In Vitro Conditions Research of Sophora koreensis Nakai for Shoot Elongation
Abstract
1. Introduction
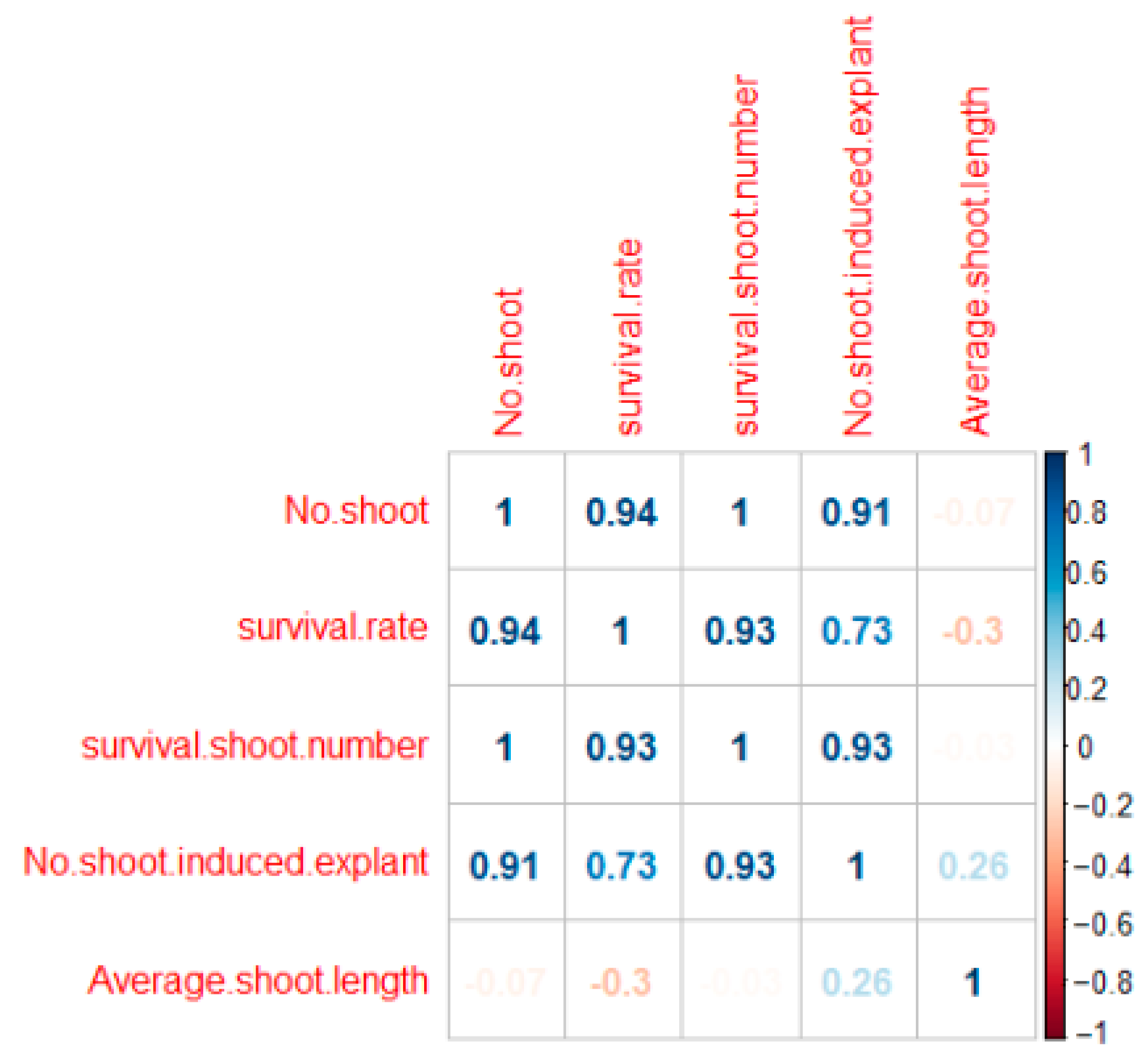
2. Results
2.1. Effects of Growth Regulator Combinations on Shoot Multiplication
2.2. Shoot Elongation as Affected by Environmental and Cultural
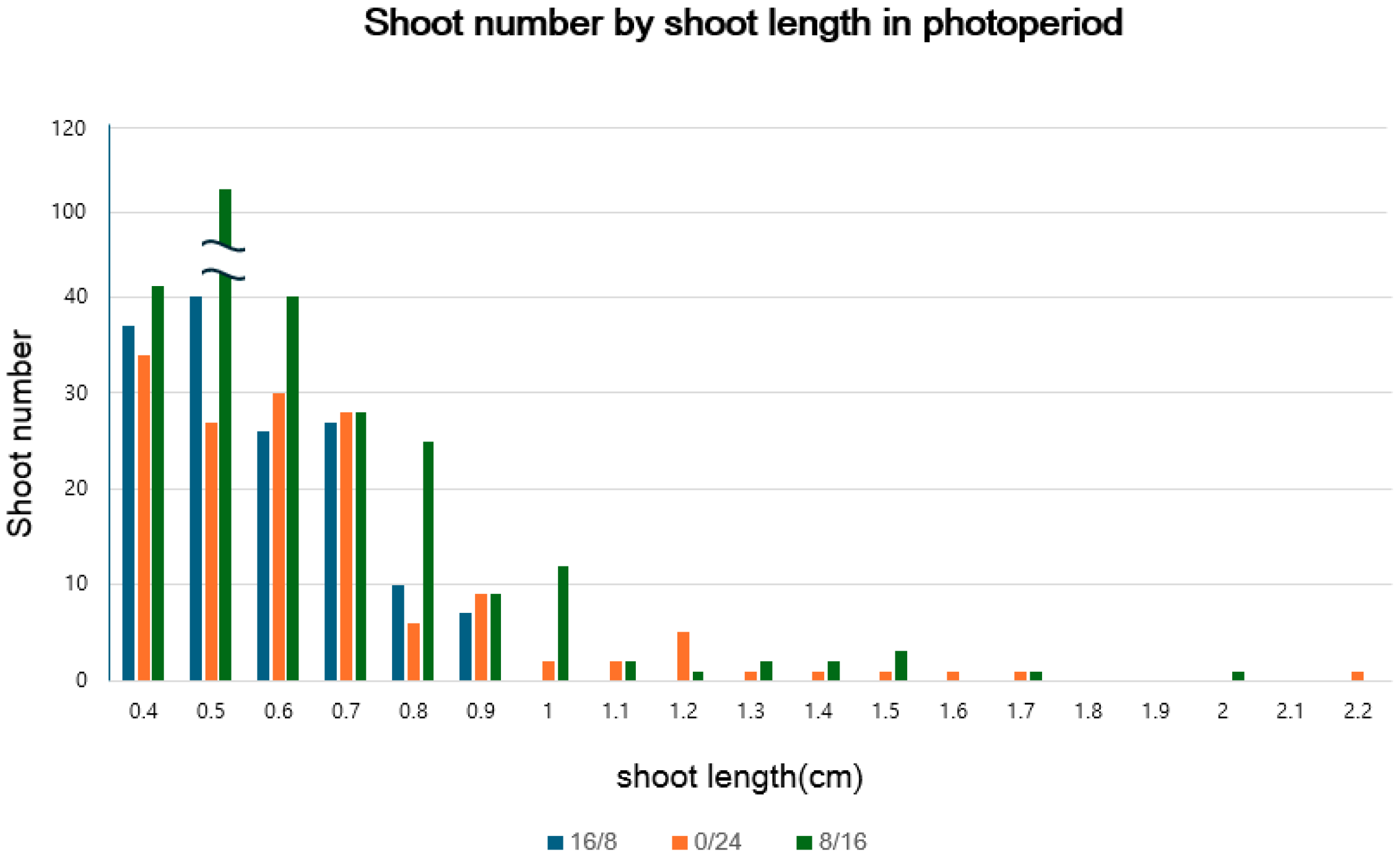
2.2.1. Photoperiod
2.2.2. Subculture
2.2.3. The Effect of Shoot Cluster Size in Subculture
2.3. Effect of Auxin Treatments on Root Induction
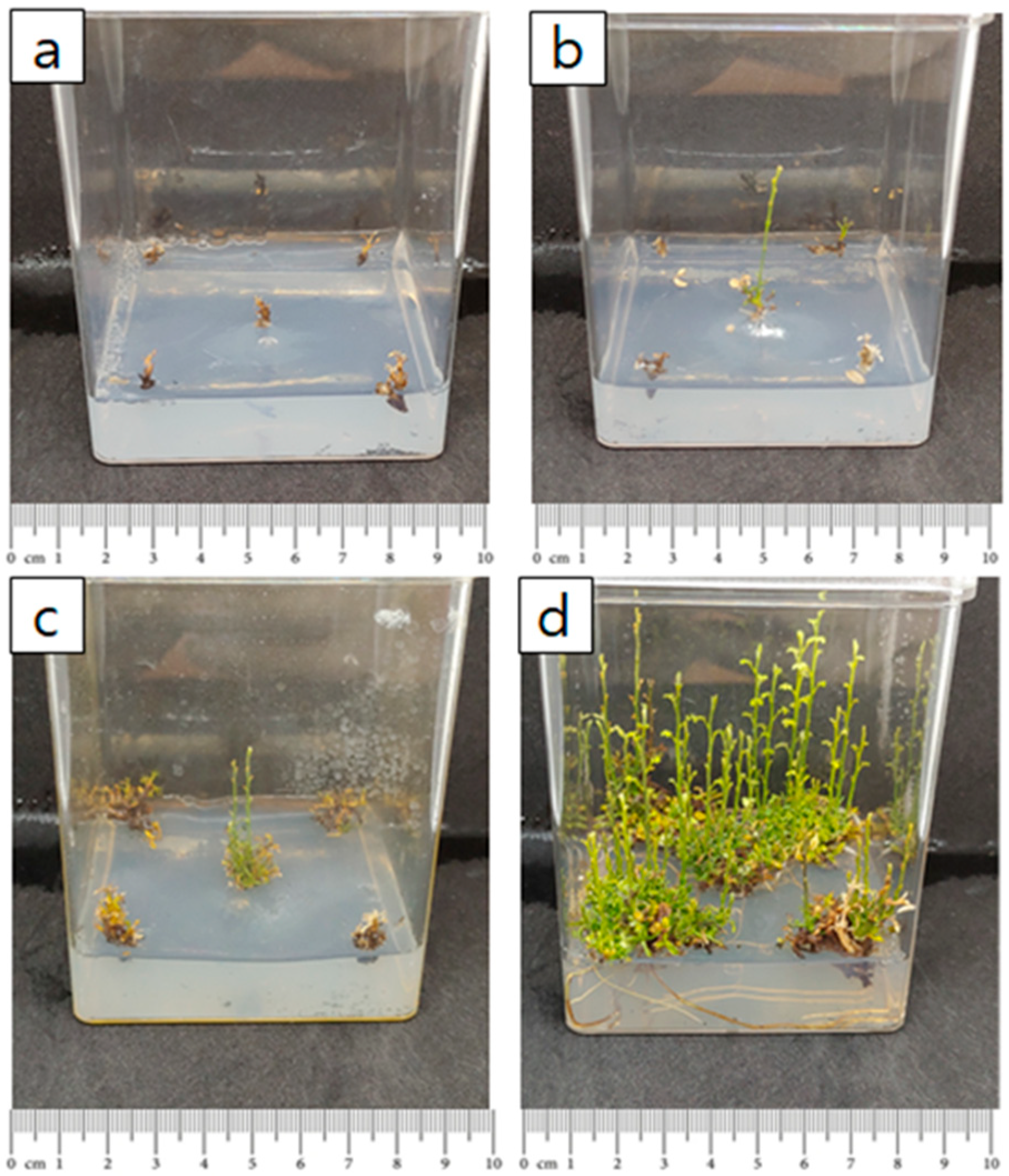
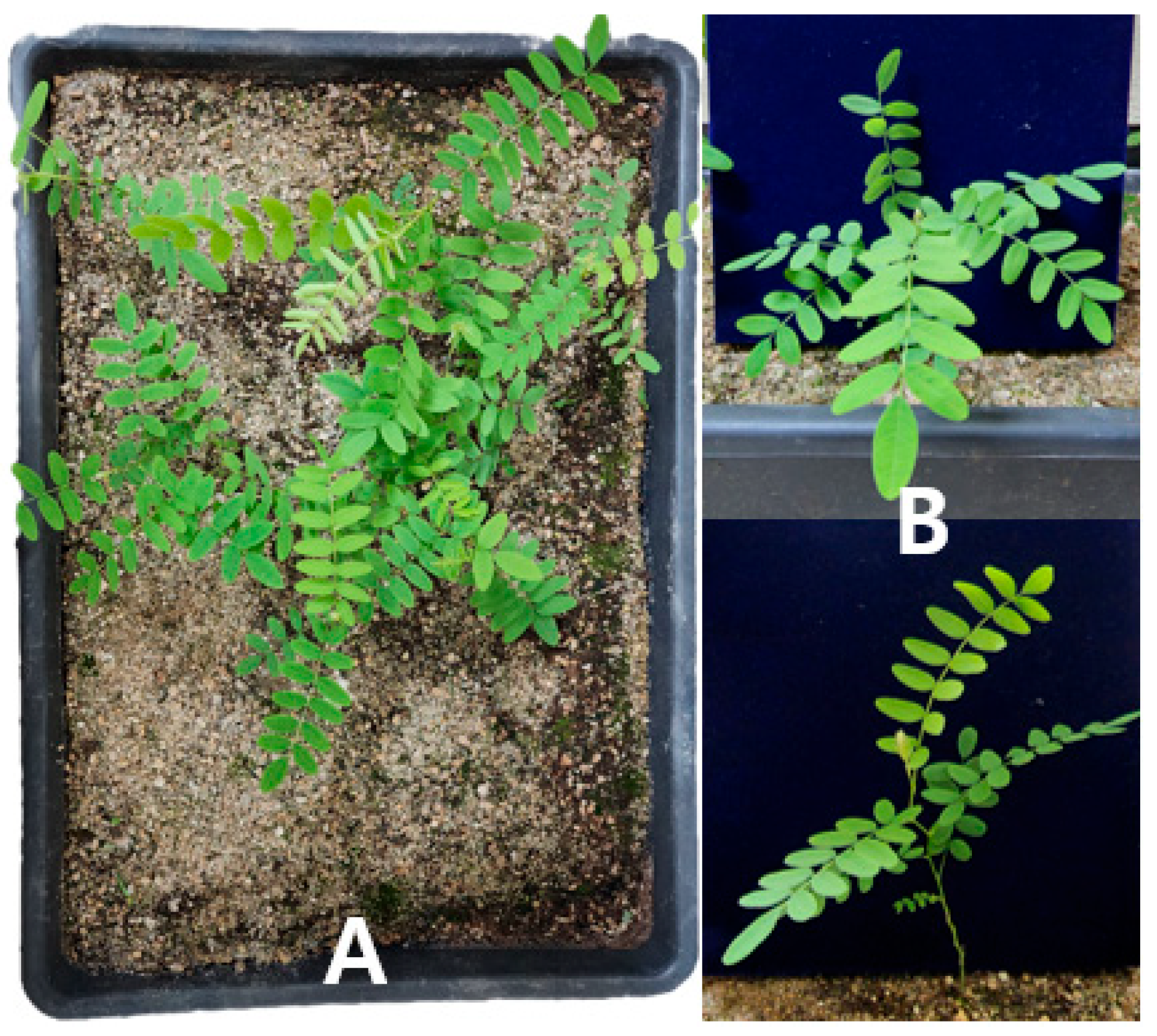
2.4. Acclimatization in Soil
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Synergistic Effect of Gibberellic Acid and Auxins with Cytokinins for Shoot Multiplication
4.3. Factors Influencing Shoot Elongation
4.3.1. Gibberellic Acid in the Subsequent Culture Medium for Shoot Growth
4.3.2. Photoperiod on Shoot Growth
4.3.3. Shoot Growth on Shoot Clump Numbers
4.4. Root Induction by Auxin Treatments
4.5. Soil Acclimatization
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheon, K.S.; Jang, S.K.; Lee, W.T.; Yoo, K.O. The natural habitat and distribution of Echinosophora koreensis (Nakai) Nakai in Korea. Korean J. Plant Taxon. 2009, 39, 254–263. [Google Scholar] [CrossRef]
- Lee, W.K.; Tokuoka, T.; Heo, K. Molecular evidence for the inclusion of the Korean endemic genus “Echinosophora” in Sophora (Fabaceae), and embryological features of the genus. J. Plant Res. 2004, 117, 209–219. [Google Scholar] [CrossRef]
- Ramekar, R.V.; Cheong, E.J.; Lee, H.; Park, K.C.; Kwak, M.; Choi, I.Y. The complete chloroplast genome of a Korean endemic species Sophora koreensis, Nakai. Mitochondrial DNA Part B 2020, 5, 3067–3068. [Google Scholar] [CrossRef]
- Kang, K. Find Echinosophora koreensis NAKAI: Yellow flowers adorn the barren land. Endem. Plant 1992, 24, 274–275. [Google Scholar]
