The analyses carried out enabled the investigation of variations in the content of amino acids, caffeine, organic acids, phenolic compounds, and other miscellaneous molecules in matcha tea samples. Specifically, three different matcha tea samples were analyzed, belonging to three different quality categories, ranging from ceremonial grade to food grade.
The study was based on the combination of two complementary analytical techniques: HPTLC and NMR spectroscopy. The combination of these methods enabled a comprehensive assessment of the chemical composition, leveraging the sensitivity of HPTLC and the detailed structural insights provided by NMR. This dual approach ensures robust and accurate profiling of the bioactive compounds, offering valuable insights into the quality and functionality of matcha tea across different grades. The combined use of these two techniques has been reported for the analysis of a few specific compounds in C. sinensis leaves [
17], but to the best of our knowledge, it has never been applied to matcha tea or to such a large number of metabolites. HPTLC is an extensively used chromatographic technique in food analysis, which has been specifically applied in the study of tea [
29,
30]. It enables the simultaneous analysis of extracts and standards, verifying the presence of the latter within the sample. The CAMAG TLC Scanner 4 measures the absorbance and fluorescence of compounds, also providing reliable quantitative data through densitometric peak profiles for individual tracks. The instrument operates within a spectral range of 190–900 nm and records the UV-VIS spectra of the detected peaks, allowing for a comprehensive evaluation of the data [
31]. Unlike HPTLC, nuclear magnetic resonance (NMR) is a spectroscopic method based on the magnetic properties of the nuclei of certain atoms and isotopes and, therefore, does not require a direct comparison with standards. The identification of different substances within an extract is based on the interpretation of information related to the resonance frequency of active atoms’ nuclei.
The HPTLC analysis made it possible to verify the presence of various metabolites within the samples and to evaluate how their individual concentration varied from G1 to FG.
2.1. Amino Acids Analysis
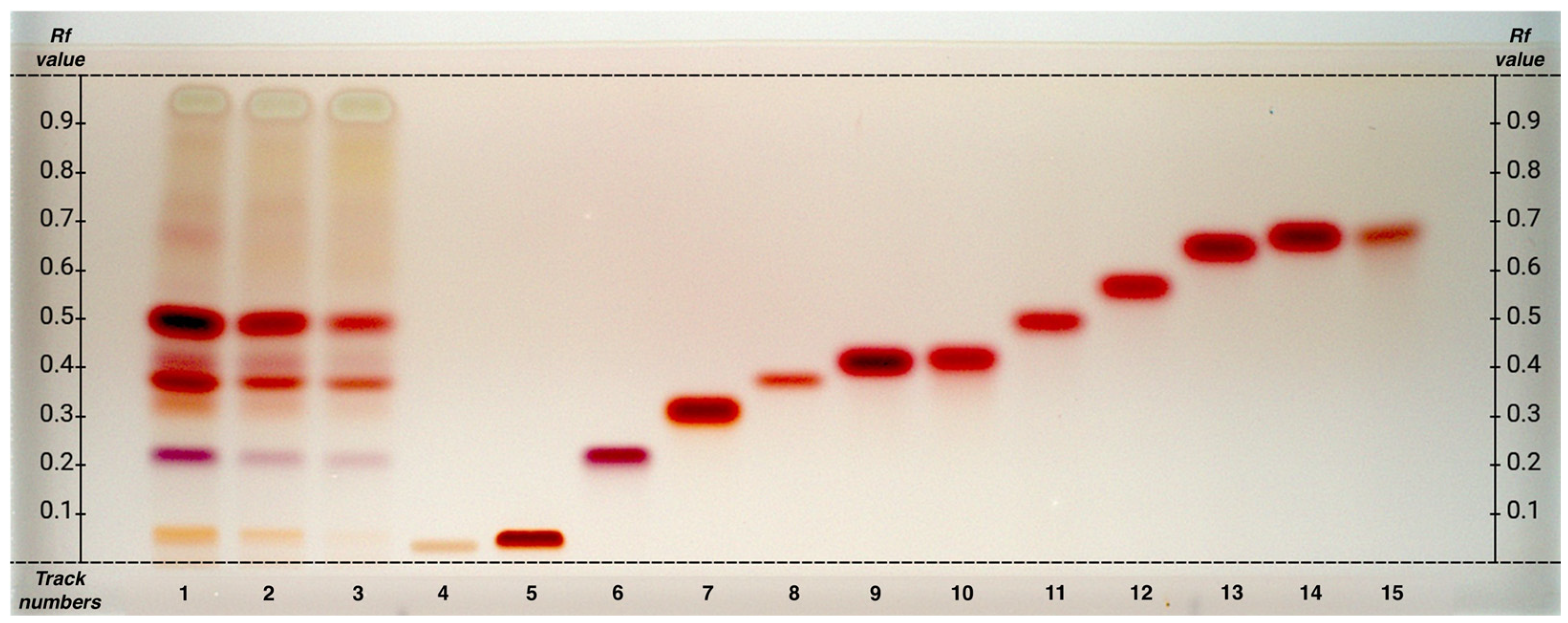
The HPTLC analysis from the hydroalcoholic phase of matcha tea grades G1, G4, and FG (
Figure 1) examined a wide range of amino acids, including not only theanine but also several proteinogenic amino acids identified in previous studies [
5,
32,
33]. Aromatic amino acids were also analyzed since, given the abundance of molecules containing aromatic moieties, their quantification through NMR is hindered by resonance superimposition. The amino acids analyzed include alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, theanine, threonine, tryptophan, tyrosine, and valine.
