Araçá-Boi Extract and Gallic Acid Reduce Cell Viability and Modify the Expression of Tumor Suppressor Genes and Genes Involved in Epigenetic Processes in Ovarian Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Capacity of Araçá-Boi Extract
2.2. Phytochemical Profile by UHPLC-Q-Orbitrap-MS/MS of Araçá-Boi Extract
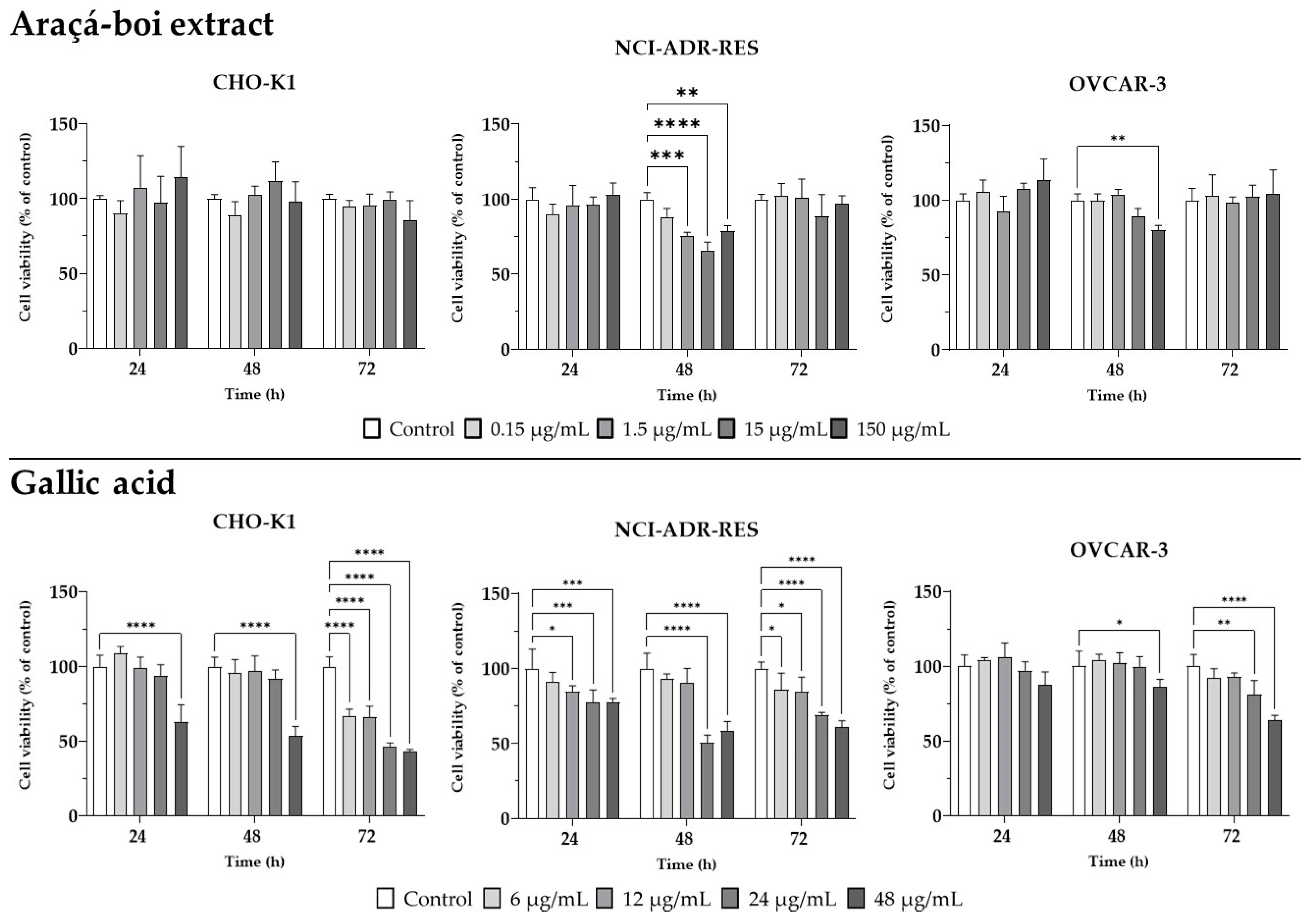
2.3. Cell Viability in Healthy Chinese Hamster Ovary Cells and Human Ovarian Tumor Cells
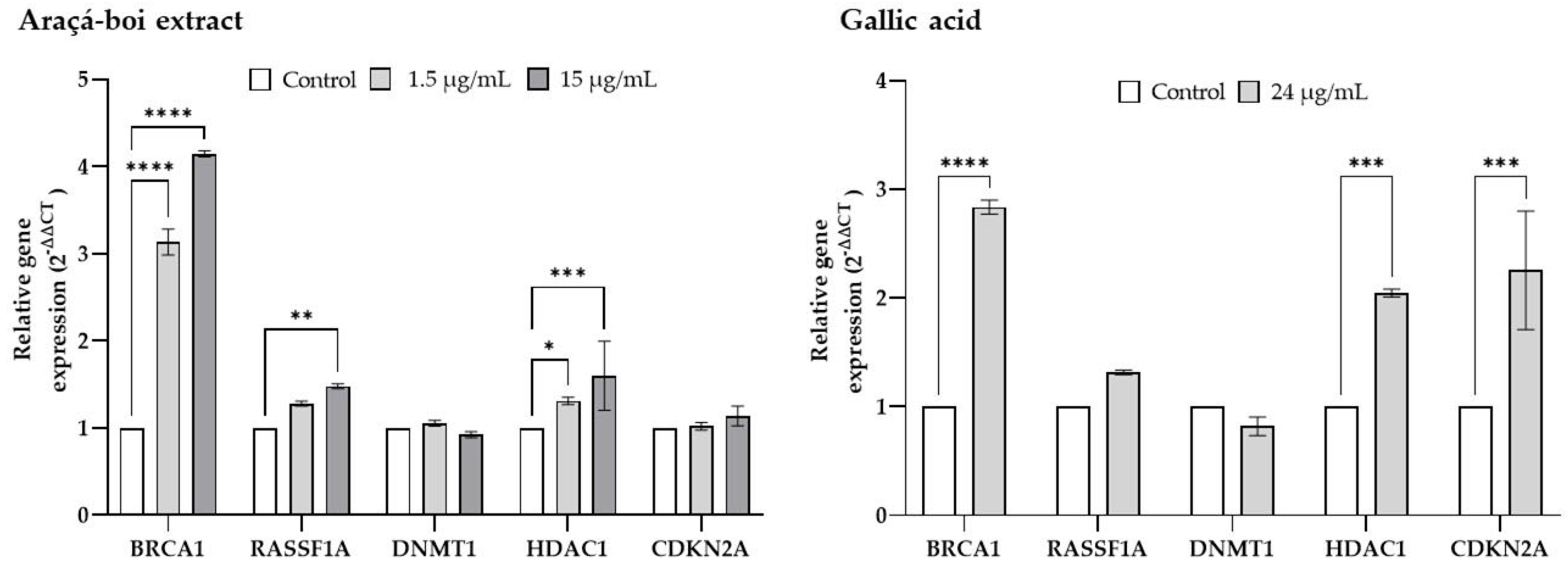
2.4. Relative Gene Expression of Tumor Suppressor Genes and Epigenetic Enzymes
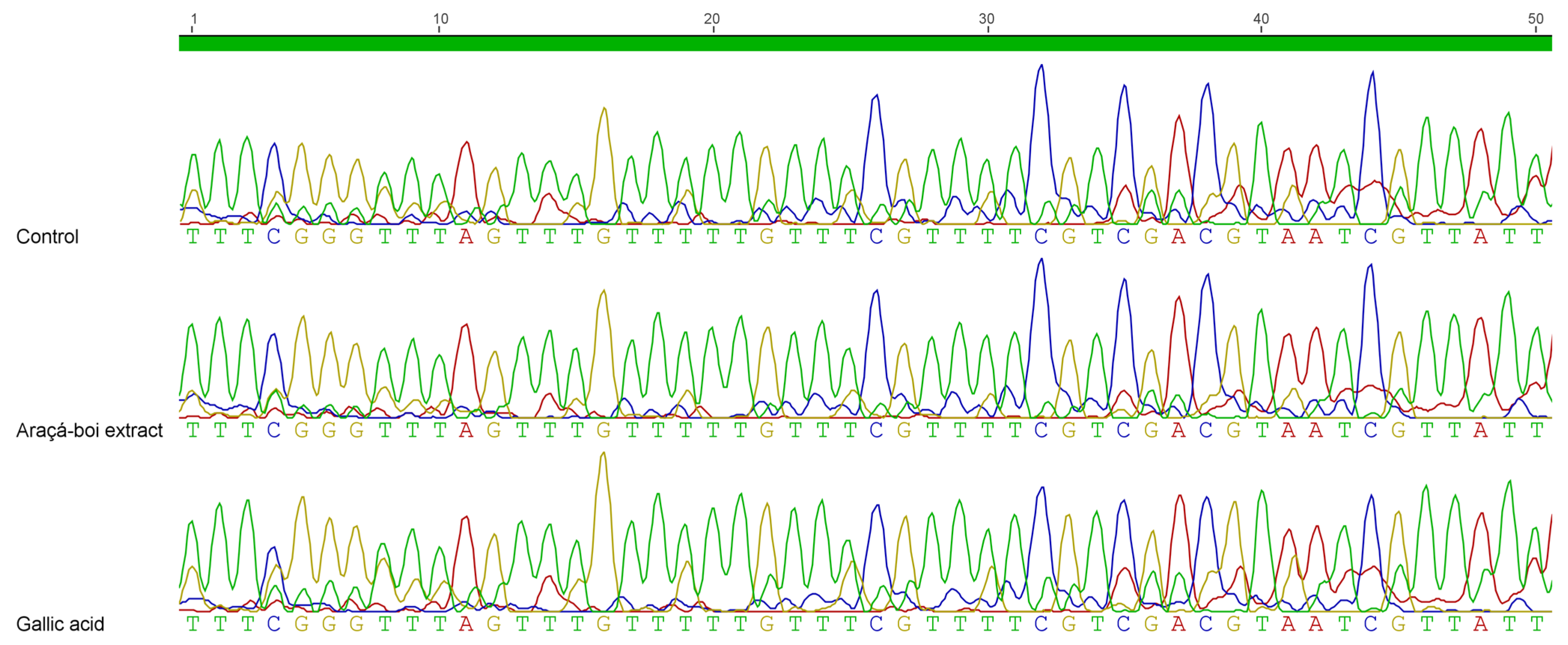
2.5. DNA Methylation Profiling of BRCA1 Promoter
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material, Sample Preparation, and Ultrasound-Assisted Extraction
3.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.4. Phytochemical Profile by UHPLC-Q-Orbitrap-MS/MS
3.5. Antioxidant Capacity Against Synthetic Free Radicals and Reactive Oxygen Species (ROS)
3.5.1. Scavenging of Synthetic Free Radicals DPPH•, ABTS•+, and Ferric-Reducing Antioxidant Power (FRAP)
3.5.2. Reactive Oxygen Species (ROS): Peroxyl Radicals (ROO•), Hydroxyl Radical (OH•), Superoxide Anion (O2•−), and Hypochlorous Acid (HOCl) Scavenging Assays
3.6. Cell Lines and Culture Conditions
3.7. Cell Viability by MTT Assay
3.8. Gene Expression Analysis
3.8.1. Total RNA Isolation
3.8.2. cDNA Synthesis and Primer Design
3.8.3. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
3.9. DNA Methylation Analysis
3.9.1. Bisulfite Conversion of DNA
3.9.2. Primer Design and PCR-Amplification of the Bisulfite-Treated DNA
3.9.3. Purification and Sanger Sequencing
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Webb, P.M.; Jordan, S.J. Global Epidemiology of Epithelial Ovarian Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN Global Cancer Observatory. Available online: https://gco.iarc.fr/en (accessed on 29 April 2025).
