Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
2.2. Relative Growth Rate
2.3. Chlorophyll Content
2.4. Measurement of Chlorophyll a Fluorescence Parameters
2.5. Measurement of Key Enzyme Activities in the Calvin Cycle
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.7. Data Analysis
3. Results
3.1. Effects of Melatonin on the Growth of Tomato Seedlings Under Salt Stress
3.2. Effects of Melatonin on Photosynthetic Pigments in Tomato Seedlings Under Salt Stress
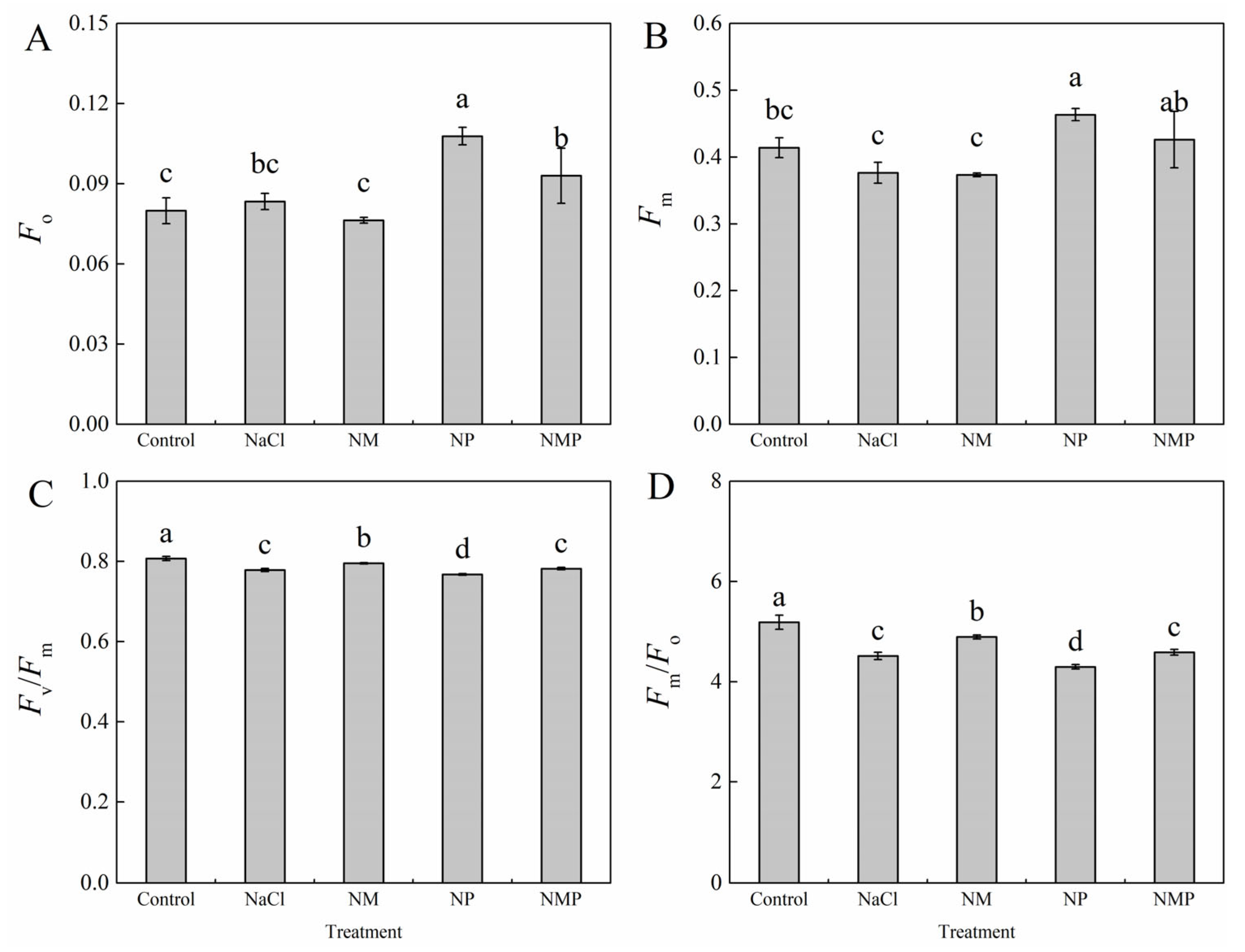
3.3. Effects of Melatonin on Dark-Adapted Chlorophyll a Fluorescence Parameters in Tomato Seedlings Under Salt Stress
3.4. Effects of Melatonin on Light-Adapted Chlorophyll a Fluorescence Parameters in Tomato Seedlings Under Salt Stress
3.5. Effects of Melatonin on the Distribution of Excitation Energy Between PSI and PSII in Tomato Seedlings Under Salt Stress
3.6. Effects of Melatonin on the Non-Cyclic Electron Transport Rate, Photochemical Reaction Rate, Thermal Dissipation Rate, and Photosynthetic Function Limitation in Tomato Seedlings Under Salt Stress
3.7. Effects of Melatonin on the Distribution of Absorbed Light Energy in PSII of Tomato Seedlings Under Salt Stress
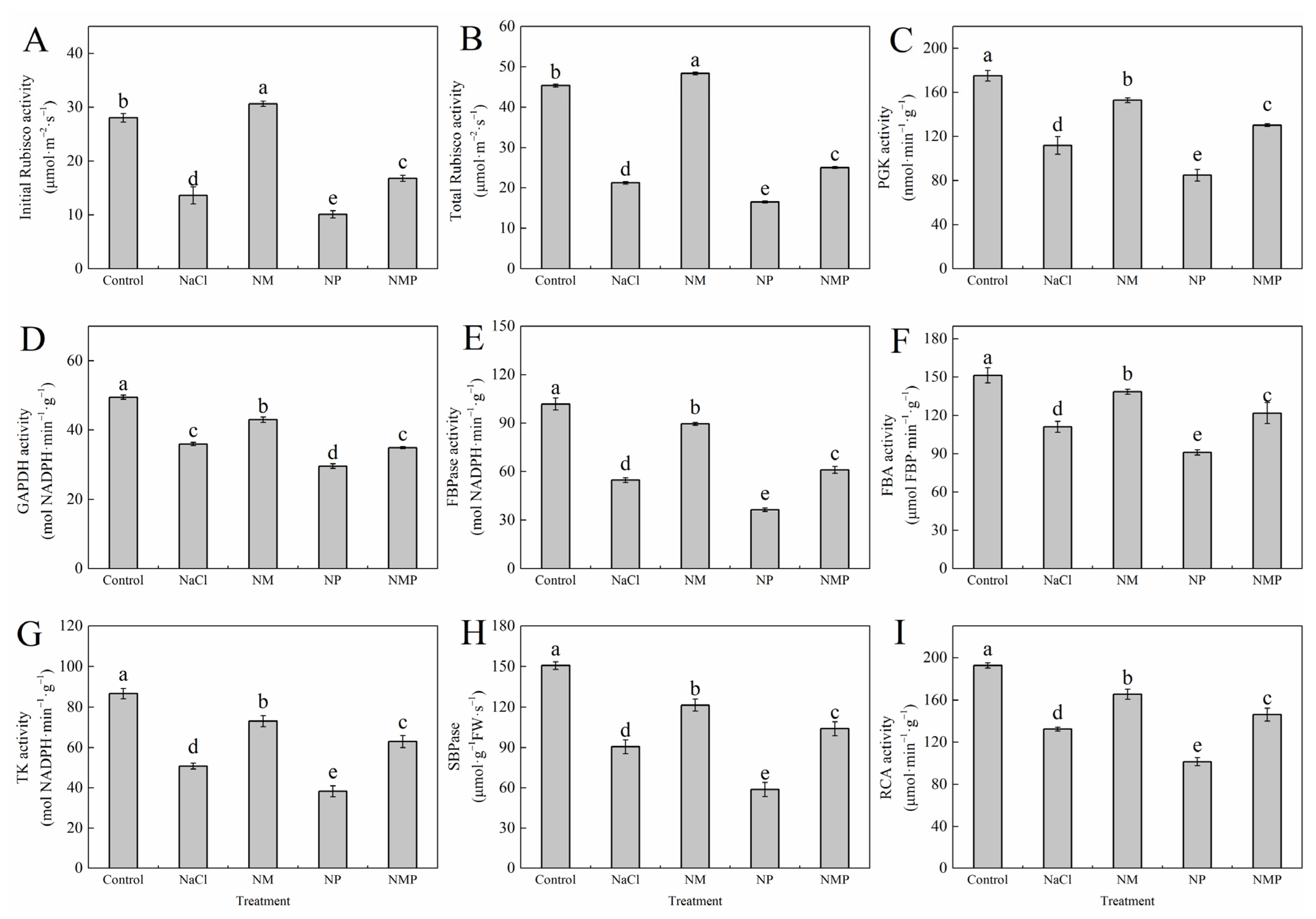
3.8. Effects of Melatonin on the Activity and Gene Expression of Key Enzymes in the Calvin Cycle of Tomato Seedlings Under Salt Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.; Zhu, H.; Wang, Y.; Kang, J.; Hu, L.; Li, L.; Zhu, K.; Yan, J.; Bu, X.; Wang, X.; et al. Revisiting the Role of Light Signaling in Plant Responses to Salt Stress. Horticulturae 2025, 12, uhae262. [Google Scholar] [CrossRef] [PubMed]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil Salinity, a Serious Environmental Issue and Plant Responses: A Metabolomics Perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaadi, K.A.; Tola, E.; Madugundu, R.; Zeyada, A.M.; Alameen, A.A.; Edrris, M.K.; Edrees, H.F.; Mahjoop, O. Response of Leaf Photosynthesis, Chlorophyll Content and Yield of Hydroponic Tomatoes to Different Water Salinity Levels. PLoS ONE 2024, 19, e0293098. [Google Scholar] [CrossRef] [PubMed]
- Borbély, P.; Iqbal, N.; Czékus, Z.; Tari, I.; Poór, P. Exogenous Sodium Nitroprusside Alleviates Salt-Induced Changes in Photosynthesis of Greenhouse Tomato Plants by Leaf Age-Dependent Manner. J. Plant Growth Regul. 2025, 1–13. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Li, J. The Effect of Salinity Stress on Tomato Defense Mechanisms and Exogenous Application of Salicylic Acid, Abscisic Acid, and Melatonin to Reduce Salinity Stress. Soil Sci. Plant Nutr. 2025, 71, 93–110. [Google Scholar] [CrossRef]
