Ginsenoside-Enriched Panax ginseng Sprouts Cultivated from Aquaponic System with a Novel Nutrient Solution Regulate LPS-Induced Inflammatory Cytokines and UVB-Induced Photoaging Responses via MAPK/AP-1 Signaling Pathways
Abstract
1. Introduction
2. Results and Discussion
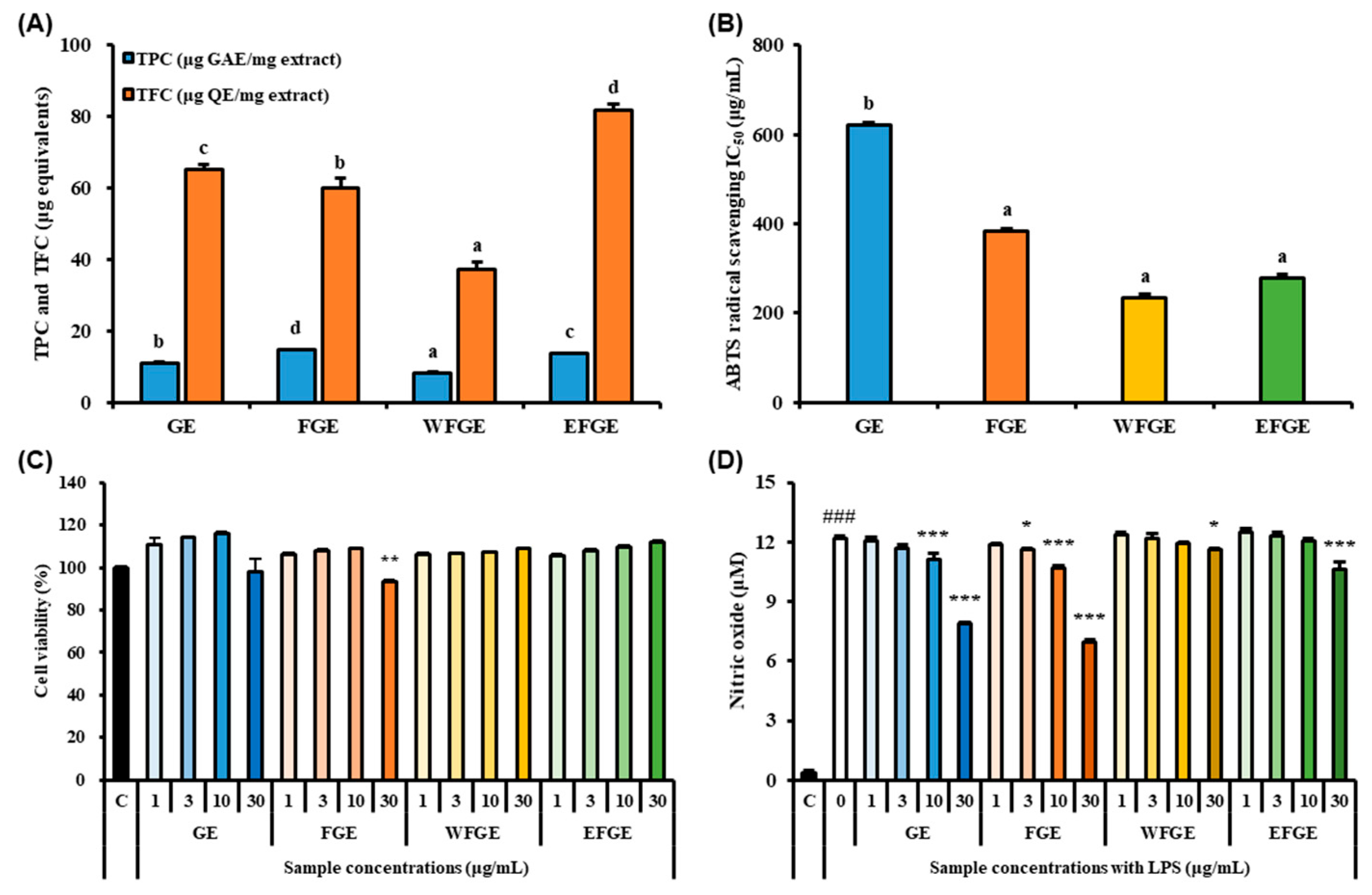
2.1. Bioactive Compounds Contents in Ginseng Sprouts Cultivated with Kelp Fermentates and Its Antioxidant Activities
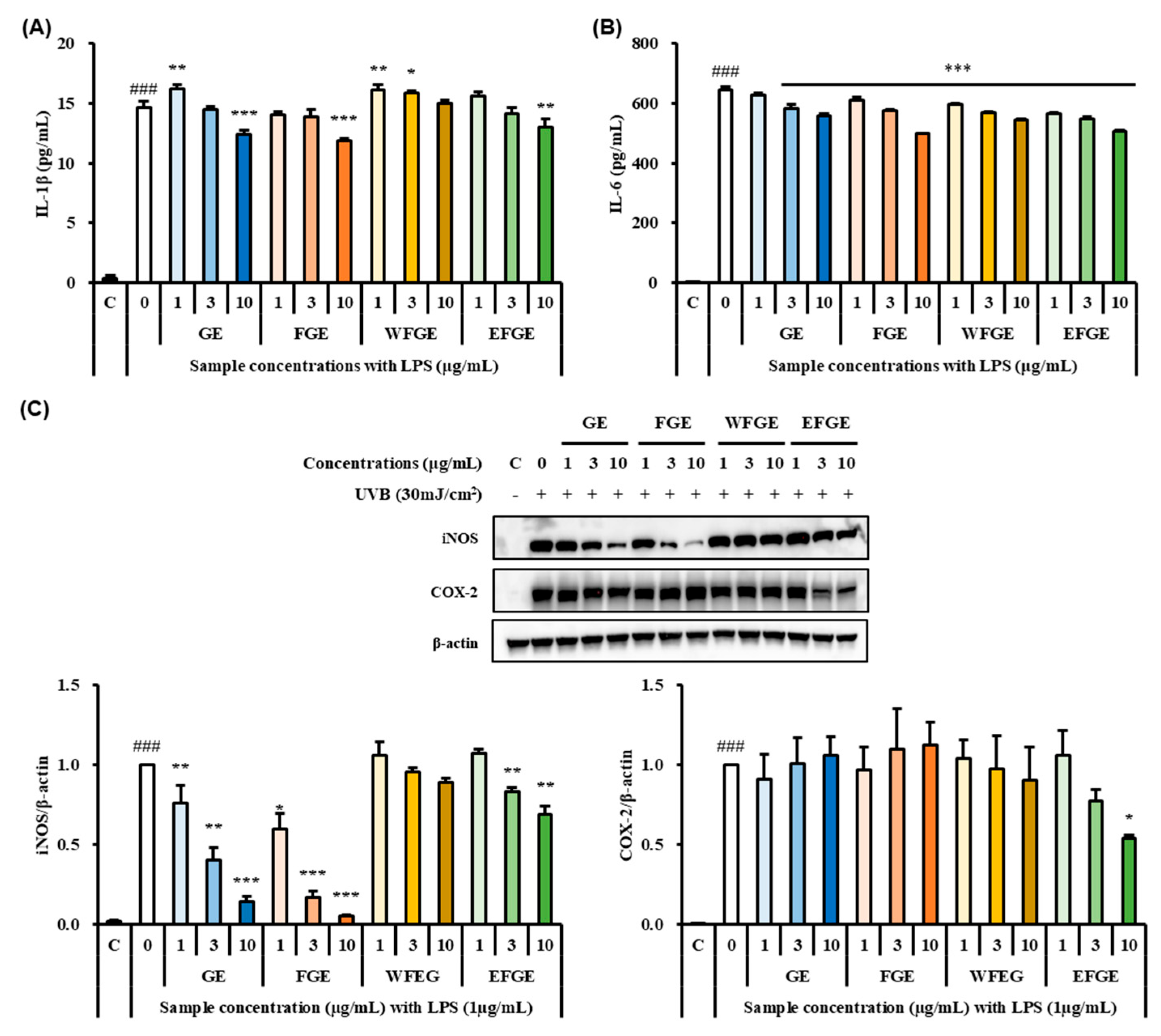
2.2. Effect of Ginseng Sprouts Cultivated with Kelp Fermentates on LPS-Induced Inflammatory Responses in Macrophages
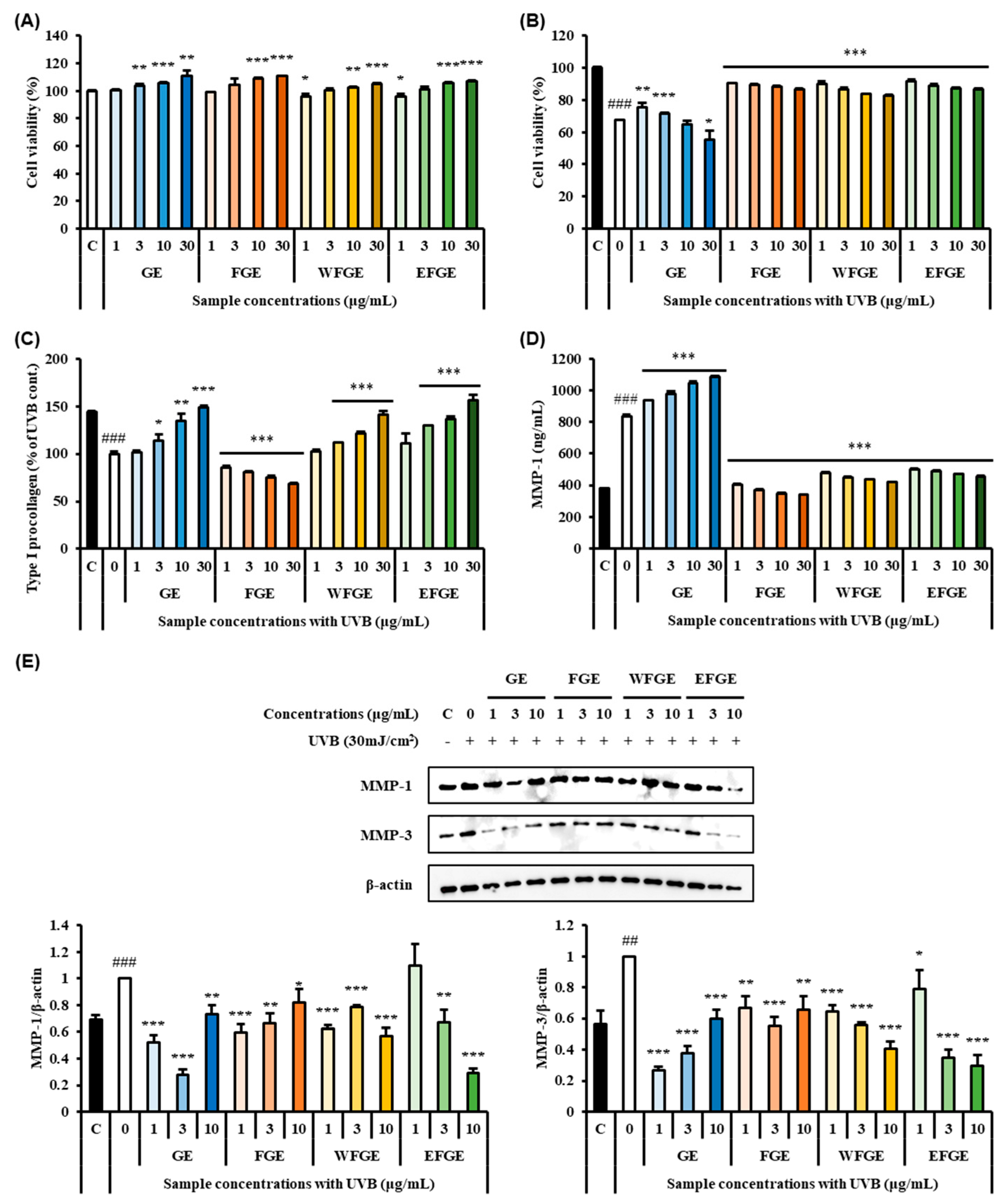
2.3. Protective Effect of Ginseng Sprouts Cultivated with Kelp Fermentates on Extracellular Matrix and MMPs in UVB-Irradiated Fibroblasts
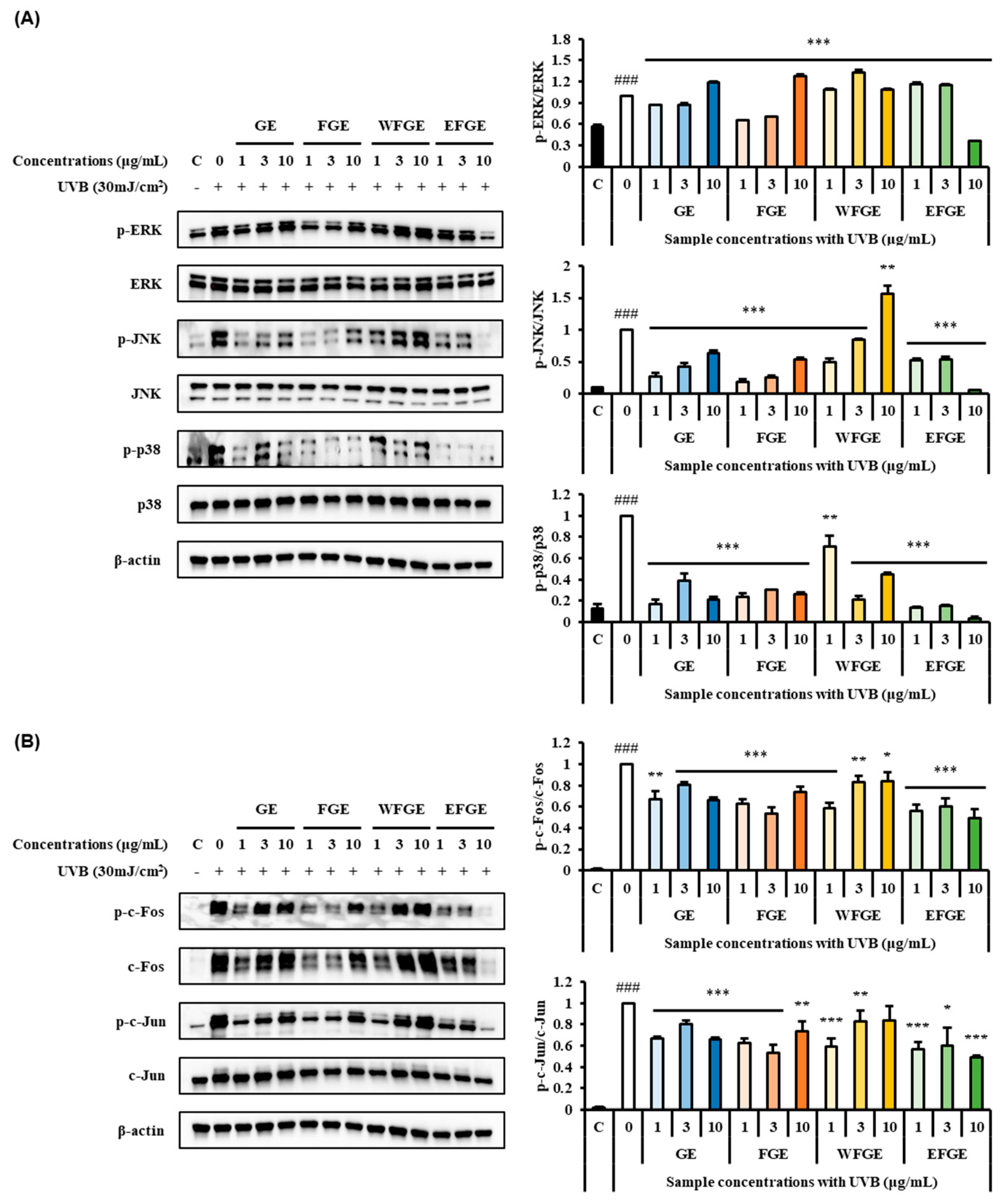
2.4. Regulation of Ginseng Sprouts Cultivated with Kelp Fermentates on MAPK/AP-1 Signaling Pathways in UVB-Irradiated Hs68 Cells
2.5. Ginsenoside Contents in Ginseng Sprout Extract and Its Crude Saponins Fractions Cultivated with Kelp Fermentates
3. Materials and Methods
3.1. Preparation of Extracts and Crude Saponin Fractions
3.2. Phytochemicals in Ginseng Sprouts
3.3. ABTS Radical Scavenging Assay
3.4. Cell Culture
3.5. Cell Viability
3.6. Nitric Oxide Assay
3.7. Determination of Cytokine Levels
3.8. Measurement of Intracellular Procollagen
3.9. Matrix Metalloproteinase-1 Inhibition Activity
3.10. Western Blot Analysis
3.11. Identification and Quantification of Ginsenosides
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

| Ginsenosides | Retention Time (min) | Calibration Curve | Correlation Coefficient (r2) | Test-Range (μg/mL) | LOD (μg) | LOQ (μg) |
|---|---|---|---|---|---|---|
| Rg1 | 13.91 | y = 6.1815x + 12.324 | 0.9996 | 10–200 | 1.13 | 11.34 |
| Rf | 18.38 | y = 5.6109x + 12.12 | 0.9997 | 10–200 | 2.81 | 28.11 |
