Silicon Reduce Structural Carbon Components and Its Potential to Regulate the Physiological Traits of Plants
Abstract
1. Introduction
2. Results
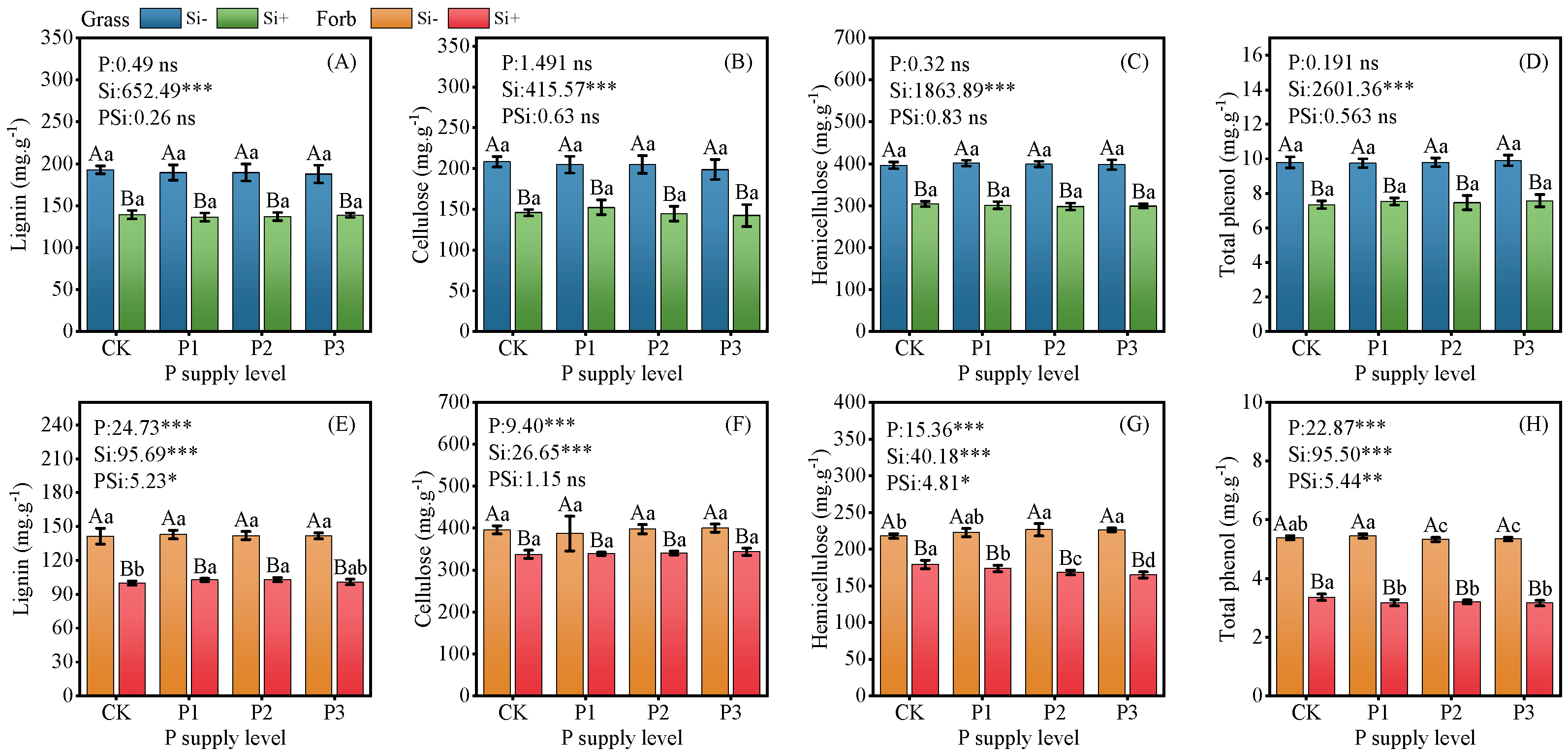
2.1. Structural Carbon Components and Phenol Content
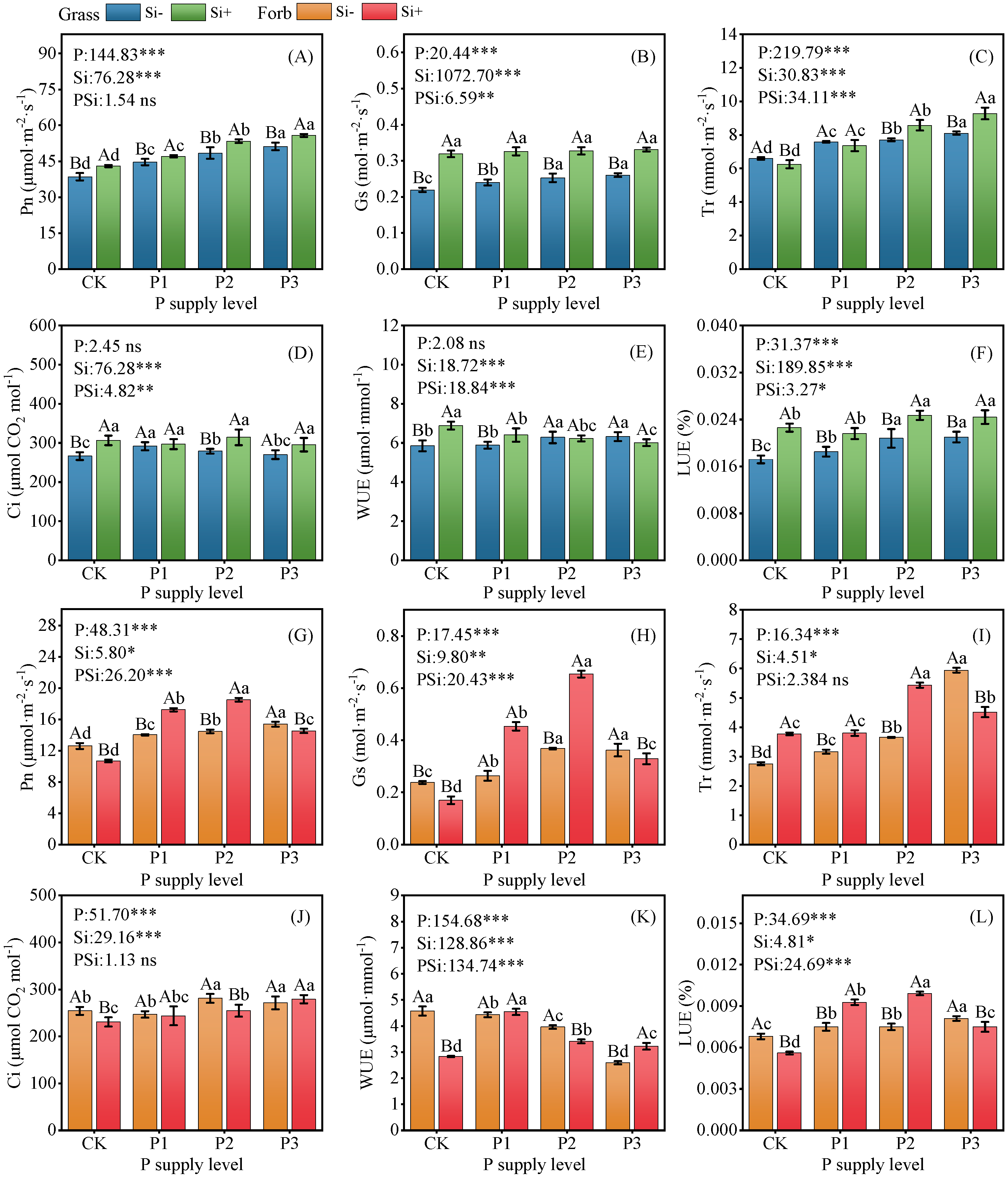
2.2. Photosynthetic Performance
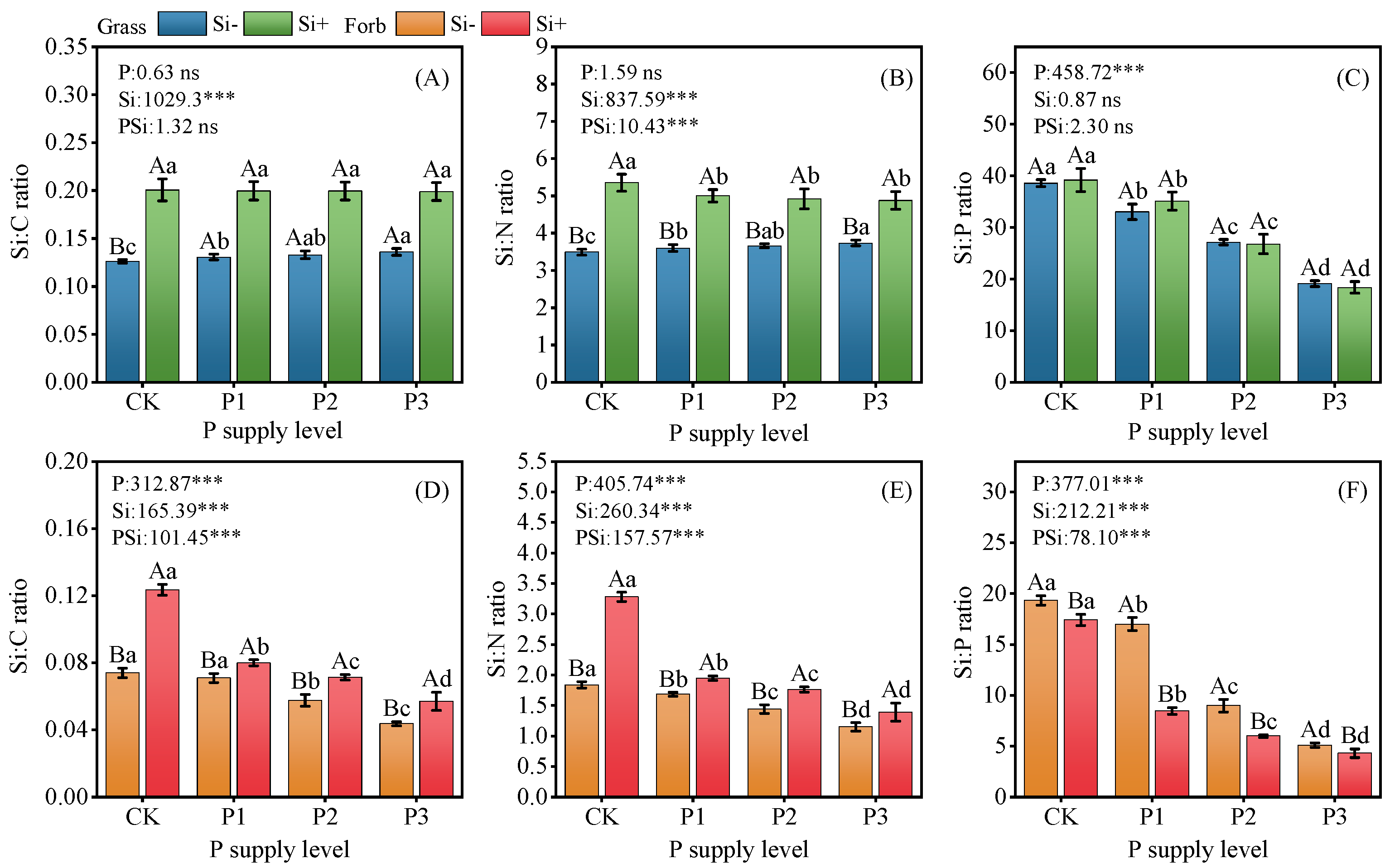
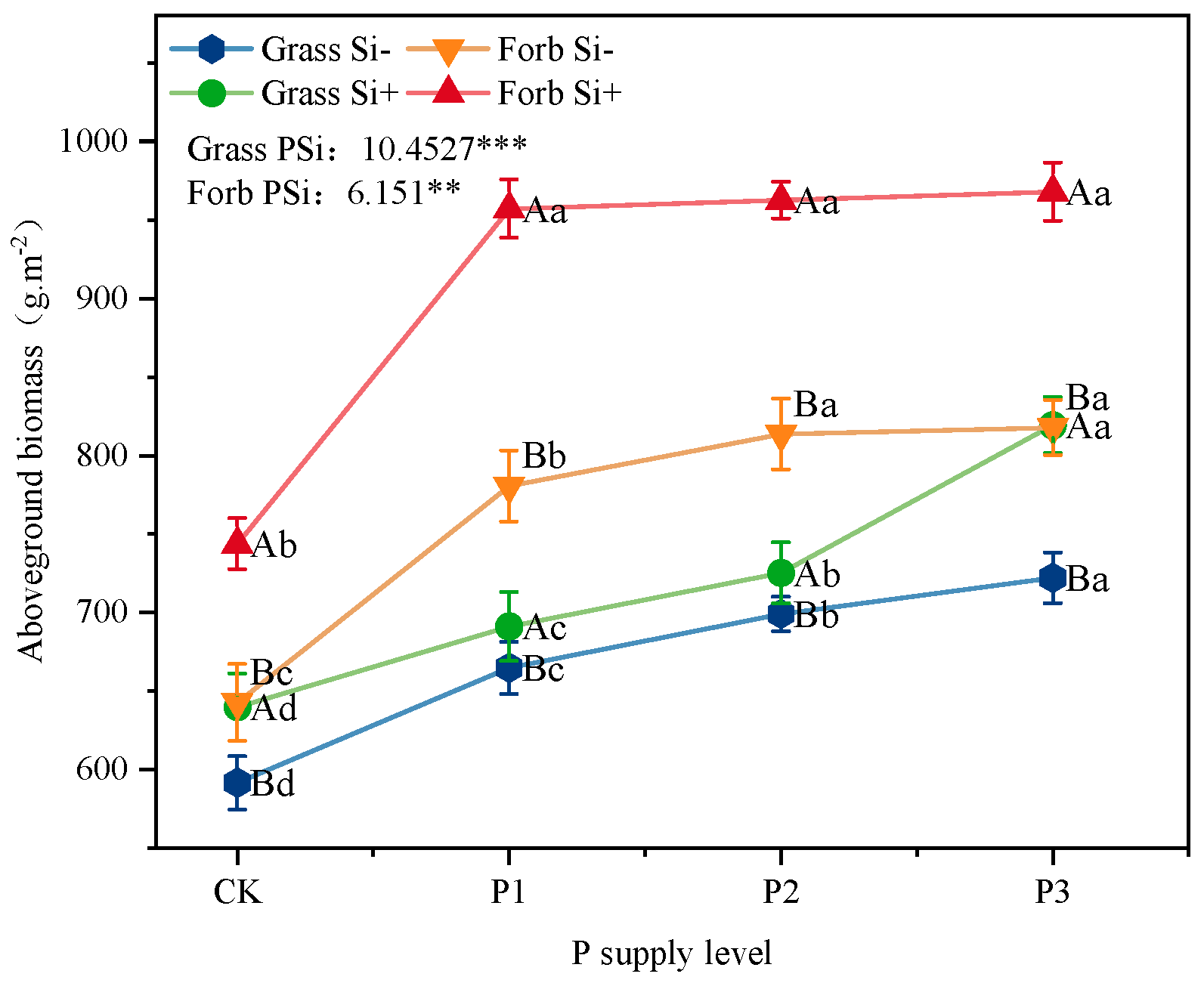
2.3. Stoichiometric Ratios and Biomass
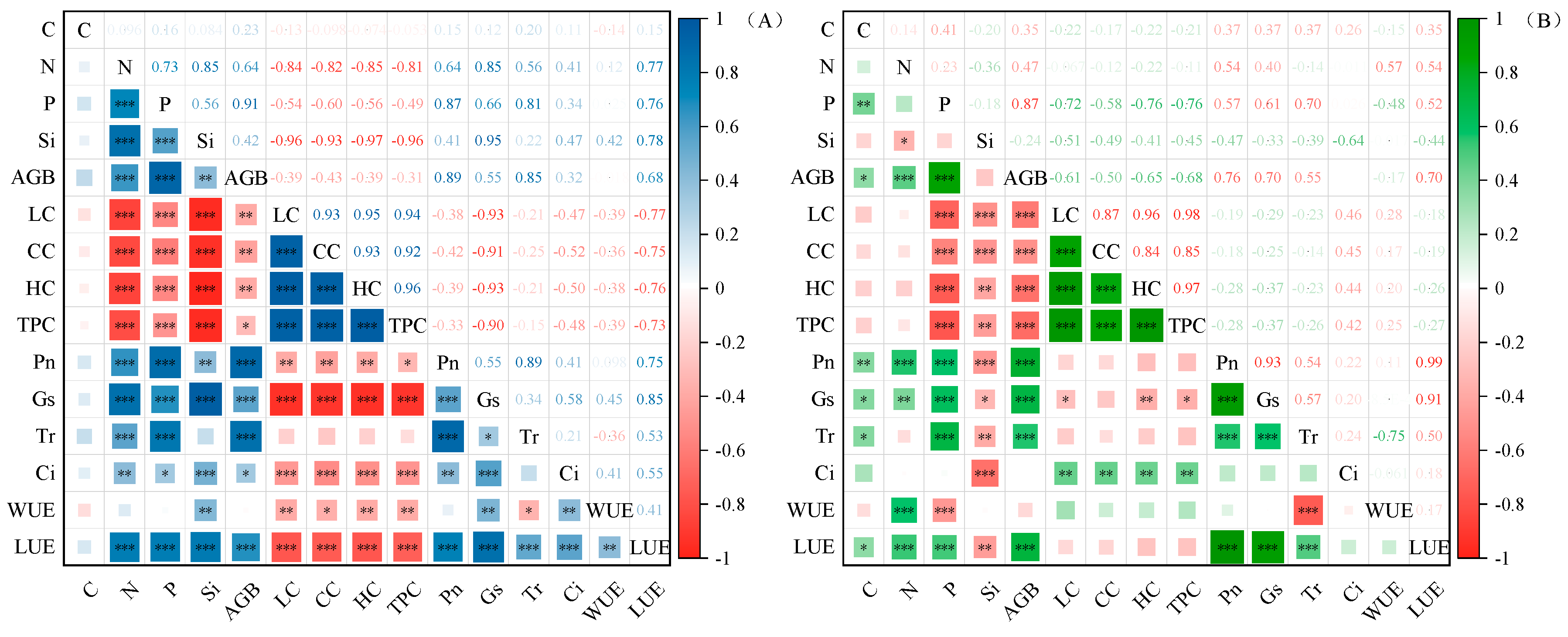
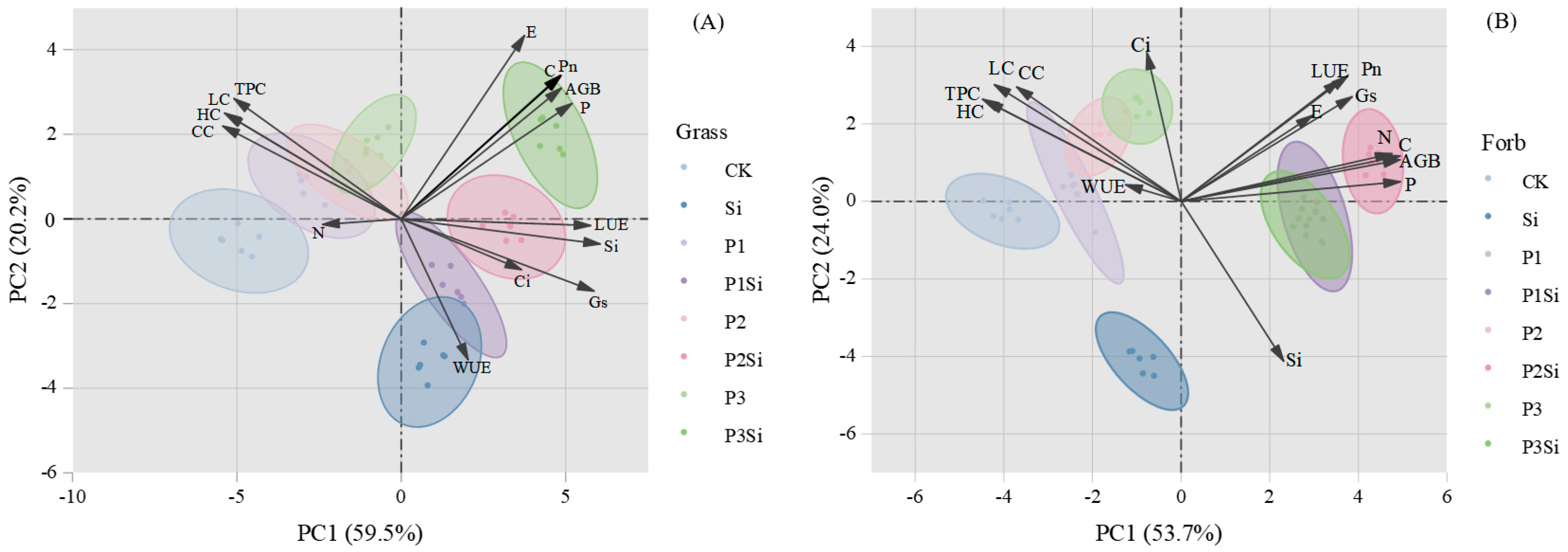
2.4. Correlation and Principal Component Analysis
3. Discussion
3.1. Silicon Decreased Plant Structural Carbon Components and Phenols
3.2. Silicon Increased Plant Photosynthesis
3.3. Si Increased Biomass and Changed Plant Stoichiometry
4. Materials and Methods
4.1. Study Location
4.2. Experimental Design
4.3. Determination of Photosynthetic Parameters
4.4. Plant Materials
4.5. Determination of Soil Characteristics
4.6. Leaf C, N, P, and Si Contents
4.7. Lignin, Cellulose, Hemicellulose, and Total Phenol Contents
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, X.; Qin, W.K.; Xu, H.; Zhang, Z.H.; Zhou, H.K.; Zhu, B. Sensitivity of soil carbon dynamics to nitrogen and phosphorus enrichment in an alpine meadow. Soil Biol. Biochem. 2020, 150, 107984. [Google Scholar] [CrossRef]
- Cui, H.; Sun, W.; Delgado-Baquerizo, M.; Song, W.; Ma, J.Y.; Wang, K.; Ling, X. Phosphorus addition regulates the responses of soil multifunctionality to nitrogen over–fertilization in a temperate grassland. Plant Soil. 2022, 473, 73–87. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Lambers, H. Phosphorus Metabolism in Plants PREFACE. Annu. Plant Rev. 2015, 48, XXIII–XXIV. [Google Scholar]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Hernández, I.; Munné-Bosch, S. Linking phosphorus availability with photooxidative stress in plants. J. Exp. Bot. 2015, 66, 2889–2900. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B. Soil processes drive the biological silicon feedback loop. Funct. Ecol. 2016, 30, 1298–1310. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Oberson, A.; Frossard, E. Phosphorus in action: Biological processes in soil phosphorus cycling. Soil Biol. 2011, 26, 447. [Google Scholar]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019, 9, 449. [Google Scholar] [CrossRef]
- Kostic, L.; Nikolic, N.; Bosnic, D.; Samardzic, J.; Nikolic, M. Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil 2017, 419, 447–455. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Z.; Yang, X.; Song, A.; Yu, C.; Wang, T.; Xia, S.P.; Liang, Y.C. Impacts of silicon on biogeochemical cycles of carbon and nutrients in croplands. J. Integr. Agric. 2018, 17, 2182–2195. [Google Scholar] [CrossRef]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W. Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Schaller, J.; Heimes, R.; Ma, J.F.; Meunier, J.D.; Shao, J.F.; Fujii-Kashino, M.; Knorr, K.H. Silicon accumulation in rice plant aboveground biomass affects leaf carbon quality. Plant Soil 2019, 444, 399–407. [Google Scholar] [CrossRef]
- de Tombeur, F.; Cooke, J.; Collard, L.; Cisse, D.; Saba, F.; Lefebvre, D.; Burgeon, V.; Nacro, H.B.; Cornelis, J.T. Biochar affects silicification patterns and physical traits of rice leaves cultivated in a desilicated soil (Ferric lixisol). Plant Soil 2021, 460, 375–390. [Google Scholar] [CrossRef]