- Choi, E.J.; Kwon, H.C.; Sohn, Y.C.; Nam, C.W.; Park, H.B.; Kim, C.Y.; Yang, H.O. Four flavonoids from Echinosophora koreensis and their effects on alcohol metabolizing enzymes. Arch. Pharmacal Res. 2009, 32, 851–855. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Son, K.; Kwon, C.-S.; Kwon, G.S.; Kang, S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. The Conservation Strategy for the Endangered and Reserved Plants Based on the Ecological and Genetic Characteristics; National Institute of Environmental Research: Inchon, Republic of Korea, 2005; Volume (V). [Google Scholar]
- Bhagwat, B.; Vieiral, L.G.; Erickson, L.R. Stimulation of in vitro shoot proliferation from nodal explants of cassava by thidiazuron, benzyladenine and gibberellic acid. Plant Cell Tissue Organ Cult. 1996, 46, 1–7. [Google Scholar] [CrossRef]
- Quraishi, A.; Koche, V.; Mishra, S. In vitro micropropagation from nodal segments of Cleistanthus collinus. Plant Cell Tissue Organ Cult. 1996, 45, 87–91. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhattacharyya, S. Rapid multiplication of Jasminum officinale L. by in vitro culture of nodal explants. Plant Cell Tissue Organ Cult. 1997, 51, 57–60. [Google Scholar] [CrossRef]
- Krishnan, P.; Seeni, S. Rapid micropropagation of Woodfordia fruticosa (L.) Kurz (Lythraceae), a rare medicinal plant. Plant Cell Rep. 1994, 14, 55–58. [Google Scholar] [CrossRef]
- Yi, J.-S.; Lee, H.; An, C. Effects of Plant Growth Regulators on in vitro Propagation of Echinosophora koreensis Nakai. J. For. Environ. Sci. 2013, 29, 275–281. [Google Scholar] [CrossRef]
