Physiological Responses and the Dust Retention Ability of Different Turfgrass Mixture Ratios Under Continuous Drought
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Measurement Indexes and Methods
2.4. Data Analysis
3. Results
3.1. The Changes in the Physiological and Ecological Variables of Six Groups of Lawn Grasses Under Drought Stress
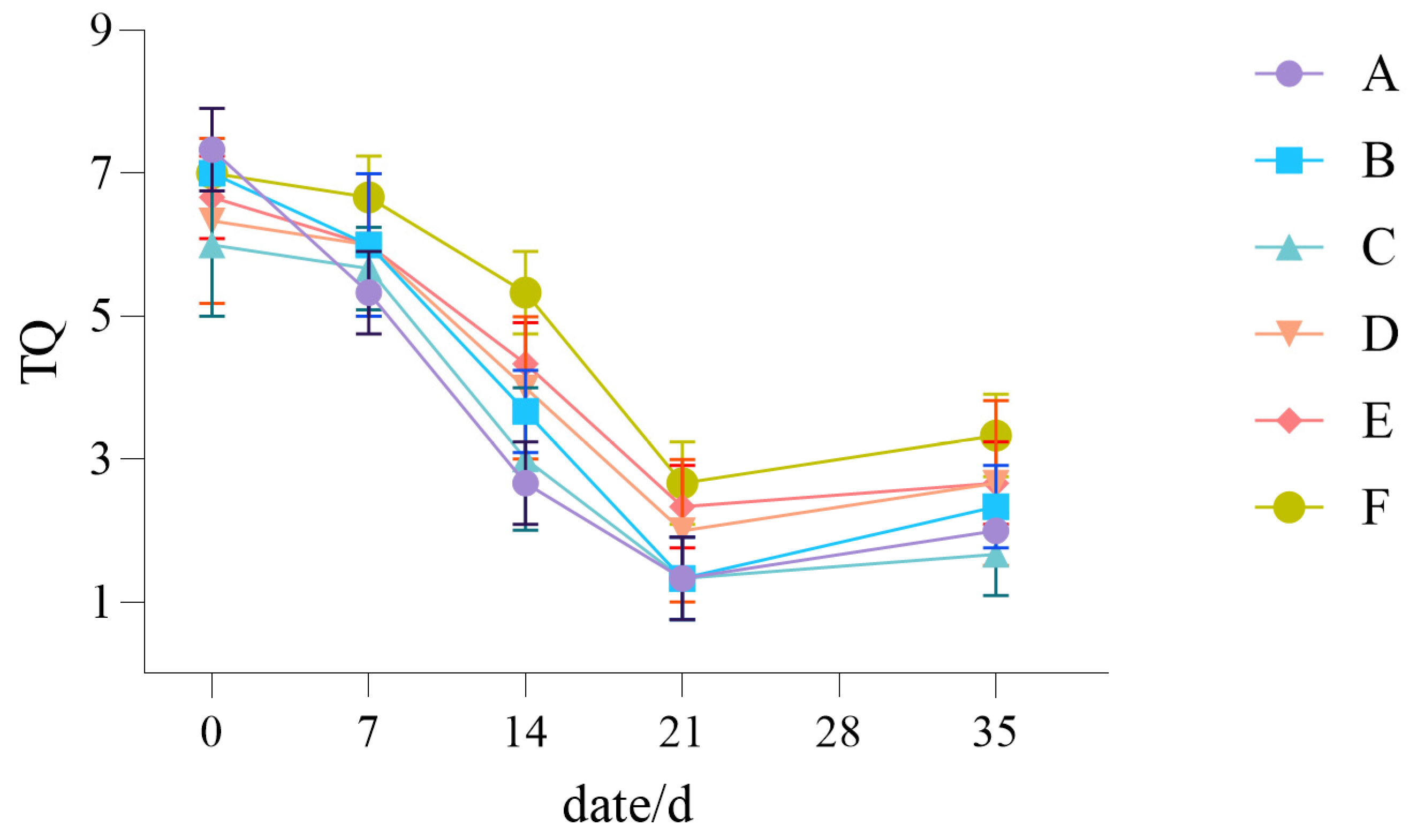
3.1.1. The Influence of Drought Stress on the TQ of Lawn Grass
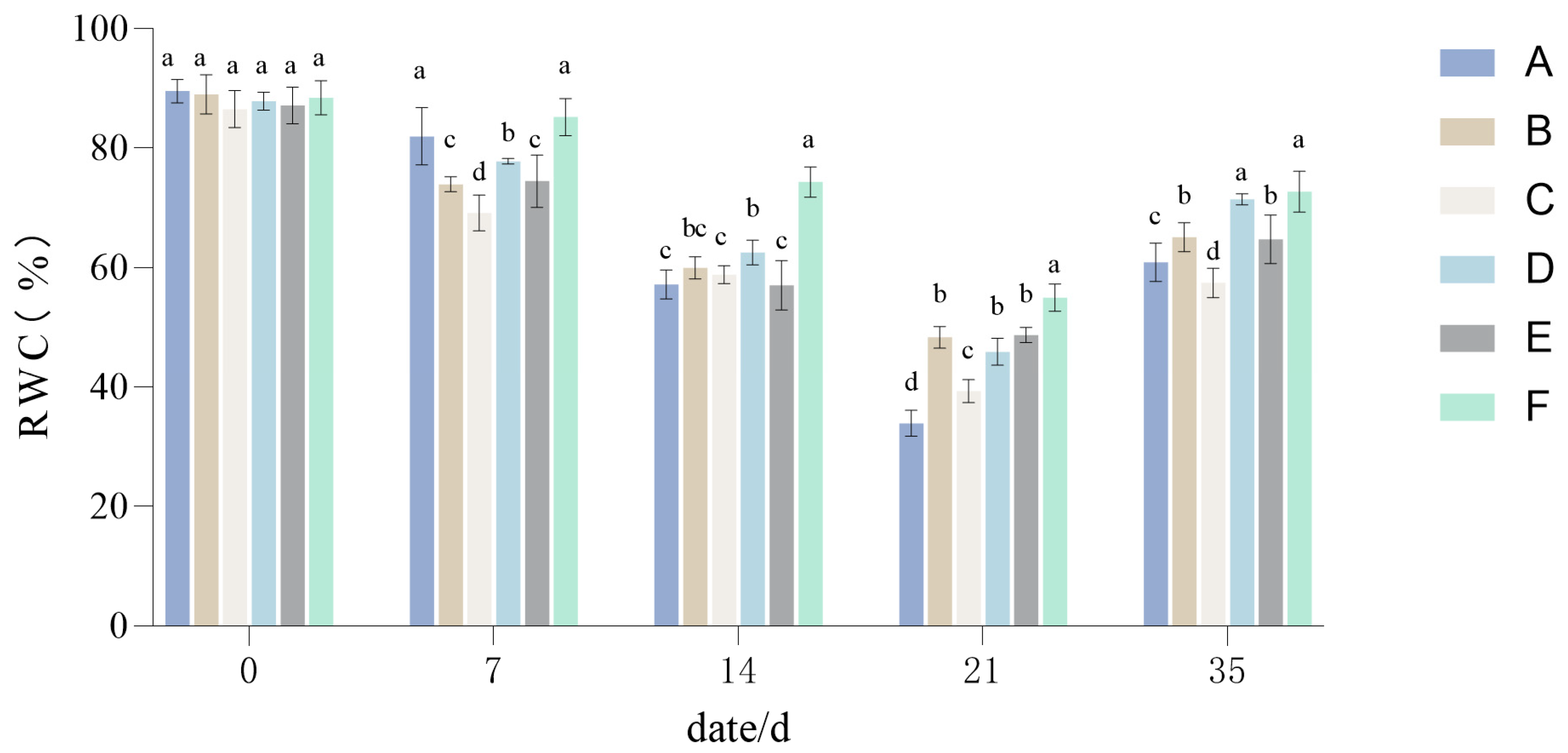
3.1.2. The Influence of Drought Stress on the RWC of Lawn Grass
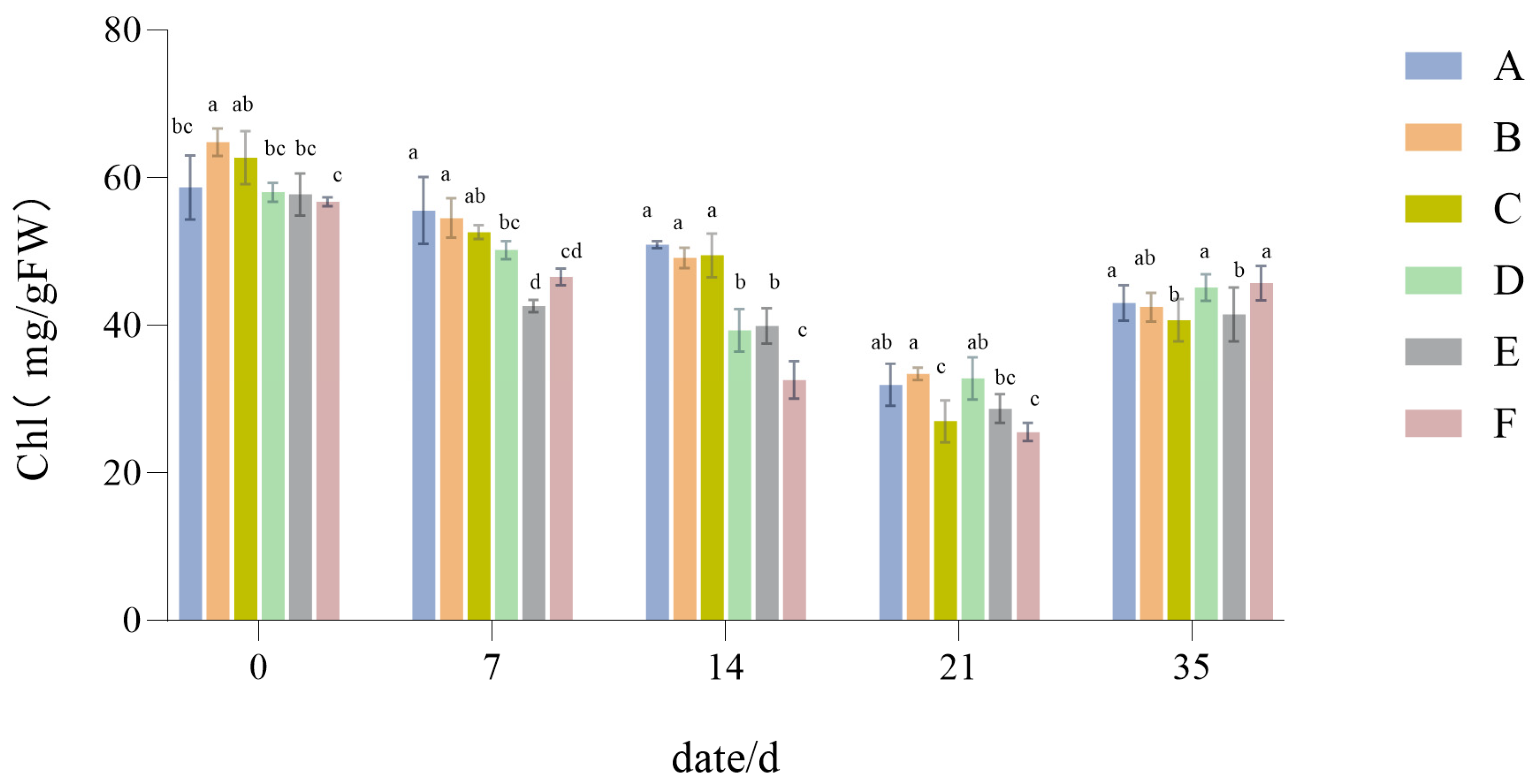
3.1.3. The Influence of Drought Stress on the Chl Content of Lawn Grass
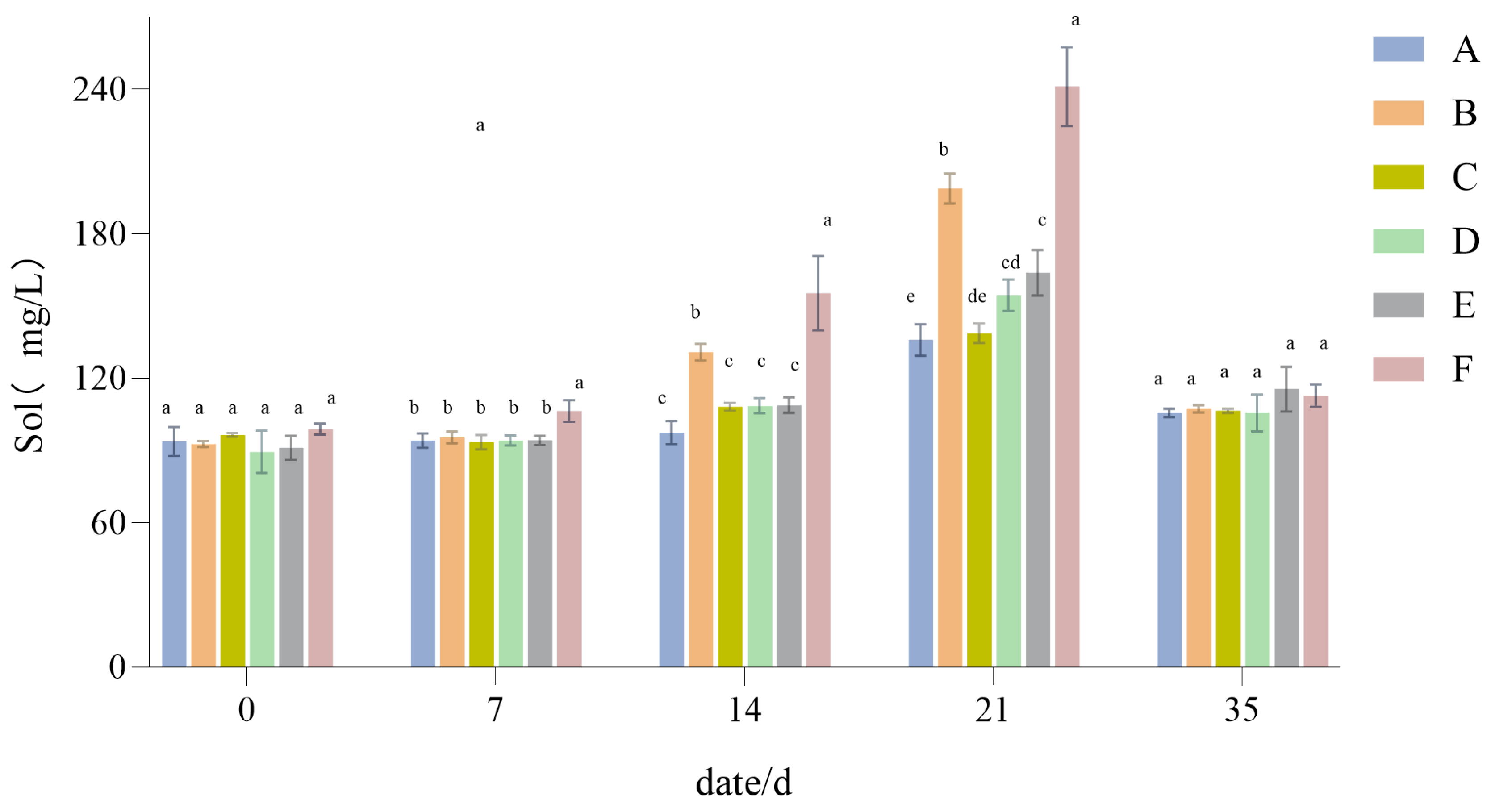
3.1.4. The Influence of Drought Stress on the Sol Content of Lawn Grass
3.1.5. The Influence of Drought Stress on the Pro Content of Lawn Grass
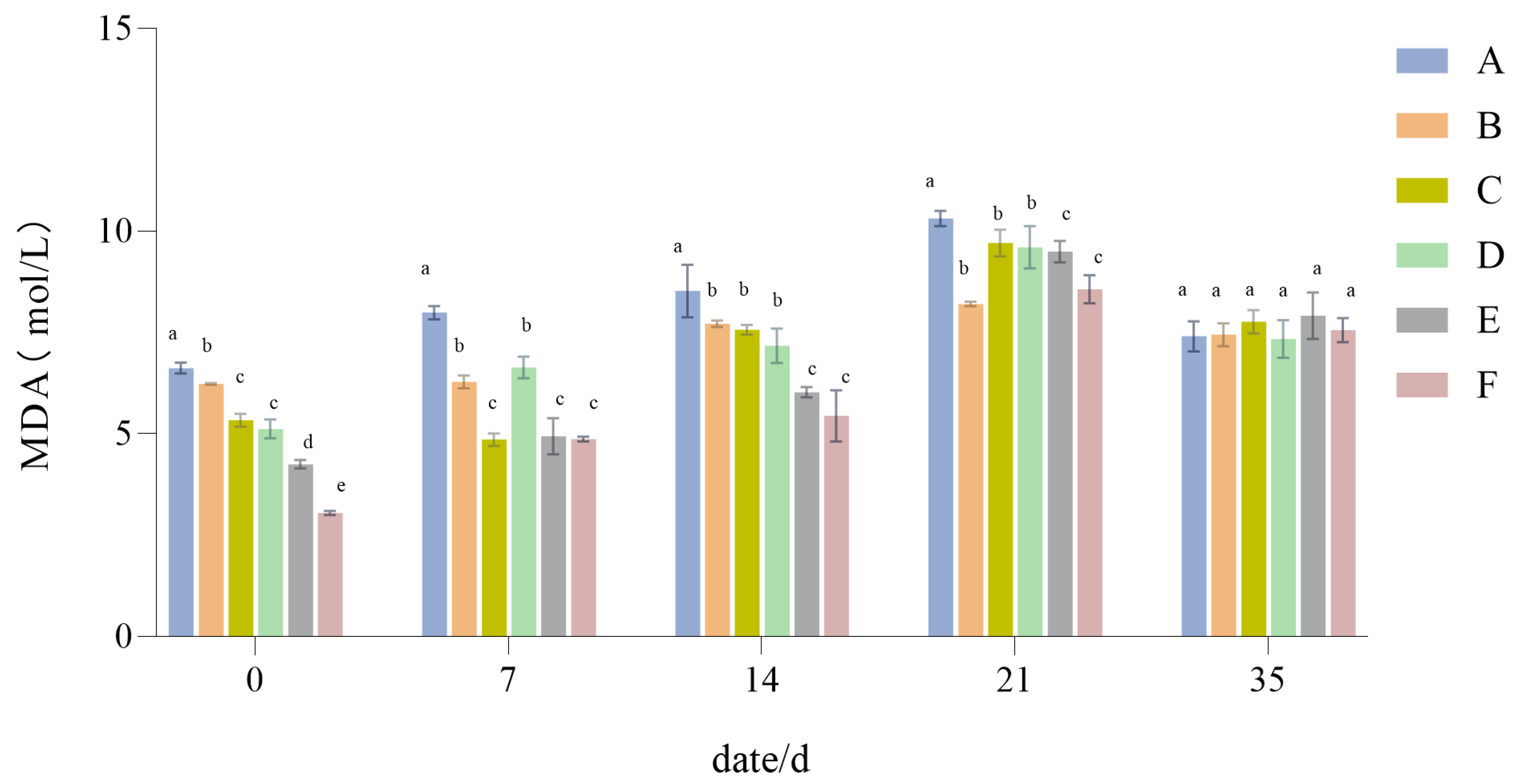
3.1.6. The Influence of Drought Stress on the MDA Content of Lawn Grass
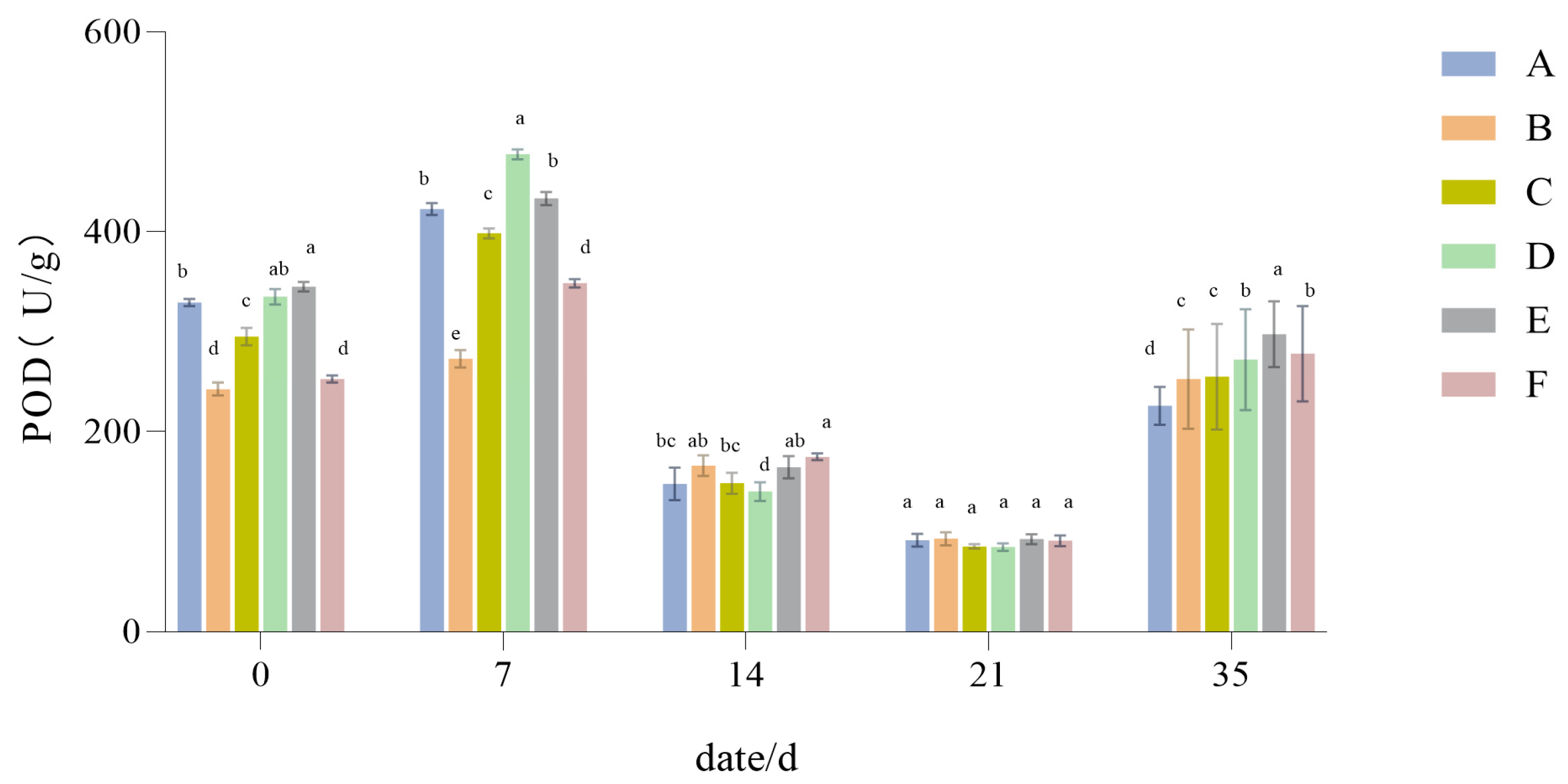
3.1.7. The Influence of Drought Stress on the POD Activity of Lawn Grass
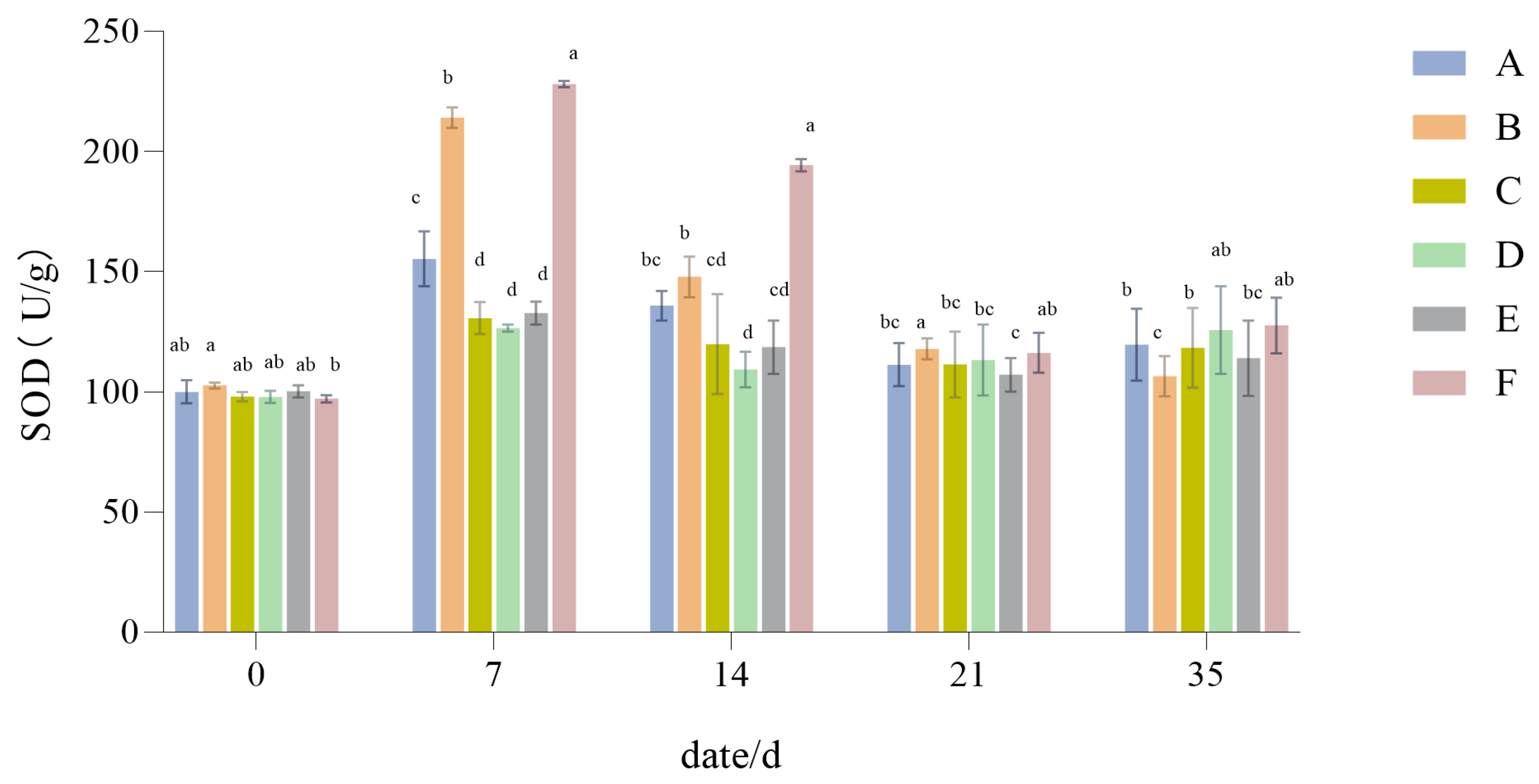
3.1.8. The Influence of Drought Stress on the SOD Activity of Lawn Grass
3.1.9. Changes in Dust Retention
3.2. Data Analysis
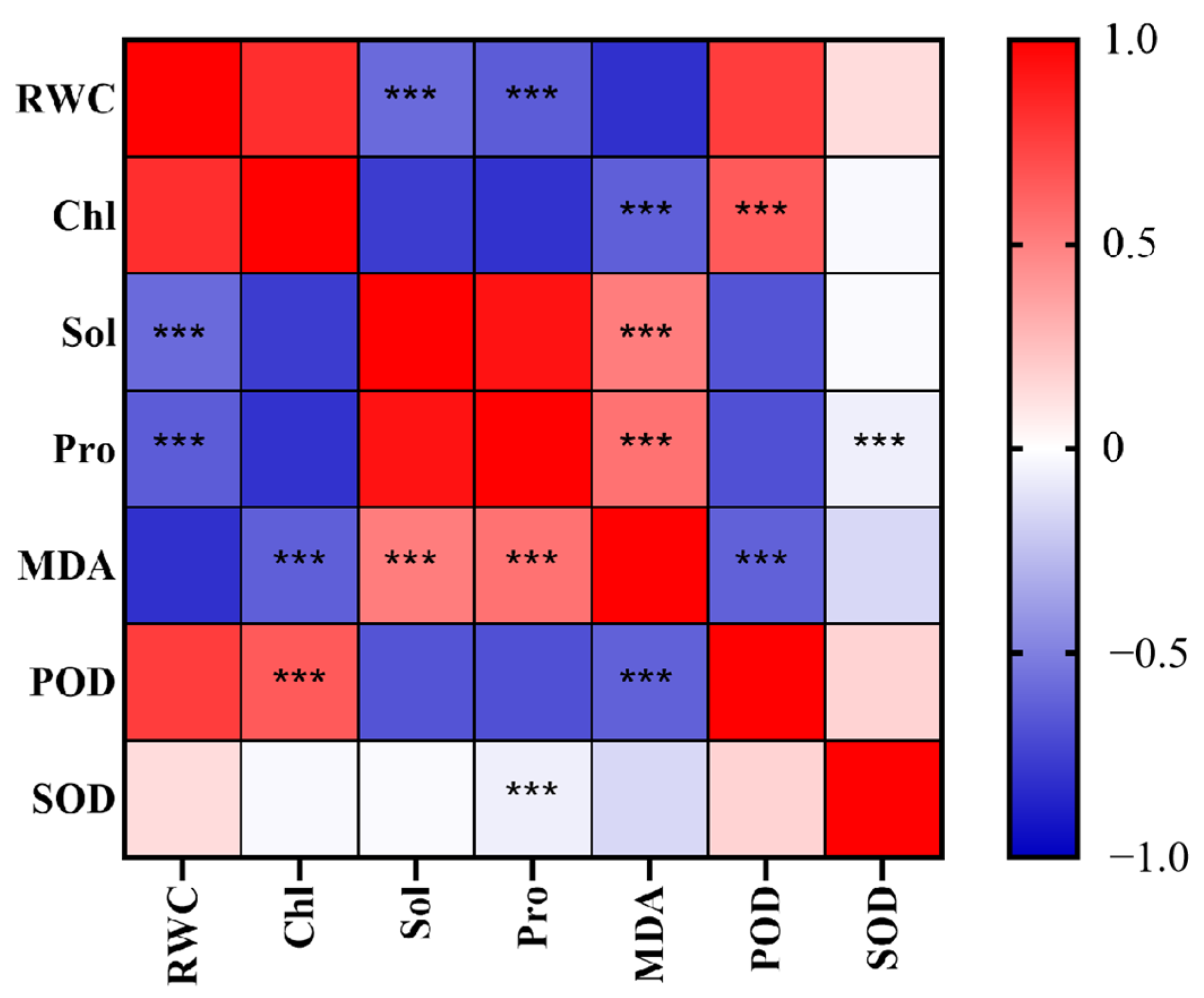
3.2.1. Correlation Analysis of the Effects of Drought Stress on Physiological Indicators of Lawn Grass
3.2.2. Membership Function Analysis of Drought Resistance of Turfgrass in Six Treatments
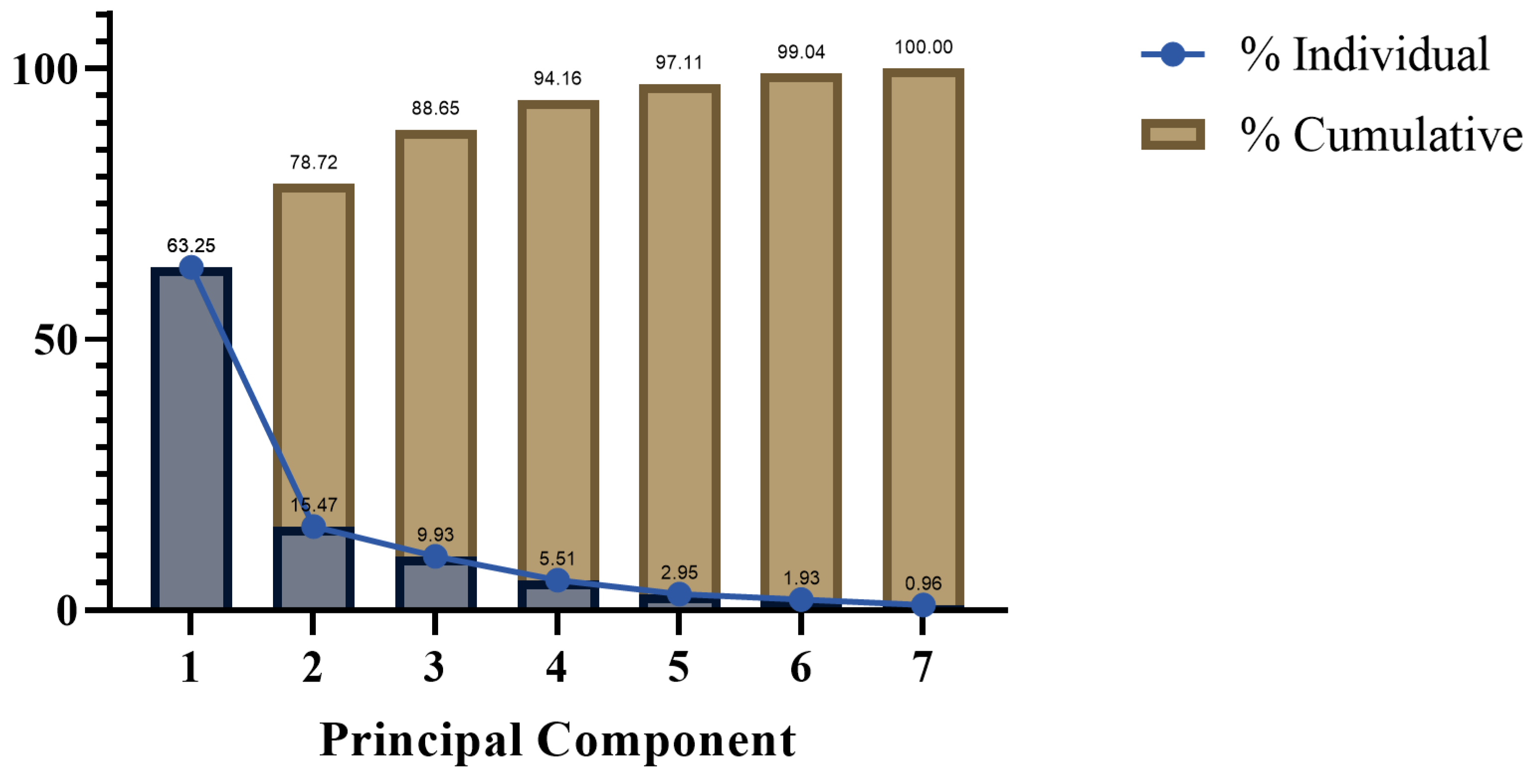
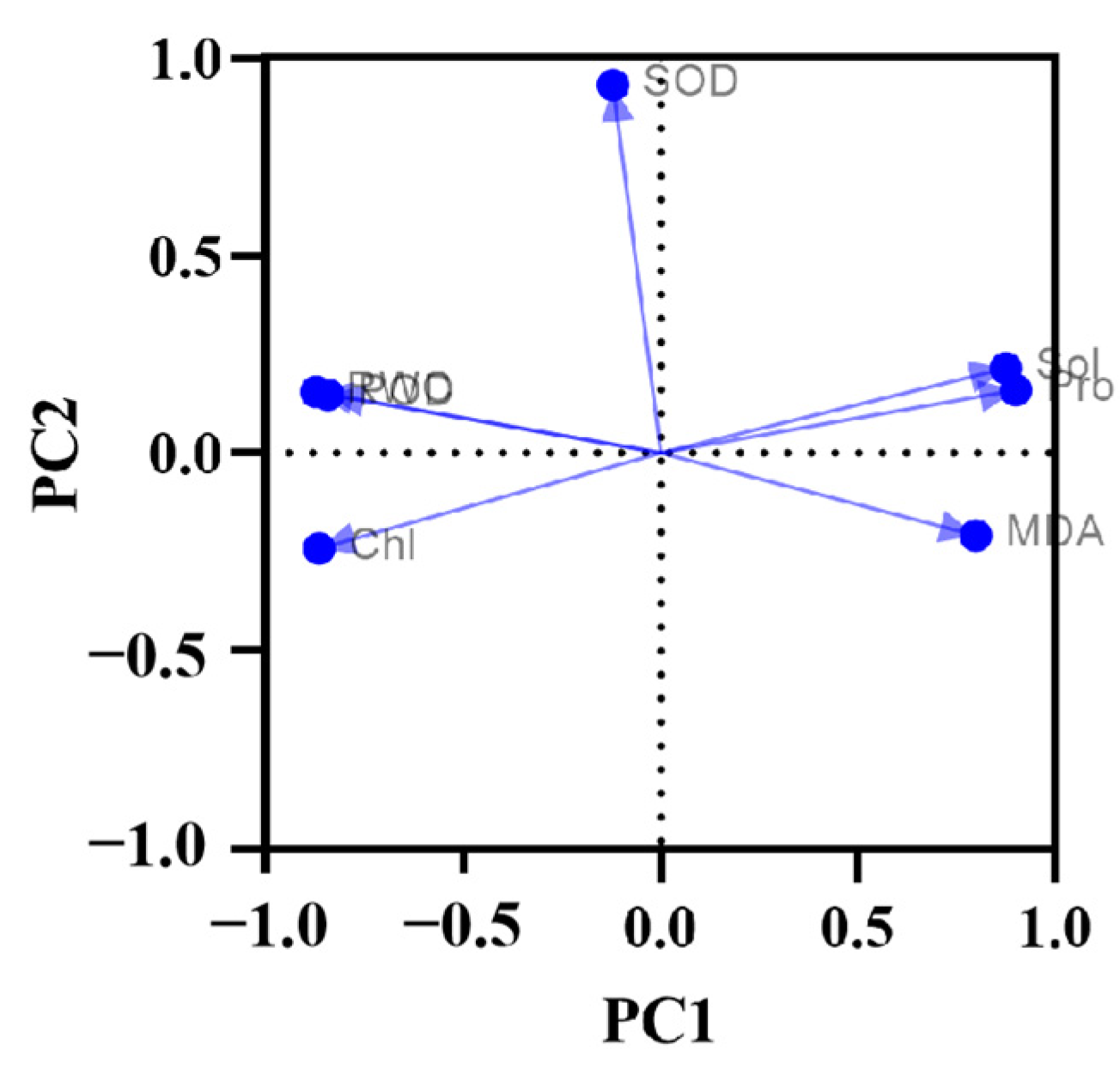
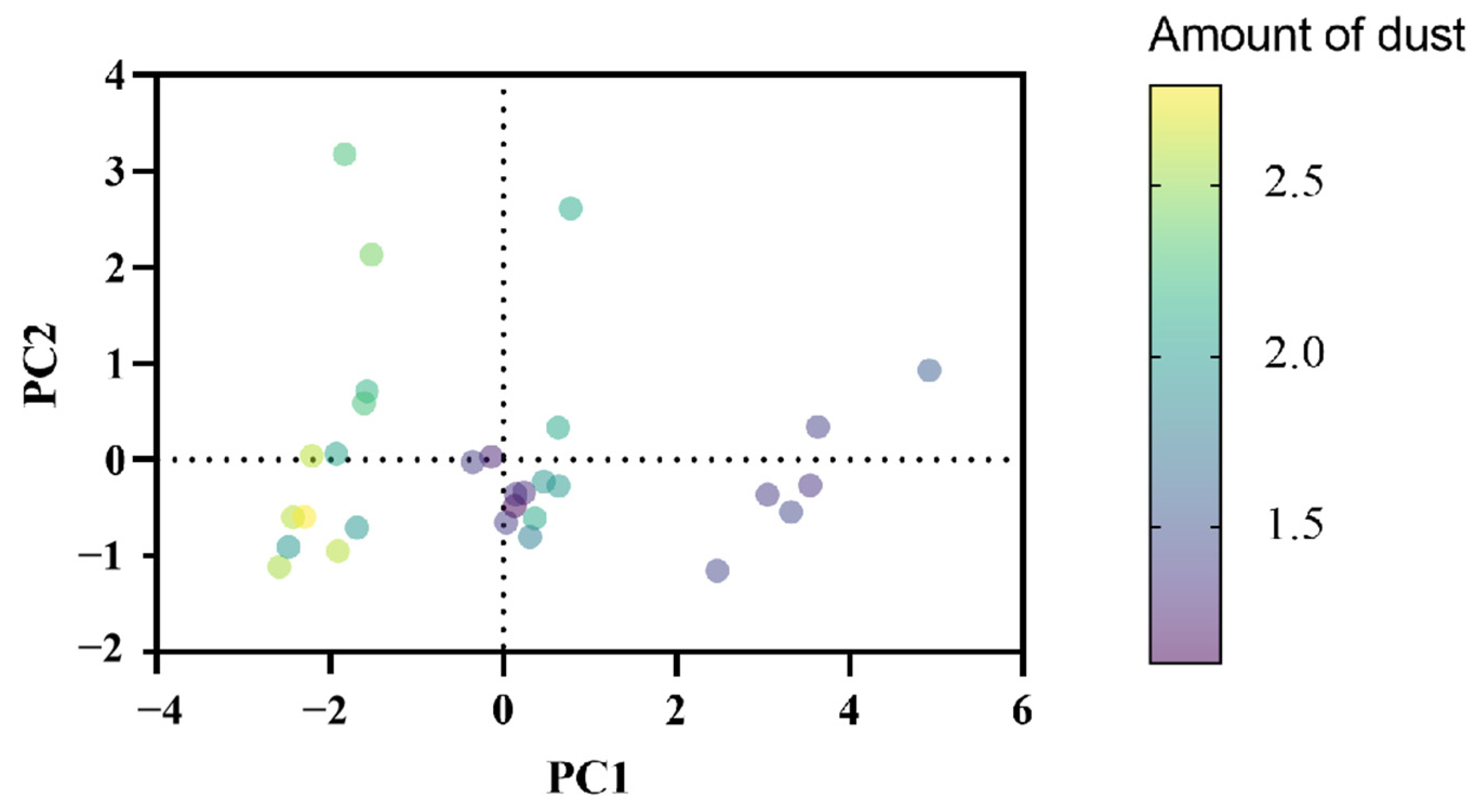
3.2.3. Principal Component Analysis (PCA) of the Effects of Drought Stress on Physiological Indicators of Lawn Grass
4. Discussion
4.1. The Responses of TQ of Six Groups of Lawn Grasses to Drought Stress
4.2. The Responses of Chlorophyll Content of Six Groups of Lawn Grasses to Drought Stress
4.3. The Responses of Osmotic Regulatory Substances and Antioxidation of Six Groups of Lawn Grasses to Drought Stress
4.4. The Responses of the Dust Retention Abilities of Six Groups of Lawn Grasses to Drought Stress