- Hodson, M.J.; Guppy, C.N. Some thoughts on silicon and carbon trade-offs in plants. Plant Soil 2022, 477, 233–239. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Gessner, M.O.; Bäuker, E.; Gert Dudel, E. Silicon supply modifies C:N:P stoichiometry and growth of Phragmites australis. Plant Biol. 2012, 14, 392–396. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Dudel, E.G. Silicon availability changes structural carbon ratio and phenol content of grass. Environ. Exp. Bot. 2012, 77, 283–287. [Google Scholar] [CrossRef]
- Jung, H.J.G.; Varel, V.H.; Weimer, P.J.; Ralph, J. Accuracy of Klason lignin and acid detergent lignin methods as assessed by bomb calorimetry. J. Agric. Food Chem. 1999, 47, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. The transport and function of silicon in plants. Biol. Rev. 1983, 58, 179–207. [Google Scholar] [CrossRef]
- de Tombeur, F.; Raven, J.A.; Toussaint, A.; Violle, C.; Cooke, J.; Hartley, S.E.; Johnson, S.N.; Coq, S.; Katz, O.; Schaller, J.; et al. Why do plants silicify? Trends Ecol. Evol. 2023, 38, 275–288. [Google Scholar] [CrossRef]
- Schoelynck, J.; Bal, K.; Backx, H.; Okruszko, T.; Meire, P.; Struyf, E. Silica uptake in aquatic and wetland macrophytes: A strategic choice between silica, lignin and cellulose? New Phytol. 2010, 186, 385–391. [Google Scholar] [CrossRef]
- Ulloa, M.; Nunes-Nesi, A.; da Fonseca-Pereira, P.; Poblete-Grant, P.; Reyes-Díaz, M.; Cartes, P. The effect of silicon supply on photosynthesis and carbohydrate metabolism in two wheat (Triticum aestivum L.) cultivars contrasting in response to phosphorus nutrition. Plant Physiol. Bioch. 2021, 169, 236–248. [Google Scholar] [CrossRef]
- Guo, N.; Degen, A.A.; Deng, B.; Shi, F.Y.; Bai, Y.F.; Zhang, T.; Long, R.J.; Shang, Z.H. Changes in vegetation parameters and soil nutrients along degradation and recovery successions on alpine grasslands of the Tibetan plateau. Agric. Ecosyst. Environ. 2019, 284, 106593. [Google Scholar] [CrossRef]
