Abstract
The interactive effects between nano-silicon dioxide (n-SiO2) and elevated CO2 (eCO2; 645 ppm) on soybean physiology, nitrogen fixation, and nutrient dynamics under climate stress remain underexplored. This study elucidates their combined effects under ambient (aCO2; 410 ppm) and eCO2 conditions. eCO2 + n-SiO2 synergistically enhanced shoot length (30%), total chlorophyll (112.15%), and photosynthetic rate (103.23%), alongside improved stomatal conductance and intercellular CO2 (17.19%), optimizing carbon assimilation. Nodulation efficiency increased, with nodule number and biomass rising by 48.3% and 53.6%, respectively, under eCO2 + n-SiO2 versus aCO2. N-assimilation enzymes (nitrate reductase, nitrite reductase, glutamine synthetase, glutamate synthase) surged by 38.5–52.1%, enhancing nitrogen metabolism. Concurrently, phytohormones (16–21%) and antioxidant activities (15–22%) increased, reducing oxidative markers (18–22%), and bolstering stress resilience. Nutrient homeostasis improved, with P, K, Mg, Cu, Fe, Zn, and Mn elevating in roots (13–41%) and shoots (13–17%), except shoot Fe and Zn. These findings demonstrate that n-SiO2 potentiates eCO2-driven benefits, amplifying photosynthetic efficiency, nitrogen fixation, and stress adaptation through enhanced biochemical and nutrient regulation. This synergy underscores n-SiO2 role in optimizing crop performance under future CO2-rich climates, advocating nano-fertilizers as sustainable tools for climate-resilient agriculture.
1. Introduction
In soybean, elevated CO2 can stimulate growth by increasing photosynthetic rates, but it also induces physiological and biochemical changes that may negatively impact seed quality. Research indicates that while carbohydrate accumulation may increase under elevated CO2, nitrogen and mineral concentrations tend to decline due to reduced transpiration and altered nutrient uptake [1,2]. Additionally, interactions between elevated CO2 and other environmental stressors, such as drought, can exacerbate these effects, leading to fluctuations in seed composition, including protein and fatty acid profiles [3,4]. Given these challenges, innovative strategies are required to balance the benefits of CO2 fertilization with the need to maintain nutrient integrity in crops.
Nanotechnology has emerged as a transformative tool to enhance crop resilience under climatic stressors [5]. Previous studies have demonstrated the potential of nanomaterials (NMs) in enhancing plant responses under elevated CO2 conditions. For instance, chitosan nanoparticles have been shown to upregulate carbon and nitrogen metabolism in soybean [6], while foliar application of CeO2 nanoparticles improved growth and nutritional quality in Spinacia oleracea L. [7], highlighting their role in promoting food security under future climatic conditions. Similarly, Se-NMs enhanced nutrient concentrations and biomass in wheat, demonstrating their potential to optimize growth conditions under increased CO2 levels [8]. Similarly, Silicon nanoparticles (n-SiO2), in particular, exhibit unique physicochemical properties that improve plant water-use efficiency, nutrient uptake, and stress tolerance [9]. Silicon, though non-essential for most plants, amplifies defense mechanisms against biotic and abiotic stresses by reinforcing cell walls, modulating antioxidant systems, and enhancing photosynthetic efficiency [10]. In legumes, silicon supplementation strengthens root architecture, promotes nodulation, and stabilizes nitrogenase activity under drought and salinity [11]. However, the interplay between n-SiO2 and eCO2 in soybean remains unexplored, despite the potential for synergistic effects on carbon assimilation and nitrogen dynamics.
In the current work, soybean plants were employed as the model leguminous species to thoroughly investigate how soybean responds to n-SiO2 exposure under elevated CO2. The detailed studies on soybean plants are particularly necessary because soybean (Glycine max) serves as a vital crop for food, animal feed, and biofuel, contributing significantly to global legume production. Its symbiotic relationship with N2-fixing bacteria enables soybeans to fix approximately 16.4 million metric tons of nitrogen annually, significantly reducing reliance on synthetic nitrogenous fertilizers. This study examines how n-SiO2 influences soybean physiology and nitrogen fixation under elevated CO2, focusing on four key aspects: (1) physiological adaptation, assessing n-SiO2 role in enhancing mesophyll conductance and chloroplast stability; (2) net photosynthetic activity, evaluating its impact on Rubisco activity and electron transport efficiency; (3) root and nodule health, investigating how n-SiO2 stabilizes nodule function amid shifting C:N ratios; and (4) biochemical and nitrogen metabolism, exploring its role in mitigating oxidative stress and optimizing nitrogen assimilation. By addressing these dimensions, this research provides critical insights into n-SiO2 potential to enhance soybean resilience and productivity in a CO2-enriched environment.
2. Results
2.1. n-SiO2 Alters Soybean Physiology Under Elevated CO2
The increasing atmospheric CO2 levels due to climate change pose significant challenges to agricultural systems, particularly in enhancing crop growth and nutritional quality. The current study aimed to investigate how n-SiO2 influences soybean physiology, including shoot growth, biomass accumulation, leaf development, and photosynthetic efficiency, under ambient (aCO2) and elevated CO2 levels (Figure 1). The results indicate that eCO2 alone significantly enhanced soybean growth, with a 30% increase in shoot length and a 58% increase in shoot fresh weight compared to aCO2 conditions. These findings align with previous reports that elevated CO2 improves photosynthesis and biomass accumulation in C3 plants like soybean [12]. However, the addition of n-SiO2 under eCO2 further boosted shoot length by an additional 38%, indicating that n-SiO2 may enhance cell elongation and chloroplast stability, optimizing photosynthetic efficiency [13]. Similarly, shoot fresh weight increased by an additional 11% with n-SiO2, reflecting its role in improving nutrient uptake and water-use efficiency, which are critical for maximizing plant productivity under stress. These results are consistent with studies showing that n-SiO2 can promote biomass accumulation through enhanced nutrient acquisition and improved physiological responses to environmental stress [14,15].
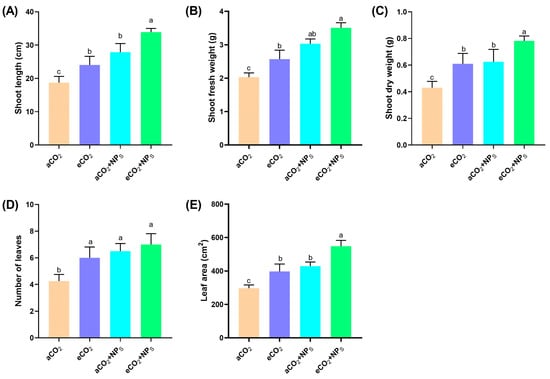
Figure 1.
The effects of n-SiO2 on soybean physiological traits under ambient (aCO2) and elevated (eCO2) CO2 conditions. Data are presented for (A) shoot length, (B) shoot fresh weight, (C) shoot dry weight, (D) number of leaves, and (E) leaf area. Soybean plants exposed to elevated CO2 showed significant improvements in all measured traits compared to ambient CO2, with n-SiO2 supplementation further enhancing these parameters. Different letters above the bars indicate significant differences (p < 0.05) between treatments.