4.5. The Shortcomings of the Experiment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, J.; Wang, D.; Chang, S.; Li, Z.; Tang, X. Effects of cold plasma treatment on alfalfa seed growth under simulated drought stress. Plasma Sci. Technol. 2018, 20, 035505. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Y.; Ma, J.Y.; Wang, H.; Wang, K.L.; Wang, T.; Jiang, S.Y.; Jiao, J.B.; Sun, Y.K.; Jiang, X.L.; et al. Nitrogen Deposition Effects on Invasive and Native Plant Competition: Implications for Future Invasions. Ecotoxicol. Environ. Saf. 2023, 259, 115029. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Change 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Torre, F.D.; Ferreira, B.G.; Lima, J.E.; Lemos-Filho, J.P.; Rossiello, R.O.; França, M.G. Leaf morphophysiological changes induced by long-term drought in Jatropha curcas plants explain the resilience to extreme drought. J. Arid. Environ. 2021, 185, 104381. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and Biochemical Responses in Two Ornamental Shrubs to Drought Stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef]
- Go, T.; Kim, J.; Lee, S.J. Three-dimensional volumetric monitoring of settling particulate matters on a leaf using digital in-line holographic microscopy. J. Hazard. Mater. 2021, 404, 124116. [Google Scholar] [CrossRef]
- Popek, R.; Przybysz, A.; Gawronska, H.; Klamkowski, K.; Gawronski, S.W. Impact of particulate matter accumulation on the photosynthetic apparatus of roadside woody plants growing in the urban conditions. Ecotoxicol. Environ. Safe 2018, 163, 56–62. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Beckett, K.P.; Taylor, G. Deposition velocities to Sorbus aria, Acer campestre, Populus deltoides X trichocarpa ‘Beaupre’, Pinus nigra and X Cupressocyparis leylandii for coarse, fine and ultra-fine particles in the urban environment. Environ. Pollut. 2005, 133, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Hofman, J.; Bartholomeus, H.; Calders, K.; Van Wittenberghe, S.; Wuyts, K.; Samson, R. On the relation between tree crown morphology and particulate matter deposition on urban tree leaves: A ground-based LiDAR approach. Atmos. Environ. 2014, 99, 130–139. [Google Scholar] [CrossRef]
- Dzierzanowski, K.; Popek, R.; Gawrońska, H.; Saebø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediat. 2011, 13, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Daresta, B.E.; Italiano, F.; de Gennaro, G.; Trotta, M.; Tutino, M.; Veronico, P. Atmospheric particulate matter (PM) effect on the growth of Solanum lycopersicum cv. Roma plants. Chemosphere 2015, 119, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.T.; Odsuren, U.; Kwon, K.J.; Kim, S.Y.; Yang, J.C.; Jeong, N.R.; Park, B.J. Assessment of Air Pollution Tolerance and Particulate Matter Accumulation of 11 Woody Plant Species. Atmosphere 2021, 12, 1067. [Google Scholar] [CrossRef]
