Allelopathy and Identification of Allelochemicals in the Leaves of Hakea decurrens subsp. physocarpa W.R. Barker
Abstract
1. Introduction
2. Results
2.1. Bioactivity Test on Lactuca sativa
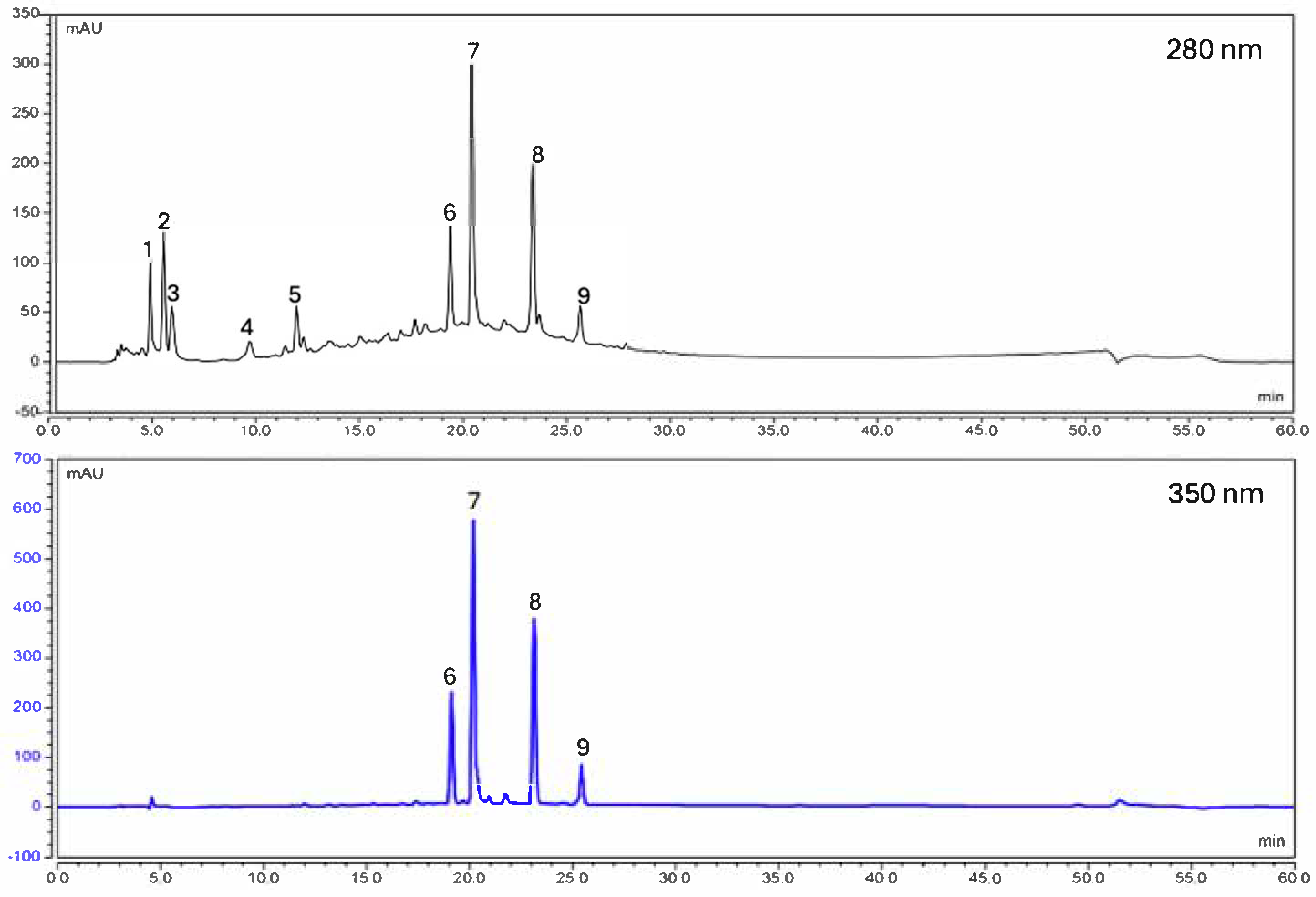
2.2. HPLC Phytochemical Analysis of the Aqueous Extract of H. decurrens subsp. physocarpa
2.3. Correlation Between the Compounds Quantified in the Aqueous Extracts of H. decurrens subsp. physocarpa and Bioactivity Parameters: Germination, Germination Rate, and Root Size
3. Discussion
4. Materials and Methods
4.1. Gathering of Materials and Sample Treatment
4.2. Preparation of the Aqueous Extracts
4.3. Bioassays
4.3.1. Bioassays on Lactuca sativa Germination
- -
- Germination: Number of germinated seeds.