Shoot dry weight followed a similar pattern, increasing by 38% under eCO2 and by an additional 28% with n-SiO2 supplementation (Figure 1). This suggests that n-SiO2 helps in improving the plant’s metabolic activity and stress tolerance, thereby enhancing its ability to accumulate dry matter under elevated CO2 conditions [16]. Moreover, leaf number increased by approximately 27% with n-SiO2 supplementation, likely due to enhanced auxin signaling and root function [17]. These changes in leaf morphology are crucial for optimizing the plant’s ability to capture light and perform photosynthesis effectively. The leaf area, a critical factor for photosynthetic capacity, increased by 53% under eCO2, with an additional 50% increase observed when n-SiO2 was applied (Figure 1). These results suggest that n-SiO2 enhances the plant’s capacity for light capture and carbon fixation, improving overall growth under elevated CO2 conditions. The significant increase in leaf area aligns with previous findings that n-SiO2 can enhance photosynthetic efficiency by improving leaf expansion and reducing oxidative stress [14,15]. A recent study reported that nanoparticles (CeO2-NPs) significantly increased plant physiological growth indicators (shoot and root length, shoot and root weight) by 40.5% to 84.6% at 100 ppm under the eCO2 conditions [7]. Another study documented that chitosan nanoparticles (CS-NPs) significantly enhanced soybean shoot and root biomass by ~1.6-fold under elevated CO2 conditions compared to the control group [6]. SiO2-NPS significantly increased (36%) the cherry radish fresh weight [18]. Regarding the soybean growth, ionic Si significantly increased the shoot dry weight by 23% compared to the control group [19]. These results collectively highlight that n-SiO2 plays a crucial role in optimizing soybean growth under elevated CO2 conditions. The observed enhancements in shoot elongation, biomass accumulation, leaf development, and photosynthetic potential demonstrate the potential of n-SiO2 to mitigate the negative effects of elevated CO2 and improve soybean productivity. This aligns with the growing body of literature emphasizing the role of nanomaterials, like n-SiO2, in enhancing crop resilience and performance under future climate scenarios with higher CO2 levels [6,7].
2.2. Enhancement of Photosynthesis Activity in Soybean Under Elevated CO2 Induced by n-SiO2
The application of n-SiO2 significantly enhanced soybean photosynthetic performance under eCO2 conditions, demonstrating their potential to optimize plant physiology in response to climate change. Under eCO2 (645 ppm), n-SiO2 treatment led to marked improvements in key photosynthetic parameters compared to aCO2, highlighting a strong synergistic effect between elevated CO2 and n-SiO2. A substantial increase in chlorophyll content was observed, with chlorophyll a and b rising by 106.16% and 136.92%, respectively, and a 112.15% increase in total chlorophyll (Figure 2). These results align with previous studies [6,7] and suggest enhanced chlorophyll biosynthesis and stabilization of the photosynthetic apparatus, likely due to more efficient nutrient uptake, enhanced nitrogen metabolism, and reduced oxidative stress. These changes facilitate greater light absorption and energy conversion, which are critical for maintaining high photosynthetic activity under eCO2. Further supporting this, Ahsan et al. documented that n-SiO2 nanoparticles penetrate chloroplasts, reaching photosystem II reaction centers, stimulating electron transmission and light absorption, and thus enhancing photosynthetic efficacy. This mechanism contributes to the elevated chlorophyll concentration, leading to improved photosynthesis and overall plant performance [20].
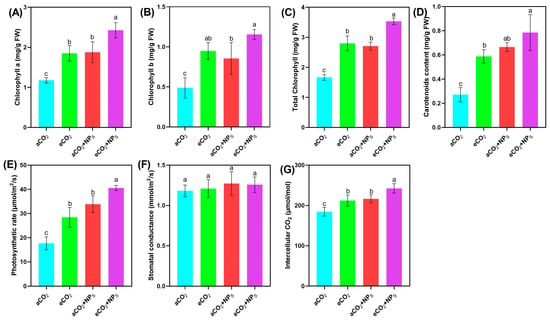
Figure 2.
Effects of n-SiO2 on soybean photosynthetic parameters under ambient (aCO2) and elevated (eCO2) CO2 conditions. Data represent (A) chlorophyll a, (B) chlorophyll b, (C) total chlorophyll, (D) carotenoid content, (E) photosynthetic rate, (F) stomatal conductance, and (G) intercellular CO2 concentration. Different letters above the bars indicate significant differences (p < 0.05) between treatments.
Additionally, carotenoid content, crucial for light harvesting and photoprotection, increased by 41.96% under eCO2 with n-SiO2 treatment. This suggests strengthened antioxidant defenses, mitigating photooxidative stress commonly associated with high CO2 levels and intense light exposure. The elevated carotenoid levels also stabilize chlorophyll molecules, supporting sustained photosynthetic efficiency. In parallel, photosynthetic efficiency, as indicated by the net photosynthetic rate (Pn), stomatal conductance (Gs), and intercellular CO2 concentration (Ci), showed significant improvements. Previous studies have documented that elevated CO2 initially boosts Pn but can lead to down-regulation over time, accompanied by reduced Gs and increased Ci [21,22]. Another study reported that nanoparticles (CeO2-NPs) significantly enhanced photosynthetic indicators (chlorophyll a, b, and total chlorophyll content) by 85.3% to 398.8% at 50–100 mg L−1 (CeO2-NPs) under the elevated CO2 conditions [7]. Additionally, CS-NPs at 40–65 μg mL−1 improved the soybean photosynthetic activities by 1.5 to 2.9 folds more than that ambient CO2 group [6]. SiO2-NPs, as a nano-fertilizer, increased the plant’s (cherry radish) total chlorophyll and carotenoids contents by 14.2–18.7% [18]. Wang et al. comparing the potential of silicate and SiO2-NPs, observed that SiO2-NPs significantly improved soybean photosynthesis related indicators compared to both the control and silicate groups [13]. In our study, the photosynthetic rate increased by 103.23% under eCO2 + n-SiO2, reflecting enhanced carbon assimilation. This was supported by improved stomatal conductance, which facilitated more efficient CO2 influx, and a 17.19% rise in intercellular CO2 concentration, indicating better CO2 diffusion and utilization within the leaf tissues. These physiological changes suggest that n-SiO2 helps optimize gas exchange processes, thereby enhancing photosynthetic performance and overall plant productivity [23]. This is consistent with previous reports that nanomaterials can enhance Pn, counteract the reduction of Gs, and improve overall photosynthetic performance under elevated CO2 [6,7]. Overall, the integration of n-SiO2 with elevated CO2 conditions significantly boosts soybean growth by enhancing chlorophyll synthesis, optimizing gas exchange, and reinforcing photoprotective mechanisms. These findings underscore the potential of n-SiO2 as an effective strategy to improve crop performance and resilience in the face of rising atmospheric CO2 concentrations.
2.3. Synergistic Effects of Silicon Nanoparticles on Root Morphology and in Soybean Under Elevated CO2
The application of n-SiO2 under eCO2 significantly improved various root physiology indicators in soybean plants. Root fresh biomass (Figure 3A) showed a clear enhancement with a 48.3% upregulation under eCO2 + n-SiO2 compared to aCO2, with values increasing from 2.3 g to 3.5 g. This increase suggests that n-SiO2 plays a role in improving overall root growth and biomass accumulation under eCO2 conditions. Similarly, root dry biomass (Figure 3B) increased by 43.3% under eCO2 + n-SiO2, further confirming the positive impact of n-SiO2 on root system development under stress conditions. Root length (Figure 3C) followed a similar trend, with a 38.5% increase under eCO2 + n-SiO2 compared to aCO2. This finding is consistent with enhanced root elongation and expansion, which is crucial for optimizing nutrient and water uptake in challenging environmental conditions. The improvement in root length may be attributed to the combined effects of elevated CO2 and n-SiO2, which likely enhance root cell division and elongation processes. Root surface area (Figure 3D) increased by 28.5% under eCO2 + n-SiO2, further supporting the notion that n-SiO2 facilitates greater root surface area, which is vital for improving nutrient and water absorption. The average root diameter (Figure 3E) also showed a 13.5% increase under eCO2 + n-SiO2, indicating that n-SiO2 not only boosts root surface area but also enhances root structure and thickness, contributing to a more robust root system. Total root volume (Figure 3F) exhibited a 23.4% increase under eCO2 + n-SiO2, reinforcing the overall improvements in root morphology and physiology. The increase in root volume is likely to enhance the plant’s ability to absorb water and nutrients [24,25], which is critical for maintaining plant growth under elevated CO2 conditions [26].