- Mariën, B.; Mariën, J.; Nguyen, X.H.; Nguyen, T.C.; Nguyen, V.S.; Samson, R. Particulate matter accumulation capacity of plants in Hanoi, Vietnam. Environ. Pollut. 2019, 253, 1079–1088. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, W.; Mo, L.; Heal, M.R.; Xu, X.; Yu, X. Quantifying particulate matter accumulated on leaves by 17 species of urban trees in Beijing, China. Environ. Sci. Pollut. Res. 2018, 25, 12545–12556. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhang, Y.Z.; Gong, J.R.; Yang, B.; Zhang, Z.H.; Wang, B.; Zhu, C.C.; Shi, J.Y.; Yue, K.X. Comparison of the suitability of plant species for greenbelt construction based on particulate matter capture capacity, air pollution tolerance index, and antioxidant system. Environ. Pollut. 2020, 263, 12. [Google Scholar] [CrossRef]
- Guerrero-Leiva, N.; Castro, S.A.; Rubio, M.A.; Ortiz-Calderon, C. Retention of Atmospheric Particulate by Three Woody Ornamental Species in Santiago, Chile. Water Air Soil Pollut. 2016, 227, 9. [Google Scholar] [CrossRef]
- Leonard, R.J.; Mcarthur, C.; Hochuli, D.F. Particulate matter deposition on roadside plants and the importance of leaf trait combinations. Urban For. Urban Green. 2016, 20, 249–253. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.H.; Sun, F.B.; Wang, Y.J.; Wu, H.T.; Hu, Z.W.; Zhang, B.B.; Yu, L.; Yan, H.; Shao, F. Capacity of Landscaping Plants to Accumulate Airborne Particulate Matter in Hangzhou China. Pol. J. Environ. Stud. 2020, 29, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Li, H.M.; Guo, X.; Sun, Y.K.; Li, S.M. Capacity of six shrub species to retain atmospheric particulates with different diameters. Environ. Sci. Pollut. Res. 2018, 25, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Germade, I.; Mohamed, K.J.; Rey, D.; Rubio, B.; Garcia, A. The influence of weather and climate on the reliability of magnetic properties of tree leaves as proxies for air pollution monitoring. Sci. Total Environ. 2014, 468–469, 892–902. [Google Scholar] [CrossRef]
- Liu, L.; Bai, W.; Li, H.; Tian, Y.; Yuan, H.; Garant, T.M.; Liu, H.; Zhang, J.; Bao, A.; Rowland, O.; et al. ZxABCG11 from the xerophyte Zygophyllum xanthoxylum enhances drought tolerance in Arabidopsis thaliana through modulating cuticular wax accumulation. Environ. Exp. Bot. 2021, 190, 104570. [Google Scholar] [CrossRef]