- -
4.3.2. Bioassays on L. sativa Root Size
4.4. Identification and Quantification of Phenolic Compounds
4.4.1. Identification: UHPLC/Q-TOF MS Method
4.4.2. Quantification: HPLC-DAD Method
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPBES. Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and Their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Roy, H.E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B.S., Hulme, P.E., Ikeda, T., Sankaran, K.V., McGeoch, M.A., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2023. [Google Scholar] [CrossRef]
- Barker, W.R. Novelties and taxonomic notes relating to Hakea Sect. Hakea (Proteaceae), mainly of Eastern Australia. J. Adel. Bot. Gard. 1996, 17, 177–209. [Google Scholar]
- van Valkenburg, J.L.C.H.; Beyer, J.; Champion, P.; Coetzee, J.; Diadema, K.; Kritzinger-Klopper, S.; Marchante, E.; Piet, L.; Richardson, D.M.; Schönberger, I. Naturalised Hakea. What Species Are We Actually Talking about in Europe? Bot. Lett. 2024, 171, 357–370. [Google Scholar] [CrossRef]
- Gerber, D.; Azevedo, J.C.; Nereu, M.; de Oliveira, A.S.; Marchante, E.; Jacobson, T.K.B.; Silva, J.S. Hakea decurrens invasion increases fire hazard at the landscape scale. Biol. Invasions 2024, 26, 3779–3793. [Google Scholar] [CrossRef]
- Enright, N.J.; Goldblum, D. Demography of a non-sprouting and resprouting Hakea species (Proteaceae) in fire-prone Eucalyptus woodlands of southeastern Australia in relation to stand age, drought and disease. Plant Ecol. 1999, 144, 71–82. [Google Scholar] [CrossRef]
- Plumanns-Pouton, E.; Swan, M.; Penman, T.; Kelly, L.T. How do intervals between fires influence canopy seed production and viability? Funct. Ecol. 2024, 38, 1915–1930. [Google Scholar] [CrossRef]
- Shawn, A. The ecological role of fire in the northern Grampians National Park: Management of serotinous species. Appl. Ecol. 2007, 2, 121–030. [Google Scholar]
- Caldwell, E.; Read, J.; Sanson, G.D. Which leaf mechanical traits correlate with insect herbivory among feeding guilds? Ann. Bot. 2016, 117, 349–361. [Google Scholar] [CrossRef]
- Hayes, P.E.; Nge, F.J.; Cramer, M.D.; Finnegan, P.M.; Fu, P.; Hopper, S.D.; Oliveira, R.S.; Turner, B.L.; Zemunik, G.; Zhong, H. Traits related to efficient acquisition and use of phosphorus promote diversification in Proteaceae in phosphorus-impoverished landscapes. Plant Soil 2021, 462, 67–88. [Google Scholar] [CrossRef]
- Enright, N.J.; Agne, M.C. Climate drying reduces serotinous seedbanks and threatens persistence in two fire-killed shrubs. Int. J. Wildland Fire 2025, 34, WF24046. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on Allelopathy of Exotic Invasive Plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984; p. 422. [Google Scholar]
- Hierro, J.L.; Callaway, R.M. Allelopathy and Exotic Plant Invasion. Plant Soil 2003, 256, 29–39. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Bossdorf, O.; Dawson, W. The Ecology and Evolution of Alien Plants. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 25–47. [Google Scholar] [CrossRef]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy Is Pervasive in Invasive Plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel Weapons: Invasive Success and the Evolution of Increased Competitive Ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Catalán, P.; Vázquez-De-Aldana, B.R.; De Las Heras, P.; Fernández-Seral, A.; Pérez Corona, M.E. Comparing the Allelopathic Potential of Exotic and Native Plant Species on Understory Plants: Are Exotic Plants Better Armed? An. Biol. 2013, 35, 65–74. [Google Scholar] [CrossRef]