- Ali, A.T.; Al-Ani, O.; Al-Ani, F. Epidemiology and Risk Factors for Ovarian Cancer. Menopause Rev. 2023, 22, 93–104. [Google Scholar] [CrossRef]
- Xiao, Y.; Bi, M.; Guo, H.; Li, M. Multi-Omics Approaches for Biomarker Discovery in Early Ovarian Cancer Diagnosis. EBioMedicine 2022, 79, 104001. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Deng, F.; Chen, J.; Fu, L.; Lei, J.; Xu, T.; Chen, Y.; Zhou, J.; Gao, Q.; Ding, H. Current Data and Future Perspectives on DNA Methylation in Ovarian Cancer (Review). Int. J. Oncol. 2024, 64, 62. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Alves, L.F.; Neri-Numa, I.A.; Geraldo, M.V.; Pastore, G.M. A Multi-Omics Approach to Understand the Influence of Polyphenols in Ovarian Cancer for Precision Nutrition: A Mini-Review. Crit. Rev. Food Sci. Nutr. 2023, 65, 1037–1054. [Google Scholar] [CrossRef]
- Chang, Y.; Guo, H.; Li, X.; Zong, L.; Wei, J.; Li, Z.; Luo, C.; Yang, X.; Fang, H.; Kong, X.; et al. Development of a First-in-Class DNMT1/HDAC Inhibitor with Improved Therapeutic Potential and Potentiated Antitumor Immunity. J. Med. Chem. 2024, 67, 16480–16504. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, L.J.; Ulloa, E.V.; Sahlgren, C.; Lizano, M.; de la Cruz-Hernández, E.; Contreras-Paredes, A. Modulating Epigenetic Modifications for Cancer Therapy (Review). Oncol. Rep. 2023, 49, 59. [Google Scholar] [CrossRef]
- Dorna, D.; Grabowska, A.; Paluszczak, J. Natural Products Modulating Epigenetic Mechanisms by Affecting Histone Methylation/Demethylation: Targeting Cancer Cells. Br. J. Pharmacol. 2023, 182, 2137–2158. [Google Scholar] [CrossRef]
- Rathee, S.; Patil, U.K.; Jain, S.K. Exploring the Potential of Dietary Phytochemicals in Cancer Prevention: A Comprehensive Review. J. Explor. Res. Pharmacol. 2024, 9, 34–47. [Google Scholar] [CrossRef]
- Pavithra, R.; Khan, M.R.; Khan, M.S. Recent Advancements in Natural Compounds for Cancer Therapy and Prevention. Phytochem. Rev. 2024, 23, 1835–1859. [Google Scholar] [CrossRef]
- Nanakali, N.M.Q.; Dana, P.M.; Sadoughi, F.; Asemi, Z.; Sharifi, M.; Asemi, R.; Yousefi, B. The Role of Dietary Polyphenols in Alternating DNA Methylation in Cancer. Crit. Rev. Food Sci. Nutr. 2023, 63, 12256–12269. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Vega, L.; Moreno, D.A.; Cuéllar Álvarez, L.N. Arazá: Eugenia stipitata Mc Vaught as a Potential Functional Food. Foods 2024, 13, 2310. [Google Scholar] [CrossRef]
- De Araújo, F.F.; de Farias, D.P.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; do Sacramento, C.K.; Pastore, G.M. Chemical Characterization of Eugenia stipitata: A Native Fruit from the Amazon Rich in Nutrients and Source of Bioactive Compounds. Food Res. Int. 2021, 139, 109904. [Google Scholar] [CrossRef] [PubMed]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Maróstica Junior, M.R.; Pastore, G.M. Evaluation of the Antioxidant, Antiproliferative and Antimutagenic Potential of Araçá-Boi Fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Borsoi, F.T.; da Silva, G.; Manica, D.; Bagatini, M.D.; Pastore, G.M.; Arruda, H.S. Extract of Araçá-Boi and Its Major Phenolic Compound, Trans-Cinnamic Acid, Reduce Viability and Inhibit Migration of Human Metastatic Melanoma Cells. Nutrients 2024, 16, 2929. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Rosalen, P.L.; Lazarini, J.G.; Massarioli, A.P.; da Silva, C.F.; Nani, B.D.; Franchin, M.; de Alencar, S.M. Comprehensive Characterization of Bioactive Phenols from New Brazilian Superfruits by LC-ESI-QTOF-MS, and Their ROS and RNS Scavenging Effects and Anti-Inflammatory Activity. Food Chem. 2019, 281, 178–188. [Google Scholar] [CrossRef]
- He, Z.; Liu, X.; Wu, F.; Wu, S.; Rankin, G.O.; Martinez, I.; Rojanasakul, Y.; Chen, Y.C. Gallic Acid Induces S and G2 Phase Arrest and Apoptosis in Human Ovarian Cancer Cells In Vitro. Appl. Sci. 2021, 11, 3807. [Google Scholar] [CrossRef]
- He, Z.; Chen, A.Y.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Gallic Acid, a Phenolic Compound, Exerts Anti-Angiogenic Effects via the PTEN/AKT/HIF-1α/VEGF Signaling Pathway in Ovarian Cancer Cells. Oncol. Rep. 2016, 35, 291–297. [Google Scholar] [CrossRef]