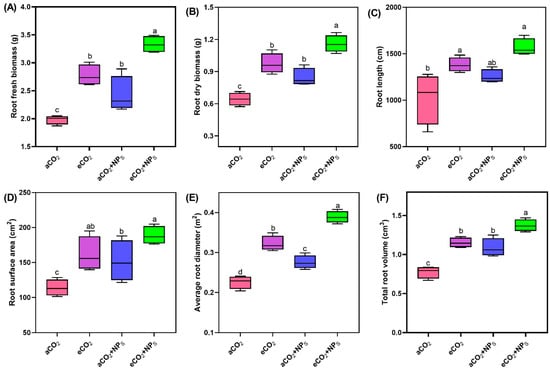
Figure 3.
Effects of SiO2-NPs under aCO2 and eCO2 conditions on root physiological traits of soybean. (A) Root fresh biomass, (B) root dry biomass, (C) root length, (D) root surface area, (E) average root diameter, and (F) total root volume in soybean plants exposed to different CO2 concentrations and SiO2-NPs treatments. Box plots represent the median (line), interquartile range (box), and minimum/maximum values (whiskers). Different letters indicate significant differences among treatments (p < 0.05).
The observed enhancement of root physiology in soybean plants under eCO2 induced by n-SiO2 can be attributed to several synergistic effects. Elevated CO2 typically stimulates plant growth by enhancing photosynthesis, leading to increased carbon allocation to roots [22,27,28]. Mukarram et al. investigated the impact of SiNPs on crop health through their direct influence on various biochemical and physiological processes, such as increased photosynthetic activity, enhanced nutrient uptake, improved nitrogen metabolism, and elevated enzymatic activity, all of which directly enhanced plant growth and yield [29]. Another recent study documented that n-SiO2 at 100 mg kg−1 significantly increased maize root weight, length, and number of roots by 4.5% to 20% compared to a control group [30]. The addition of n-SiO2 further amplifies this effect by improving root architecture, increasing root biomass, surface area, and volume. The improvement in root growth can be attributed to enhanced photosynthesis, which facilitates belowground carbon sequestration [31]. Larger root systems with optimized architecture are also expected to improve water and nutrient acquisition by plants, as well as indirectly stimulate photosynthetic CO2 capture. These findings are consistent with our study, where the enhancement of root physiological traits was evident under eCO2 + n-SiO2 treatment.
The increase in root diameter and surface area suggests that n-SiO2 promotes root cell expansion and tissue development, improving the plant’s ability to absorb nutrients and water more efficiently. Such changes are crucial for optimizing plant growth under water-limited conditions and improving stress resilience. Previous studies have highlighted that n-SiO2 promotes secondary and lateral root development, further improving nutrient uptake and overall root function [11,20,32]. This supports our observation that n-SiO2 enhances root structure and function, making the plant more resilient under challenging environmental conditions. Overall, these results underscore the potential of n-SiO2 to optimize plant growth and nutrient uptake under elevated CO2, making it a promising tool for enhancing crop resilience and ensuring food security in the face of climate change. The improvement in root physiology and nitrogen metabolism demonstrates that n-SiO2 could be a key strategy in enhancing crop productivity, particularly under elevated CO2 conditions.
2.4. Modulation in Biochemical Response in Soybean Under n-SiO2-Induced by Elevated CO2
The combined application of n-SiO2 and eCO2 significantly influenced key phytohormones that regulate plant growth, stress adaptation, and development. Abscisic acid (ABA) content (Figure 4A) increased by 16.5% under eCO2 + n-SiO2 compared to aCO2, indicating enhanced plant resilience through improved stomatal regulation. ABA, which plays a crucial role in mitigating water loss under elevated CO2, regulates stomatal closure, and the addition of n-SiO2 further strengthens this effect by improving water retention. Similarly, jasmonic acid (JA) levels (Figure 4B) showed a 35.2% increase under eCO2 + n-SiO2, suggesting a reinforced defense response. JA is involved in systemic resistance and stress signaling, and its enhanced levels indicate a stronger adaptive response to oxidative stress. Indole-3-acetic acid (IAA) content (Figure 4C) increased by 21.7% under eCO2 + n-SiO2, signifying improved root and shoot development. As a primary auxin responsible for cell elongation and differentiation, the upregulation of IAA suggests that n-SiO2 enhances cell expansion, optimizing root architecture for better nutrient and water uptake under eCO2. Likewise, gibberellic acid (GA3) levels (Figure 4D) increased by 18.9%, indicating enhanced shoot elongation and biomass accumulation. GA3, essential for stem elongation and seed germination, likely promotes growth pathways under eCO2, supporting improved plant performance under stress conditions. The enhanced phytohormone levels observed in soybean under eCO2 and n-SiO2 treatments suggest a synergistic mechanism that could improve plant resilience and productivity. Previous studies have shown that nanoparticles like chitosan can induce a similar phytohormone accumulation under elevated CO2, which aligns with our findings [6]. Phytohormones, particularly auxins (IAA) and gibberellins (GA), play pivotal roles in regulating plant physiological and biochemical processes, including photosynthesis [33]. These hormones are synthesized within chloroplasts and influence chloroplast development, size, thylakoid membrane organization, and chlorophyll content, ultimately determining photosynthetic capacity [33]. Furthermore, phytohormones like IAA and GA are involved in a crosstalk with sugar metabolism, linking growth regulation and energy production [34,35]. Similar to our findings, elevated CO2 has been shown to increase phytohormone levels in Arabidopsis thaliana and soybean, reflecting biochemical adjustments to enhanced CO2 availability [36]. These observations confirm that improvements in IAA and GA levels under eCO2 and n-SiO2 treatments help enhance photosynthesis and overall growth, highlighting the potential of n-SiO2 to boost plant physiological functions under changing environmental conditions.
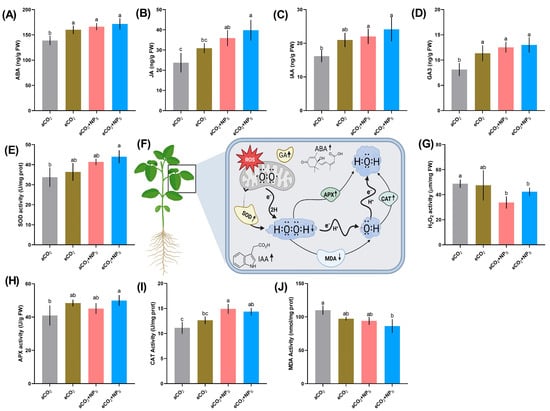
Figure 4.
Effects of elevated CO2 and nanoparticle treatments on phytohormone levels and antioxidant enzyme activities in plants. (A–D) Changes in abscisic acid (ABA), jasmonic acid (JA), indole-3-acetic acid (IAA), and gibberellic acid (GA3) levels under ambient CO2 (aCO2), elevated CO2 (eCO2), eCO2 with nanoparticles (eCO2 + NPs), and aCO2 with nanoparticles (aCO2 + NPs). (E–J) Antioxidant enzyme activities, including superoxide dismutase (SOD), hydrogen peroxide (H2O2), ascorbate peroxidase (APX), catalase (CAT), and malondialdehyde (MDA), under the same treatments. (F) Schematic representation of the antioxidant defense system in plants, illustrating the role of reactive oxygen species (ROS), enzymatic detoxification pathways (SOD, APX, and CAT), and interactions with phytohormones. Different letters indicate statistically significant differences among treatments (p < 0.05). Error bars represent standard deviations (SD).