- Inderjit; Seastedt, T.R.; Callaway, R.M.; Pollock, J.L.; Kaur, J. Allelopathy and Plant Invasions: Traditional, Congeneric, and Bio-Geographical Approaches. Biol. Invasions 2008, 10, 875–890. [Google Scholar] [CrossRef]
- Thorpe, A.S.; Callaway, R.M. Biogeographic Differences in the Effects of Centaurea stoebe on the Soil Nitrogen Cycle: Novel Weapons and Soil Microbes. Biol. Invasions 2011, 13, 1435–1445. [Google Scholar] [CrossRef]
- Kim, Y.O.; Lee, E.J. Comparison of Phenolic Compounds and the Effects of Invasive and Native Species in East Asia: Support for the Novel Weapons Hypothesis. Ecol. Res. 2011, 26, 87–94. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Feng, Y.L.; Zhang, L.Q.; Callaway, R.M.; Valiente-Banuet, A.; Lei, Y.B.; Luo, D.Q.; Liao, Z.Y.; Barclay, G.F.; Silva-Pereyra, C. Integrating Novel Chemical Weapons and Evolutionarily Increased Competitive Ability in Success of a Tropical Invader. New Phytol. 2015, 205, 1350–1359. [Google Scholar] [CrossRef]
- Valares, C.; Sosa, T.; Alías, J.C.; Chaves, N. Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L. at different ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef]
- Jandová, K.; Dostál, P.; Cajthaml, T.; Kameník, Z. Intraspecific variability in allelopathy of Heracleum mantegazzianum is linked to the metabolic profile of root exudates. Ann. Bot. 2014, 115, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Escudero, J.C.; Gutierrez-Merino, C. Seasonal variation of exudate of Cistus ladanifer. J. Chem. Ecol. 1993, 19, 2577–2591. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Escudero, J.C.; Gutierrez-Merino, C. Role of ecological variables in the seasonal variation of flavonoid content of Cistus ladanifer exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C. Variation of flavonoid synthesis induced by ecological factors. In Principles and Practices in Plant Ecology. Allelochemicals Interactions; Dakshini, K.M.N., Chester, F.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 267–285. [Google Scholar]
- Pramanik, M.; Nagai, M.; Asao, T.; Matsui, Y. Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol. 2000, 26, 1953–1967. [Google Scholar] [CrossRef]
- Chaves Lobón, N.; González Félix, M.; Alías Gallego, J.C. Comparison of the Allelopathic Potential of Non-Native and Native Species of Mediterranean Ecosystems. Plants 2023, 12, 972. [Google Scholar] [CrossRef]
- Franco, D.M.; Silva, E.M.; Saldanha, L.L.; Adachi, S.A.; Schley, T.R.; Rodrigues, T.M.; Dokkedal, A.L.; Nogueira, F.T.S.; De Almeida, L.F.R. Flavonoids modify root growth and modulate expression of SHORT-ROOT and HD-ZIP III. J. Plant Physiol. 2015, 188, 89–95. [Google Scholar] [CrossRef]
- Franco, D.M.; Saldanha, L.L.; Silva, E.M.; Nogueira, F.T.S.; Dokkedal, A.L.; Almeida, L.F.R. Effects of leaf extracts of Myrcia guianensis (Aubl.) DC.: On growth and gene expression during root development of Sorghum bicolor (L.) Moench. Allelopathy J. 2015, 35, 237–248. [Google Scholar]
- Franco, D.M.; Almeida, L.F.R.; Poletto, R.S. Allelopathic potential of Equisetum giganteum L. and Nephrolepis exaltata L. on germination and growth of cucumber and lettuce. J. Plant Sci. 2014, 2, 237–241. [Google Scholar] [CrossRef]
- Inderjit; Nilsen, E. Bioassays and field studies for allelopathy in terrestrial plants: Progress and Problems. Crit. Rev. Plant Sci. 2003, 22, 221–238. [Google Scholar] [CrossRef]
- Plan Forestal de Extremadura. Análisis y Estudio del Paisaje Vegetal y su Dinámica en la Región de Extremadura. Available online: http://extremambiente.juntaex.es/index.php?option=com_content&view=article&id=3609&Itemid=307 (accessed on 18 May 2024).