- Räsänen, J.; Yli-Pirilä, P.; Holopainen, T.; Joutsensaari, J.; Pasanen, P.; Kivimäenpää, M. Soil drought increases atmospheric fine particle capture efficiency of Norway spruce. Boreal. Environ. Res. 2012, 17, 21–30. [Google Scholar]
- Kong, Q.; Zhang, J.; Chen, S.; Zhang, J.; Ren, Y.; Jin, X.; Chen, J. Effects of periodic drought with severe exhaust exposure on particle retention capacity and physiological responses of Photinia × fraseri Dress. Ecotoxicol. Environ. Saf. 2022, 241, 113807. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S. Associations between winter atmospheric teleconnections in drought and haze pollution over Southwest China. Sci. Total Environ. 2021, 766, 142599. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Yang, X.; Kang, Y.; Ning, G.; Du, H. Impact of winter droughts on air pollution over Southwest China. Sci. Total Environ. 2019, 664, 724–736. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Lüscher, A. Grass―legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 2011, 140, 155–163. [Google Scholar] [CrossRef]
- Guo, H.; Bao, A.; Ndayisaba, F.; Liu, T.; Jiapaer, G.; El-Tantawi, A.M.; De Maeyer, P. Space-time characterization of drought events and their impacts on vegetation in central asia. J. Hydrol. 2018, 564, 1165–1178. [Google Scholar] [CrossRef]
- Picotte, J.J.; Rhode, J.M.; Cruzan, M.B. Leaf morphological responses to variation in water availability for plants in the Piriqueta caroliniana complex. Plant Ecol. 2009, 200, 267–275. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Huang, B. Elevated CO2-mitigation of high temperature stress associated with maintenance of positive carbon balance and carbohydrate accumulation in Kentucky bluegrass. PLoS ONE 2014, 9, e89725. [Google Scholar] [CrossRef] [PubMed]
- Gasch, C.K.; Toledo, D.; Kral-O’Brien, K.C.; Baldwin, C.; Bendel, C.R.; Fick, W.H.; Gerhard, L.; Harmon, J.P.; Hendrickson, J.R.; Hovick, T.J.; et al. Poa pratensis Invaded Rangeland: Ecosystem Implications and Adaptive Management Approaches. Rangelands 2020, 42, 106–116. [Google Scholar] [CrossRef]
- Becker, T.; Kayser, M.; Isselstein, J. Feed quality of modern varieties of Festuca arundinacea and Phleum pratense as an alternative to Lolium perenne in intensively managed grassland with different defoliation schemes. J. Agric. Sci. 2023, 161, 645–653. [Google Scholar] [CrossRef]
- Lai, J.; Han, L. Progress and Challenges in China Turfgrass Abiotic Stress Resistance Research. Front. Plant Sci. 2022, 13, 922175. [Google Scholar] [CrossRef]