- Alphonse, M.; Chandrasekaran, R.; Ramamoorthy, S.; Fulzele, D.P.; Raina, R.; Thiagarajan, K. Optimizing shoot formation in Gentiana kurroo Royle for gentiopicroside production. J. Plant Growth Regul. 2021, 41, 983–992. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Pang, Y.; Hu, J.; Kang, X.; Qian, C. Thidiazuron Enhances Strawberry Shoot Multiplication by Regulating Hormone Signal Transduction Pathways. Int. J. Mol. Sci. 2025, 26, 4060. [Google Scholar] [CrossRef]
- Shri, M.; Pandey, V. Developing in-vitro cultivation techniques for Psoralea corylifolia and optimizing seed germination methods to investigate the impact of elicitors on seedling growth. Sci. Rep. 2025, 15, 15530. [Google Scholar] [CrossRef]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro manipulation and propagation of medicinal plants. Biotechnol. Adv. 2000, 18, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Yue, K.J.; Liu, H.X.; Tian, X.P. Regeneration of in vitro plants through direct and indirect organogenesis from Dracocephalum rupestre leaf explants. Plant Cell Tissue Organ Cult. 2025, 161, 7. [Google Scholar] [CrossRef]
- Brassard, N.; Brissette, L.; Lord, D.; Laliberté, S. Elongation, rooting and acclimatization of micropropagated shoots from mature material of hybrid larch. Plant Cell Tissue Organ Cult. 1996, 44, 37–44. [Google Scholar] [CrossRef]
- Jana, S.; Sivanesan, I.; Jeong, B.R. Effect of cytokinins on in vitro multiplication of Sophora tonkinensis. Asian Pac. J. Trop. Biomed. 2013, 3, 549–553. [Google Scholar] [CrossRef]
- Lu, C.Y. The use of thidiazuron in tissue culture. Vitr. Cell. Dev. Biol.-Plant 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Malik, K.A.; Saxena, P. Thidiazuron induces high-frequency shoot regeneration in intact seedlings of pea (Pisum sativum), chickpea (Cicer arietinum) and lentil (Lens culinaris). Funct. Plant Biol. 1992, 19, 731–740. [Google Scholar] [CrossRef]