- Varela-Rodríguez, L.; Sánchez-Ramírez, B.; Hernández-Ramírez, V.I.; Varela-Rodríguez, H.; Castellanos-Mijangos, R.D.; González-Horta, C.; Chávez-Munguía, B.; Talamás-Rohana, P. Effect of Gallic Acid and Myricetin on Ovarian Cancer Models: A Possible Alternative Antitumoral Treatment. BMC Complement. Med. Ther. 2020, 20, 110. [Google Scholar] [CrossRef]
- Do Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Navarro, M.; Ortíz, J.; Angós, I.; Carrillo, W. Effect of Modified Atmosphere Packaging (MAP) in the Antioxidant Capacity of Arazá (Eugenia stipitata McVaugh), Naranjilla (Solanum quitoense Lam.), and Tree Tomato (Solanum betaceum Cav.) Fruits from Ecuador. J. Food Process. Preserv. 2020, 44, e14757. [Google Scholar] [CrossRef]
- Popescu, D.I.; Botoran, O.R.; Cristea, R.; Mihăescu, C.; Șuțan, N.A. Effects of Geographical Area and Harvest Times on Chemical Composition and Antibacterial Activity of Juniperus communis L. Pseudo-Fruits Extracts: A Statistical Approach. Horticulturae 2023, 9, 325. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Borsoi, F.T.; Neri-Numa, I.A.; de Oliveira, W.Q.; de Araújo, F.F.; Pastore, G.M. Dietary Polyphenols and Their Relationship to the Modulation of Non-Communicable Chronic Diseases and Epigenetic Mechanisms: A Mini-Review. Food Chem. Mol. Sci. 2023, 6, 100155. [Google Scholar] [CrossRef]
- De Araújo, F.F.; de Farias, D.P.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; do Sacramento, C.K.; Pastore, G.M. Gastrointestinal Bioaccessibility and Bioactivity of Phenolic Compounds from Araçá-Boi Fruit. LWT 2021, 135, 110230. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; AL-Farga, A.M. Mode of Action and Determination of Antioxidant Activity in the Dietary Sources: An Overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Recent Advances in Reactive Oxygen Species Scavenging Nanomaterials for Wound Healing. Exploration 2024, 4, 20230066. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer IV, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS Systems Are a New Integrated Network for Sensing Homeostasis and Alarming Stresses in Organelle Metabolic Processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V. Role of Organic Acids in the Integration of Cellular Redox Metabolism and Mediation of Redox Signalling in Photosynthetic Tissues of Higher Plants. Free Radic. Biol. Med. 2018, 122, 74–85. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic Acid: The Chemistry Underlying Its Antioxidant Properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Chen, H.; Yu, F.; Kang, J.; Li, Q.; Warusawitharana, H.K.; Li, B. Quality Chemistry, Physiological Functions, and Health Benefits of Organic Acids from Tea (Camellia sinensis). Molecules 2023, 28, 2339. [Google Scholar] [CrossRef]
- Arruda, H.S.; Angolini, C.F.F.; Eberlin, M.N.; Pastore, G.M.; Marostica Junior, M.R. UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity. Foods 2023, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, M.; Ghasemnezhad Targhi, R.; Soleimani, M. Anti-Proliferative and Anti-Apoptotic Effects of Grape Seed Extract on Chemo-Resistant OVCAR-3 Ovarian Cancer Cells. Res. Pharm. Sci. 2020, 15, 390–400. [Google Scholar] [CrossRef]
- Russo, M.; Sogari, A.; Bardelli, A. Adaptive Evolution: How Bacteria and Cancer Cells Survive Stressful Conditions and Drug Treatment. Cancer Discov. 2021, 11, 1886–1895. [Google Scholar] [CrossRef]
- Castañeda, A.M.; Meléndez, C.M.; Uribe, D.; Pedroza-Díaz, J. Synergistic Effects of Natural Compounds and Conventional Chemotherapeutic Agents: Recent Insights for the Development of Cancer Treatment Strategies. Heliyon 2022, 8, e09519. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The Synergistic and Antagonistic Antioxidant Interactions of Dietary Phytochemical Combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef] [PubMed]
- Al Balushi, N.; Hassan, S.I.; Abdullah, N.; Al Dhahli, B.; Al Bahlani, S.; Ahmed, I.; Tsang, B.K.; Dobretsov, S.; Tamimi, Y.; Burney, I.A. Addition of Gallic Acid Overcomes Resistance to Cisplatin in Ovarian Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2022, 23, 2661. [Google Scholar] [CrossRef]
