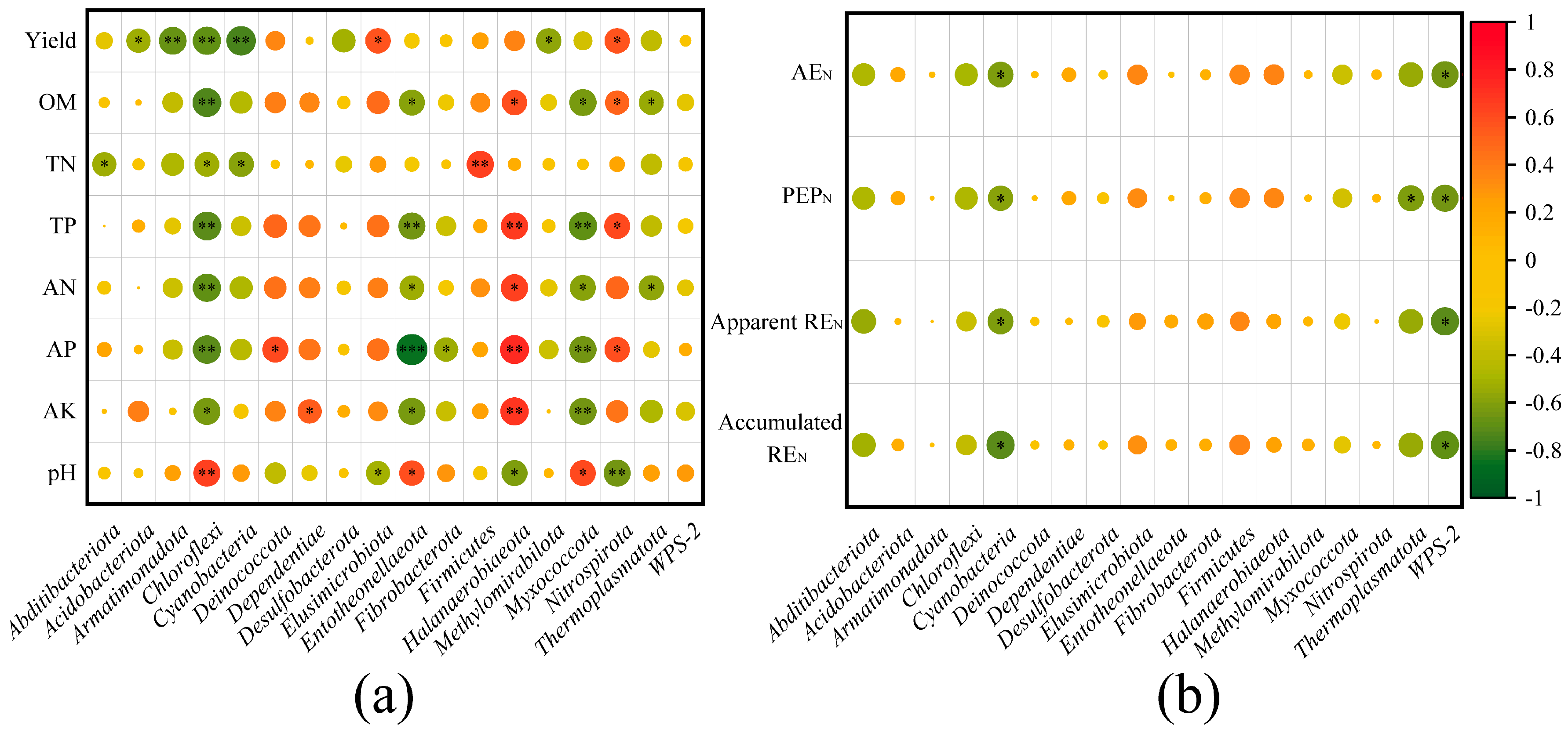
2.5. Bacterial Community Relationships with Soil Properties, Wheat Yield, and NUE
3. Discussion
3.1. Effects of Long-Term Straw Incorporation and Manure on Wheat Yield and N Use Efficiency
3.2. Effects of Long-Term Straw Incorporation and Manure on Soil Properties
3.3. Effects of Long-Term Straw Incorporation and Manure on Soil Bacterial Diversity
3.4. Effects of Long-Term Straw Incorporation and Manure on Soil Bacterial Communities
3.5. Bacterial Community Relationships with Soil Properties, Yield, and NUE
4. Materials and Methods
4.1. Sampling Collection and Chemical Analysis
4.2. DNA Extraction and Illumina MiSeqsequencing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Z.L.; Chen, D.L. Nitrogen fertilizer use in China–Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.E.; Poulton, P.R. The importance of long-term experiments in agriculture: Their management to ensure continued crop production and soil fertility; the Rothamsted experience. Eur. J. Soil Sci. 2018, 69, 113–125. [Google Scholar] [CrossRef]
- Baier, C.; Modersohn, A.; Jalowy, F.; Glaser, B.; Gross, A. Effects of recultivation on soil organic carbon sequestration in abandoned coal mining sites: A meta-analysis. Sci. Rep. 2022, 12, 20090. [Google Scholar] [CrossRef]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, Y.; Wei, Y.; Cai, C. Impacts of long-term organic manure inputs on cultivated soils with various degradation degrees. Soil Tillage Res. 2024, 236, 105950. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Li, Y.; Jiao, Y.; Fan, L.; Jiang, Y.; Qu, C.; Filimonenko, E.; Jiang, Y.; Tian, X. Nitrogen fertilizer builds soil organic carbon under straw return mainly via microbial necromass formation. Soil Biol. Biochem. 2024, 188, 109223. [Google Scholar] [CrossRef]
- Wang, Z.G.; Li, H.X.; Yue, M.C.; Li, P.; Jiao, J.G. Livestock manure resources and their replace potential fertilizer in China. Chin. Agric. Sci. Bull. 2019, 35, 121–128. [Google Scholar]
- Li, T.; Wei, G.; Liu, H.; Zhu, Y.; Lin, Y.; Han, Q. Comparative Assessment of the Environmental and Economic Performance of Two Straw Utilization Pathways in China. BioEnergy Res. 2024, 17, 2164–2176. [Google Scholar] [CrossRef]
- Varvel, G.E.; Wilhelm, W.W. No-tillage increases soil profile carbon and nitrogen under long-term rainfed cropping systems. Soil Tillage Res. 2011, 114, 28–36. [Google Scholar] [CrossRef]
- Cao, B.; Qu, C.; Guo, Y.; Liu, C.; Liang, Z.; Jiao, Y.; Shi, J.; Tian, X. Long-term nitrogen and straw application improves wheat production and soil organic carbon sequestration. J. Soil Sci. Plant Nutr. 2022, 22, 3364–3376. [Google Scholar] [CrossRef]
- Cui, H.; Luo, Y.; Chen, J.; Jin, M.; Li, Y.; Wang, Z. Straw return strategies to improve soil properties and crop productivity in a winter wheat-summer maize cropping system. Eur. J. Agron. 2022, 133, 126436. [Google Scholar] [CrossRef]
- Khan, A.; Jan, M.T.; Afzal, M.; Muhammad, I.; Jan, A.; Shah, Z. An integrated approach using organic amendments under a range of tillage practices to improve wheat productivity in a cereal based cropping system. Int. J. Agric. Biol. 2015, 17, 467–474. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Li, W.; Zhang, D.; Fang, W.; Li, Y.; Wang, Q.; Cao, A.; Yan, D. Long-term effects of chloropicrin fumigation on soil microbe recovery and growth promotion of Panax notoginseng. Front. Microbiol. 2023, 14, 1225944. [Google Scholar] [CrossRef]
