Engineering Oilseed Microbiome Synergy for Saline Alkaline Soil Restoration
Abstract
1. Introduction
2. Oilseed–Microbiome Synergy Under Saline–Alkali Stress
3. Rhizosphere Microbiota Drive Soil Remediation
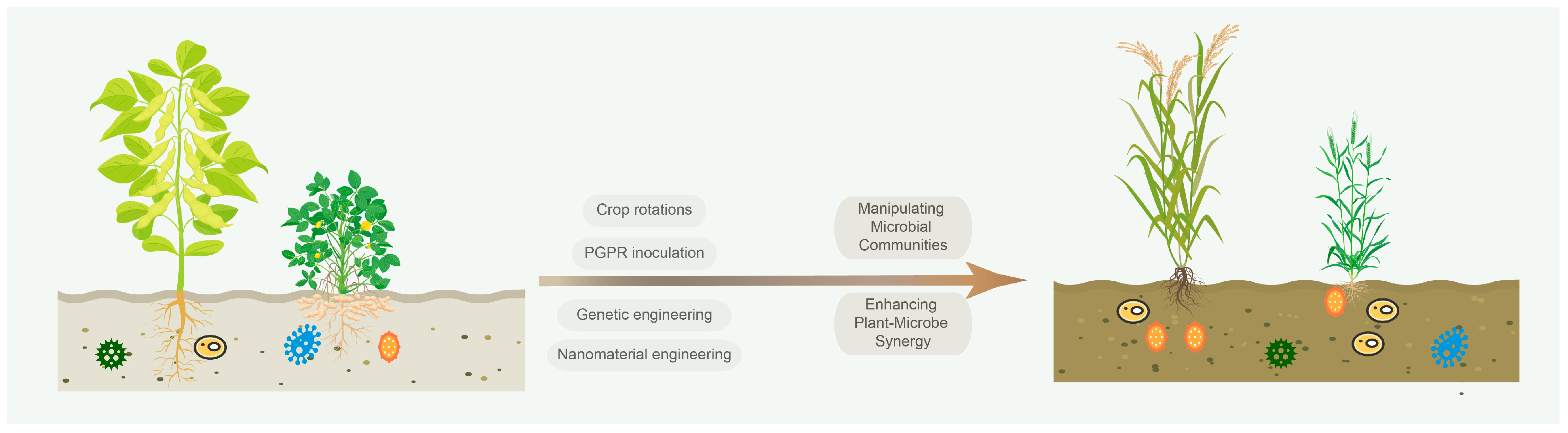
4. From Insights to Impact: Engineering Sustainable Solutions
4.1. Microbiome-Based Technologies
4.2. Nanomaterial-Enhanced Remediation
4.3. AI-Driven Precision Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-scale soil salinization dynamics from global to pore scale: A review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Bui, E.N. Causes of soil salinization, sodification, and alkalinization. In Oxford Research Encyclopedia of Environmental Science; Oxford AResearch Encyclopedias: Oxford, UK, 2017. [Google Scholar]
- Wang, J.; Li, Z.; Qin, X.; Yang, X.; Qin, Q.; Zhang, N. Research on Dynamic Evolution of Soil Salinization in Tianjin Costal Area Using Remote Sensing. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Melbourne, VIC, Australia, 21–26 July 2013; IEEE: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-Tolerant Plant Growth Promoting Rhizobacteria for Improving Productivity and Remediation of Saline Soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Cerdà, A.; Santos, F.D.; Schmidt, L. Current and Future Salinity Intrusion in the South-Western Coastal Region of Bangladesh. Span. J. Soil Sci. 2022, 12, 10017. [Google Scholar] [CrossRef]
- Tang, H.; Zhong, Z.; Hou, J.; You, L.; Zhao, Z.; Kwok, L.Y.; Bilige, M. Metagenomic Analysis Revealed the Potential of Lactic Acid Bacteria in Improving Natural Saline-Alkali Land. Int. Microbiol. 2023, 27, 311–324. [Google Scholar] [CrossRef]
- Ntanasi, T.; Karavidas, I.; Zioviris, G.; Ziogas, I.; Karaolani, M.; Fortis, D.; Conesa, M.À.; Schubert, A.; Savvas, D.; Ntatsi, G. Assessment of Growth, Yield, and Nutrient Uptake of Mediterranean Tomato Landraces in Response to Salinity Stress. Plants 2023, 12, 3551. [Google Scholar] [CrossRef] [PubMed]
- Perri, S.; Molini, A.; Hedin, L.O.; Porporato, A. Contrasting effects of aridity and seasonality on global salinization. Nat. Geosci. 2022, 15, 375–381. [Google Scholar] [CrossRef]
- Singh, C.; Kumari, G.; Lalita, L.; Gandhi, V.; Jain, A.; Madaan, S.; Saini, S.; Kumar, A.; Mahaveer; Kumar, R. Remediation of Saline Soils Using Halo-Tolerant Plant Growth Promoting Rhizobacteria. Int. J. Environ. Clim. Change 2024, 14, 24–35. [Google Scholar] [CrossRef]
- Shokri-Kuehni, S.M.S.; Raaijmakers, B.; Kurz, T.; Or, D.; Helmig, R.; Shokri, N. Water Table Depth and Soil Salinization: From Pore-Scale Processes to Field-Scale Responses. Water Resour. Res. 2020, 56, e2019WR026707. [Google Scholar] [CrossRef]
- Awad-Allah, E.F.A.; Attia, M.; Mahdy, A. Salinity Stress Alleviation by Foliar Bio-Stimulant, Proline and Potassium Nutrition Promotes Growth and Yield Quality of Garlic Plant. Open J. Soil Sci. 2020, 10, 443–458. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Sen, D.O.U.; Jun, S.; Xiangyun, S.; Rui, C.A.O.; Meng, W.U.; Chenglin, L.I.; Song, G. Are humic substances soil microbial residues or unique synthesized compounds? A perspective on their distinctiveness. Pedosphere 2020, 30, 159–167. [Google Scholar]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the restoration of polluted soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef]