- Yılmaz, M.T. The effects of different combinations of combined fertilizer doses on some turfgrass performances of turf mixture. Pak. J. Bot. 2019, 51, 1357–1364. [Google Scholar] [CrossRef]
- Qian, Y.; Fu, J. Response of creeping bentgrass to salinity and mowing management: Carbohydrate availability and ion accumulation. Hortscience 2005, 40, 2170–2174. [Google Scholar] [CrossRef]
- Räsänen, J.V.; Holopainen, T.; Joutsensaari, J.; Pasanen, P.; Kivimäenpää, M. Particle capture efficiency of different-aged needles of Norway spruce under moderate and severe drought. Can. J. For. Res. 2014, 44, 831–835. [Google Scholar] [CrossRef]
- Wang, J.; Kong, W.; Li, H.; Sun, X.; Sun, Y.; Liu, Y. Effects of meteorological factors on the retention of particulate matter in lawn grass blades. Front. Plant Sci. 2025, 16, 1495212. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Yu, W.; Liu, Y.; Huang, B. Differential metabolic responses of perennial grass Cynodon transvaalensis × Cynodon dactylon (C4) and Poa Pratensis (C3) to heat stress. Physiol. Plant. 2011, 141, 251–264. [Google Scholar] [CrossRef]
- Turgeon, A.J. Tuffgrass Management; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Hou, Y.; Su, S.; Li, Y.; Chong, P.; Ma, P.; Wei, B. Effects of exogenous abscisic acid on the content of osmotic adjustment substances and antioxidant enzyme activity in the leaves of Reaumuria soongorica. Pratacult. Sci. 2020, 37, 245–255. [Google Scholar]
- Bandara, W.A.; Dissanayake, C.T. Most tolerant roadside tree species for urban settings in humid tropics based on Air Pollution Tolerance Index. Urban Clim. 2021, 37, 100848. [Google Scholar] [CrossRef]
- Chong, P.; Li, P.; Li, Y.; Li, Z.; Su, S. Screening and analysis of drought resistance indexes for 18 families of Reaumuria soongorica. Acta Agrestia Sin. 2020, 28, 412–419. [Google Scholar]
- Guo, X.; Sun, Z.; Gao, Y.; Zhang, H.; Wang, Q.; Guo, X.; Li, M.; Liu, L.; Lu, J.; Guo, S.; et al. Haplotype-specific interactions of Phragmites australis with Spartina alterniflora under salt stress. J. Environ. Manag. 2025, 384, 125506. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Ling, H.; Wang, Z.; Gai, J. A Hyperspectral Image-Based Method for Estimating Water and Chlorophyll Contents in Maize Leaves under Drought Stress. Smart Agric. 2023, 5, 142–153. [Google Scholar]