- Hyder, P.W.; Fredrickson, E.L.; Estell, R.E.; Lucero, M.E. Transport of Phenolic Compounds from Leaf Surface of Creosotebush and Tarbush to soil Surface by Precipitation. J. Chem. Ecol. 2002, 28, 2475–2482. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: London, UK, 1983. [Google Scholar]
- Kobayashi, A.; Kato-Noguchi, H. The seasonal variations of allelopathic activity and allelopathic substances in Brachiaria brizantha. Bot. Stud. 2015, 56, 25. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Kato-Noguchi, H. Seasonal Variation in Allelopathic Activity of Japanese Red Pine Needles. Environ. Control Biol. 2014, 52, 249–251. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ravnjak, E.; Rusjan, D. Identification of Phenolic Compounds in the Invasive Plants Staghorn Sumac and Himalayan Balsam: Impact of Time and Solvent on the Extraction of Phenolics and Extract Evaluation on Germination Inhibition. Plants 2024, 13, 3339. [Google Scholar] [CrossRef]
- Hoang Anh, L.; Van Quan, N.; Tuan Nghia, L.; Dang Xuan, T. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Sidari, M. The effect of phenols on respiratory enzymes in seed germination respiratory enzyme activities during germination of Pinus laricio seeds treated with phenols extracted from different forest soils. Plant Growth Regul. 2001, 35, 31–35. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Sorgona, A.; Albano, S.; Cacco, G. Coumarin differentially affects the morphology of different root types of maize seedlings. J. Chem. Ecol. 2004, 30, 1871–1883. [Google Scholar] [CrossRef]
- Macias, F.A.; Castellano, D.; Molinillo, J.M.G. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.T.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.A.; Stansley, P.D.; Holmes, E. Application on NMR-based metabolomics to the investigation of salt stress in maize (Zea mays). Phytochem. Anal. 2011, 22, 214–224. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Yu, C.Y.; Ghimire, B.; Seong, E.S.; Chung, I.M. Allelopathic Potential of Phenolic Compounds in Secale Cereale Cultivars and Its Relationship with Seeding Density. Appl. Sci. 2019, 9, 3072. [Google Scholar] [CrossRef]
- Dufall, L.A.; Solomon, P.S. Role of cereal secondary metabolites involved in mediating the outcome of plant-pathogen interaction. Metabolites 2011, 1, 64–78. [Google Scholar] [CrossRef]