- Bradbury, A.; O’Donnell, R.; Drew, Y.; Curtin, N.J.; Sharma Saha, S. Characterisation of Ovarian Cancer Cell Line NIH-OVCAR3 and Implications of Genomic, Transcriptomic, Proteomic and Functional DNA Damage Response Biomarkers for Therapeutic Targeting. Cancers 2020, 12, 1939. [Google Scholar] [CrossRef]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cell Mol. Biol. Lett. 2022, 27, 1–26. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Vrânceanu, M.; Galimberti, D.; Banc, R.; Dragoş, O.; Cozma-Petruţ, A.; Hegheş, S.C.; Voştinaru, O.; Cuciureanu, M.; Stroia, C.M.; Miere, D.; et al. The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression. Plants 2022, 11, 2524. [Google Scholar] [CrossRef]
- Nowrasteh, G.; Zand, A.; Raposa, L.B.; Szabó, L.; Tomesz, A.; Molnár, R.; Kiss, I.; Orsós, Z.; Gerencsér, G.; Gyöngyi, Z.; et al. Fruit Extract, Rich in Polyphenols and Flavonoids, Modifies the Expression of DNMT and HDAC Genes Involved in Epigenetic Processes. Nutrients 2023, 15, 1867. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Useros, J.; Martin-Galan, M.; Florez-Cespedes, M.; Garcia-Foncillas, J. Epigenetics of Most Aggressive Solid Tumors: Pathways, Targets and Treatments. Cancers 2021, 13, 3209. [Google Scholar] [CrossRef]
- Al-Yousef, N.; Shinwari, Z.; Al-Shahrani, B.; Al-Showimi, M.; Al-Moghrabi, N. Curcumin Induces Re-Expression of BRCA1 and Suppression of γ Synuclein by Modulating DNA Promoter Methylation in Breast Cancer Cell Lines. Oncol. Rep. 2020, 43, 827–838. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.; Zhang, H.; Xiao, L. Prognostic Effects of RASSF1A, BRCA1, APC, and P16 Promoter Methylation in Ovarian Cancer: A Meta-Analysis. Gynecol. Obstet. Invest. 2024, 89, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, Y.; Zhao, D.; Zou, Q.; Yu, F.; Zhang, L.; Xu, L. Comprehensive Analysis Revealed That CDKN2A Is a Biomarker for Immune Infiltrates in Multiple Cancers. Front. Cell Dev. Biol. 2021, 9, 808208. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Li, B.; Liu, L.; Huang, C. The Emerging Roles and Therapeutic Implications of Epigenetic Modifications in Ovarian Cancer. Front. Endocrinol. 2022, 13, 863541. [Google Scholar] [CrossRef]
- Weng, Y.P.; Hung, P.F.; Ku, W.Y.; Chang, C.Y.; Wu, B.H.; Wu, M.H.; Yao, J.Y.; Yang, J.R.; Lee, C.H. The Inhibitory Activity of Gallic Acid against DNA Methylation: Application of Gallic Acid on Epigenetic Therapy of Human Cancers. Oncotarget 2017, 9, 361. [Google Scholar] [CrossRef]
- Khan, H.; Labanca, F.; Ullah, H.; Hussain, Y.; Tzvetkov, N.T.; Akkol, E.K.; Milella, L. Advances and Challenges in Cancer Treatment and Nutraceutical Prevention: The Possible Role of Dietary Phenols in BRCA Regulation. Phytochem. Rev. 2022, 21, 385–400. [Google Scholar] [CrossRef]
- Fatima, N.; Baqri, S.S.R.; Bhattacharya, A.; Koney, N.K.K.; Husain, K.; Abbas, A.; Ansari, R.A. Role of Flavonoids as Epigenetic Modulators in Cancer Prevention and Therapy. Front. Genet. 2021, 12, 758733. [Google Scholar] [CrossRef]
- Letsiou, S.; Kapazoglou, A.; Tsaftaris, A.; Spanidi, E.; Gardikis, K. Transcriptional and Epigenetic Effects of Vitis vinifera L. Leaf Extract on UV-Stressed Human Dermal Fibroblasts. Mol. Biol. Rep. 2020, 47, 5763–5772. [Google Scholar] [CrossRef]
- Jasek, K.; Kubatka, P.; Samec, M.; Liskova, A.; Smejkal, K.; Vybohova, D.; Bugos, O.; Biskupska-Bodova, K.; Bielik, T.; Zubor, P.; et al. DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions. Biomolecules 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Kischkel, F.; Hoheise, J.D. High-Definition DNA Methylation Profiles from Breast and Ovarian Carcinoma Cell Lines with Differing Doxorubicin Resistance. PLoS ONE 2010, 5, e11002. [Google Scholar] [CrossRef] [PubMed]
- Gull, N.; Jones, M.R.; Peng, P.-C.; Coetzee, S.G.; Silva, T.C.; Plummer, J.T.; Reyes, A.L.P.; Davis, B.D.; Chen, S.S.; Lawrenson, K.; et al. DNA Methylation and Transcriptomic Features Are Preserved throughout Disease Recurrence and Chemoresistance in High Grade Serous Ovarian Cancers. J. Exp. Clin. Cancer Res. 2022, 41, 232. [Google Scholar] [CrossRef] [PubMed]