- Polisetty, R.; Paul, V.; Deveshwar, J.; Khetarpal, S.; Suresh, K.; Chandra, R. Multiple shoot induction by benzyladenine and complete plant regeneration from seed explants of chickpea (Cicer arietinum L.). Plant Cell Rep. 1997, 16, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Distabanjong, K.; Geneve, R.L. Multiple shoot formation from cotyledonary node segments of Eastern redbud. Plant Cell Tissue Organ Cult. 1997, 47, 247–254. [Google Scholar] [CrossRef]
- Smith, T.C.; Weathers, P.J.; Cheetham, R.D. Effects of gibberellic acid on hairy root cultures of Artemisia annua: Growth and artemisinin production. Vitr. Cell. Dev. Biol.-Plant 1997, 33, 75–79. [Google Scholar] [CrossRef]
- Davidonis, G.H. Gibberellic acid-induced cell elongation in cotton suspension cultures. J. Plant Growth Regul. 1990, 9, 243–246. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Venkatachalam, P. Large scale in vitro propagation of Stevia rebaudiana (bert) for commercial application: Pharmaceutically important and antidiabetic medicinal herb. Ind. Crops Prod. 2012, 37, 111–117. [Google Scholar] [CrossRef]
- Silva de Oliveira, L.; Brondani, G.E.; Batagin-Piotto, K.D.; Calsavara, R.; Gonçalves, A.N.; de Almeida, M. Micropropagation of Eucalyptus cloeziana mature trees. Aust. For. 2015, 78, 219–231. [Google Scholar] [CrossRef]
- Sugimoto, K.; Gordon, S.P.; Meyerowitz, E.M. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011, 21, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, T.; Tanno, S. In vitro propagation of Humulus lupulus through the induction of axillary bud development. Plants 2022, 11, 1066. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates, Inc.: Sunderland, MA, USA, 2002. [Google Scholar]