The antioxidant defense system plays a crucial role in mitigating oxidative stress caused by reactive oxygen species (ROS) [37]. Our study demonstrates that the application of n-SiO2 under elevated CO2 (eCO2) significantly enhanced antioxidant enzyme activities, thereby improving the plant’s ability to counteract oxidative stress. Superoxide dismutase (SOD) activity (Figure 4E) increased by 19.4% under eCO2 + n-SiO2 compared to aCO2, highlighting an improved ROS scavenging capacity. Since SOD is the first line of defense against oxidative stress by converting superoxide radicals into hydrogen peroxide (H2O2) [38], its enhanced activity suggests that n-SiO2 helps reduce ROS accumulation, preventing cellular damage and maintaining redox homeostasis. As a consequence of increased SOD activity, H2O2 content (Figure 4G) decreased by 22.1% under eCO2 + n-SiO2, confirming the effective mitigation of oxidative stress. Lower H2O2 levels indicate that the antioxidative system efficiently neutralized excess ROS, thereby reducing oxidative damage to cellular structures [39]. To further strengthen ROS detoxification, ascorbate peroxidase (APX) activity (Figure 4H) exhibited a 15.8% increase. APX plays a critical role in converting H2O2 into water, preventing its accumulation and minimizing oxidative damage. Similarly, catalase (CAT) activity (Figure 4I) showed a 23.7% increase, reinforcing the plant’s ability to decompose excess H2O2 into water and oxygen. Higher CAT activity further supports the role of n-SiO2 in enhancing antioxidative defense, allowing plants to better cope with oxidative stress under eCO2 conditions. Additionally, malondialdehyde (MDA) content (Figure 4J) decreased by 18.6%, demonstrating lower lipid peroxidation and improved membrane stability. Since MDA is a key indicator of oxidative membrane damage, its reduction suggests that n-SiO2 contributes to protecting plant cells from oxidative stress by maintaining membrane integrity. While n-SiO2 application significantly enhanced the antioxidant defense response, the effects of cerium oxide nanoparticles (CeO2 NPs) under eCO2 exhibited a different trend. The exposure of plants to CeO2 NPs under eCO2 conditions did not induce oxidative stress, as evidenced by stable MDA and H2O2 levels. Similarly, no oxidative stress was observed under CeO2 NPs treatment at any concentration under both ambient CO2 (aCO2) and eCO2 conditions [7]. This suggests that, unlike some metal-based nanoparticles that induce ROS accumulation, CeO2 NPs do not generate excessive ROS, maintaining cellular homeostasis. Despite the absence of oxidative stress, CeO2 NPs altered antioxidant enzyme activities, potentially enhancing plant stress resilience [7]. At low concentrations, CeO2 NPs positively impacted the overall antioxidant capacity of plants, likely due to their enzyme-mimicking properties. CeO2 NPs exhibit both oxidant and antioxidant behavior in plants, particularly by mimicking superoxide dismutase (SOD) activity, which enhances plant tolerance to abiotic stress. However, previous studies have reported mixed responses regarding the effect of CeO2 NPs on antioxidant enzymes. In spinach plants, no significant change in SOD activity was observed across all CeO2 NPs treatments under both aCO2 and eCO2 conditions. In contrast, CAT activity increased under both CO2 conditions, while APX activity exhibited a significant upregulation at 100 mg/L CeO2 NPs under eCO2 [7]. These findings suggest that the effects of CeO2 NPs on antioxidant enzyme activity are enzyme specific and influenced by environmental conditions. Furthermore, contradictory findings have been reported concerning oxidative stress markers. Some studies indicate that higher concentrations of NMs lead to increased MDA content and ion membrane leakage, while others found no significant oxidative damage. These discrepancies could be attributed to variations in plant growth conditions, species-specific responses, application methods, and differences in concentration and duration of exposure.
2.5. n-SiO2 Enhances Nodule Health and Biological Nitrogen Fixation Potential in Soybean Under Elevated CO2
The impact of eCO2 on nodulation and biological nitrogen fixation in soybean, particularly when influenced by n-SiO2, has not been thoroughly investigated. Our study shows that the application of n-SiO2 under eCO2 significantly enhances nodule health and nitrogen fixation potential in soybean plants compared to aCO2 conditions. The number of nodules per plant (Figure 5A) significantly increased under eCO2 + n-SiO2, showing a 48.3% upregulation compared to aCO2 and a 22.5% increase relative to eCO2 alone. Similarly, nodule biomass (Figure 5B) was enhanced by 53.6% under eCO2 + n-SiO2, suggesting that n-SiO2 supplementation amplifies the beneficial effects of eCO2 on nodulation efficiency. Previous studies have documented the positive influence of eCO2 on root physiology, including increased root nodule growth, nutrient uptake efficiency, and enhanced water absorption capacity [22,27,28,40]. Our findings indicate that n-SiO2 further strengthens these effects, potentially by promoting secondary and lateral root development, leading to improved overall nutrient acquisition and stress resilience [11,20,32].
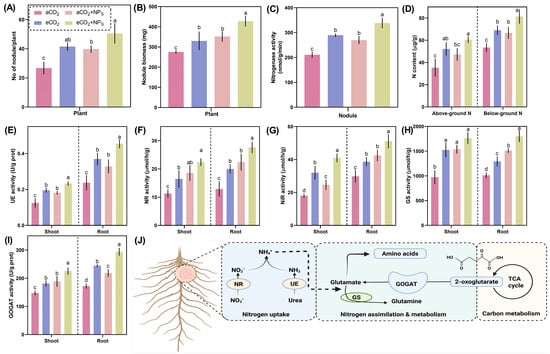
Figure 5.
Effects of n-SiO2 on soybean root and nodule physiological parameters under ambient (aCO2) and elevated (eCO2) CO2 conditions. Data represent (A) number of nodules per plant, (B) nodule biomass, (C) nitrogenase activity, (D) nitrogen content in above- and below-ground tissues, (E) Urease (UE) activity, (F) nitrate reductase (NR) activity, (G) nitrite reductase (NIR) activity, (H) glutamine synthetase (GS) activity, and (I) glutamate synthase (GOGAT) activity in soybean shoots and roots. (J) Schematic representation of nitrogen uptake, assimilation, and metabolism pathways in response to n-SiO2 and elevated CO2. Different letters indicate significant differences (p < 0.05) among treatments.
Nitrogenase activity (Figure 5C), a key determinant of biological nitrogen fixation, was significantly enhanced in response to n-SiO2 supplementation. Compared to aCO2, nitrogenase activity exhibited a 65.2% increase under eCO2 + n-SiO2, confirming that n-SiO2 plays a crucial role in enhancing nitrogen fixation efficiency. Likewise, total nitrogen content (Figure 5D) in both above-ground and below-ground tissues increased markedly, with a 34.7% rise in above-ground nitrogen and a 41.2% increase in below-ground nitrogen under eCO2 + n-SiO2 compared to aCO2. These results suggest that n-SiO2 enhances nitrogen assimilation by facilitating nitrogen uptake and transport throughout the plant.
The impact of n-SiO2 on key nitrogen metabolism enzymes was also evident. Urease (UE) activity (Figure 5E), which plays a critical role in nitrogen remobilization, increased by 43.8% in root nodule tissues under eCO2 + n-SiO2. Similarly, nitrate reductase (NR) and nitrite reductase (NIR) activities (Figure 5F,G) exhibited significant upregulation, increasing by 38.5% and 52.1%, respectively, under eCO2 + n-SiO2 compared to aCO2. These enhancements indicate an improved nitrate assimilation pathway, leading to more efficient nitrogen utilization. Furthermore, glutamine synthetase (GS) and glutamate synthase (GOGAT) activities (Figure 5H,I), both essential for nitrogen assimilation, were significantly enhanced under eCO2 + n-SiO2. GS activity increased by 47.2%, while GOGAT activity exhibited a 58.3% upregulation, reinforcing the improved nitrogen metabolism and assimilation associated with n-SiO2 supplementation under eCO2 conditions. The schematic representation (Figure 5J) provides an overview of nitrogen uptake and assimilation pathways, highlighting the mechanisms through which n-SiO2 improves nitrogen fixation and metabolic assimilation under eCO2. Several studies have reported that increased CO2 levels can stimulate nitrogen fixation, potentially due to enhanced photosynthetic rates and improved root architecture [41] Additionally, root exudate composition under eCO2 can influence microbial communities in the rhizosphere, with beneficial rhizobacteria playing a crucial role in promoting root nodule health and nitrogen fixation efficiency [42]. Given that eCO2 typically enhances nodule biomass and nitrogen fixation in legumes like soybean, our results suggest that n-SiO2 further strengthens this effect by optimizing carbon allocation to nodules and improving overall plant health [28]. It was previously documented that Si (100–800 mg kg−1) significantly increased the soybean nodulation by 25–46% compared to the control group [19]. Abuelsod et al. reported that nanoparticles (CS-NPs) under the elevated CO2 conditions (645 ppm) significantly increased the nitrogen assimilation enzymatic activities (GDH, GS, and GOGAT) as compared to the control group [6]. Silicon has also been found to enhance growth, nodulation, and nitrogen fixation in leguminous plants through various mechanisms [43]. For example, Si has been shown to increase leghemoglobin formation, thereby enhancing the N2-fixation potential of legume root nodules [44]. It also augments the abundance of bacteroides and symbiosomes within the nodules, further promoting N2-fixation potential [45]. Moreover, Si promotes cell wall thickness in nodules and reduces the size of peri-bacteroid spaces, facilitating solute transport and rapid dissolving in O2 diffusion [45]. Additionally, Si reduces the plant requirement for lignin, a carbon-intensive compound, allowing more carbon to be allocated to bacteroid respiration and nodule organogenesis, ultimately increasing N2-fixation efficiency [43,45]. Recent studies have reported that SiO2-NPs significantly increased the module health and N2-fixation potential [46,47]. Collectively, these findings highlight the role of n-SiO2 in mitigating nitrogen limitations under elevated CO2. The observed improvements in nodule health, nitrogen fixation, and nitrogen metabolism support the use of n-SiO2 as a strategy to enhance crop productivity under future climate scenarios characterized by elevated CO2.