| Rb1 | 18.94 | y = 3.5427x + 5.6434 | 0.9999 | 10–200 | 2.39 | 23.90 |
| Rc | 19.81 | y = 3.9614x + 7.4171 | 1.0000 | 10–200 | 6.82 | 68.22 |
| Rg2 | 20.31 | y = 6.3725x + 9.4992 | 0.9996 | 10–200 | 1.38 | 13.80 |
| Rb2 | 20.75 | y = 4.0161x − 0.5434 | 0.9994 | 10–200 | 1.86 | 18.56 |
| Rd | 23.24 | y = 3.7723x + 4.7266 | 0.9993 | 10–200 | 3.04 | 30.40 |
| F2 | 30.48 | y = 4.6498x + 11.965 | 0.9996 | 10–200 | 1.56 | 15.57 |
| Rg3 | 32.38 | y = 5.7352x + 13.632 | 0.9999 | 10–200 | N.D. | N.D. |
| CK | 37.72 | y = 6.5322x + 16.706 | 0.9996 | 10–200 | N.D. | N.D. |
References
- Hwa, C.; Bauer, E.A.; Cohen, D.E. Skin biology. Dermatol. Ther. 2011, 24, 464–470. [Google Scholar] [PubMed]
- Choi, J.W.; Lee, J.S.; Park, Y.I. 7,8-Dihydroxyflavone attenuates TNF-α-induced skin aging in Hs68 human dermal fibroblast cells via down-regulation of the MAPKs/Akt signaling pathways. Biomed. Pharmacother. 2017, 95, 1580–1587. [Google Scholar] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar]
- Han, H.S.; Shin, J.S.; Myung, D.B.; Ahn, H.S.; Lee, S.H.; Kim, H.J.; Lee, K.T. Hydrangea serrata (Thunb.) Ser. extract attenuate UVB-induced photoaging through MAPK/AP-1 inactivation in human skin fibroblasts and hairless mice. Nutrients 2019, 11, 533. [Google Scholar]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar]
- Lee, H.J.; Hwang, E.S.; Park, B.; Zhang, M.Y.; Sun, Z.W.; Lee, D.G.; Park, S.Y.; Yi, T.H. Methanol extract of bitter melon alleviates UVB-induced MMPs expression via MAP Kinase and AP-1 signaling in human dermal fibroblasts in vitro. Phytother. Res. 2016, 30, 1519–1526. [Google Scholar]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduc. Target. Ther. 2023, 8, 239. [Google Scholar]
- Sun, B.S.; Gu, L.J.; Fang, Z.M.; Wang, C.Y.; Wang, Z.; Lee, M.R.; Li, Z.; Li, J.J.; Sung, C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef]