- Wang, W.-N.; Ge, J.-Z.; Yang, H.-C.; Yin, F.-T.; Huang, T.-L.; Kuai, J.; Wang, J.; Wang, B.; Zhou, G.-S.; Fu, T.-D. Adaptation of Feed Crops to Saline-Alkali Soil Stress and Effect of Improving Saline-Alkali Soil; Science Press: Beijing, China, 2022; Volume 48, pp. 1451–1462. [Google Scholar]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.; Shalaby, T.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches From Leaching to Nano-Management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and arid soils: Impact on bacteria, plants, and their interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.K.; Mamet, S.D.; Helgason, B.L.; Siciliano, S.D. Brassica Napus Bacterial Assembly Processes Vary with Plant Compartment and Growth Stage but Not Between Lines. Appl. Environ. Microbiol. 2022, 88, e0027322. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Yang, X.; Dai, Z.; Yuan, R.; Guo, Z.; Xi, H.; He, Z.; Wei, M. Effects of salinity on assembly characteristics and function of microbial communities in the phyllosphere and rhizosphere of salt-tolerant Avicennia marina mangrove species. Microbiol. Spectr. 2023, 11, e03000–e03022. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Novinscak, A.; Filion, M. Inoculation with the plant-growth-promoting rhizobacterium Pseudomonas fluorescens LBUM677 impacts the rhizosphere microbiome of three oilseed crops. Front. Microbiol. 2020, 11, 569366. [Google Scholar] [CrossRef]
- Ashkanani, Z.; Mohtar, R.; Al-Enezi, S.; Smith, P.K.; Calabrese, S.; Ma, X.; Abdullah, M. AI-assisted systematic review on remediation of contaminated soils with PAHs and heavy metals. J. Hazard. Mater. 2024, 468, 133813. [Google Scholar] [CrossRef]
- Maaz, T.M.; Dobermann, A.; Lyons, S.E.; Thomson, A.M. Review of research and innovation on novel fertilizers for crop nutrition. NPJ Sustain. Agric. 2025, 3, 1–12. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yu, K.; Liu, H.; Sheng, Q.; Zhang, Y. Effects of Bacillus Subtilis on Rose Growth Promotion and Rhizosphere Microbial Community Changes Under Saline–Alkaline Stress. Agronomy 2024, 14, 730. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Wei, T.; Feng, G.; Zhao, H.; Wei, W.; Wang, M.; Zhao, Y. Oilseed Rape Cultivation Increases the Microbial Richness and Diversity in Soils Contaminated with Cadmium. J. Soils Sediments 2018, 18, 2451–2462. [Google Scholar] [CrossRef]
- Qin, Y.; Pan, X.; Kubicek, C.; Druzhinina, I.S.; Chenthamara, K.; Labbé, J.; Yuan, Z. Diverse Plant-Associated Pleosporalean Fungi from Saline Areas: Ecological Tolerance and Nitrogen-Status Dependent Effects on Plant Growth. Front. Microbiol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Picot, E.; Hale, C.C.; Hilton, S.; Teakle, G.R.; Schäfer, H.; Huang, Y.; Perryman, S.A.M.; West, J.S.; Bending, G.D. Contrasting Responses of Rhizosphere Bacterial, Fungal, Protist, and Nematode Communities to Nitrogen Fertilization and Crop Genotype in Field Grown Oilseed Rape (Brassica napus). Front. Sustain. Food Syst. 2021, 5, 613269. [Google Scholar] [CrossRef]
- Louvieaux, J.; Spanoghe, M.; Hermans, C. Root Morphological Traits of Seedlings Are Predictors of Seed Yield and Quality in Winter Oilseed Rape Hybrid Cultivars. Front. Plant Sci. 2020, 11, 568009. [Google Scholar] [CrossRef]
- Qin, T.; Ali, K.; Wang, Y.; Dormatey, R.; Yao, P.; Bi, Z.; Liu, Y.; Sun, C.; Bai, J. Root-Related Genes in Crops and Their Application Under Drought Stress Resistance—A Review. Int. J. Mol. Sci. 2022, 23, 11477. [Google Scholar] [CrossRef]