- Xu, J.; Han, H.; Ning, T.; Li, Z.; Lal, R. Long-term effects of tillage and straw management on soil organic carbon, crop yield, and yield stability in a wheat-maize system. Field Crops Res. 2019, 233, 33–40. [Google Scholar] [CrossRef]
- Yang, L.; Chen, T.Y.; Li, Z.Y.; Muhammad, I.; Chi, Y.X.; Zhou, X.B. Straw incorporation and nitrogen fertilization regulate soil quality, enzyme activities and maize crop productivity in dual maize cropping system. BMC Plant Biol. 2024, 24, 729. [Google Scholar] [CrossRef]
- Yang, J.Y.; Tung, S.A.; Xu, J.T.; Pan, Y.Q.; Yang, L.; Zhou, X.B. Effects of straw and nitrogenous fertilizers on the soil aggregate stability and quality in subtropical regions of China. J. Soil Sci. Plant Nutr. 2024, 24, 5988–5999. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Hu, S.; Zhang, X.; Ouyang, Z.; Zhang, G.; Huang, B.; Zhao, S.; Wu, J.; Xie, D. Economics-and policy-driven organic carbon input enhancement dominates soil organic carbon accumulation in Chinese croplands. Proc. Natl. Acad. Sci. USA 2018, 115, 4045–4050. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ejue, W.S.; Olayanju, A.; Dunsin, O.; Aboyeji, C.M.; Aremu, C.; Adegbite, K.; Akinpelu, O. Different organic manure sources and NPK fertilizer on soil chemical properties, growth, yield and quality of okra. Sci. Rep. 2020, 10, 16083. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Chandra, S.; Singh, R.D.; Kundu, S.; Srivastva, A.K.; Gupta, H.S. Long-term farmyard manure application effects on properties of a silty clay loam soil under irrigated wheat–soybean rotation. Soil Tillage Res. 2007, 94, 386–396. [Google Scholar] [CrossRef]
- Chen, M.; Yang, H.; Yang, Q.; Li, Y.; Wang, H.; Wang, J.; Fan, Q.; Yang, N.; Wang, K.; Zhang, J. Different Impacts of Long-Term Tillage and Manure on Yield and N Use Efficiency, Soil Fertility, and Fungal Community in Rainfed Wheat in Loess Plateau. Plants 2024, 13, 3477. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Yang, W.; Sun, H.; Kong, S.; Xu, H. Effects of animal manure and nitrification inhibitor on N2O emissions and soil carbon stocks of a maize cropping system in Northeast China. Sci. Rep. 2022, 12, 15202. [Google Scholar] [CrossRef]
- Du, W.Y.; Tang, S.; Wang, H. The status of organic fertilizer industry and organic fertilizer resources in China. Soil Fertil. Sci. China 2020, 3, 210–219. [Google Scholar]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Panainte-Lehadus, M.; Mosnegutu, E.; Tomozei, C.; Chitimus, D.; Irimia, O. Assessment of manure compost used as soil amendment—A review. Processes 2023, 11, 1167. [Google Scholar] [CrossRef]
- Muhammad, I.; Khan, F.; Khan, A.; Wang, J. Soil fertility in response to urea and farmyard manure incorporation under different tillage systems in Peshawar, Pakistan. Int. J. Agric. Biol 2018, 20, 1539–1547. [Google Scholar]
- Sun, R.; Guo, X.; Wang, D.; Chu, H. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, M.; Zhai, Z.; Dai, H.; Yang, M.; Zhang, Y.; Liang, T. Soil organic carbon, carbon fractions, and microbial community under various organic amendments. Sci. Rep. 2024, 14, 25431. [Google Scholar] [CrossRef]