- Subhash, N.; Mohanan, C.N.; Mallia, R.J.; Muralidharan, V. Quantification of stress adaptation by laser-induced fluorescence spectroscopy of plants exposed to engine exhaust emission and drought. Funct. Plant Biol. FPB 2004, 31, 709–719. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. PPB 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Maqsood, Y.; Abdin, Z.U.; Manzoor, A.; Hassan, M.; Jamil, A. SSR Markers Associated with Proline in Drought Tolerant Wheat Germplasm. Appl. Biochem. Biotechnol. 2016, 178, 1042–1052. [Google Scholar] [CrossRef]
- Galmés, J.; Ochogavía, J.M.; Gago, J.; Roldán, E.J.; Cifre, J.; Conesa, M.À. Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: Anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ. 2013, 36, 920–935. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fang, Z.; Liu, H.N.; Zhao, D.; Tao, J. Exogenous calcium-induced physiological and biochemical changes in tree peony (Paeonia section Moutan DC.) under drought stress. Photosynthetica 2019, 57, 904–911. [Google Scholar] [CrossRef]
- Hura, T.; Dziurka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. Different allocation of carbohydrates and phenolics in dehydrated leaves of triticale. J. Plant Physiol. 2016, 202, 1–9. [Google Scholar] [CrossRef]
- Hanslin, H.M.; Przybysz, A.; Slimestad, R.; Sæbø, A. Stress acclimation and particulate matter accumulation in Pinus sylvestris saplings affected by moderate combinations of urban stressors. Sci. Total Environ. 2017, 593–594, 581–591. [Google Scholar] [CrossRef]
- Pariyar, S.; Noga, G. Rainfall does not impair particulate matter accumulation on peri-urban field crops, but improves photosynthetic activity at UV exposure. Environ. Exp. Bot. 2018, 156, 288–297. [Google Scholar] [CrossRef]
- Luisde la Fuente, J.; Zunzunegui, M.; Barradas, M.C. Physiological Responses to Water Stress and Stress Memory in Argania Spinosa. SSRN Electron. J. 2023, 7, 100133. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.X.; Li, Y.; Liu, X. Leaf surface microstructure of Ligustrum lucidum and Viburnum odoratissimum observed by Atomic force microscopy(AFM). Acta Ecol. Sin. 2011, 31, 1471–1477. [Google Scholar]
- Lu, L.; Dongsheng, G.; Yongqin, C. Morphological structure of leaves and dust-retaining capability of common street trees in Guangzhou Municipality. Acta Ecol. Sin. 2013, 33, 2604–2614. [Google Scholar] [CrossRef]