2.6. Synergistic Effects of Elevated CO2 and Nano-Silica on Soybean Uptake and Nutrient Homeostasis
The effects of various treatments, including aCO2, aCO2 + n-SiO2, eCO2, and eCO2 + n-SiO2, on plant Si content, translocation factor, bioconcentration factor, and elemental uptake were analyzed to understand the synergistic impacts of n-SiO2 under different CO2 conditions. The results revealed distinct trends and enhancements in all parameters tested, particularly when n-SiO2 was applied under elevated CO2 conditions. In terms of shoot silicon content (Figure 6A), both aCO2 + n-SiO2 and eCO2 + n-SiO2 treatments resulted in marked increases compared to the control (aCO2). Specifically, aCO2 + n-SiO2 exhibited a ~50% increase, while eCO2 + n-SiO2 showed the most substantial enhancement, with a ~100% increase, demonstrating the amplified effect of elevated CO2 in combination with n-SiO2 on silicon accumulation in shoots. This trend was similarly observed in root silicon content (Figure 6B), where the aCO2 + n-SiO2 treatment resulted in a moderate increase (~40%) compared to the control, and eCO2 + n-SiO2 exhibited the highest value with an ~80% increase. These findings suggest that the addition of n-SiO2 under elevated CO2 conditions significantly boosts the plant’s ability to accumulate Si, both in the shoots and roots. Soil silicon content (Figure 6C) also showed positive changes, although to a lesser extent. The aCO2 + n-SiO2 treatment led to a slight increase (~10%), while eCO2 + n-SiO2 resulted in a more pronounced enhancement of ~30%. This suggests that elevated CO2, in conjunction with n-SiO2, improves the availability of silicon in the soil, which is subsequently taken up by plants. Correspondingly, the translocation factor (Figure 6D), which measures the efficiency of Si movement from soil to plant tissues, was significantly increased in both n-SiO2 treatments. While aCO2 + n-SiO2 showed a moderate increase (~20%) compared to the control, eCO2 + n-SiO2 exhibited the highest increase (~50%), indicating that elevated CO2 conditions further enhance the transport of Si within the plant system. Interestingly, the bioconcentration factor (Figure 6E), which reflects the plant’s capacity to concentrate Si from the soil, was slightly decreased in the aCO2 + n-SiO2 treatment (~10% decrease), whereas eCO2 + n-SiO2 resulted in the most significant increase (~30%) compared to the control. This highlights the greater efficiency of Si accumulation in plants under elevated CO2 conditions when combined with n-SiO2. In addition to silicon content, the elemental content analysis (Figure 6F,G) revealed substantial upregulation of several essential nutrients under the eCO2 + n-SiO2 treatment. Notable increases were observed in phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), and zinc (Zn), with eCO2 + n-SiO2 showing increases of ~74%, ~141%, ~91%, ~87%, ~70%, and ~49%, respectively, compared to the control. These results suggest that the application of n-SiO2 under elevated CO2 conditions not only boosts Si content but also enhances the uptake of other essential nutrients, thus contributing to improved overall plant health and growth.
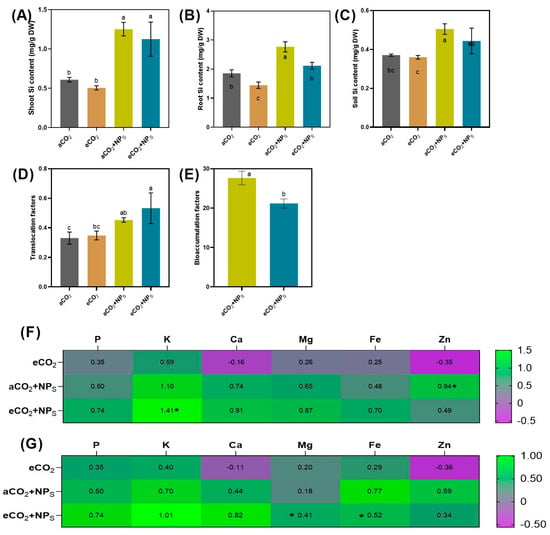
Figure 6.
Effects of elevated CO2 and nano-silica on soybean uptake and nutrient dynamics. (A) Shoot silicon content, (B) root silicon content, (C) soil silicon content, (D) translocation factor, (E) bioconcentration factor, (F,G) heatmaps showing overall nutrient homeostasis. Bars represent mean ± standard deviation, asterisks (*) and letters indicate a significant difference between treatments.
Previous studies have shown that plants tightly regulate, or in some cases downregulate [48], nutrient accumulation at sub-cellular and cellular levels in a CO2-dependent manner in Oryza sativa L. [49], soybean (Glycine max L.), and common bean (Phaseolus vulgaris L.) [50], which is consistent with our current study. It has been previously observed that the presence of Si in soil enhances the availability of macro- and micronutrients to plants. Nano SiO2 increased root growth, along with its unique surface properties and catalytic activity, may promote nutrient retention and transport in the rhizosphere, thereby improving nutrient uptake by plants [30,51]. Xu et al., documented that SiO2-NPs significantly increased (~23.7%) the nutritional elements (P, K, Mn, Cu, and Zn) [18]. Previous studies have also documented that nano-SiO2 not only improves Si uptake capacity but also upregulates nutrient homeostasis under both normal [52] and stressed conditions [53,54]. However, we are the first to study the interactive effects of nano-SiO2 with elevated CO2 exposure. Overall, our findings consistently demonstrate that the combination of elevated CO2 and n-SiO2 significantly enhances silicon uptake and translocation, as well as the accumulation of key nutrients in plants.
3. Materials and Methods
3.1. Experimental Conditions and Plant Growth
The experimental soil (surface soil, 0–20 cm) was collected from the university experimental station. The air-dried soil was sieved through 2 mm mesh to remove the debris and plant residues, ensuring homogenization. The physiochemical properties of the sieved experimental soil are presented in Supplementary Table S1. We characterized the n-SiO2 following methodologies outlined in recently published studies [55,56]. Nanoscale SiO2 with a purity of 99.98% (17 ± 2 nm) was purchased from “Shanghai Pantian Powder Material Co., Ltd., Shanghai, China” in powder form. The characterization and sizes of the SiO2-NPs were analyzed using SEM and TEM (Figure S1). The size distribution and surface charge of the SiO2-NPs in distilled water were assessed through using dynamic light scattering (DLS) and zeta potential analysis with a Zetasizer (Nano ZS90, Malvern, UK) equipped with a He–Ne laser beam at 25 °C. The zeta-potential of used SiO2-NPs was −40 ± 3.7 MV, and the hydrodynamic diameter was 258.9 ± 9.87 nm. The SiO2-NPs were thoroughly mixed with the soil, while untreated soil served as the control (Ctrl). The treatments included soil contaminated with 100 mg kg−1 SiO2-NPs. The amended soil was left in pots for a period of two weeks to allow for stabilization. The applied SiO2-NPs concentrations represent realistic environmental levels.