- Yao, J.; Shengzhe, E.; Yuan, J.; Shi, X.; Che, Z. Effects of different organic matters on crop yields, soil quality and heavy metal content in irrigated desert soil. Chin. J. Eco-Agric. 2020, 28, 813–825. [Google Scholar]
- Zheng, S.; Wu, J.-G.; Li, J.-R.; Hu, J.; He, L. Effects of different conditioners on humus composition and humic acid structural characteristics in Black Soil under the combined application of pig manure and straw. J. Soil Sci. Plant Nutr. 2023, 23, 6246–6256. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems: A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, J.; Liu, W.; Cheng, W.; Shaaban, M.; Jiang, Y. The effects of continuous straw returning strategies on SOC balance upon fresh straw incorporation. Environ. Res. 2023, 232, 116225. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Peng, H.; Chen, J.; Wang, X.; Wei, M.; Li, W.; Yang, L.; Zhang, Q.; Wang, W.; Mellouki, A. An estimation of CO2 emission via agricultural crop residue open field burning in China from 1996 to 2013. J. Clean. Prod. 2016, 112, 2625–2631. [Google Scholar] [CrossRef]
- Zhang, Y.; Zang, G.-Q.; Tang, Z.-H.; Chen, X.-H.; Yu, Y.-S. Burning straw, air pollution, and respiratory infections in China. Am. J. Infect. Control 2014, 42, 815. [Google Scholar] [CrossRef]
- Kennedy, A.C. Bacterial diversity in agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 65–76. [Google Scholar]
- Zhao, S.; Li, K.; Zhou, W.; Qiu, S.; Huang, S.; He, P. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, S.; Cao, C.; Li, C. Tillage practices and straw-returning methods affect topsoil bacterial community and organic C under a rice-wheat cropping system in central China. Sci. Rep. 2016, 6, 33155. [Google Scholar] [CrossRef]
- Wu, G.; Ling, J.; Zhao, D.-Q.; Liu, Z.-X.; Xu, Y.-P.; Kuzyakov, Y.; Marsden, K.; Wen, Y.; Zhou, S.-L. Straw return counteracts the negative effects of warming on microbial community and soil multifunctionality. Agric. Ecosyst. Environ. 2023, 352, 108508. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Gebremikael, M.T.; Wu, H.; Cai, D.; Wang, B.; Li, B.; Zhang, J.; Li, Y.; Xi, J. Response of soil organic carbon fractions, microbial community composition and carbon mineralization to high-input fertilizer practices under an intensive agricultural system. PLoS ONE 2018, 13, e0195144. [Google Scholar] [CrossRef]
- Yu, D.; Wen, Z.; Li, X.; Song, X.; Wu, H.; Yang, P. Effects of straw return on bacterial communities in a wheat-maize rotation system in the North China Plain. PLoS ONE 2018, 13, e0198087. [Google Scholar] [CrossRef]
- Pan, H.; Chen, M.; Feng, H.; Wei, M.; Song, F.; Lou, Y.; Cui, X.; Wang, H.; Zhuge, Y. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Tillage Res. 2020, 198, 104540. [Google Scholar] [CrossRef]