- Yao, F.; Xue, Q.; Li, K.; Cao, X.; Sun, L.; Liu, Y. Phenolic compounds and ginsenosides in ginseng shoots and their antioxidant and anti-inflammatory capacities in LPS-induced RAW264.7 mouse macrophages. Int. J. Mol. Sci. 2019, 20, 2951. [Google Scholar] [CrossRef]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Shen, T.; Hu, W.; Cho, J.Y. Protopanaxatriol activates EGFR and HER2 to strengthen the molecule of skin protection and human keratinocytes. Phytomedicine 2024, 123, 155167. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Jung, J.M.; Seo, J.Y.; Chang, S.E. Anti-melanogenic property of ginsenoside Rf from Panax ginseng via inhibition of CREB/MITF pathway in melanocytes and ex vivo human skin. J. Ginseng Res. 2021, 45, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Xiao, Y.K.; Hwang, E.S.; Jeong, J.H.; Yi, T.H. Antiphotoaging and antimelanogenesis properties of ginsenoside C-Y, a ginsenoside Rb2 metabolite from american ginseng PDD-ginsenoside. Photochem. Photobiol. 2019, 95, 1412–1423. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Jang, S.N.; Lee, G.O.; Sim, H.S.; Kang, M.J. Comprehensive comparison of chemical composition and antioxidant activity of Panax ginseng sprouts by different cultivation systems in a plant factory. Plants 2022, 11, 1818. [Google Scholar] [CrossRef]
- Zamora-Izquierdo, M.A.; Santa, J.; Martinez, J.A.; Martinez, V.; Skarmeta, A.F. Smart farming IoT platform based on edge and cloud computing. Biosyst. Eng. 2019, 177, 4–17. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.Y.; Seo, C.M.; Kim, H.J.; Choi, G.S.; Kim, M.H.; Kim, B.J.; Lee, H.W. Sustainable and inflatable aeroponics smart farm system for water efficiency and high-value crop production. Appl. Sci. 2024, 14, 4931. [Google Scholar] [CrossRef]