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Particulate pollution capture by urban trees: Effect of species and windspeed. Glob. Change Biol. 2000, 6, 995–1003. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Yang, L.; Zhao, Y.; Zhang, Q.; Cheng, L.; Han, M.; Ren, Y.; Yang, L. Effects of drought-re-watering-drought on the photosynthesis physiology and secondary metabolite production of Bupleurum chinense DC. Plant Cell Rep. 2019, 38, 1181–1197. [Google Scholar] [CrossRef]
- Laio, F. A vertically extended stochastic model of soil moisture in the root zone. Water Resour. Res. 2006, 420. [Google Scholar] [CrossRef]
- Laio, F.; Tamea, S.; Ridolfi, L.; D’Odorico, P.; Rodriguez-Iturbe, I. Ecohydrology of groundwater-dependent ecosystems: 1. stochastic water table dynamics. Water Resour. Res. 2009, 45, 207–213. [Google Scholar] [CrossRef]
- Tamea, S.; Laio, F.; Ridolfi, L.; D’Odorico, P.; Rodriguez-Iturbe, I. Ecohydrology of groundwater-dependent ecosystems: 2. Stochastic soil moisture dynamics. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef]
- Jamil, S.; Abhilash, P.C.; Singh, A.; Singh, N.; Behl, H.M. Fly ash trapping and metal accumulating capacity of plants: Implication for green belt around thermal power plants. Landsc. Urban Plan. 2009, 92, 136–147. [Google Scholar] [CrossRef]
- Dall’Osto, M.; Beddows, D.C.S.; Gietl, J.K.; Olatunbosun, O.A.; Yang, X.; Harrison, R.M. Characteristics of tyre dust in polluted air: Studies by single particle mass spectrometry (ATOFMS). Atmos. Environ. 2014, 94, 224–230. [Google Scholar] [CrossRef]
- Klara, S.; Simone, M.; Maria do Carmo, P. Atmospheric Nanoparticles and Their Impacts on Public Health. In Current Topics in Public Health; Rodriguez-Morales, A.J., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 23. [Google Scholar]
- Chaudhary, I.J.; Rathore, D. Suspended particulate matter deposition and its impact on urban trees. Atmos. Pollut. Res. 2018, 9, 1072–1082. [Google Scholar] [CrossRef]
- Gallego-Hernández, A.L.; Meza-Figueroa, D.; Tanori, J.; Acosta-Elías, M.; González-Grijalva, B.; Maldonado-Escalante, J.F.; Rochín-Wong, S.; Soto-Puebla, D.; Navarro-Espinoza, S.; Ochoa-Contreras, R.; et al. Identification of inhalable rutile and polycyclic aromatic hydrocarbons (PAHs) nanoparticles in the atmospheric dust. Environ. Pollut. 2020, 260, 114006. [Google Scholar] [CrossRef]




| Turfgrass Species and Proportion | ||||
|---|---|---|---|---|
| Codename for the Mix | Lolium perenne | Festuca elata | Poa pratensis | |
| Group A | A1 | 10 | 0 | 0 |