- Baldini, T.F.; Neri-Numa, I.A.; Do Sacramento, C.K.; Schmiele, M.; Bolini, H.M.A.; Pastore, G.M.; Bicas, J.L. Elaboration and Characterization of Apple Nectars Supplemented with Araçá-Boi (Eugenia stipitata Mac Vaugh—Myrtaceae). Beverages 2017, 3, 59. [Google Scholar] [CrossRef]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G. Antioxidant Activity of Annona crassiflora: Characterization of Major Components by Electrospray Ionization Mass Spectrometry. Food Chem. 2007, 104, 1048–1054. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Sustainable Pectin-Based Film for Carrying Phenolic Compounds and Essential Oil from Citrus Sinensis Peel Waste. Food Biosci. 2024, 61, 104526. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of High-Intensity Ultrasound Process Parameters on the Phenolic Compounds Recovery from Araticum Peel. Ultrason. Sonochem. 2019, 50, 82–95. [Google Scholar] [CrossRef]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Maróstica Júnior, M.R. Antioxidant Potential of Rat Plasma by Administration of Freeze-Dried Jaboticaba Peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Arruda, H.S.; Reguengo, L.M.; Neri Numa, I.A.; Pastore, G.M. Understanding the Gastrointestinal Behavior of Phytochemicals and Antioxidants from Araçá-Boi (Eugenia stipitata- McVaugh) Extract: An in Vitro and in Silico Approach. Food Chem. 2025, 483, 144254. [Google Scholar] [CrossRef]
- Saliba, A.S.M.C.; Quirino, D.J.G.; Favaro-Trindade, C.S.; de Sartori, A.G.O.; Massarioli, A.P.; Lazarini, J.G.; de Souza Silva, A.P.; de Alencar, S.M. Effects of Simulated Gastrointestinal Digestion/Epithelial Transport on Phenolics and Bioactivities of Particles of Brewer’s Spent Yeasts Loaded with Brazilian Red Propolis. Food Res. Int. 2023, 173, 113345. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.; Borsoi, F.T.; Sofia, A.; Chaib Saliba, M.; Matias De Alencar, S.; Pastore, G.M.; Arruda, H.S. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Araticum Peel Using Response Surface Methodology. Plants 2024, 13, 2560. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
| Analysis | Parameters | Araçá-Boi Extract |
|---|---|---|
| Phytochemicals | Total phenolics (mg GAE/g dw) | 25.90 ± 1.37 |
| Total flavonoids (mg CE/g dw) | 6.53 ± 0.19 | |
| Synthetic free radical | DPPH (μmol TE/g dw) | 68.78 ± 7.03 |
| ABTS (μmol TE/g dw) | 155.52 ± 8.08 | |
| FRAP (μmol TE/g dw) | 161.77 ± 10.21 | |
| Reactive oxygen species (ROS) | ROO• (μmol TE/g dw) | 366.13 ± 19.39 |
| OH• (IC50 µg/mL dw) | 1.91 ± 0.20 | |
| O2•− (IC50 µg/mL dw) | 3534.33 ± 111.99 | |
| HOCl (IC50 µg/mL dw) | 307.66 ± 30.08 |
| N. ° | R.T. (min) | Identified/Tentatively Annotated Compound | Molecular Formula | Observed m/z Value | Theoretical m/z Value | Error (ppm) | Characteristic MS/MS Fragments |
|---|---|---|---|---|---|---|---|
| Organic acid and derivatives | |||||||
| 1 | 0.68 | Quinic acid | C6H12O6 | 191.0566 | 191.0556 | 5.23 | 191.0568, 173.0456, 127.0408, 93.0347, 85.0294 |
| 2 | 0.74 | Malic acid | C4H6O5 | 133.0144 | 133.0137 | 5.26 | 115.0038, 71.0135 |
| 3 | 0.83 | Citric acid | C6H8O7 | 191.0201 | 191.0192 | 4.71 | 129.0192, 111.0090 |
| 4 | 0.90 | Shikimic acid | C7H10O5 | 173.0452 | 173.0450 | 1.16 | 155.0004, 111.0087, 93.0350 |
| 5 | 0.90 | Succinic acid | C4H6O4 | 117.0180 | 117.0188 | −6.84 | 99.0087, 73.0295 |
| 6 | 0.93 | Hydroxyadipic acid | C6H10O5 | 161.0457 | 161.0450 | 4.35 | 101.0245, 99.0451 |
| 7 | 1.38 | Ascorbic acid | C6H8O6 | 175.0251 | 175.0243 | 4.57 | 115.0037, 87.0090, 71.0138 |
| 8 | 1.23 | Pantothenic acid (vitamin B5) | C9H17NO5 | 218.1033 | 218.1028 | 2.29 | 146.0829, 88.0402 |
| 9 | 4.43 | Tuberonic acid hexoside | C18H28O9 | 387.1662 | 387.1655 | 1.81 | 207.1024, 163.1134 |
| 10 | 10.87 | 12-hydroxyjasmonoyl-isoleucine | C18H29NO5 | 338.1984 | 338.1967 | 5.03 | 130.0876 |
| Phenolic acids and derivatives | |||||||
| 11 | 0.99 | Gallic acid glucoside | C13H16O10 | 331.0678 | 331.0665 | 3.93 | 271.0489, 211.0272, 169.0155 |
| 12 | 1.14 | Gallic acid | C7H6O5 | 169.0146 | 169.0137 | 5.33 | 169.0146, 125.0245, 107.0137, 97.0299, 79.0188 |
| 13 | 1.39 | Salicylic acid isomer 1 | C7H6O3 | 137.0244 | 137.0239 | 3.65 | 93.0349 |
| 14 | 1.62 | Hydroxybenzoic acid hexoside | C13H16O8 | 299.0775 | 299.0767 | 2.67 | 137.0252 |
| 15 | 1.70 | Peduncalagin isomer 1 | C34H24O22 | 783.0698 | 783.0681 | 2.17 | 300.9982, 275.0201 |
| 16 | 1.99 | Vanillic acid hexoside isomer 1 | C14H18O9 | 329.0886 | 329.0872 | 4.25 | 329.0946, 167.0350, 152.0115, 123.0455, 108.0220 |
| 17 | 2.06 | Protocatechuic acid xyloside | C12H14O8 | 285.0620 | 285.0610 | 3.51 | 153.0192, 152.0112, 123.4724, 109.0290, 108.0222 |
| 18 | 2.12 | Vanillic acid hexoside isomer 2 | C14H18O9 | 329.0887 | 329.0872 | 4.56 | 329.0908, 167.0358, 123.0451 |