- Bittman, S.; Forge, T.A.; Kowalenko, C.G. Responses of the bacterial and fungal biomass in a grassland soil to multi-year applications of dairy manure slurry and fertilizer. Soil Biol. Biochem. 2005, 37, 613–623. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Diao, T.; Sun, Y.; Sun, T.; Wang, C. Influence of continuous fertilization on heavy metals accumulation and microorganism communities in greenhouse soils under 22 years of long-term manure organic fertilizer experiment. Sci. Total Environ. 2025, 959, 178294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, W.; Yu, H.; He, X. Linking organic carbon accumulation to microbial community dynamics in a sandy loam soil: Result of 20 years compost and inorganic fertilizers repeated application experiment. Biol. Fertil. Soils 2015, 51, 137–150. [Google Scholar] [CrossRef]
- Tian, J.; Lou, Y.; Gao, Y.; Fang, H.; Liu, S.; Xu, M.; Blagodatskaya, E.; Kuzyakov, Y. Response of soil organic matter fractions and composition of microbial community to long-term organic and mineral fertilization. Biol. Fertil. Soils 2017, 53, 523–532. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Zhou, D. Application of cattle manure increased the stability of organic carbon in the subsoil in Mollisols. Plant Soil 2024, 504, 861–877. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Rong, X.; Zhou, X.; Fei, J.; Peng, J.; Luo, G. Biochar and organic fertilizer applications enhance soil functional microbial abundance and agroecosystem multifunctionality. Biochar 2024, 6, 3. [Google Scholar] [CrossRef]
- Wen, M.; Liu, Y.; Yang, C.; Dou, Y.; Zhu, S.; Tan, G.; Wang, J. Effects of manure and nitrogen fertilization on soil microbial carbon fixation genes and associated communities in the Loess Plateau of China. Sci. Total Environ. 2024, 954, 176581. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Wen, J.; Mo, F.; Liu, Y. Continuous manure application strengthens the associations between soil microbial function and crop production: Evidence from a 7-year multisite field experiment on the Guanzhong Plain. Agric. Ecosyst. Environ. 2022, 338, 108082. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yang, B.; Zhang, M.; Song, D.; Xu, X.; Ai, C.; Liang, G.; Zhou, W. Investigating the effects of organic amendments on soil microbial composition and its linkage to soil organic carbon: A global meta-analysis. Sci. Total Environ. 2023, 894, 164899. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, H.; Ren, Z.; Ding, Y.; Long, M.; Zhang, G.; Qin, X.; Siddique, K.H.M.; Liao, Y. Response of soil microbial community parameters to plastic film mulch: A meta-analysis. Geoderma 2022, 418, 115851. [Google Scholar] [CrossRef]
- Li, J.; Han, G.; Wang, G.; Liu, X.; Zhang, Q.; Chen, Y.; Song, W.; Qu, W.; Chu, X.; Li, P. Imbalanced nitrogen–phosphorus input alters soil organic carbon storage and mineralisation in a salt marsh. Catena 2022, 208, 105720. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Tian, W.; Wang, L.; Li, Y.; Zhuang, K.; Li, G.; Zhang, J.; Xiao, X.; Xi, Y. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric. Ecosyst. Environ. 2015, 213, 219–227. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, M.; Li, Y.; Wu, J.; Tian, W.; Li, J.; Hou, Y.; Wu, S.; Zhang, J.; Zhang, Z. Combined organic fertilizer and straw return enhanced summer maize productivity and optimized soil nitrate–N distribution in rainfed summer maize–winter wheat rotation on the Southeast Loess Plateau. J. Soil Sci. Plant Nutr. 2023, 23, 938–952. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Lu, X.; Zhu, Y.; Han, X.; Yan, J.; Yan, L.; Zou, W. Impact of combined organic amendments and chemical fertilizers on soil microbial limitations, soil quality, and soybean yield. Plant Soil 2024, 507, 317–334. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Liang, Z.; Li, Y.; Wang, J.; Hao, J.; Jiang, Y.; Shi, J.; Meng, X.; Tian, X. Effects of the combined application of livestock manure and plant residues on soil organic carbon sequestration in the southern Loess Plateau of China. Agric. Ecosyst. Environ. 2024, 368, 109011. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Kang, G.; Zhu, B.; Li, H.; Hu, F.; Jiao, J. Soil quality response to organic amendments on dryland red soil in subtropical China. Geoderma 2020, 373, 114416. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Gu, Y.; Guo, L.; Huang, Y.; Zhang, J.; Xu, Z.; Tan, B.; Zhang, L.; Chen, L. Ecoenzymatic stoichiometry and microbial nutrient limitations in rhizosphere soil along the Hailuogou Glacier forefield chronosequence. Sci. Total Environ. 2020, 704, 135413. [Google Scholar] [CrossRef] [PubMed]
- Barak, P.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 1997, 197, 61–69. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, G.; Liu, H.; Dai, X. Application of composted lipstatin fermentation residue as organic fertilizer: Temporal changes in soil characteristics and bacterial community. Chemosphere 2022, 306, 135637. [Google Scholar] [CrossRef]
- Bier, R.L.; Daniels, M.; Oviedo-Vargas, D.; Peipoch, M.; Price, J.R.; Omondi, E.; Smith, A.; Kan, J. Agricultural soil microbiomes differentiate in soil profiles with fertility source, tillage, and cover crops. Agric. Ecosyst. Environ. 2024, 368, 109002. [Google Scholar] [CrossRef]
- Li, J.; Cooper, J.M.; Li, Y.; Yang, X.; Zhao, B. Soil microbial community structure and function are significantly affected by long-term organic and mineral fertilization regimes in the North China Plain. Appl. Soil Ecol. 2015, 96, 75–87. [Google Scholar] [CrossRef]
- Hu, J.; Lin, X.; Wang, J.; Dai, J.; Chen, R.; Zhang, J.; Wong, M.H. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soils Sediments 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Jiang, S.; Xue, D.; Feng, W.; Wang, K.; Wang, S.; Wang, T.; Lv, M.; Han, Y.; Lv, Y.; Hu, A. Long-term organic fertilization alters soil microbial community structure and its influence on faba bean production in a six-crop rotation system. Plant Soil 2024, 1–17. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Zhu, P.; Wei, D.; Jin, J.; Liu, X.; Wang, G. Long-term manure addition reduces diversity and changes community structure of diazotrophs in a neutral black soil of northeast China. J. Soils Sediments 2018, 18, 2053–2062. [Google Scholar] [CrossRef]
- Xu, P.; Liu, Y.; Zhu, J.; Shi, L.; Fu, Q.; Chen, J.; Hu, H.; Huang, Q. Influence mechanisms of long-term fertilizations on the mineralization of organic matter in Ultisol. Soil Tillage Res. 2020, 201, 104594. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, C.; Qiao, Y.; Du, S.; Li, W.; Zhang, X.; Zhao, Z.; Cao, C.; Zhang, W. Long-term organic and inorganic fertilization alters the diazotrophic abundance, community structure, and co-occurrence patterns in a vertisol. Sci. Total Environ. 