Among the essential amino acids, lysine was clearly identified, whereas isoleucine and leucine could not be reliably distinguished. Using the mobile phase 1-butanol/acetone/acetic acid/water (7:7:2:4 v/v), these two amino acids show very similar Rf values (0.68 and 0.69), as does tyrosine (Rf 0.69), making it difficult to determine whether they are present individually or as a mixture. In the samples, a broad spot is observed in this Rf range. A similar pattern was also observed for alanine and threonine, which show closely spaced Rf values (0.42 and 0.43).
Preliminary analyses using an alternative mobile phase (1-butanol/acetic acid/formic acid/water, 28:9:8:2 v/v) excluded the presence of tyrosine but did not improve the distinction between leucine, isoleucine, and other amino acids.
Therefore, the mobile phase 1-butanol/acetone/acetic acid/water (7:7:2:4 v/v) is considered the most effective for identifying the largest number of amino acids, although it presents some limitations in separating compounds with very similar Rf values.
This analysis, in addition to enabling the identification of amino acids, allowed us to observe a common decreasing trend of concentration from G1 to FG of all detected amino acids. In fact, G1 appears to be particularly rich in amino acids, with theanine being the most abundant compound. This decreasing trend maintains the concentration ratios of the different substances, indicating that the reduction occurs proportionally across the different tea samples.
Preliminary analyses have excluded the presence of asparagine, cysteine, glycine, histidine, methionine, phenylalanine, proline, tryptophan, and valine.
The amino acid contents evaluated by densitometric scanning analysis of HPTLC plates are compared in
Table 1 while the quantification by NMR spectroscopy is reported in
Table 2.
NMR analysis allowed the identification of the following amino acids: alanine, dimethylglycine, glutamine, isoleucine, leucine, lysine, theanine, threonine, and valine.
Dimethylglycine (DMG) is a naturally occurring derivative of glycine, characterized by the presence of two methyl groups attached to the nitrogen atom. As an
N,
N-dimethylated amino acid, it plays a role as an intermediate in the metabolic conversion of choline to glycine betaine, a pathway often linked to cellular stress responses. In plants, DMG is typically associated with osmoprotective functions and methylation cycles. Although not widely studied, its presence has been reported in several species [
34,
35], including
C. sinensis [
36], where it may contribute to adaptive responses and secondary metabolism. Univariate analysis showed significant differences among the amino acids between G1 and G4 versus FG, with generally higher levels observed in G1 and G4, in general agreement with HPTLC data. In particular, higher amounts of glutamine, isoleucine, leucine, and lysine were reported in G1.
The HPTLC analysis of amino acids in the hydroalcoholic extracts of matcha tea G1, G4, and FG allowed the definitive identification of theanine, along with proteinogenic amino acids: arginine, aspartic acid, glutamine, glutamic acid, and serine. Additionally, several essential amino acids were identified, including lysine, isoleucine, and leucine.
Aromatic amino acids were also tested but were not identified in the samples. This test was performed because, in the corresponding spectral region of NMR experiments, the most abundant resonances belonged to the phenols and polyphenols, thus making a necessity to employ a complementary targeted approach.
As previously noted, theanine—a γ-amino acid—was the most abundant amino acid, especially in sample G1. Known for its health benefits, such as promoting relaxation, enhancing learning ability, and boosting the immune system [
22], theanine played a key role in distinguishing tea quality. The analysis revealed a clear trend: amino acid concentrations decreased progressively from G1 to FG grade, with G1 standing out for its richness in amino acids, particularly theanine. This pattern aligns with existing literature [
5].
The most plausible explanation lies in leaf aging. The observed general decrease in amino acid content from younger to older leaves can be attributed to the higher metabolic activity and protein synthesis in young tissues, combined with the remobilization of nitrogenous compounds from senescing leaves. This dynamic reflects the plant’s strategy to optimize nitrogen use and support growth and development in metabolically active sink tissues.
This phenomenon is particularly evident in the case of theanine, the most abundant non-proteinogenic amino acid in
C. sinensis, which plays a central role in nitrogen storage and transport. Synthesized primarily in the roots, theanine is translocated to young leaves, where it contributes to nitrogen buffering and supports active protein biosynthesis. As leaves mature and enter senescence, theanine, along with other nitrogenous compounds, is remobilized toward younger organs, thereby explaining its lower accumulation in older leaves [
37].