| 19 | 2.19 | Salicylic acid isomer 2 | C7H6O3 | 137.0243 | 137.0239 | 2.92 | 93.0341 |
| 20 | 2.26 | Galloyl shikimic acid | C14H14O9 | 325.0573 | 325.0560 | 4.00 | 169. 0154, 125.0252 |
| 21 | 2.33 | Caffeic acid hexoside isomer 1 | C15H18O9 | 341.0885 | 341.0873 | 3.52 | 179.0360, 161.0255, 135.0455 |
| 22 | 2.59 | Peduncalagin isomer 2 | C34H24O22 | 783.0710 | 783.0681 | 3.70 | 300.9995, 275.0200 |
| 23 | 2.62 | Coumaric acid isomer 1 | C9H8O3 | 163.0403 | 163.0395 | 4.91 | 162.8391, 119.0504 |
| 24 | 2.62 | p-coumaric acid hexoside isomer 1 | C15H18O8 | 325.0933 | 325.0923 | 3.08 | 163.0403, 119.0504 |
| 25 | 2.74 | Syringic acid hexoside isomer 1 | C15H20O10 | 359.0995 | 359.0978 | 4.73 | 197.0839, 138.3060, 123.0091 |
| 26 | 2.78 | Caffeic acid hexoside isomer 2 | C15H18O9 | 341.0886 | 341.0873 | 3.81 | 179.0361, 135.0459 |
| 27 | 2.95 | Vanillic acid hexoside isomer 3 | C14H18O9 | 329.0887 | 329.0872 | 4.56 | 167.0352, 123.0456 |
| 28 | 3.10 | Coumaric acid isomer 2 | C9H8O3 | 163.0403 | 163.0395 | 4.91 | 162.8406, 119.0507 |
| 29 | 3.12 | p-coumaric acid hexoside isomer 2 | C15H18O8 | 325.0939 | 325.0923 | 4.92 | 163.0410, 145.0301, 119.0498 |
| 30 | 3.22 | Caffeic acid hexoside isomer 3 | C15H18O9 | 341.0883 | 341.0873 | 2.93 | 179.0356, 135.0453 |
| 31 | 3.28 | Coumaric acid isomer 3 | C9H8O3 | 163.0402 | 163.0395 | 4.29 | 162.8410, 119.0506 |
| 32 | 3.31 | p-coumaric acid hexoside isomer 3 | C15H18O8 | 325.0938 | 325.0923 | 4.61 | 163.0408, 145.0292, 119.0506 |
| 33 | 3.72 | Vanillic acid hexoside isomer 4 | C14H18O9 | 329.0887 | 329.0872 | 4.56 | 167.0357, 123.0456, 108.0218 |
| 34 | 3.81 | Digalloyl hexoside isomer 1 | C20H20O14 | 483.0797 | 483.0775 | 4.55 | 313.0592, 271.0473, 211.0240, 169.0148 |
| 35 | 4.10 | Digalloyl hexoside isomer 2 | C20H20O14 | 483.0796 | 483.0775 | 4.35 | 169.0144, 125.0241 |
| 36 | 4.14 | p-coumaric acid hexoside isomer 4 | C15H18O8 | 325.0939 | 325.0923 | 4.92 | 163.0392, 119.0498 |
| 37 | 4.15 | Ferulic acid hexoside | C16H20O9 | 355.1041 | 355.1029 | 3.38 | 193.0498, 175.0413, 134.0370 |
| 38 | 4.15 | Di-O-galloyl-rhamnose | C20H20O13 | 467.0806 | 467.0826 | −4.28 | 315.0174, 169.0143, 125.0251 |
| 39 | 4.16 | Ferulic acid | C10H10O4 | 193.0508 | 193.0501 | 3.63 | 134.0376 |
| 40 | 5.11 | Coumaric acid isomer 4 | C9H8O3 | 163.0402 | 163.0395 | 4.29 | 162.8396, 119.0506 |
| 41 | 5.17 | Trans-cinnamic acid | C9H8O2 | 147.0453 | 147.0446 | 4.76 | 147.0457, 103.0549 |
| 42 | 5.88 | Syringic acid hexoside isomer 1 | C15H20O10 | 359.0964 | 359.0978 | −3.90 | 197.0831, 153.0923 |
| 43 | 6.10 | Caffeoylshikimic acid | C16H16O8 | 335.0784 | 335.0770 | 4.18 | 179.0359, 161.0258, 135.0447 |
| 44 | 6.34 | Tri-O-galloyl-glucose | C27H24O18 | 635.0926 | 635.0884 | 6.61 | 465.0686, 313.0588, 169.0142, 125.0249 |
| 45 | 7.77 | Mirciaphenone B | C21H22O13 | 481.0994 | 481.0982 | 2.49 | 313.0557, 169.0147 |
| 46 | 9.25 | Cis-Cinnamic acid | C9H8O2 | 147.0452 | 147.0446 | 4.08 | 147.0459, 103.0549 |
| Flavonoids and derivatives | |||||||
| 47 | 3.24 | Taxifolin isomer 1 | C15H12O7 | 303.0514 | 303.0505 | 2.97 | 285.0428, 217.0512, 175.0395, 125.0245 |
| 48 | 3.44 | Taxifolin isomer 2 | C15H12O7 | 303.0513 | 303.0505 | 2.64 | 285.0403, 217.0499, 175.0410, 125.0243 |
| 49 | 4.74 | (Epi)catechin | C15H14O6 | 289.0723 | 289.0712 | 3.81 | 245.0483, 221.0465, 151.0033, 137.0254, 125.0251 |
| 50 | 5.28 | Dihydroquercetin hexoside | C21H22O12 | 465.1045 | 465.1068 | −4.95 | 285.0390, 151.0038 |
| 51 | 6.99 | Taxifolin isomer 3 | C15H12O7 | 303.0515 | 303.0505 | 3.30 | 285.0417, 175.0397, 125.0250 |
| 52 | 7.52 | Myricetin-3-O-galactoside | C21H20O13 | 479.0848 | 479.0826 | 4.59 | 317.0311, 316.0230 |
| 53 | 8.33 | Quercetin-3-O-galloyl hexoside isomer 1 | C28H24O16 | 615.1002 | 615.0986 | 2.60 | 463.0884, 301.0350, 300.0306, 169.0145, 151.0046 |
| 54 | 8.68 | Quercetin-3-O-galloyl hexoside isomer 2 | C28H24O16 | 615.1002 | 615.0986 | 2.60 | 463.0849, 301.0344, 300.0313, 169.0144, 125.2322 |
| 55 | 8.74 | Myricetin-3-O-rhamnoside (myricetrin) | C21H20O12 | 463.0895 | 463.0877 | 3.89 | 316.0224, 137.0305 |
| 56 | 8.80 | Quercetin maloyl hexoside | C25H24O16 | 579.1018 | 579.0986 | 5.53 | 301.0336, 300.0305 |
| 57 | 8.98 | Quercetin-3-O-galactoside | C21H20O12 | 463.0899 | 463.0877 | 4.75 | 301.0342, 300.0311, 179.1588, 151.0037 |