2021, 766, 142441. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, W.; Zhu, C.; Luo, G.; Kong, Y.; Ling, N.; Wang, M.; Dai, J.; Shen, Q.; Guo, S. Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Lu, M.; Qin, C.; Chen, Y.; Yang, L.; Huang, Q.; Wang, J.; Shen, Z.; Shen, Q. Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol. Fertil. Soils 2016, 52, 455–467. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhao, J.; Wang, L.; Yang, D.; Li, G.; Xiu, W. Variation of soil bacterial and fungal communities from fluvo-aquic soil under chemical fertilizer reduction combined with organic materials in North China Plain. J. Soil Sci. Plant Nutr. 2021, 21, 349–363. [Google Scholar] [CrossRef]
- Wan, J.; Wang, X.; Yang, T.; Wei, Z.; Banerjee, S.; Friman, V.-P.; Mei, X.; Xu, Y.; Shen, Q. Livestock manure type affects microbial community composition and assembly during composting. Front. Microbiol. 2021, 12, 621126. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Wu, G.; Liu, J.; Ye, Z. Characterization of phosphobacteria isolated from eutrophic aquatic ecosystems. Microbiology 2009, 78, 769–775. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Knapp, B.A.; Fuchs, J.; Kaufmann, R.; Insam, H. Application of COMPOCHIP microarray to investigate the bacterial communities of different composts. Microb. Ecol. 2009, 57, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Sun, Y.; Wu, S.; Liang, W.; Yang, S. Effects of mechanical weeding on soil fertility and microbial community structure in star anise (Illicium verum Hook. f.) plantations. PLoS ONE 2022, 17, e0266949. [Google Scholar] [CrossRef]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Li, G.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Lian, J.; Wang, H.; Deng, Y.; Xu, M.; Liu, S.; Zhou, B.; Jangid, K.; Duan, Y. Impact of long-term application of manure and inorganic fertilizers on common soil bacteria in different soil types. Agric. Ecosyst. Environ. 2022, 337, 108044. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Li, S.; Zhong, L.; Zhang, B.; Fan, C.; Gao, Y.; Wang, M.; Xiao, H.; Tang, X. Microplastics induced the differential responses of microbial-driven soil carbon and nitrogen cycles under warming. J. Hazard. Mater. 2024, 465, 133141. [Google Scholar] [CrossRef]
- Koch, H.; Lücker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.-G.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, J.; Gao, F.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Integrated agronomic practices management improve yield and nitrogen balance in double cropping of winter wheat-summer maize. Field Crops Res. 2018, 221, 196–206. [Google Scholar] [CrossRef]
- Huang, S.; He, P.; Jia, L.; Ding, W.; Ullah, S.; Zhao, R.; Zhang, J.; Xu, X.; Liu, M.; Zhou, W. Improving nitrogen use efficiency and reducing environmental cost with long-term nutrient expert management in a summer maize-winter wheat rotation system. Soil Tillage Res. 2021, 213, 105117. [Google Scholar] [CrossRef]
| Treatments | Grain Yield (kg/ha) | AEN (kg/kg) | PEPN (kg/kg) | Apparent REN (%) | Accumulated REN (%) |
|---|---|---|---|---|---|
| NP | 5402.7 ± 353.17 c | 8.64 ± 1.98 c | 24.01 ± 1.57 c | 33.80 ± 5.87 b | 45.59 ± 5.09 c |