- Heo, J.W.; Lee, C.W.; Murthy, H.; Paek, K.Y. Influence of light quality and photoperiod on flowering of Cyclamen persicum Mill. cv. ‘Dixie White’. Plant Growth Regul. 2003, 40, 7–10. [Google Scholar] [CrossRef]
- Stadler, R.; Buttner, M.; Ache, P.; Hedrich, R.; Ivashikina, N.; Melzer, M.; Shearson, S.M.; Smith, S.M.; Sauer, N. Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol. 2003, 133, 528–537. [Google Scholar] [CrossRef]
- Jo, E.A.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Effect of photoperiod and light intensity on in vitro propagation of Alocasia amazonica. Plant Biotechnol. Rep. 2008, 2, 207–212. [Google Scholar] [CrossRef]
- Chin-Atkins, A.N.; Craig, S.; Hocart, C.H.; Dennis, E.S.; Chaudhury, A.M. Increased endogenous cytokinin in the Arabidopsis amp1 mutant corresponds with de-etiolation responses. Planta 1996, 198, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Fei, S.Z.; Riordan, T.; Read, P. Stepwise decrease of 2,4-D and addition of BA in subculture medium stimulated shoot regeneration and somatic embryogenesis in buffalograss. Plant Cell Tissue Organ Cult. 2002, 70, 275–279. [Google Scholar] [CrossRef]
- Kaburu M’Ribu, H.; Veilleux, R.E. Effect of genotype, explant, subculture interval and environmental conditions on regeneration of shoots from in vitro monoploids of a diploid potato species, Solanum phureja Juz. & Buk. Factors affecting shoot regeneration of monoploid potato. Plant Cell Tissue Organ Cult. 1990, 23, 171–179. [Google Scholar]
- Wijerathna-Yapa, A.; Hiti-Bandaralage, J. Tissue culture—A sustainable approach to explore plant stresses. Life 2023, 13, 780. [Google Scholar] [CrossRef]
- Sugandh Suman, S.S. Plant tissue culture: A promising tool of quality material production with special reference to micropropagation of banana. Biochem. Cell. Arch. 2017, 17, 1–26. [Google Scholar]