- Oñez, L.J.P.; Catubis, K.M.L.; Cabillo, R.A.; Pascual, P.R.L. Improved morphological and yield responses of green lettuce (Lactuca sativa L.) grown with seaweed extract as a hydroponic nutrient solution. J. Plant Nutr. 2024, 48, 658–669. [Google Scholar] [CrossRef]
- Jalali, P.; Roosta, H.R.; Khodadadi, M.; Torkashvand, A.M.; Jahromi, M.G. Effects of brown seaweed extract, silicon, and selenium on fruit quality and yield of tomato under different substrates. PLoS ONE 2022, 17, e0277923. [Google Scholar] [CrossRef]
- Park, K.W.; Kim, J.H.; Jeong, B.G.; Park, J.K.; Jang, H.Y.; Oh, Y.S.; Kang, K.Y. Increased accumulation of ginsenosides in Panax ginseng sprouts cultivated with kelp fermentates. Plants 2024, 13, 463. [Google Scholar] [CrossRef]
- Jiang, R.; Xu, X.H.; Wang, K.; Yang, X.Z.; Bi, Y.F.; Yan, Y.; Liu, J.Z.; Chen, X.N.; Wang, Z.Z.; Guo, X.L.; et al. Ethyl acetate extract from Panax ginseng C.A. Meyer and its main constituents inhibit α-melanocyte-stimulating hormone-induced melanogenesis by suppressing oxidative stress in B16 mouse melanoma cells. J. Ethnopharmacol. 2017, 208, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Investigation of phenolic, flavonoid, and vitamin contents in different parts of Korean ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Chung, S.I.; Lee, S.C.; Kang, M.Y. Influence of Aging Process on the Bioactive Components and Antioxidant Activity of Ginseng (Panax ginseng L.). J. Food Sci. 2014, 79, H2127–H2131. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Hwang, C.E.; Kim, B.O.L.; Lee, K.H.; Lee, J.H.; Cho, K.M.; Joo, O.S. Comparison of ginsenoside (Rg1, Rb1) content and radical-scavenging activities of wild-simulated ginseng extract with respect to the solvent. Korean J. Food Preserv. 2021, 28, 261–269. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Chung, Y.R.; Park, J.Y.; Lee, J.E.; Kim, K.T.; Paik, H.D. Antioxidant activity and inhibitory effect on nitric oxide production of hydroponic ginseng fermented with Lactococcus lactis KC24. Antioxidants 2021, 10, 1614. [Google Scholar] [CrossRef]