| NPM | 6118.61 ± 224.71 b | 11.82 ± 0.8 b | 27.19 ± 1.00 b | 39.77 ± 2.36 b | 49.83 ± 1.76 b |
| SNP | 7301.40 ± 575.01 a | 17.07 ± 2.88 a | 32.45 ± 2.56 a | 64.98 ± 9.08 a | 61.70 ± 3.84 a |
| SNPM | 7412.59 ± 512.56 a | 17.57 ± 2.6 a | 32.94 ± 2.28 a | 59.62 ± 7.69 a | 59.68 ± 3.69 a |
| CK | 3459.00 ± 144.90 d | - | - | - | - |
| Treatments | pH | OM g/kg | TN g/kg | TP g/kg | AN mg/kg | AP mg/kg | AK mg/kg |
|---|---|---|---|---|---|---|---|
| NP | 8.05 ± 0.02 ab | 9.50 ± 0.50 c | 13.10 ± 6.50 a | 4.03 ± 0.56 c | 60.67 ± 10.69 c | 68.79 ± 4.89 c | 187.99 ± 5.63 b |
| NPM | 7.98 ± 0.04 b | 11.50 ± 0.10 b | 17.70 ± 4.30 a | 6.33 ± 0.36 b | 102.33 ± 10.69 b | 270.42 ± 6.24 b | 260.88 ± 34.24 c |
| SNP | 7.99 ± 0.02 b | 11.20 ± 0.30 b | 18.70 ± 5.80 a | 3.35 ± 0.17 cd | 102.50 ± 14.57 b | 55.71 ± 3.85 c | 220.82 ± 12.65 c |
| SNPM | 7.86 ± 0.03 c | 16.50 ± 1.20 a | 18.70 ± 1.60 a | 15.54 ± 0.72 a | 165.67 ± 14.57 a | 345.30 ± 22.50 a | 447.30 ± 29.13 a |
| CK | 8.07 ± 0.08 a | 9.08 ± 0.40 c | 12.10 ± 5.80 a | 2.66 ± 0.15 d | 67.33 ± 14.57 c | 24.02 ± 2.37 d | 140.89 ± 8.22 d |
| Treatments | Community Richness Index | Chao1 Index | Shannon Index | ACE Index |
|---|---|---|---|---|
| NP | 4320.33 ± 8.50 a | 4993.09 ± 73.30 a | 10.45 ± 0.03 a | 4851.89 ± 381.37 a |
| NPM | 4152.33 ± 209.09 ab | 4773.96 ± 147.46 ab | 10.27 ± 0.05 ab | 4869.02 ± 161.74 a |
| SNP | 3866.33 ± 266.27 bc | 4784.61 ± 5.42 ab | 10.22 ± 0.25 ab | 4696.24 ± 183.87 a |
| SNPM | 3989.00 ± 128.95 abc | 4659.32 ± 151.57 b | 10.14 ± 0.10 ab | 4735.98 ± 163.06 a |
| CK | 3619.00 ± 303.85 c | 4304.08 ± 252.27 c | 10.01 ± 0.21 b | 4365.85 ± 195.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Yang, Z.; Yang, N.; Wang, H.; Li, Y.; Wang, K.; Wang, J.; Fan, Q.; Zhang, J.; Yuan, J.; et al. Wheat Yield, N Use Efficiency, Soil Properties, and Soil Bacterial Community as Affected by Long-Term Straw Incorporation and Manure Under Wheat–Summer Maize Cropping System in Southern Shanxi Province, China. Plants 2025, 14, 1795. https://doi.org/10.3390/plants14121795
Chen M, Yang Z, Yang N, Wang H, Li Y, Wang K, Wang J, Fan Q, Zhang J, Yuan J, et al. Wheat Yield, N Use Efficiency, Soil Properties, and Soil Bacterial Community as Affected by Long-Term Straw Incorporation and Manure Under Wheat–Summer Maize Cropping System in Southern Shanxi Province, China. Plants. 2025; 14(12):1795. https://doi.org/10.3390/plants14121795
Chicago/Turabian StyleChen, Mengni, Zhiguo Yang, Na Yang, Hui Wang, Yongshan Li, Ke Wang, Jian Wang, Qiaolan Fan, Jiancheng Zhang, Jiawei Yuan, and et al. 2025. "Wheat Yield, N Use Efficiency, Soil Properties, and Soil Bacterial Community as Affected by Long-Term Straw Incorporation and Manure Under Wheat–Summer Maize Cropping System in Southern Shanxi Province, China" Plants 14, no. 12: 1795. https://doi.org/10.3390/plants14121795
APA StyleChen, M., Yang, Z., Yang, N., Wang, H., Li, Y., Wang, K., Wang, J., Fan, Q., Zhang, J., Yuan, J., Dong, P., & Wang, L. (2025). Wheat Yield, N Use Efficiency, Soil Properties, and Soil Bacterial Community as Affected by Long-Term Straw Incorporation and Manure Under Wheat–Summer Maize Cropping System in Southern Shanxi Province, China. Plants, 14(12), 1795. https://doi.org/10.3390/plants14121795