- Burgos, N.A.; Talbert, R.E.; Kim, K.S.; Kuk, Y.I. Growth inhibition and root ultrastructure of cucumber seedlings exposed to allelochemicals from rye (Secale cereale). J. Chem. Ecol. 2004, 30, 671–689. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, T.; Đurović-Pejčev, R.; Stevanović, M.; Sarić-Krsmanović, M.; Radivojević, L.; Šantrić, L.; Gajić-Umiljendić, J. Phytotoxicity and allelopathic potential of Juglans regia L. leaf extract. Front. Plant Sci. 2022, 13, 986740. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Al-Groshi, A.; Kumar, A.; Sarker, S.D. Arbutin: Occurrence in Plants, and Its Potential as an Anticancer Agent. Molecules 2022, 27, 8786. [Google Scholar] [CrossRef]
- Xu, K.-X.; Xue, M.-G.; Li, Z.; Ye, B.-C.; Zhang, B. Recent progress on feasible strategies for arbutin production. Front. Bioeng. Biotechnol. 2022, 10, 914280. [Google Scholar] [CrossRef]
- Yang, Z.K.; Shi, H.Y.; Chinnathambi, A.; Salmen, S.H.; Alharbi, S.A.; Veeraraghavan, V.P.; Surapaneni, K.M.; Arulselvan, P. Arbutin exerts anticancer activity against rat C6 glioma cells by inducing apoptosis and inhibiting the inflammatory markers and P13/Akt/mTOR cascade. J. Biochem. Mol. Toxicol. 2021, 35, e22857. [Google Scholar] [CrossRef]
- Erenler, R.; Sen, O.; Aksit, H.; Demirtas, I.; Yaglioglu, A.S.; Elmastas, M.; Telci, I. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016, 96, 822–836. [Google Scholar] [CrossRef]
- Zeng, X.T.; Liu, H.P.; Huang, Z.P.; Dong, P.; Chen, X. Anticancer effect of arbutin on diethylnitrosamine-induced liver carcinoma in rats via the GRP and GADD pathway. J. Environ. Pathol. Toxicol. Oncol. 2022, 41, 15–26. [Google Scholar] [CrossRef]
- Hazman, O.; Evin, H.; Bozkurt, M.F.; Cigerci, I.H. Two faces of arbutin in hepatocellular carcinoma (HepG2) cells: Anticarcinogenic effect in high concentration and protective effect against cisplatin toxicity through its antioxidant and anti-inflammatory activity in low concentration. Biologia 2022, 77, 225–239. [Google Scholar] [CrossRef]
- Kang, M.; Ha, H.W.; Kim, H.G.; Lee, D.H.; Kong, M.; Ahn, Y.T.; Kim, D.H.; Shin, B.S.; Kang, W.; Jeong, H.G. Role of metabolism by intestinal bacteria in arbutin-induced toxicity in vitro. Arch. Pharm. Res. 2011, 34, 687–693. [Google Scholar] [CrossRef]