- Liu, L.; Yahaya, B.S.; Li, J.; Wu, F. Enigmatic role of auxin response factors in plant growth and stress tolerance. Front. Plant Sci. 2024, 15, 1398818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; de Zeeuw, T.; Duijts, K.; Kawa, D.; Lamers, J.; Munzert, K.S.; Li, H.; Zou, Y.; Meyer, A.J.; et al. Root branching under high salinity requires auxin-independent modulation of Lateral Organ Boundary Domain 16 function. Plant Cell 2024, 36, 899–918. [Google Scholar] [CrossRef]
- Li, Z.; An, M.; Hong, D.; Chang, D.; Wang, K.; Fan, H. Transcriptomic and Metabolomic Analyses Reveal the Differential Regulatory Mechanisms of Compound Material on the Responses of Brassica Campestris to Saline and Alkaline Stresses. Front. Plant Sci. 2022, 13, 820540. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Feng, N.; Zheng, D. Regulation of Photosynthetic Capacity and Ion Metabolism of Oilseed Rape Under Salt Stress by Prohexadione-Calcium Priming; PrePrints: Charlottesville, VA, USA, 2024. [Google Scholar] [CrossRef]
- Van Breemen, N.; Mulder, J.; Driscoll, C.T. Acidification and alkalinization of soils. Plant Soil 1983, 75, 283–308. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants Under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, M.J.; Ucros, J.M.; Wickings, K.; Wilhelm, R.C.; Sparks, J.P.; Buckley, D.H.; Bauerle, T.L. Prevalent Root-Derived Phenolics Drive Shifts in Microbial Community Composition and Prime Decomposition in Forest Soil. Soil Biol. Biochem. 2020, 145, 107797. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Hannula, S.E.; Berg, M.v.d.; Hundscheid, M.P.J.; Boer, W.d. Evaluation of Phenolic Root Exudates as Stimulants of Saptrophic Fungi in the Rhizosphere. Front. Microbiol. 2021, 12, 644046. [Google Scholar] [CrossRef]
- Khan, A.; Awan, A.A.; Yasin, M.; Ramzan, A.; Cheema, M.W.A.; Jan, A. Edible Oilseeds: Historical Perspectives, Recent Advances, and Future Directions. In Edible Oilseeds Research-Updates and Prospects; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Kefale, H.; You, J.; Zhang, Y.; Getahun, S.; Berhe, M.; Abbas, A.F.; Ojiewo, C.O.; Wang, L. Metabolomic Insights Into the Multiple Stress Responses of Metabolites in Major Oilseed Crops. Physiol. Plant. 2024, 176, e14596. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Hodson, J.N.; Williams, J.P.; Blumwald, E. Engineering salt-tolerant Brassica plants: Characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc. Natl. Acad. Sci. USA 2001, 98, 12832–12836. [Google Scholar] [CrossRef]
- He, Y.; Dong, Y.; Yang, X.; Guo, D.; Qian, X.; Yan, F.; Wang, Y.; Li, J.; Wang, Q. Functional activation of a novel R2R3-MYB protein gene, GmMYB68, confers salt-alkali resistance in soybean (Glycine max L.). Genome 2020, 63, 13–26. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Jia, X.; Liu, S.; Wang, P.; Zhu, Z.; Wan, S.; Duan, W. Transcriptome and metabolome analysis of the responses of salt resistance of different Helianthus annuus germplasms to melatonin. Front. Plant Sci. 2025, 16, 1558877. [Google Scholar] [CrossRef]
- Li, C.; Duan, Y.; Miao, H.; Ju, M.; Wei, L.; Zhang, H. Identification of candidate genes regulating the seed coat color trait in sesame (Sesamum indicum L.) using an integrated approach of QTL mapping and transcriptome analysis. Front. Genet. 2021, 12, 700469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Sun, Q.; Yan, C.; Wang, J.; Yuan, C.; Li, C.; Shan, S.; Liu, F. Comparative transcriptome analysis reveals molecular defensive mechanism of Arachis hypogaea in response to salt stress. Int. J. Genom. 2020, 2020, 6524093. [Google Scholar] [CrossRef] [PubMed]