- Ilari, S.; Dagostino, C.; Malafoglia, V.; Lauro, F.; Giancotti, L.A.; Spila, A.; Proietti, S.; Ventrice, D.; Rizzo, M.; Gliozzi, M.; et al. Protective effect of antioxidants in nitric oxide/COX-2 interaction during inflammatory pain: The role of nitration. Antioxidants 2020, 9, 1284. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Yu, G.J.; Choi, I.H.; Kim, G.Y.; Kim, B.W.; Park, C.; Hong, S.H.; Moon, S.W.; Cha, H.J.; Chang, Y.C.; Paek, K.Y.; et al. Anti-inflammatory potential of saponins derived from cultured wild ginseng roots in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2015, 35, 1690–1698. [Google Scholar]
- Gao, H.; Kang, N.; Hu, C.; Hu, C.; Zhang, Z.; Xu, Q.; Liu, Y.; Yang, S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine 2020, 69, 153197. [Google Scholar] [CrossRef]
- Kumagai, C.M.; Martinez, R.M.; Colombo, B.B.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Vale, D.L.; Matos, R.L.N.; Bracarense, A.P.F.R.L.; Baracat, M.M.; et al. Topical administration of 15-deoxy-Δ12,14-prostaglandin J2 using a nonionic cream: Effect on UVB-induced skin oxidative, inflammatory, and histopathological modifications in mice. Mediators Inflamm. 2021, 2021, 9330596. [Google Scholar] [CrossRef]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immonol. 2010, 10, 712–723. [Google Scholar] [CrossRef]
- Mariné-Casadó, R.; Teichenné, J.; Tobajas, Y.; Caimari, A.; Villar, A.; Zangara, A.; Mulà, A.; Bas, J.M.B. Pomegranate natural extract Pomanox® positively modulates skin health-related parameters in normal and UV-induced photoaging conditions in Hs68 human fibroblast cells. Int. J. Food Sci. Nutr. 2023, 74, 51–63. [Google Scholar] [CrossRef]