- Safari, H.; Zabihi, E.; Pouramir, M.; Morakabati, P.; Abedian, Z.; Karkhah, A.; Nouri, H.R. Decrease of intracellular ROS by arbutin is associated with apoptosis induction and downregulation of IL-1β and TNF-α in LNCaP prostate cancer. J. Food Biochem. 2020, 44, e13360. [Google Scholar] [CrossRef]
- Castaldi, S.; Carfora, A.; Fiorentino, A.; Natale, A.; Messere, A.; Miglietta, F.; Cotrufo, M.F. Inhibition of net nitrification activity in a Mediterranean woodland: Possible role of chemicals produced by Arbutus unedo. Plant Soil 2009, 315, 273–283. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Schneider, B. Identification of Potential Allelochemicals From Donor Plants and Their Synergistic Effects on the Metabolome of Aegilops geniculata. Front. Plant Sci. 2020, 11, 1046. [Google Scholar] [CrossRef] [PubMed]
- Weidenhamer, J.D.; Romeo, J.T. Allelochemicals of Polygonella myriophylla: Chemistry and soil degradation. J. Chem. Ecol. 2004, 30, 1067–1082. [Google Scholar] [CrossRef]
- Manners, G.D.; Galitz, D.S. Allelopathy of small everlasting (Antennaria microphylla): Identification of constituents phytotoxic to leafy spurge (Euphorbia esula). Weed Sci. 1985, 34, 8–12. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Mushtaq, W. Biological Control of Weeds by Allelopathic Compounds from Different Plants: A Bioherbicide Approach. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 107–117. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Ding, L.; Kong, C.-H. Allelopathy and Allelochemicals in Grasslands and Forests. Forests 2023, 14, 562. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Janusauskaite, D. The Allelopathic Activity of Aqueous Extracts of Helianthus annuus L., Grown in Boreal Conditions, on Germination, Development, and Physiological Indices of Pisum sativum L. Plants 2023, 12, 1920. [Google Scholar] [CrossRef]
- Adhikari, B.; Olorunwa, O.J.; Barickman, T.C. Seed Priming Enhances Seed Germination and Morphological Traits of Lactuca sativa L. under Salt Stress. Seeds 2022, 1, 74–86. [Google Scholar] [CrossRef]
- Macías, F.A.; Simonet, A.M.; Pacheco, P.C.; Barrero, A.F.; Cabrera, E.; Jiménez-González, D. Natural and Synthetic Podolactones with Potential Use as Natural Herbicide Models. J. Agric. Food Chem. 2000, 48, 3003–3007. [Google Scholar] [CrossRef]