- Gkarmiri, K.; Mahmood, S.; Ekblad, A.; Alström, S.; Högberg, N.; Finlay, R. Identifying the Active Microbiome Associated with Roots and Rhizosphere Soil of Oilseed Rape. Appl. Environ. Microbiol 2017, 83, e01938. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. J. Adv. Res. 2019, 19, 67–73. [Google Scholar] [CrossRef]
- Hossain, M.S.; Frith, C.; Bhattacharyya, S.S.; DeLaune, P.B.; Gentry, T.J. Isolation and Characterization of Bacterial Endophytes from Small Nodules of Field-Grown Peanut. Microorganisms 2023, 11, 1941. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. Bacterial community structure of the sunflower (Helianthus annuus) endosphere. Plant Signal. Behav. 2021, 16, 1974217. [Google Scholar] [CrossRef]
- Eşítken, A.; Yıldız, H.; Erċışlı, S.; Dönmez, M.F.; Turan, M.; Güneş, A. Effects of Plant Growth Promoting Bacteria (PGPB) on Yield, Growth and Nutrient Contents of Organically Grown Strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Pindi, P.K.; Sultana, T.; Vootla, P.K. Plant Growth Regulation of Bt-Cotton Through Bacillus Species. 3 Biotech 2013, 4, 305–315. [Google Scholar] [CrossRef]
- Mulissa, J.M.; Löscher, C.R.; Albert, R.; Assefa, F. Phosphate Solubilization and Multiple Plant Growth Promoting Properties of Rhizobacteria Isolated from Chickpea (Cicer Aeritinum L.) Producing Areas of Ethiopia. Afr. J. Biotechnol. 2016, 15, 1899–1912. [Google Scholar] [CrossRef]
- McInnes, A.; Haq, K. Contributions of rhizobia to soil nitrogen fertility. In Soil Biological Fertility: A Key to Sustainable Land Use in Agriculture; Springer: Berlin/Heidelberg, Germany, 2007; pp. 99–128. [Google Scholar]
- Wang, Q.; Sheng, J.; Pan, L.; Cao, H.; Li, C.; Lambers, H.; Wang, X. Soil property determines the ability of rhizobial inoculation to enhance nitrogen fixation and phosphorus acquisition in soybean. Appl. Soil Ecol. 2022, 171, 104346. [Google Scholar] [CrossRef]
- Döölotkeldieva, T.; Konurbaeva, M.; Bobusheva, S. Microbial Communities in Pesticide-Contaminated Soils in Kyrgyzstan and Bioremediation Possibilities. Environ. Sci. Pollut. Res. 2017, 25, 31848–31862. [Google Scholar] [CrossRef]
- Lacombe-Harvey, M.-È.; Brzezinski, R.; Beaulieu, C. Chitinolytic functions in actinobacteria: Ecology, enzymes, and evolution. Appl. Microbiol. Biotechnol. 2018, 102, 7219–7230. [Google Scholar] [CrossRef]
- Pezeshknejad, P.; Ghorbani Nasrabadi, R.; Etesami, H.; Khormali, F. Isolation and characterization of salt tolerant-plant growth promoting actinobacteria from the rhizosphere of crop plants. J. Soil Manag. Sustain. Prod. 2024, 14, 121–142. [Google Scholar]
- Nazari, M.T.; Schommer, V.A.; Braun, J.C.A.; dos Santos, L.F.; Lopes, S.T.; Simon, V.; Machado, B.S.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Using Streptomyces spp. as plant growth promoters and biocontrol agents. Rhizosphere 2023, 27, 100741. [Google Scholar] [CrossRef]
- Al-Quwaie, D.A. The role of Streptomyces species in controlling plant diseases: A comprehensive review. Australas. Plant Pathol. 2024, 53, 1–14. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, S.; Wang, L.; Chen, S.; Li, S.; Lei, X.; Sun, X.; Qin, L. Salt stress-induced changes in microbial community structures and metabolic processes result in increased soil cadmium availability. Sci. Total Environ. 2021, 782, 147125. [Google Scholar] [CrossRef]