- Chen, J.J.; Yi, S.H.; Qin, Y. The contribution of plateau pika disturbance and erosion on patchy alpine grassland soil on the Qinghai-Tibetan Plateau: Implications for grassland restoration. Geoderma 2017, 297, 1–9. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmulowicz, B.; Dabrowski, P.; Szara, E.; Perzanowska, A.; Wrobel, B. Can the application the silicon improve the productivity and nutritional value of grass-clover sward in conditions of rainfall shortage in organic management? Agronomy 2020, 10, 14. [Google Scholar] [CrossRef]
- Xu, D.H.; Gao, T.P.; Fang, X.W.; Bu, H.Y.; Li, Q.X.; Wang, X.N.; Zhang, R.Y. Silicon addition improves plant productivity and soil nutrient availability without changing the grass: Legume ratio response to N fertilization. Sci. Rep. 2020, 10, 9. [Google Scholar]
- Tran, H.; Ficke, A.; Asiimwe, T.; Hofte, M.; Raaijmakers, J.M. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol. 2007, 175, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C.; Nesselrode, B.H.W.A.; Miller, J.G.; Mewburn, B.R. Mixed-linkage (1→3,1→4)-β-D-glucan is a major hemicellulose of Equisetum (Horsetail) cell walls. New Phytol. 2008, 179, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Valencise, C.A.; Fernandes, J.; Carnier, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar]
- Tamai, K.; Ma, J.F. Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 2008, 307, 21–27. [Google Scholar] [CrossRef]