- Meftahizade, H.; Lotfi, M.; Moradkhani, H. Optimization of micropropagation and establishment of cell suspension culture in Melissa officinalis L. Afr. J. Biotechnol. 2010, 9, 4314–4321. [Google Scholar]
- Thorpe, T.A. In vitro organogenesis and somatic embryogenesis: Physiological and biochemical aspects. Morphog. Plants Mol. Approaches 1993, 253, 19–38. [Google Scholar]
- Zhao, D.; Guo, G.; Wang, X.; Zheng, G. In vitro micropropagation of a medicinal plant species Sophora flavescens. Biol. Plant. 2003, 47, 117–120. [Google Scholar] [CrossRef]
- do Vale, P.A.A.; Júnior, J.B.O.; Costa, F.H.S.; Scherwinski-Pereira, J.E. Height and Number of Shoots on the Survival and Development of Micropropagated Bamboo Plantlets during Pre-Acclimatization. Pesq. Agropec. Trop. 2019, 49, e53751. [Google Scholar]



| Treatment (µM) | Survival Rate (%) | No. of Shoot Induced/Explant | Average Shoot Length (cm) |
|---|---|---|---|
| Control | 90 | 3.70 ± 0.76 ab* | 0.47 ± 0.05 a |
| MS B2 | 100 | 2.37 ± 0.29 a | 0.70 ± 0.09 b |
| MS B2T2 | 100 | 6.97 ± 0.50 bc | 0.57 ± 0.03 ab |
| MS B2T2G1 | 100 | 6.93 ± 0.82 bc | 0.57 ± 0.03 ab |
| MS B2T2G2 | 100 | 8.97 ± 0.78 cd | 0.52 ± 0.04 a |
| MS B2T2G5 | 100 | 9.27 ± 0.40 cd | 0.52 ± 0.03 a |
| MS B2T2G10 | 100 | 11.30 ± 0.87 de | 0.50 ± 0.02 a |
| MS B2T2NAA0.5 | 100 | 16.70 ± 1.43 fg | 0.47 ± 0.01 a |
| MS B2T2NAA1 | 100 | 18.37 ± 1.41 g | 0.50 ± 0.01 a |
| MS B2T2NAA2 | 100 | 15.33 ± 1.00 efg | 0.54 ± 0.02 ab |
| MS B2T2IBA0.5 | 100 | 13.80 ± 1.00 efg | 0.70 ± 0.05 b |
| MS B2T2IBA1 | 100 | 15.40 ± 1.26 fg | 0.59 ± 0.02 ab |
| MS B2T2IBA2 | 100 | 14.23 ± 1.43 efg | 0.50 ± 0.02 a |
| Photoperiod (Light/Dark h) | No. of Shoot Induced/Explant | Average Shoot Length(cm) | Longest Shoot Length(cm) |