- Aliyu, G.O.; Ezugworie, F.N.; Onwosi, C.O.; Nnamchi, C.I.; Ekwealor, C.C.; Igbokwe, V.C.; Sani, R.K. Multi-stress adaptive lifestyle of acidophiles enhances their robustness for biotechnological and environmental applications. Sci. Total Environ. 2024, 954, 176190. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Dai, Y.; Tian, H.; Zhou, W.; Lv, J. Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci. Total Environ. 2021, 750, 141719. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Shade, A. Chapter 3-Soil bacteria and archaea. In Soil Microbiology, Ecology and Biochemistry, 5th ed.; Paul, E.A., Frey, S.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 41–74. [Google Scholar]
- Ouni, Y.; Ghnaya, T.; Montemurro, F.; Abdelly, C.; Lakhdar, A. The role of humic substances in mitigating the harmful effects of soil salinity and improve plant productivity. Int. J. Plant Prod. 2014, 8, 353–374. [Google Scholar]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, L.V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genom. 2019, 20, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, R.; Siddiqui, Z.S. Physiological Responses of Crop Plants Against Trichoderma Harzianum in Saline Environment. Acta Bot. Croat. 2017, 76, 154–162. [Google Scholar] [CrossRef]
- Boamah, S.; Zhang, S.; Xu, B.; Li, T.; Inayat, R.; Calderón-Urrea, A. The Role of Trichoderma Species in Plants Response to Salt Stress. Asian J. Res. Crop Sci. 2021, 6, 28–43. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.-J. Penicillium–sesame interactions: A remedy for mitigating high salinity stress effects on primary and defense metabolites in plants. Environ. Exp. Bot. 2015, 116, 47–60. [Google Scholar] [CrossRef]
- Chaudhary, S.; Shankar, A.; Singh, A.; Prasad, V. Usefulness of Penicillium in enhancing plants resistance to abiotic stresses: An overview. New Future Dev. Microb. Biotechnol. Bioeng. 2018, 277–284. [Google Scholar] [CrossRef]
- Yang, G.; Liu, N.; Lu, W.; Wang, S.; Kan, H.; Zhang, Y.; Xu, L.; Chen, Y. The Interaction Between Arbuscular Mycorrhizal Fungi and Soil Phosphorus Availability Influences Plant Community Productivity and Ecosystem Stability. J. Ecol. 2014, 102, 1072–1082. [Google Scholar] [CrossRef]
- Geo, J.A. Association of Glomus Intraradices in Sorghum Bicolor. Int. J. Agric. Sci. Food Technol. 2018, 4, 3–6. [Google Scholar] [CrossRef]
- Agnihotri, R.; Sharma, M.P.; Prakash, A.; Ramesh, A.; Bhattacharjya, S.; Patra, A.K.; Manna, M.C.; Kurganova, I.; Kuzyakov, Y. Glycoproteins of arbuscular mycorrhiza for soil carbon sequestration: Review of mechanisms and controls. Sci. Total Environ. 2022, 806, 150571. [Google Scholar] [CrossRef]
- Gleason, F.H.; Midgley, D.J.; Letcher, P.M.; McGee, P.A. Can soil Chytridiomycota survive and grow in different osmotic potentials? Mycol. Res. 2006, 110, 869–875. [Google Scholar] [CrossRef]