- Mekkaoui, F.; Ait-El-Mokhtar, M.; Zaari Jabri, N.; Amghar, I.; Essadssi, S.; Hmyene, A. The Use of Compost and Arbuscular Mycorrhizal Fungi and Their Combination to Improve Tomato Tolerance to Salt Stress. Plants 2024, 13, 2225. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; He, Z.; Liu, Y.; Chen, Z.; Ottosen, C.-O.; Mittler, R.; Wu, Z.; Zhou, R. Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants. Antioxidants 2024, 13, 448. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A Master Regulator of Plant Development and Stress Responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An Evolutionary View of Melatonin Synthesis and Metabolism Related to Its Biological Functions in Plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, J.; Li, J.; Lin, X.; Xia, X.; Yan, W.; Zhang, Y.; Deng, X.; Ke, Q. Meta-analysis of the effect of melatonin application on abiotic stress tolerance in plants. Plant Biotechnol. Rep. 2023, 17, 39–52. [Google Scholar] [CrossRef]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Jang, Y.H.; Kim, E.G.; Kim, N.; Kim, K.M. Exogenous melatonin induces salt and drought stress tolerance in rice by promoting plant growth and defense system. Sci. Rep. 2024, 14, 1214. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Wang, F.; Liu, A.; Chen, S.; Li, X. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Wang, G.; Dou, J.; Zhang, Y.; Yang, Q.; He, Y.; Tang, Z.; Yu, J. Protective mechanisms of exogenous melatonin on chlorophyll metabolism and photosynthesis in tomato seedlings under heat stress. Front. Plant Sci. 2025, 16, 1519950. [Google Scholar] [CrossRef]
- Shah, S.H.A.; Wang, H.; Xu, H.; Yu, Z.; Hou, X.; Li, Y. Comparative transcriptome analysis reveals the protective role of melatonin during salt stress by regulating the photosynthesis and ascorbic acid metabolism pathways in Brassica campestris. Int. J. Mol. Sci. 2024, 25, 5092. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, S.; Wang, M.; Zeb, S.; Khan, M.N.; Chen, Y.; Zhu, G.; Zhu, Z. Enhancement of sweetpotato tolerance to chromium stress through melatonin and glutathione: Insights into photosynthetic efficiency, oxidative defense, and growth parameters. Plant Physiol. Biochem. 2024, 208, 108509. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Cong, Y.; Jiang, Y.; Zhang, J.; Yang, Q.; Liu, H. Melatonin alleviates photosynthetic injury in tomato seedlings subjected to salt stress via OJIP chlorophyll fluorescence kinetics. Plants 2025, 14, 824. [Google Scholar] [CrossRef]
- Taghvimi, P.; Golfazani, M.M.; Taghvaei, M.M.; Lahiji, H.S. Investigating the effect of drought stress and methanol spraying on the influential genes in the Calvin cycle and photorespiration of rapeseed (Brassica napus). Funct. Plant Biol. 2024, 51, 23–36. [Google Scholar] [CrossRef]