- Xu, D.H.; Fang, X.W.; Zhang, R.Y.; Gao, T.P.; Bu, H.Y.; Du, G.Z. Influences of nitrogen, phosphorus and silicon addition on plant productivity and species richness in an alpine meadow. AoB Plants 2015, 7, plv125. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Xia, J.; Wilcox, K.R.; Cao, J.; Zhou, X.; Jiang, L.; Niu, S.; Estera, K.Y.; Huang, R.; et al. Warming effects on ecosystem carbon fluxes are modulated by plant functional types. Ecosystems 2017, 20, 515–526. [Google Scholar] [CrossRef]
- Wang, S.; Duan, J.; Xu, G.; Wang, Y.; Zhang, Z.; Rui, Y.; Luo, C.; Xu, B.; Zhu, X.; Chang, X.; et al. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 2012, 93, 2365–2376. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Ahmed, M.; Kamran, A.; Asif, M.; Qadeer, U.; Ahmed, Z.I.; Goyal, A. Silicon priming: A potential source to impart abiotic stress tolerance in wheat: A review. Aust. J. Crop Sci. 2013, 7, 484–491. [Google Scholar]
- Feng, M.J. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. J. Plant Nutr. Soil Sc. 2004, 50, 11–18. [Google Scholar]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Sajad, H.; Li, S.; Maryam, M.; Iram, S.; Nasir, I.; Marian, B.; Muhammad, S.; Qin, S.; Wang, L.; Xu, M.; et al. Foliar application of silicon improves stem strength under low light stress by regulating lignin biosynthesis genes in soybean (Glycine max (L.) Merr.). J. Hazard Mater. 2021, 401, 123256. [Google Scholar]
- Meunier, J.D.; Barboni, D.; Anwar-Ul-Haq, M.; Levard, C.; Chaurand, P.; Vidal, V.; Grauby, O.; Huc, R.; Laffont-Schwob, I.; Rabier, J.; et al. Effect of phytoliths for mitigating water stress in durum wheat. New Phytol. 2017, 215, 229–239. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, D.H.M.; Mesquita, M.; Bueno, A.M.; Flores, R.A.; de Oliveira, H.F.E.; de Lima, F.S.R.; Prado, R.D.; Battisti, R. Combined effects of induced water deficit and foliar application of silicon on the gas exchange of tomatoes for processing. Agronomy 2020, 10, 1715. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Cabrera, J.C.B.; Hirl, R.T.; Schaufele, R.; Macdonald, A.; Schnyder, H. Stomatal conductance limited the CO2 response of grassland in the last century. BMC Biol. 2021, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Iturrate-Garcia, M.; Heijmans-Monique, M.P.D.; Cornelissen, J.H.C.; Schweingruber, F.H.; Niklaus, P.A.; Schaepman-Strub, G. Plant trait response of tundra shrubs to permafrost thaw and nutrient addition. Biogeosciences 2020, 17, 4981–4998. [Google Scholar] [CrossRef]
- Soons, M.B.; Hefting, M.M.; Dorland, E.; Lamers, L.M.P.; Versteeg, C.; Bobbink, R. Nitrogen effects on plant species richness in herbaceous communities are more widespread and stronger than those of phosphorus. Biol. Conserv. 2017, 212, 390–397. [Google Scholar] [CrossRef]
- He, Y.E.; Hong, M.; Liang, Z.; Tu, N.R.; Wu, Z.D.; Wang, L.Q.; Bao, M.Z.; Zhao, B. Effects of precipitation and nitrogen deposition on litter decomposition of two perennial grass in a desert steppe. Acta Ecolo. Sin. 2021, 42, 2910–2920. [Google Scholar]
- Mitani-Ueno, N.; Ma, J.F. Linking transport system of silicon with its accumulation in different plant species. J. Soil Sci. Plant Nut. 2021, 67, 10–17. [Google Scholar] [CrossRef]
- Mayor, J.R.; Wright, S.J.; Turner, B.L. Species-specific responses of foliar nutrients to longterm nitrogen and phosphorus additions in a lowland tropical forest. J. Ecol. 2014, 102, 36–44. [Google Scholar] [CrossRef]