Soybean (Glycine max L.) seeds (Zhonghuang-13) were purchased from the “Shouguang Seed and Seedling Co. Ltd., Shandong, China”. Uniformly sized seeds were selected and sterilized with a 3% hypochlorite sodium solution (v/v) for 10 min, then thoroughly washed with deionized (DI) water 3–4 times. The sterilized seeds were then transferred to wet filter paper and kept at 25 °C for germination. Uniform seedlings were subsequently moved into plastic pots containing SiO2-treated soil (100 mg kg−1). The experiment followed a completely randomized design (CRD), with four replicates for each treatment. Ambient CO2 (aCO2) (400 ± 15 ppm) and elevated CO2 (eCO2) (600 ± 20 ppm) levels were maintained as growth conditions. The elevated CO2 concentration was chosen based on projections from the IPCC-SRES B2 scenario for the year 2100 [57]. The CO2 for all chambers was supplied from a compressed gas tank containing liquid CO2. An inline fan equipped with a variable damper controlled the airflow through an external soda lime unit to regulate CO2 levels. The gas was supplied into the airflow of the growth chamber, and its concentration was continuously monitored using a CO2 analyzer (WMA-4, PP Systems, Hitchin, UK). The experiment was conducted in a climate-controlled chamber under a 16/8 h day/night photoperiod, with a photosynthetically active radiation (PAR) intensity of 350 μmol m−2 s−1, 60% relative humidity, and a constant temperature of 25 °C. Throughout the experiment, soil moisture was maintained at 60% of field capacity using DI water for on demand. The phenotypic and physiological analyses were measured after 28 days before harvesting. Four weeks after planting, plant roots and shoots were harvested, flash-frozen in liquid nitrogen, and stored at −80 °C for further analysis. The fresh and dry biomass of shoots and roots was measured.
3.2. Photosynthetic Pigments and Activity Assessment
Photosynthetic pigments and activity were analyzed after 27 days of exposure by measuring the fully expanded second leaf of soybean plants. Key parameters, including intercellular CO2 concentration (Ci), transpiration rate (Tr), net photosynthesis rate (Pn), and stomatal conductance (Gs), were evaluated using an open gas exchange system (IRGA) (LI-38 COR Biosciences, Lincoln, NE, USA). Measurements were obtained between 09:00 and 11:30 AM under standardized conditions, with a photosynthetically active radiation (PAR) of 1000 µmol m−2 s−1 and a CO2 molar fraction of 400 µmol mol−1. To ensure precision, the instrument was recalibrated before each use to maintain stable and reliable readings. Chlorophyll (a, b) and carotenoid contents were quantified by extracting pigments from the second leaf using 95% ethanol. The absorbance of the extracts was measured with a plate reader spectrophotometer (Beckman DU-640, Ramsey, MN, USA) at wavelengths of 665 nm, 649 nm, and 470 nm, respectively, following the method outlined in our previous study [52,54].
3.3. Biochemical Enzymes and Phytohormones Analysis
For the determination of antioxidant enzymes, 100 mg of fresh soybean leaves was ground and homogenized with a phosphorus buffer solution (50 nM, pH 7.8) at a 1:9 ratio. The homogenized samples were centrifuged at 8000–10,000 rpm for ten minutes, and the supernatants were collected for further analysis. The activities of superoxide dismutase (SOD), catalase (CAT), and along with the contents of malondialdehyde (MDA), hydrogen peroxide (H2O2), and ascorbate peroxidase (APX), were quantified using commercial kits from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Phytohormone analysis was conducted on fresh soybean shoot samples collected after 28 days. These samples were frozen in liquid nitrogen and ground into a fine powder. A 0.5 g portion of the powdered sample was then mixed with 3 mL of 80% methanol. Following centrifugation at 5000 rpm for ten minutes, the supernatant was collected. Phytohormones were quantified using enzyme-linked immunosorbent assay (ELISA), with specific polyclonal or monoclonal antibodies for each hormone and alkaline phosphatase as markers, as outlined in previous studies [58].
3.4. Nitrogen Assimilation Enzymes and Nitrogenase Activity
The enzymatic activities of nitrogen assimilation, including nitrite reductase (NiR), nitrate reductase (NR), glutamate synthetase (GOGAT), and glutamine synthetase (GS), were measured in fresh soybean shoots and roots according to the assay kit protocols provided by Nanjing “Jiancheng Bioengineering Co., Ltd., Nanjing, China”. Urease (UE) activity in these tissues was determined using assay kits following the manufacturer’s instructions from “Beijing Boxbio Co., Ltd., Beijing, China”. The nitrogen content in soybean shoots and roots (in dry powder form) was analyzed using an organic elemental analyzer (Vario EL model, Elemental, Germany) [52].
The nitrogenase activity in soybean nodules was evaluated using the acetylene reduction assay. Freshly harvested nodules were placed into 50 mL micro-reaction vials. Air was initially extracted from the vials using a syringe, and 10 mL of acetylene was then injected into the vials. The mixture was incubated at 30 °C for 30 min. Afterward, 500 µL of the gas mixture was sampled and analyzed for ethylene content using gas chromatography (Agilent 7890, Stevens Creek, CA, USA). Nitrogenase activity was quantified by measuring the rate of ethylene production per gram of nodule tissue [54].
3.5. Quantity of Mineral in Soil-Plant System
Soybean shoots and roots were freeze-dried using lyophilization at −48 °C for 72 h with a freeze-dryer (TF-FD-18S, Shanghai, China). After freeze-drying, the plant tissues were finely ground into a fine powder. A 200 mg sample of the powder was subjected to digestion with 3 mL of HNO3 and 0.5 mL of H2O2 in a 75 mL tube using microwave digestion (Ultra WAVE, Italy). The final volume was diluted to 50 mL with DI water and filtered through a 0.25 µm PTFE membrane. The concentrations of Si, P, K, Cu, Zn, Mn, and Fe were measured using inductively coupled plasma mass spectrometry (ICP-MS) (DRCII, PerkinElmer, and Norwalk). The mineral content in the soybean’s planted soil was analyzed through acid-wet digestion using aqua regia (3 HCl:1 HNO3) in a microwave digestion system. Elemental concentrations were quantified by ICP-MS using calibration standards with concentrations ranging from 0.01 to 100 ppm. Recovery rates for the analyzed elements are provided in Table S2.
3.6. Statistical Analysis
The experiment followed a completely randomized design (CRD) with four replicates for each treatment. The data are presented as mean ± standard deviation (SD). Statistical analysis was performed using Statistical Software (Statistix 8.1), with significance determined by one-way analysis of variance (ANOVA). Comparisons of mean values between treatments were made using the least significant difference (LSD) test. A significance level of p < 0.05 was considered statistically significant. Graphical representations of the data were generated using GraphPad Prism (version 8.0.2).