- Gurrieri, L.; Fermani, S.; Zaffagnini, M.; Sparla, F.; Trost, P. Calvin–Benson cycle regulation is getting complex. Trends Plant Sci. 2021, 26, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Z.; Zhang, Y.; Bai, L.; Hu, X.; Li, X.; Zhang, L.; Miao, Y.; Wang, Y. Melatonin reduces photoinhibition in cucumber during chilling by regulating the Calvin–Benson cycle. Sci. Hortic. 2022, 299, 111007. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A.; Hasan, M.M. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Fatma, M.; Al-Hashimi, A.; Sofo, A.; Khan, N.A. Relative effects of melatonin and hydrogen sulfide treatments in mitigating salt damage in wheat. Front. Plant Sci. 2024, 15, 1406092. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, Z.; Zhang, Y.; Wang, X.; Shao, D.; Wang, C.; Wang, J.; Wang, B.; Zhao, J.; Xu, Z.; et al. Biochar-based pelletized seed enhances the yield of late-sown rapeseed by improving the relative growth rate and cold resistance of seedlings. Ind. Crops Prod. 2025, 223, 119993. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Cong, Y.; Liu, X.; Yang, Q.; Xing, J.; Liu, H. Ascorbic acid mitigates salt stress in tomato seedlings by enhancing chlorophyll synthesis pathways. Agronomy 2024, 14, 1810. [Google Scholar] [CrossRef]
- Chen, X.; Han, H.; Cong, Y.; Li, X.; Zhang, W.; Wan, W.; Cui, J.; Xu, W.; Diao, M.; Liu, H. The protective effect of exogenous ascorbic acid on photosystem inhibition of tomato seedlings induced by salt stress. Plants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO₂ fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, X.; Xing, J.; Li, X.; Pang, S.; Liu, H. Nitric oxide signal is required for glutathione-induced enhancement of photosynthesis in salt-stressed Solanum lycopersicum L. Front. Plant Sci. 2024, 15, 1413653. [Google Scholar] [CrossRef]
- Sherin, G.; Aswathi, K.; Puthur, J.T. Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress 2022, 4, 100079. [Google Scholar] [CrossRef]
- Sun, H.; Luan, G.; Ma, Y.; Lou, W.; Chen, R.; Feng, D.; Zhang, S.; Sun, J.; Lu, X. Engineered hypermutation adapts cyanobacterial photosynthesis to combined high light and high temperature stress. Nat. Commun. 2023, 14, 1238. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and responses of chloroplasts to salt stress in plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. The physiological and molecular mechanisms of silicon action in salt stress amelioration. Plants 2024, 13, 525. [Google Scholar] [CrossRef]