- Qasim, M.; Fujii, Y.; Zaheer, M.; Irfan Aziz, A.; Watanabe, K.N.; Ajmal Khan, M. Phytotoxic Analysis of Coastal Medicinal Plants and Quantification of Phenolic Compounds Using HPLC. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2019, 153, 767–774. [Google Scholar] [CrossRef]
- Pece, M.G.; De Benítez, C.G.; Acosta, M.; Bruno, C.; Saavedra, S.; Buvenas, O. Germinación de Tipuana tipu (Benth.) O. Kuntze (tipa blanca) en condiciones de laboratorio. Quebracho 2010, 18, 5–15. [Google Scholar]
- Nakagawa. Teste de Vigor Baseados no Desempenho das Plântulas. In Vigor de Sementes: Conceitos e Testes; Abrates: Londrinas, Brasil, 1999. [Google Scholar]
- Brenton, A.G.; Godfrey, A.R. Accurate mass measurement: Terminology and treatment of data. J. Am. Soc. Mass Spectrom. 2010, 21, 1821–1835. [Google Scholar] [CrossRef]

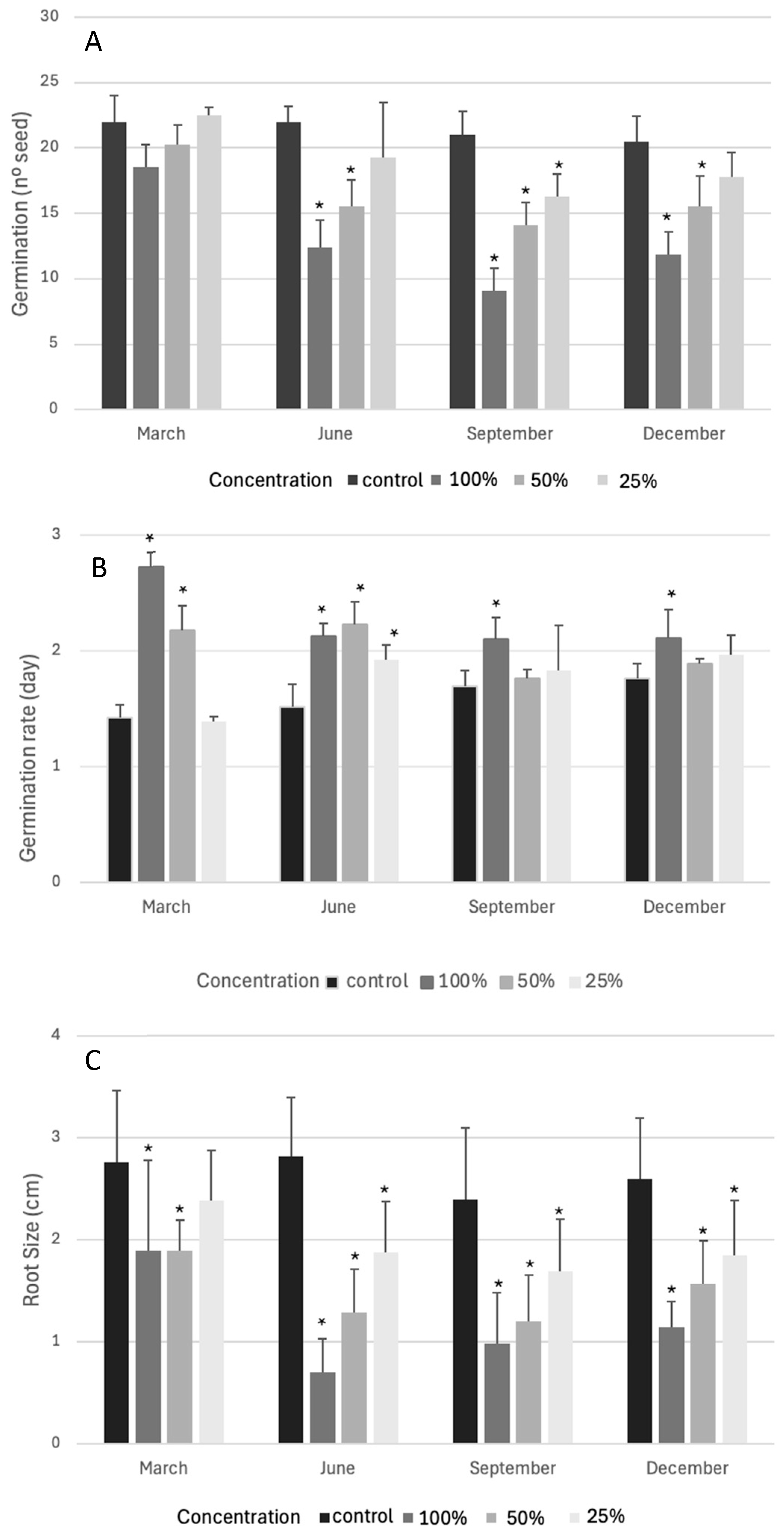
| I50 (g/mL) | ||
|---|---|---|
| Germination | Root Size | |
| March | 0.33 | 0.13 |
| June | 0.1 | 0.05 |
| September | 0.08 | 0.07 |
| December | 0.1 | 0.08 |
| Compounds | Molecular Formula | Rt HPLC-DAD (min) | Measured [M-H]- | Exact Mass (calc.) | Ppm Value | Peak Area % |
|---|---|---|---|---|---|---|
| Arbutin | C12H16O7 | 4.5 | 271.0821 | 271.0823 | 0.73 | 8.4 |
| Mesaconic acid | C5H6O4 | 5.2 | 129.2000 | 129.1930 | −5.14 | 9.1 |
| Isotachioside | C13H18O8 | 5.6 | 301.0936 | 301.0929 | −2.35 | 5.7 |
| 1-O-vanilloyl-beta-D-glucose | C14H18O9 | 9.4 | 329.0883 | 329.0878 | −1.5 | 3.2 |
| Syringic acid-4-beta-D-glucopyranoside | C15H20O10 | 11.7 | 359.0984 | 359.0984 | −0.8 | 6.7 |
| Quercetin 3-robinobioside-7-glucoside | C33H40O21 | 19.1 | 771.2015 | 771.1989 | −3.33 | 11.9 |
| Quercetin 3-rhamninoside | C33H40O20 | 20.2 | 755.2049 | 755.204 | −1.17 | 29.3 |
| Rutin | C27H30O26 | 23.1 | 609.1467 | 609.1461 | −0.97 | 20.2 |
| Isorhamnetin-3-O-rutinoside | C28H32O16 | 25.4 | 623.1626 | 623.1618 | −1.35 | 5.4 |
| Compounds (mg/gDW) | March | June | September | December |
|---|---|---|---|---|
| Arbutin | 0.27 ± 0.01 | 0.22 ± 0.015 | 0.27 ± 0.001 | 0.25 ± 0.016 |
| Mesaconic acid | 0.29 ± 0.009 | 0.23 ± 0.012 | 0.23 ± 0.009 | 0.35 ± 0.011 |
| Isotachioside | 0.16 ± 0.0002 | 0.14 ± 0.003 | 0.17 ± 0.005 | 0.23 ± 0.009 |
| 1-O-vanilloyl-beta-D-glucose | 0.10 ± 0.001 | 0.08 ± 0.0003 | 0.11 ± 0.004 | 0.09 ± 0.0002 |
| Syringic acid-4-beta-D-glucopyranoside | 0.18 ± 0.009 | 0.19 ± 0.0001 | 0.28 ± 0.008 | 0.17 ± 0.006 |
| Quercetin 3-robinobioside-7-glucoside | 0.37 ± 0.013 | 0.21 ± 0.0003 | 0.67 ± 0.01 | 0.90 ± 0.015 |