|---|---|---|---|
| Light (16/8) | 8.40 ± 1.01 | 0.46 ± 0.02 a* | 0.9 |
| Darkness (0/24) | 8.60 ± 0.95 | 0.49 ± 0.02 a | 2.2 |
| Light (8/16) | 8.90 ± 0.88 | 0.60 ± 0.02 b | 1.1 |
| Treatment (µM) | Survival Rate (%) | No. of Shoot Induced/Explant | Average Shoot Length (cm) | |
|---|---|---|---|---|
| Shoot Initiation | Subculture Medium | |||
| B2 | MS hormone-free | 70 | 0.70 ± 0.11 abc* | 0.38 ± 0.06 abc |
| MS G1 | 60 | 0.70 ± 0.13 abc | 0.33 ± 0.06 ab | |
| MS G2 | 40 | 0.35 ± 0.11 a | 0.22 ± 0.06 a | |
| MS G5 | 35 | 0.35 ± 0.11 a | 0.28 ± 0.10 ab | |
| 51 | 0.53 ± 0.06 a | 0.30 ± 0.04 a | ||
| B2T2 | MS hormone-free | 35 | 0.55 ± 0.11 ab | 0.20 ± 0.06 a |
| MS G1 | 60 | 0.80 ± 0.12 abc | 0.47 ± 0.07 abc | |
| MS G2 | 60 | 1.00 ± 0.22 abc | 0.44 ± 0.09 abc | |
| MS G5 | 50 | 0.60 ± 0.17 ab | 0.58 ± 0.19 abc | |
| 53 | 0.74 ± 0.08 ab | 0.45 ± 0.06 ab | ||
| B2T2G1 | MS hormone-free | 90 | 0.95 ± 0.11 abc | 0.91 ± 0.16 c |
| MS G1 | 75 | 0.85 ± 0.11 abc | 0.63 ± 0.10 abc | |
| MS G2 | 90 | 1.25 ± 0.20 bc | 0.81 ± 0.10 bc | |
| MS G5 | 45 | 0.90 ± 0.24 abc | 0.42 ± 0.09 abc | |
| 77 | 0.99 ± 0.09 bc | 0.77 ± 0.07 c | ||
| B2T2G2 | MS hormone-free | 45 | 0.95 ± 0.18 abc | 0.55 ± 0.12 abc |
| MS G1 | 40 | 1.10 ± 0.24 abc | 0.65 ± 0.15 abc | |
| MS G2 | 75 | 0.85 ± 0.11 abc | 0.57 ± 0.09 abc | |
| MS G5 | 90 | 1.50 ± 0.21 c | 0.78 ± 0.10 bc | |
| 70 | 1.10 ± 0.10 c | 0.73 ± 0.07 bc | ||
| B2T2G5 | MS hormone-free | 80 | 1.25 ± 0.19 bc | 0.66 ± 0.09 abc |
| MS G1 | 65 | 0.80 ± 0.14 abc | 0.40 ± 0.07 abc | |
| MS G2 | 60 | 0.85 ± 0.18 abc | 0.42 ± 0.09 abc | |
| MS G5 | 45 | 0.65 ± 0.18 ab | 0.38 ± 0.10 abc | |
| 62 | 0.89 ± 0.09 bc | 0.54 ± 0.06 ab | ||
| B2T2G10 | MS hormone-free | 30 | 0.35 ± 0.13 a | 0.40 ± 0.17 abc |
| MS G1 | 40 | 0.40 ± 0.13 a | 0.27 ± 0.08 ab | |