| A2 | 10 | 0 | 0 | |
| Group B | B1 | 0 | 10 | 0 |
| B2 | 0 | 10 | 0 | |
| Group C | C1 | 0 | 0 | 10 |
| C2 | 0 | 0 | 10 | |
| Group D | D1 | 2 | 3 | 5 |
| D2 | 2 | 3 | 5 | |
| Group E | E1 | 2 | 2 | 6 |
| E2 | 2 | 2 | 6 | |
| Group F | F1 | 2 | 6 | 2 |
| F2 | 2 | 6 | 2 | |
| RWC | Chl | Sol | Pro | MDA | POD | SOD | |
|---|---|---|---|---|---|---|---|
| RWC | 1 | 0.8187 | −0.5869 | −0.6404 | −0.8155 | 0.7574 | 0.1345 |
| Chl | 0.8187 | 1 | −0.7668 | −0.8069 | −0.6310 | 0.6457 | −0.0271 |
| Sol | −0.5869 | −0.7668 | 1 | 0.9285 | 0.5095 | −0.6741 | −0.0217 |
| Pro | −0.6404 | −0.8069 | 0.9285 | 1 | 0.5509 | −0.688 | −0.0648 |
| MDA | −0.8155 | −0.6311 | 0.5095 | 0.5509 | 1 | −0.6226 | −0.1567 |
| POD | 0.7574 | 0.6457 | −0.6741 | −0.6881 | −0.6226 | 1 | 0.1716 |
| SOD | 0.1345 | −0.0271 | −0.0217 | −0.0648 | −0.1567 | 0.1716 | 1 |
| Group | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| RWC | 0.19063 | 0.33385 | 0 | 0.53069 | 0.32391 | 1 |
| Chl | 0.88625 | 1 | 0.68068 | 0.49266 | 0.08918 | 0 |
| Sol | 0 | 0.52411 | 0.08904 | 0.13611 | 0.24904 | 1 |
| Pro | 0.01415 | 0.48894 | 0 | 0.22715 | 0.56265 | 1 |
| MDA | 0 | 0.43768 | 0.49434 | 0.43838 | 0.72375 | 1 |
| POD | 0.62159 | 0 | 0.50791 | 0.92349 | 1 | 0.38569 |
| SOD | 0.25931 | 0.61044 | 0.02905 | 0 | 0.00039 | 1 |
| Average | 0.2817 | 0.485007 | 0.25729 | 0.39264 | 0.42127 | 0.76938 |
| sequence | 5 | 2 | 6 | 4 | 3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, H.; Gong, D.; Liu, X.; Liu, B.; Guo, X. Physiological Responses and the Dust Retention Ability of Different Turfgrass Mixture Ratios Under Continuous Drought. Plants 2025, 14, 1667. https://doi.org/10.3390/plants14111667
Wang J, Li H, Gong D, Liu X, Liu B, Guo X. Physiological Responses and the Dust Retention Ability of Different Turfgrass Mixture Ratios Under Continuous Drought. Plants. 2025; 14(11):1667. https://doi.org/10.3390/plants14111667
Chicago/Turabian StyleWang, Junrui, Haimei Li, Dehong Gong, Xiujun Liu, Bingqi Liu, and Xiao Guo. 2025. "Physiological Responses and the Dust Retention Ability of Different Turfgrass Mixture Ratios Under Continuous Drought" Plants 14, no. 11: 1667. https://doi.org/10.3390/plants14111667
APA StyleWang, J., Li, H., Gong, D., Liu, X., Liu, B., & Guo, X. (2025). Physiological Responses and the Dust Retention Ability of Different Turfgrass Mixture Ratios Under Continuous Drought. Plants, 14(11), 1667. https://doi.org/10.3390/plants14111667