- Chen, F.S.; Niklas, K.J.; Liu, Y.; Fang, X.M.; Wan, S.Z.; Wang, H. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, F.B. Effect of Increasing Levels of Monosilicic Acid on Arsenic and Phosphorus Sorption in Soil. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2018. [Google Scholar]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Hu, K.W.; Yan, L.; Guan, L.Z. Interaction of silicon and phosphorus in soils. Chin. J. Soil Sci. 2004, 2, 230–233. [Google Scholar]
- Teixeira, G.C.M.; Prado, R.D.; Rocha, A.M.S.; Piccolo, M.D. Silicon as a Sustainable Option to Increase Biomass with Less Water by Inducing Carbon: Nitrogen: Phosphorus Stoichiometric Homeostasis in Sugarcane and Energy Cane. Front. Plant Sci. 2022, 13, 826512. [Google Scholar] [CrossRef]
- Yan, Z.B.; Hou, X.H.; Han, W.X.; Ma, S.H.; Shen, H.H.; Guo, Y.L.; Fang, J.Y. Effects of nitrogen and phosphorus supply on stoichiometry of six elements in leaves of Arabidopsis thaliana. Ann. Bot. 2018, 123, 441–450. [Google Scholar] [CrossRef]
- Carey, J.; Fulweiler, R. Watershed land use alters riverine silica cycling. Biogeochemistry 2013, 113, 525–544. [Google Scholar] [CrossRef]
- Hao, Q.; Yang, S.; Song, Z.; Li, Z.; Ding, F.; Yu, C.; Hu, G.; Liu, H. Silicon affects plant stoichiometry and accumulation of C, N, and P in Grasslands. Front. Plant Sci. 2020, 11, 1304. [Google Scholar] [CrossRef]
- Hillebrand, H.; Cowles, J.M.; Lewandowska, A.; Van de Waal, D.B.; Plum, C. Think ratio! A stoichiometric view on biodiversity–ecosystem functioning research. Basic Appl. Ecol. 2014, 15, 465–474. [Google Scholar] [CrossRef]
- Lucas, P.W.; Turner, I.M.; Dominy, N.J.; Yamashita, N. Mechanical defences to herbivory. Ann. Bot. 2000, 86, 913–920. [Google Scholar] [CrossRef]
- Borawska-Jarmulowicz, B.; Mastalerczuk, G.; Janicka, M.; Wróbel, B. Effect of Silicon-Containing Fertilizers on the Nutritional Value of Grass–Legume Mixtures on Temporary Grasslands. Agriculture 2022, 12, 145. [Google Scholar] [CrossRef]