- Chaurasia, S.; Sapna, S.; Padhy, A.K.; Bhatia, S. Emerging roles of melatonin in mitigating salinity stress of legumes. S. Afr. J. Bot. 2023, 163, 181–190. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Y.; Zhu, G.; Zhou, G. Melatonin mitigated salinity stress on alfalfa by improving antioxidant defense and osmoregulation. Agronomy 2023, 13, 1727. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Shahzadi, A.; Noreen, Z.; Alamery, S.; Zafar, F.; Haroon, A.; Rashid, M.; Aslam, M.; Younas, A.; Attia, K.A.; Mohammed, A.A.; et al. Effects of biochar on growth and yield of wheat (Triticum aestivum L.) under salt stress. Sci. Rep. 2024, 14, 20024. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Varone, L.; Gratani, L. Physiological response of eight Mediterranean maquis species to low air temperatures during winter. Photosynthetica 2007, 45, 385–391. [Google Scholar] [CrossRef]
- Ahmad, I.; Munsif, F.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Babar, S.; Fawad, M.; Zia, A. Beneficial effect of melatonin on growth and chlorophyll content in wheat (Triticum aestivum L.) grown under salt stress conditions. Gesunde Pflanzen 2022, 74, 997–1009. [Google Scholar] [CrossRef]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F.; et al. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Y.; Qin, X.; Ding, C.; Chen, Y.; Tang, Z.; Huang, Y.; Reiter, R.J.; Yuan, S.; Yuan, M. New insights into the role of melatonin in photosynthesis. J. Exp. Bot. 2022, 73, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta (BBA)—Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Yamamoto, H.Y. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 1991, 96, 635–643. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Lv, Y.; Li, T.; Tang, J.; Yang, X.; Zhou, H. Effects of drought stress during critical periods on the photosynthetic characteristics and production performance of naked oat (Avena nuda L.). Sci. Rep. 2022, 12, 11199. [Google Scholar] [CrossRef]
- Jat, M.; Ray, M.; Ahmad, M.A.; Prakash, P. Unravelling the photosynthetic dynamics and fluorescence parameters under ameliorative effects of 24-epibrassinolide in wheat (Triticum aestivum L.) grown under heat stress regime. Sci. Rep. 2024, 14, 30745. [Google Scholar] [CrossRef]
- Madireddi, S.K.; Nama, S.; Devadasu, E.R.; Subramanyam, R. Photosynthetic membrane organization and role of state transition in cyt, cpii, stt7 and npq mutants of Chlamydomonas reinhardtii. J. Photochem. Photobiol. B Biol. 2014, 137, 77–83. [Google Scholar] [CrossRef]
- Chen, J.H.; Tang, M.; Jin, X.Q.; Li, H.; Chen, L.S.; Wang, Q.L.; Sun, A.Z.; Guo, F.Q. Regulation of Calvin–Benson cycle enzymes under high temperature stress. Abiotech 2022, 3, 65–77. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Chen, X.; Cong, Y.; Cui, J.; Shi, Q.; Liu, H.; Diao, M. The positive effects of exogenous sodium nitroprusside on the plant growth, photosystem II efficiency and Calvin cycle of tomato seedlings under salt stress. Sci. Hortic. 2022, 299, 111016. [Google Scholar] [CrossRef]