| Quercetin 3-rhamninoside | 1.05 ± 0.01 | 0.49 ± 0.002 | 1.62 ± 0.003 | 2.14 ± 0.016 |
| Rutin | 0.90 ± 0.009 | 0.51 ± 0.0001 | 0.74 ± 0.017 | 1.50 ± 0.005 |
| Isorhamnetin-3-O-rutinoside | 0.22 ± 0.002 | 0.15 ± 0.0006 | 0.24 ± 0.01 | 0.37 ± 0.014 |
| Total compounds | 3.54 | 2.20 | 4.32 | 5.99 |
| Germination | Germination Rate | Root Size | ||||
|---|---|---|---|---|---|---|
| Pearson Correlation | Sig | Pearson Correlation | Sig | Pearson Correlation | Sig | |
| Quercetin 3-robinobioside-7-glucoside | −0.644 * | 0.024 | 0.245 | 0.443 | −0.370 | 0.236 |
| Quercetin 3-rhamninoside | −0.613 * | 0.034 | 0.291 | 0.358 | −0.335 | 0.287 |
| Rutin | −0.445 | 0.147 | 0.440 | 0.152 | −0.321 | 0.309 |
| Isorhamnetin-3-O-rutinoside | −0.583 * | 0.047 | 0.443 | 0.149 | −0.427 | 0.166 |
| Arbutin | −0.642 * | 0.024 | 0.635 * | 0.027 | −0.578 * | 0.049 |
| Mesaconic acid | −0.522 | 0.081 | 0.601 * | 0.039 | −0.493 | 0.103 |
| Isotachioside | −0.645 * | 0.024 | 0.446 | 0.146 | −0.523 | 0.081 |
| 1-O-vanilloyl-beta-D-glucose | −0.612 * | 0.035 | 0.597 * | 0.041 | −0.530 | 0.076 |
| Syringic acid-4-beta-D-glucopyranoside | −0.795 ** | 0.002 | 0.485 | 0.110 | −0.689 * | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogales, L.; Alías, J.C.; Blanco-Salas, J.; Montero-Fernández, I.; Chaves, N. Allelopathy and Identification of Allelochemicals in the Leaves of Hakea decurrens subsp. physocarpa W.R. Barker. Plants 2025, 14, 1646. https://doi.org/10.3390/plants14111646
Nogales L, Alías JC, Blanco-Salas J, Montero-Fernández I, Chaves N. Allelopathy and Identification of Allelochemicals in the Leaves of Hakea decurrens subsp. physocarpa W.R. Barker. Plants. 2025; 14(11):1646. https://doi.org/10.3390/plants14111646
Chicago/Turabian StyleNogales, Laura, Juan Carlos Alías, José Blanco-Salas, Ismael Montero-Fernández, and Natividad Chaves. 2025. "Allelopathy and Identification of Allelochemicals in the Leaves of Hakea decurrens subsp. physocarpa W.R. Barker" Plants 14, no. 11: 1646. https://doi.org/10.3390/plants14111646
APA StyleNogales, L., Alías, J. C., Blanco-Salas, J., Montero-Fernández, I., & Chaves, N. (2025). Allelopathy and Identification of Allelochemicals in the Leaves of Hakea decurrens subsp. physocarpa W.R. Barker. Plants, 14(11), 1646. https://doi.org/10.3390/plants14111646