4. Conclusions
Our study demonstrates that the interactive application of n-SiO2 and eCO2 significantly enhances soybean growth, nitrogen fixation, and nutrient homeostasis, offering promising insights for sustainable agriculture under future climate conditions. The synergistic effects of eCO2 + n-SiO2 resulted in substantial improvements in photosynthetic efficiency, stomatal conductance, and carbon assimilation, leading to a 30% increase in shoot length and over 100% enhancement in chlorophyll content and photosynthetic rate. Enhanced nitrogen assimilation, evident from the upregulation of NR, NIR, GS, and GOGAT, contributed to improved biological nitrogen fixation, with nodule biomass and number increasing by 53.6% and 48.3%, respectively. Furthermore, phytohormone regulation and antioxidant defense systems were significantly boosted under eCO2 + n-SiO2, increasing resilience against oxidative stress while maintaining lower levels of oxidative markers. The uptake and homeostasis of essential macronutrients and micronutrients improved by 13–41% in roots and 13–17% in shoots, with Fe and Zn levels remaining stable in shoots. These findings highlight the potential role of n-SiO2 as a sustainable nano-fertilizer, capable of mitigating the physiological and biochemical challenges posed by rising CO2 levels while improving overall plant health and productivity. This study provides a strong foundation for future research on nano-enabled agriculture, emphasizing the potential of Si-based nanomaterials in enhancing crop performance under climate change scenarios. Integrating nano-fertilizers with elevated CO2 conditions could pave the way for more efficient and climate-resilient crop management strategies, ensuring global food security in a changing environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14132009/s1.
Funding
This research was funded by the Horizontal project supported by the enterprise, prefabricated hydraulic structure in cold areas, 20240239.
Data Availability Statement
The original contributions presented in this study are included in the article and in the Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, S.K.; Barnaby, J.Y.; Reddy, V.R.; Sicher, R.C. Varying response of the concentration and yield of soybean seed mineral elements, carbohydrates, organic acids, amino acids, protein, and oil to phosphorus starvation and CO2 enrichment. Front. Plant Sci. 2016, 7, 1967. [Google Scholar] [CrossRef] [PubMed]
- Bredow, M.; Khwanbua, E.; Sartor Chicowski, A.; Qi, Y.; Breitzman, M.W.; Holan, K.L.; Liu, P.; Graham, M.A.; Whitham, S. Elevated CO2 alters soybean physiology and defense responses, and has disparate effects on susceptibility to diverse microbial pathogens. New Phytol. 2025, 246, 2718–2737. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J.J. Cumulative Drought Stress Leads to a Loss of Growth Resilience and Explains Higher Mortality in Planted than in Naturally Regenerated Pinus pinaster Stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Bellaloui, N.; Mengistu, A.; Abbas, H.; Kassem, M.A. Effects of Drought and Elevated Atmospheric Carbon Dioxide on Seed Nutrition and 15N and 13C Natural Abundance Isotopes in Soybean Under Controlled Environments. In Soybean-The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Shoukat, A.; Pitann, B.; Zafar, M.M.; Farooq, M.A.; Haroon, M.; Nawaz, A.; Wahab, S.W.; Saqib, Z.A. Nanotechnology for climate change mitigation: Enhancing plant resilience under stress environments. J. Plant Nutr. Soil Sci. 2024, 187, 604–620. [Google Scholar] [CrossRef]
- Abuelsoud, W.; Saleh, A.M.; Mohammed, A.E.; Alotaibi, M.O.; AbdElgawad, H. Chitosan nanoparticles upregulate C and N metabolism in soybean plants grown under elevated levels of atmospheric carbon dioxide. Int. J. Biol. Macromol. 2023, 252, 126434. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Sehrish, A.K.; Ai, F.; Zong, X.; Alomrani, S.O.; Al-Ghanim, K.A.; Alshehri, M.A.; Ali, S.; Guo, H. Morphophysiological, biochemical, and nutrient response of spinach (Spinacia oleracea L.) by foliar CeO2 nanoparticles under elevated CO2. Sci. Rep. 2024, 14, 25361. [Google Scholar] [CrossRef]
- Alsherif, E.A.; Hajjar, D.; Aldilami, M.; AbdElgawad, H. Physiological and biochemical responses of wheat to synergistic effects of selenium nanoparticles and elevated CO2 conditions. Front. Plant Sci. 2023, 14, 1183185. [Google Scholar] [CrossRef]
- Nazim, M.; Li, X.; Anjum, S.; Ahmad, F.; Ali, M.; Muhammad, M.; Shahzad, K.; Lin, L.; Zulfiqar, U. Silicon nanoparticles: A novel approach in plant physiology to combat drought stress in arid environment. Biocatal. Agric. Biotechnol. 2024, 58, 103190. [Google Scholar] [CrossRef]
- Ali, A.M.; Bijay-Singh. Silicon: A crucial element for enhancing plant resilience in challenging environments. J. Plant Nutr. 2024, 48, 486–521. [Google Scholar] [CrossRef]
- Johnson, S.N.; Ryalls, J.M.; Gherlenda, A.N.; Frew, A.; Hartley, S.E. Benefits from below: Silicon supplementation maintains legume productivity under predicted climate change scenarios. Front. Plant Sci. 2018, 9, 202. [Google Scholar]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of Elevated Carbon Dioxide on Photosynthesis and Carbon Partitioning: A Perspective on Root Sugar Sensing and Hormonal Crosstalk. Front Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, H.; Hu, X.; Xu, L.; An, X.; Jin, T.; Ma, R.; Li, Z.; Chen, S.; Du, S.; et al. Comparing the Potential of Silicon Nanoparticles and Conventional Silicon for Salinity Stress Alleviation in Soybean (Glycine max L.): Growth and Physiological Traits and Rhizosphere/Endophytic Bacterial Communities. J. Agric. Food Chem. 2024, 72, 10781–10793. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Guo, J.; Noman, M.; Lv, L.; Manzoor, N.; Qi, X.; Li, B. Metagenomic and biochemical analyses reveal the potential of silicon to alleviate arsenic toxicity in rice (Oryza sativa L.). Environ. Pollut. 2024, 345, 123537. [Google Scholar] [CrossRef]
- Hussain, S.; Mumtaz, M.; Manzoor, S.; Shuxian, L.; Ahmed, I.; Skalicky, M.; Brestic, M.; Rastogi, A.; Ulhassan, Z.; Shafiq, I.; et al. Foliar application of silicon improves growth of soybean by enhancing carbon metabolism under shading conditions. Plant Physiol. Biochem. 2021, 159, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zheng, M.; Bian, Z.; Pan, N.; Tian, H.; Zhang, X.; Qiu, Z.; Xu, J.; Gu, B. Elevated CO2 levels promote both carbon and nitrogen cycling in global forests. Nat. Clim. Change 2024, 14, 511–517. [Google Scholar] [CrossRef]
- Hachiya, T.; Sugiura, D.; Kojima, M.; Sato, S.; Yanagisawa, S.; Sakakibara, H.; Terashima, I.; Noguchi, K. High CO2 triggers preferential root growth of Arabidopsis thaliana via two distinct systems under low pH and low N stresses. Plant Cell Physiol. 2014, 55, 269–280. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Hao, Y.; Cai, Z.; Cao, Y.; Fang, W.; Zhao, B.; Haynes, C.L.; White, J.C.; Ma, C. Nano-silicon fertiliser increases the yield and quality of cherry radish. Mod. Agric. 2023, 1, 152–165. [Google Scholar] [CrossRef]
- Shamshiripour, M.; Motesharezadeh, B.; Rahmani, H.A.; Alikhani, H.A.; Etesami, H. Optimal Concentrations of Silicon Enhance the Growth of Soybean (Glycine Max L.) Cultivars by Improving Nodulation, Root System Architecture, and Soil Biological Properties. Silicon 2022, 14, 5333–5345. [Google Scholar] [CrossRef]