- Park, M.Y.; Han, J.W.; Lee, C.S.; Baek, H.S.; Lim, K.M.; Ha, H.J. Carnosic acid, a phenolic diterpene from rosemary, prevents UV-induced expression of matrix metalloproteinases in human skin fibroblasts and keratinocytes. Exp. Dermatol. 2013, 22, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Han, H.S.; Kim, J.M.; Park, G.H.; Jang, Y.P.; Shin, Y.K.; Ahn, H.S.; Lee, S.H.; Lee, K.T. Eisenia bicyclis extract repairs UVB-induced skin photoaging in vitro and in vivo: Photoprotective effects. Mar. Drugs 2021, 19, 693. [Google Scholar] [CrossRef]
- Yang, M.; Huang, C.Z. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J. Gastrpenterol. 2015, 21, 11679. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Prux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, H.N.; Yang, J.W.; Sung, J.H.; Kim, Y.H.; Jeong, H.S.; Lee, J.S. Protective activity and underlying mechanism of ginseng seeds against UVB-induced damage in human fibroblasts. Antioxidants 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Kim, H.G.; Choi, J.M.; Hwang, S.K.; Chung, Y.C.; Jeong, H.W. Cultivated ginseng suppresses ultraviolet B-induced collagenase activation via mitogen-activated protein kinases and nuclear factor κB/activator protein-1-dependent signaling in human dermal fibroblasts. Nutr. Res. 2012, 32, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in saponin diversity of Panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, R.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Panax ginseng C. A. Meyer phenolic acid extract alleviates ultraviolet B-irradiation-induced photoaging in a hairless mouse skin photodamage model. Evid. Based Complement. Alternat. Med. 2021, 2021, 9962007. [Google Scholar] [CrossRef] [PubMed]