- Waheeda, K.; Chiu, P.L. Complex formation of rubisco and rubisco activase. Biophys. J. 2022, 121, 452a. [Google Scholar] [CrossRef]
- Afamefule, C.; Raines, C.A. Insights into the regulation of the expression pattern of Calvin–Benson–Bassham cycle enzymes in C3 and C4 grasses. Front. Plant Sci. 2020, 11, 570436. [Google Scholar] [CrossRef] [PubMed]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawon, T.; Parry, M.J.; Raines, C.A. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. B 2017, 372, 20160384. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Q.; He, D.; Bai, H.; Wen, J. The ubiquity and coexistence of two FBPases in chloroplasts of photosynthetic eukaryotes and its evolutionary and functional implications. Plant Divers. 2020, 42, 120–125. [Google Scholar] [CrossRef]
- Bi, H.; Li, F.; Wang, H.; Ai, X. Overexpression of transketolase gene promotes chilling tolerance by increasing the activities of photosynthetic enzymes, alleviating oxidative damage and stabilizing cell structure in Cucumis sativus L. Physiol. Plant 2019, 167, 502–515. [Google Scholar] [CrossRef]
- Carrera, D.Á.; George, G.M.; Fischer-Stettler, M.; Galbier, F.; Eicke, S.; Truernit, E.; Streb, S.; Zeeman, S.C. Distinct plastid fructose bisphosphate aldolases function in photosynthetic and non-photosynthetic metabolism in Arabidopsis. J. Exp. Bot. 2021, 72, 3739–3755. [Google Scholar] [CrossRef]
- Cai, B.; Li, Q.; Liu, F.; Bi, H.; Ai, X. Decreasing fructose-1,6-bisphosphate aldolase activity reduces plant growth and tolerance to chilling stress in tomato seedlings. Physiol. Plant. 2018, 163, 247–258. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, B.; Jiang, Y.; Zhang, J.; Liu, M.; Yang, Q.; Liu, H. Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity. Plants 2025, 14, 1785. https://doi.org/10.3390/plants14121785
Chen X, Chen B, Jiang Y, Zhang J, Liu M, Yang Q, Liu H. Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity. Plants. 2025; 14(12):1785. https://doi.org/10.3390/plants14121785
Chicago/Turabian StyleChen, Xianjun, Bi Chen, Yao Jiang, Jianwei Zhang, Mingjie Liu, Qin Yang, and Huiying Liu. 2025. "Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity" Plants 14, no. 12: 1785. https://doi.org/10.3390/plants14121785
APA StyleChen, X., Chen, B., Jiang, Y., Zhang, J., Liu, M., Yang, Q., & Liu, H. (2025). Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity. Plants, 14(12), 1785. https://doi.org/10.3390/plants14121785