- Ahsan, M.; Radicetti, E.; Jamal, A.; Ali, H.M.; Sajid, M.; Manan, A.; Bakhsh, A.; Naeem, M.; Khan, J.A.; Valipour, M. Silicon nanoparticles and indole butyric acid positively regulate the growth performance of Freesia refracta by ameliorating oxidative stress under chromium toxicity. Front. Plant Sci. 2024, 15, 1437276. [Google Scholar] [CrossRef]
- Dakora, F.D.; Li, H.; Zhao, J. Exploring the Impacts of Elevated CO2 on Food Security: Nutrient Assimilation, Plant Growth, and Crop Quality. Engineering 2024, 44, 234–244. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Hopmans, J.W.; Bristow, K.L. Current capabilities and future needs of root water and nutrient uptake modeling. Adv. Agron. 2002, 77, 103–183. [Google Scholar]
- Wang, E.; Smith, C.J. Modelling the growth and water uptake function of plant root systems: A review. Aust. J. Agric. Res. 2004, 55, 501–523. [Google Scholar] [CrossRef]
- Madhu, M.; Hatfield, J. Dynamics of plant root growth under increased atmospheric carbon dioxide. Agron. J. 2013, 105, 657–669. [Google Scholar] [CrossRef]
- Wang, D.; Heckathorn, S.A.; Wang, X.; Philpott, S.M. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 2012, 169, 1–13. [Google Scholar] [CrossRef]
- Coker, G.T.; Schubert, K.R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. Characterization and comparison with n2 fixation and composition of xylem exudate during early nodule development. Plant Physiol. 1981, 67, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Khan, M.M.A.; Corpas, F.J. Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J. Hazard. Mater. 2021, 412, 125254. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Rani, N.; Hooda, V. Unravelling the effects of nano SiO2, nano TiO2 and their nanocomposites on Zea mays L. growth and soil health. Sci. Rep. 2024, 14, 13996. [Google Scholar] [CrossRef]
- Bach, L.; Gojon, A. Root system growth and development responses to elevated CO2: Underlying signalling mechanisms and role in improving plant CO2 capture and soil C storage. Biochem J 2023, 480, 753–771. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Mashwani, Z.-u.-R.; Hayat, R.; Yasmin, H.; Noureldeen, A.; Ahmad, P. Synergistic effects of plant growth promoting rhizobacteria and silicon dioxide nano-particles for amelioration of drought stress in wheat. Plant Physiol. Biochem. 2021, 166, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Su, L.; Cong, Y.; Chen, J.; Geng, Y.; Qian, C.; Xu, Q.; Chen, X.; Qi, X. Sugars enhance parthenocarpic fruit formation in cucumber by promoting auxin and cytokinin signaling. Sci. Hortic. 2021, 283, 110061. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Farooq, T.; Adeel, M.; He, Z.; Umar, M.; Shakoor, N.; da Silva, W.; Elmer, W.; White, J.C.; Rui, Y. Nanotechnology and Plant Viruses: An Emerging Disease Management Approach for Resistant Pathogens. ACS Nano 2021, 15, 6030–6037. [Google Scholar] [CrossRef]
- Sanyal, S.; Chakrabarti, B.; Prasanna, R.; Bhatia, A.; Kumar, S.N.; Purakayastha, T.J.; Joshi, R.; Sharma, A. Influence of cyanobacterial inoculants, elevated carbon dioxide, and temperature on plant and soil nitrogen in soybean. J. Basic Microbiol. 2022, 62, 1216–1228. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Schwinghamer, T.D.; Smith, D.L. Rhizobacteria from root nodules of an indigenous legume enhance salinity stress tolerance in soybean. Front. Sustain. Food Syst. 2021, 4, 617978. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Can interaction between silicon and non–rhizobial bacteria help in improving nodulation and nitrogen fixation in salinity–stressed legumes? A review. Rhizosphere 2020, 15, 100229. [Google Scholar] [CrossRef]
- Mali, M.; Aery, C.N. Silicon effects on nodule growth, dry-matter production, and mineral nutrition of cowpea (Vigna unguiculata). J. Plant Nutr. Soil Sci. 2008, 171, 835–840. [Google Scholar] [CrossRef]
- Nelwamondo, A.; Jaffer, M.A.; Dakora, F.D. Subcellular organization of N 2-fixing nodules of cowpea (Vigna unguiculata) supplied with silicon. Protoplasma 2001, 216, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Das, A.K.; Methela, N.J.; Yun, B.-W. Interaction Between Nitric Oxide and Silicon on Leghaemoglobin and S-Nitrosothiol Levels in Soybean Nodules. Biomolecules 2024, 14, 1417. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, L.; Wei, Z.; Qin, Q.; Bai, Q.; Zhao, C.; Zhang, S.; Wang, H. Silicon Nano-Fertilizer-Enhanced Soybean Resilience and Yield Under Drought Stress. Plants 2025, 14, 751. [Google Scholar] [CrossRef]
- Gojon, A.; Cassan, O.; Bach, L.; Lejay, L.; Martin, A. The decline of plant mineral nutrition under rising CO2: Physiological and molecular aspects of a bad deal. Trends Plant Sci. 2023, 28, 185–198. [Google Scholar] [CrossRef]
- Bouain, N.; Cho, H.; Sandhu, J.; Tuiwong, P.; Zheng, L.; Shahzad, Z.; Rouached, H. Plant growth stimulation by high CO2 depends on phosphorus homeostasis in chloroplasts. Curr. Biol. 2022, 32, 4493–4500.e4. [Google Scholar] [CrossRef]
- Deuchande, T.; Soares, J.; Nunes, F.; Pinto, E.; Vasconcelos, M.W. Short term elevated CO2 interacts with iron deficiency, further repressing growth, photosynthesis and mineral accumulation in soybean (Glycine max L.) and common bean (Phaseolus vulgaris L.). Environments 2021, 8, 122. [Google Scholar] [CrossRef]
- Mali, M.; Aery, N. Influence of silicon on growth, relative water contents and uptake of silicon, calcium and potassium in wheat grown in nutrient solution. J. Plant Nutr. 2008, 31, 1867–1876. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, Y.; Adeel, M.; Shakoor, N.; Zhao, W.; Liu, Y.; Li, Y.; Li, M.; Azeem, I.; Rui, Y.; et al. Nickel Oxide Nanoparticles Improve Soybean Yield and Enhance Nitrogen Assimilation. Environ. Sci. Technol. 2023, 57, 7547–7558. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, G.; Li, G.; Hu, G.; Fu, L.; Hu, S.; Yang, J.; Wang, Z.; Gu, W. Effects of Multi Walled Carbon Nanotubes and Nano-SiO2 on Key Enzymes for Seed Germination and Endogenous Hormone Level in Maize Seedling. Agronomy 2024, 14, 2908. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Guo, Z.; Cao, W.; Gao, L.; Li, Y.; Tian, C.F.; Chen, Q.; Shen, Y.; Ren, F.; et al. Molybdenum Nanofertilizer Boosts Biological Nitrogen Fixation and Yield of Soybean through Delaying Nodule Senescence and Nutrition Enhancement. ACS Nano 2023, 17, 14761–14774. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Deng, C.; Eggleston, I.; Gao, S.; Li, A.; Alvarez Reyes, W.R.; Cai, K.; Qiu, R.; Haynes, C.L.; et al. Optimizing SiO2 Nanoparticle Structures to Enhance Drought Resistance in Tomato (Solanum lycopersicum L.): Insights into Nanoparticle Dissolution and Plant Stress Response. J. Agric. Food Chem. 2025, 73, 9983–9993. [Google Scholar] [CrossRef]
- O’Keefe, T.L.; Deng, C.; Wang, Y.; Mohamud, S.; Torres-Gómez, A.; Tuga, B.; Huang, C.-H.; Alvarez Reyes, W.R.; White, J.C.; Haynes, C.L. Chitosan-Coated Mesoporous Silica Nanoparticles for Suppression of Fusarium virguliforme in Soybeans (Glycine max). ACS Agric. Sci. Technol. 2024, 4, 580–592. [Google Scholar] [CrossRef]
- Murray, V.; Ebi, K.L. IPCC Special Report on Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation (SREX). J. Epidemiol. Community Health 2012, 66, 759–760. [Google Scholar] [CrossRef]
- Azeem, I.; Wang, Q.; Adeel, M.; Shakoor, N.; Zain, M.; khan, A.A.; Li, Y.; Azeem, K.; Nadeem, M.; Zhu, G.; et al. Assessing the combined impacts of microplastics and nickel oxide nanomaterials on soybean growth and nitrogen fixation potential. J. Hazard. Mater. 2024, 480, 136062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).