- Chamkhi, I.; Cheto, S.; Geistlinger, J.; Zeroual, Y.; Kouisni, L.; Bargaz, A.; Ghoulam, C. Legume-based intercropping systems promote beneficial rhizobacterial community and crop yield under stressing conditions. Ind. Crops Prod. 2022, 183, 114958. [Google Scholar] [CrossRef]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective strategies for reclamation of saline-alkali soil and response mechanisms of the soil-plant system. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, Q.; Mak-Mensah, E.; Zhu, J.; Zhang, D.; Zhou, X.; Zhao, X.; Xu, Y.; Sun, Y.; Liu, Q. Effects of soil physicochemical properties on maize, wheat, and soybean yields in maize-wheat and maize-soybean intercropping systems in China: A meta-analysis. J. Soil Sci. Plant Nutr. 2024, 24, 21–29. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Q.; Zhang, K.; Sha, D.; Jiang, C.; Yang, X.; Liu, X.; Zhang, H.; Wang, X.; Guo, F. Maize-peanut rotational strip intercropping improves peanut growth and soil properties by optimizing microbial community diversity. PeerJ 2022, 10, e13777. [Google Scholar] [CrossRef]
- Nawaz, A.; Shahbaz, M.; Asadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of Salt Tolerant PGPR in Growth and Yield Augmentation of Wheat (Triticum aestivum L.) Under Saline Conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S.; Mesa-Marín, J.; Pérez-Romero, J.A.; López-Jurado, J.; García-López, J.V.; Mariscal, V.; Molina-Heredia, F.P.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Flowers, T.J.; et al. Consortia of Plant-Growth-Promoting Rhizobacteria Isolated From Halophytes Improve Response of Eight Crops to Soil Salinization and Climate Change Conditions. Agronomy 2021, 11, 1609. [Google Scholar] [CrossRef]
- Han, L. Harnessing the Power of PGPR: Unraveling the Molecular Interactions Between Beneficial Bacteria and Crop Roots. Mol. Soil Biol. 2024, 15. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic Insights of Plant Growth Promoting Bacteria Mediated Drought and Salt Stress Tolerance in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional Alterations Reveal Bacillus Amyloliquefaciens-Rice Cooperation Under Salt Stress. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, C.; Huhe, F.N.U.; Wu, B.; Gong, X.; Chuang, S.S.C.; Zheng, J. Formulating Zwitterionic, Responsive Polymers for Designing Smart Soils. Small 2022, 18, e2203899. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.W.; Huang, E.; Huang, C.C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef] [PubMed]
- Furnari, D.; Khan, N.; Delaney, M.; Hamlaoui, K.; Lagree, S.; Peace, A.; Sanchez, S.; Eyring, A.; Talyanova, E.; Milczarek, M.; et al. Assessing the Geotechnical Properties of Soils Treated with Cement and Nano-Silica Additives. JOJ Sci. 2020, 2, 1–4. [Google Scholar] [CrossRef]
- Harianja, T.S.; Amalia, D.; Mase, L.Z.; Hendry; Firuliadhim, G.; Muchtar; Pudin, A. Comparative Analysis of Laboratory Unsoaked CBR Values Between Micromaterials and Limestone Nanomaterials in Expansive Soil Stabilization. Braz. J. Dev. 2024, 10, e68636. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z.; Khan, M.; Khan, M. Engineered Nanomaterials in Soil: Their Impact on Soil Microbiome and Plant Health. Plants 2021, 11, 109. [Google Scholar] [CrossRef]
- Yang, L.; Sun, R.; Li, J.; Zhai, L.; Cui, H.; Fan, B.; Wang, H.; Liu, H. Organic-Inorganic Fertilization Built Higher Stability of Soil and Root Microbial Networks Than Exclusive Mineral or Organic. Fertilization 2021, 5, 1–14. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Zhang, Z.; Fu, H.; White, J.C.; Lynch, I. Nanomaterial Transformation in the Soil–Plant System: Implications for Food Safety and Application in Agriculture. Small 2020, 16, 2000705. [Google Scholar] [CrossRef]