- Guo, N.; Li, Y.H.; Han, L.Y.; Wang, S.P. The effects of climate change on different types of grassland in Maqu County in Northeast Tibetan Plateau. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; pp. 1139–1142. [Google Scholar]
- Pavlovic, J.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y.; et al. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.E.; Martin, T.M.; Pauly, M. Comprehensive compositional analysis of plant cell walls (Lignocellulosic biomass) part II: Carbohydrates. J. Vis. Exp. 2010, 12, e1837. [Google Scholar]
- Brinkmann, K.; Blaschke, L.; Polle, A. Comparison of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J. Chem. Ecol. 2002, 28, 2483–2501. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Xu, D.; Zhou, W.; Wu, Y.; Mou, W. Silicon Reduce Structural Carbon Components and Its Potential to Regulate the Physiological Traits of Plants. Plants 2025, 14, 1779. https://doi.org/10.3390/plants14121779
Huang B, Xu D, Zhou W, Wu Y, Mou W. Silicon Reduce Structural Carbon Components and Its Potential to Regulate the Physiological Traits of Plants. Plants. 2025; 14(12):1779. https://doi.org/10.3390/plants14121779
Chicago/Turabian StyleHuang, Baiying, Danghui Xu, Wenhong Zhou, Yuqi Wu, and Wei Mou. 2025. "Silicon Reduce Structural Carbon Components and Its Potential to Regulate the Physiological Traits of Plants" Plants 14, no. 12: 1779. https://doi.org/10.3390/plants14121779
APA StyleHuang, B., Xu, D., Zhou, W., Wu, Y., & Mou, W. (2025). Silicon Reduce Structural Carbon Components and Its Potential to Regulate the Physiological Traits of Plants. Plants, 14(12), 1779. https://doi.org/10.3390/plants14121779