- Kwok, H.H.; Yue, P.Y.-K.; Mak, N.K.; Wong, R.N.S. Ginsenoside Rb 1 induces type I collagen expression through peroxisome proliferator-activated receptor-delta. Biochem. Pharmacol. 2012, 84, 532–539. [Google Scholar] [CrossRef]
- Choi, W.R.; Cho, J.H.; Park, S.H.; Kim, D.S.; Lee, H.P.; Kim, D.H.; Kim, H.S.; Kim, J.H.; Cho, J.Y. Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species. J. Ginseng Res. 2024, 48, 211–219. [Google Scholar] [CrossRef]
- Shehzad, O.; Ha, I.J.; Park, Y.M.; Ha, Y.W.; Kim, Y.S. Development of a rapid and convenient method to separate eight ginsenosides from Panax ginseng by high-speed countercurrent chromatography coupled with evaporative light scattering detection. J. Sep. Sci. 2011, 34, 1116–1122. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Lyu, J.I.; Ryu, J.H.; Seo, K.S.; Kang, K.Y.; Park, S.H.; Ha, T.H.; Ahn, J.W.; Kang, S.Y. Comparative study on phenolic compounds and antioxidant activities of hop (Humulus lupulus L.) strobile extracts. Plants 2022, 11, 135. [Google Scholar] [CrossRef]
- Lyu, J.I.; Ryu, J.H.; Jin, C.H.; Kim, D.G.; Kim, J.M.; Seo, K.S.; Kim, J.B.; Kim, S.H.; Ahn, J.W.; Kang, S.Y.; et al. Phenolic compounds in extracts of Hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules 2020, 25, 4190. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.S.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.H.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
| Ginsenosides (μg/g) | GE | FGE | WFGE | EFGE |
|---|---|---|---|---|
| Rg1 | 57.16 ± 0.87 b | 96.89 ± 1.64 c | 1.60 ± 0.62 a | 433.39 ± 32.07 d |
| Rf | N.D. | 1.73 ± 0.62 a | N.D. | 13.90 ± 2.59 b |
| Rb1 | 14.14 ± 14.14 b | 19.21 ± 0.63 b | 3.33 ± 0.25 a | 68.48 ± 9.52 c |
| Rc | 19.37 ± 0.81 b | 32.09 ± 1.66 c | 3.09 ± 0.22 a | 117.54 ± 10.48 d |
| Rg2 | 2.86 ± 0.26 a | 4.76 ± 0.56 a | N.D. | 18.15 ± 4.67 b |
| Rb2 | 14.19 ± 0.91 a | 19.21 ± 1.18 a | 7.68 ± 0.38 a | 71.91 ± 13.79 b |