- You, Y.; Kerner, P.; Shanmugam, S.; Khodakovskaya, M.V. Differential Effects of Carbon Nanotube and Graphene on the Tomato Rhizosphere Microbiome. bioRxiv 2022, 11. [Google Scholar] [CrossRef]
- Singh, J.; Vishwakarma, K.; Ramawat, N.; Rai, P.; Singh, V.K.; Mishra, R.K.; Kumar, V.; Tripathi, D.K.; Sharma, S. Nanomaterials and Microbes’ Interactions: A Contemporary Overview. 3Biotech 2019, 9, 68. [Google Scholar] [CrossRef]
- Al-Gharrawi, A.M.B.; Hayal, A.L.; Fattah, M.Y. Effect of Nano-Carbon on Geotechnics Features of Gypseous Soils. Key Eng. Mater. 2021, 895, 20–30. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Punniyakotti, P.; Vinayagam, S.; Rajamohan, R.; Priya, S.D.; Moovendhan, M.; Sundaram, T. Environmental fate and ecotoxicological behaviour of pesticides and insecticides in non-target environments: Nanotechnology-based mitigation strategies. J. Environ. Chem. Eng. 2024, 12, 113349. [Google Scholar] [CrossRef]
- Manasa, M.; Ravinder, P.; Gopalakrishnan, S.; Srinivas, V.; Sayyed, R.Z.; Enshasy, H.A.E.; Yahayu, M.; Zuan, A.T.K.; Kassem, H.S.; Hameeda, B. Co-Inoculation of Bacillus Spp. For Growth Promotion and Iron Fortification in Sorghum. Sustainability 2021, 13, 12091. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I. Fluorescent Pseudomonas -FAP2 and Bacillus Licheniformis Interact Positively in Biofilm Mode Enhancing Plant Growth and Photosynthetic Attributes. Sci. Rep. 2019, 9, 4547. [Google Scholar] [CrossRef]
- Akkem, Y.; Biswas, S.K.; Varanasi, A. Smart farming using artificial intelligence: A review. Eng. Appl. Artif. Intell. 2023, 120, 105899. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Y.; Ma, S.; Zhang, M.; Tang, T.; Du, C. Bioengineering of long-chain polyunsaturated fatty acids in oilseed crops. Prog. Lipid Res. 2025, 99, 101333. [Google Scholar] [CrossRef]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of Indole Acetic Acid Production by Isolated Bacteria From Stevia Rebaudiana Rhizosphere and Its Effects on Plant Growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
| Plant Species | Key Microorganisms | Functional Traits | Benefits | Reference |
|---|---|---|---|---|
| Brassica napus | Proteobacteria, Actinobacteria | Organic acid production, metal chelation | P solubilization, toxicity reduction | [49] |
| Glycine max | Bradyrhizobium, Bacillus | N-fixation, biocontrol | Pathogen suppression | [50] |
| Arachis hypogaea | Pseudomonas, Rhizobium | PGPR activity, symbiosis | Growth promotion, N-fixation | [51] |
| Helianthus annuus | Acidobacteria, Saccharibacteria | Osmoregulation, aggregation | Soil structure improvement | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Tang, T.; Du, C.; Yang, Z.; Gan, B. Engineering Oilseed Microbiome Synergy for Saline Alkaline Soil Restoration. Plants 2025, 14, 2197. https://doi.org/10.3390/plants14142197
Ma S, Tang T, Du C, Yang Z, Gan B. Engineering Oilseed Microbiome Synergy for Saline Alkaline Soil Restoration. Plants. 2025; 14(14):2197. https://doi.org/10.3390/plants14142197
Chicago/Turabian StyleMa, Shijie, Tong Tang, Chang Du, Zheng Yang, and Binjie Gan. 2025. "Engineering Oilseed Microbiome Synergy for Saline Alkaline Soil Restoration" Plants 14, no. 14: 2197. https://doi.org/10.3390/plants14142197
APA StyleMa, S., Tang, T., Du, C., Yang, Z., & Gan, B. (2025). Engineering Oilseed Microbiome Synergy for Saline Alkaline Soil Restoration. Plants, 14(14), 2197. https://doi.org/10.3390/plants14142197