| 58 | 9.08 | Quercetin-3-O-glucuronide | C21H18O13 | 477.0690 | 477.0669 | 4.40 | 302.0402, 301.0370, 178.9986, 151.0045 |
| 59 | 9.23 | Phloretin-C-diglycoside | C27H34O15 | 597.1830 | 597.1820 | 1.67 | 387.1130, 357.1005, 345.0978, 315.0868, 209.0453 |
| 60 | 9.24 | Quercetin-3-O-glucoside | C21H20O12 | 463.0900 | 463.0877 | 4.97 | 301.0358, 300.0278, 178.9999, 151.0037 |
| 61 | 9.46 | Naringenin | C15H12O5 | 271.0618 | 271.0607 | 4.06 | 177.0196, 151.0033, 119.0509, 107.0135 |
| 62 | 10.08 | Quercetin-3-O-arabinoside | C20H18O11 | 433.0795 | 433.0771 | 5.54 | 301.0359, 300.0279, 271.0620, 151.0031 |
| 63 | 10.16 | Kaempferol-3-O-galactoside (trifolin) | C21H20O11 | 447.0938 | 447.0927 | 2.46 | 285.0390, 284.0330, 255.0307 |
| 64 | 10.18 | Phlorizin | C21H24O10 | 435.1302 | 435.1291 | 2.53 | 273.0791, 167.0364 |
| 65 | 10.49 | Kaempferol 7-(6′-galloyl glucoside) | C28H24O15 | 599.1061 | 599.1037 | 4.01 | 285.0420, 284.0360, 169.0143 |
| 66 | 10.69 | Kaempferol-3-O-glucoside (astragalin) | C21H20O11 | 447.0948 | 447.0927 | 4.70 | 285.0410, 284.0350, 255.0322 |
| 67 | 10.79 | Quercetin-3-O-rhamnoside (quercetrin) | C21H20O11 | 447.0948 | 447.0927 | 4.70 | 301.0341, 300.0298, 271.0249, 255.0325, 151.0048 |
| 68 | 11.85 | Kaempferol-3-O-rhamnoside (afzelin) | C21H20O10 | 431.0994 | 431.0978 | 3.71 | 286.0453, 285.0408, 284.0348, 255.0320, 227.0348 |
| 69 | 12.11 | Quercetin deoxyhesoxylhexoside | C27H30O16 | 609.1442 | 609.1456 | −2.30 | 301.0333, 300.0320 |
| 70 | 12.18 | Quercetin-3-O-acetyl rhamnoside | C23H22O12 | 489.1056 | 489.1033 | 4.70 | 301.0337, 300.0306, 271.0245, 255.0322 |
| 71 | 12.18 | Quercetin 3-O-hexuronide-7-O-hexoside | C27H28O18 | 639.1212 | 639.1197 | 2.35 | 301.0344, 300.0289, 151.0700 |
| 72 | 12.35 | Quercetin | C15H10O7 | 301.0359 | 301.0348 | 3.65 | 301.0376, 179.0004, 151.0043, 121.0305, 107.0144 |
| 73 | 12.49 | Quercetin-3,7-O-dirhamnoside | C27H30O15 | 593.1483 | 593.1506 | −3.88 | 301.0356, 300.0275, 271.0263, 255.0301, 151.0035 |
| Gene Names | Forward (5′→3′) | Reverse (5′→3′) | Amplicon (bp) |
|---|---|---|---|
| BRCA1 | CTGGACAGAGGACAATGGCT | GTGGGGGATCTGGGGTATCA | 139 |
| RASSF1A | ACCCCTCTGCCCTCATTACT | TTCTGTCTGCACCACTCCTG | 89 |
| DNMT1 | TTCAGCACAACCGTCACCAA | GTCCAGGATGTTGCCGAAGA | 147 |
| HDAC1 | TTCTTCCCCAACCCCTCAGA | GGCCTTGGTTTCTGTCCCTG | 99 |
| CDKN2A | TAAGGGGAATAGGGGAGCGG | ACTGCGAGAACCACATGTCT | 149 |
| GAPDH | ACCCACTCCTCCACCTTTGA | CTGTTGCTGTAGCCAAATTCGT | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsoi, F.T.; Arruda, H.S.; Andrade, A.C.; dos Santos, M.P.; da Silva, I.N.; Marson, L.A.; Saliba, A.S.M.C.; de Alencar, S.M.; Geraldo, M.V.; Neri Numa, I.A.; et al. Araçá-Boi Extract and Gallic Acid Reduce Cell Viability and Modify the Expression of Tumor Suppressor Genes and Genes Involved in Epigenetic Processes in Ovarian Cancer. Plants 2025, 14, 1671. https://doi.org/10.3390/plants14111671
Borsoi FT, Arruda HS, Andrade AC, dos Santos MP, da Silva IN, Marson LA, Saliba ASMC, de Alencar SM, Geraldo MV, Neri Numa IA, et al. Araçá-Boi Extract and Gallic Acid Reduce Cell Viability and Modify the Expression of Tumor Suppressor Genes and Genes Involved in Epigenetic Processes in Ovarian Cancer. Plants. 2025; 14(11):1671. https://doi.org/10.3390/plants14111671
Chicago/Turabian StyleBorsoi, Felipe Tecchio, Henrique Silvano Arruda, Amanda Cristina Andrade, Mônica Pezenatto dos Santos, Isabelle Nogueira da Silva, Leonardo Augusto Marson, Ana Sofia Martelli Chaib Saliba, Severino Matias de Alencar, Murilo Vieira Geraldo, Iramaia Angélica Neri Numa, and et al. 2025. "Araçá-Boi Extract and Gallic Acid Reduce Cell Viability and Modify the Expression of Tumor Suppressor Genes and Genes Involved in Epigenetic Processes in Ovarian Cancer" Plants 14, no. 11: 1671. https://doi.org/10.3390/plants14111671
APA StyleBorsoi, F. T., Arruda, H. S., Andrade, A. C., dos Santos, M. P., da Silva, I. N., Marson, L. A., Saliba, A. S. M. C., de Alencar, S. M., Geraldo, M. V., Neri Numa, I. A., & Pastore, G. M. (2025). Araçá-Boi Extract and Gallic Acid Reduce Cell Viability and Modify the Expression of Tumor Suppressor Genes and Genes Involved in Epigenetic Processes in Ovarian Cancer. Plants, 14(11), 1671. https://doi.org/10.3390/plants14111671