| MS G2 | 45 | 0.50 ± 0.11 ab | 0.42 ± 0.12 abc | |
| MS G5 | 50 | 0.75 ± 0.20 abc | 0.34 ± 0.09 ab | |
| 40 | 0.50 ± 0.08 a | 0.37 ± 0.06 a | ||
| No. Shoot | Survival Rate (%) | Total Number of Induced Shoots | No. of Shoot Induced/Explant | Average Shoot Length (cm) |
|---|---|---|---|---|
| 1 | 40 | 12 | 1.56 ± 0.34 a* | 0.39 ± 0.07 a |
| 2 | 50 | 30 | 1.16 ± 0.39 a | 0.40 ± 0.10 a |
| 5 | 73 | 110 | 1.56 ± 0.25 a | 0.53 ± 0.10 a |
| 10 | 83 | 249 | 4.93 ± 0.76 b | 0.95 ± 0.12 b |
| Treatment (µM) | Rooting (%) | No. of Root Induced | Average Root Length (cm) |
|---|---|---|---|
| Control | 4 | 0.04 ± 0.04 a* | 0.16 ± 0.16 a |
| IBA 1 | 8 | 0.08 ± 0.06 a | 0.02 ± 0.01 a |
| IBA 5 | 28 | 0.80 ± 0.33 ab | 0.12 ± 0.04 a |
| IBA 10 | 60 | 2.04 ± 0.49 b | 0.16 ± 0.03 a |
| NAA 1 | 24 | 2.00 ± 0.90 b | 0.14 ± 0.06 a |
| NAA 5 | 16 | 0.28 ± 0.15 ab | 0.03 ± 0.01 a |
| NAA 10 | 32 | 1.24 ± 0.49 ab | 0.07 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Han, G.I.; Cheon, K.-S.; Cheong, E.J. In Vitro Conditions Research of Sophora koreensis Nakai for Shoot Elongation. Plants 2025, 14, 1692. https://doi.org/10.3390/plants14111692
Lee H, Han GI, Cheon K-S, Cheong EJ. In Vitro Conditions Research of Sophora koreensis Nakai for Shoot Elongation. Plants. 2025; 14(11):1692. https://doi.org/10.3390/plants14111692
Chicago/Turabian StyleLee, Hwa, Gyu Il Han, Kyeong-Seong Cheon, and Eun Ju Cheong. 2025. "In Vitro Conditions Research of Sophora koreensis Nakai for Shoot Elongation" Plants 14, no. 11: 1692. https://doi.org/10.3390/plants14111692
APA StyleLee, H., Han, G. I., Cheon, K.-S., & Cheong, E. J. (2025). In Vitro Conditions Research of Sophora koreensis Nakai for Shoot Elongation. Plants, 14(11), 1692. https://doi.org/10.3390/plants14111692