| Rd | 39.48 ± 1.76 b | 79.41 ± 1.89 c | 15.14 ± 0.81 a | 272.38 ± 11.25 d |
| F2 | 2.27 ± 0.48 a | 20.57 ± 0.81 b | 3.33 ± 0.65 a | 55.19 ± 2.89 c |
| Rg3 | N.D. | N.D. | N.D. | N.D. |
| CK | N.D. | N.D. | N.D. | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Park, K.-W.; Jeong, B.-G.; Park, J.-K.; Jang, H.-Y.; Oh, Y.-S.; Choi, J.-Y.; Kang, K.-Y. Ginsenoside-Enriched Panax ginseng Sprouts Cultivated from Aquaponic System with a Novel Nutrient Solution Regulate LPS-Induced Inflammatory Cytokines and UVB-Induced Photoaging Responses via MAPK/AP-1 Signaling Pathways. Plants 2025, 14, 1712. https://doi.org/10.3390/plants14111712
Kim J-H, Park K-W, Jeong B-G, Park J-K, Jang H-Y, Oh Y-S, Choi J-Y, Kang K-Y. Ginsenoside-Enriched Panax ginseng Sprouts Cultivated from Aquaponic System with a Novel Nutrient Solution Regulate LPS-Induced Inflammatory Cytokines and UVB-Induced Photoaging Responses via MAPK/AP-1 Signaling Pathways. Plants. 2025; 14(11):1712. https://doi.org/10.3390/plants14111712
Chicago/Turabian StyleKim, Jeong-Ho, Kyung-Wuk Park, Beom-Gyun Jeong, Jun-Ki Park, Ho-Yeol Jang, Yun-Seo Oh, Jin-Yeong Choi, and Kyung-Yun Kang. 2025. "Ginsenoside-Enriched Panax ginseng Sprouts Cultivated from Aquaponic System with a Novel Nutrient Solution Regulate LPS-Induced Inflammatory Cytokines and UVB-Induced Photoaging Responses via MAPK/AP-1 Signaling Pathways" Plants 14, no. 11: 1712. https://doi.org/10.3390/plants14111712
APA StyleKim, J.-H., Park, K.-W., Jeong, B.-G., Park, J.-K., Jang, H.-Y., Oh, Y.-S., Choi, J.-Y., & Kang, K.-Y. (2025). Ginsenoside-Enriched Panax ginseng Sprouts Cultivated from Aquaponic System with a Novel Nutrient Solution Regulate LPS-Induced Inflammatory Cytokines and UVB-Induced Photoaging Responses via MAPK/AP-1 Signaling Pathways. Plants, 14(11), 1712. https://doi.org/10.3390/plants14111712