Consequently, higher-quality teas tend to contain higher levels of amino acids. In fact, according to the literature, premium green teas, including matcha, are particularly rich in amino acids. Notably, Hideki Hori et al. (2017) [
5] reported that total amino acid content may serve as a potential indicator of green tea quality.
2.2. Caffeine Analysis
To determine caffeine, an additional HPTLC analysis was performed. Although the mobile phase used for catechins determination allowed clear visualization of caffeine, catechins became distinctly visible only after derivatization (see
Section 2.3). However, catechin, epicatechin, and caffeine share the same R
f; additionally, catechins absorb slightly at 254 nm, though to a lesser extent than caffeine.
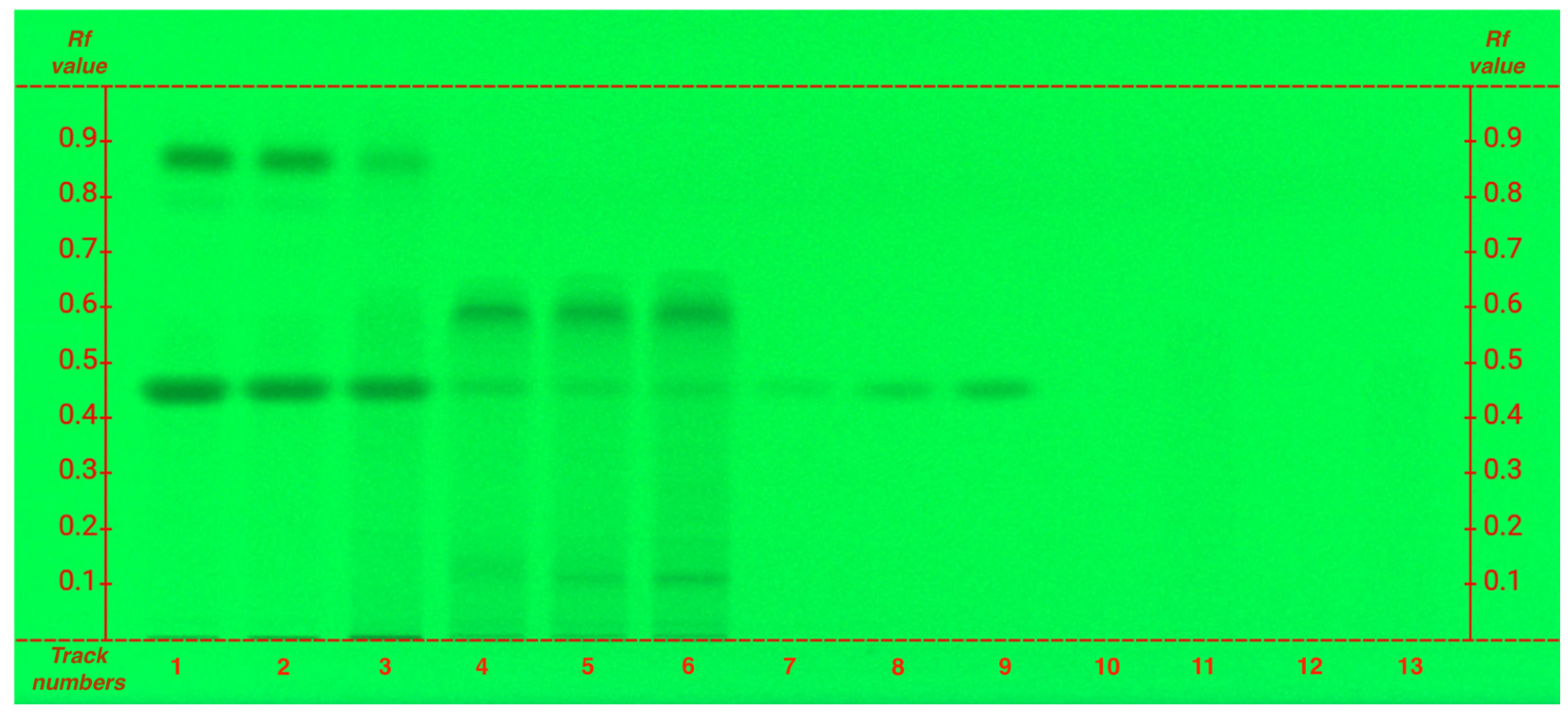
To ensure accurate data interpretation and prevent spot overlap a specific analysis for caffeine was conducted, ensuring distinct and non-overlapping R
f value (
Figure 2, caffeine R
f 0.45).
The analysis of caffeine, a xanthine alkaloid, as expected, shows that it is particularly abundant in the organic phase, while also present in lower amounts in hydroalcoholic extracts. However, unlike polyphenols, caffeine exhibits an opposite trend: grade G1 has a higher caffeine content compared to FG (
Figure 2), following the same pattern observed for amino acids.
Additional analyses (data reported in the
Supplementary Material) investigated the presence of the other two xanthine alkaloids: theobromine and theophylline. These compounds were not detected in the samples, likely due to their low concentrations or to limitations in the extraction method, which may not have effectively or fully extracted them. It is well known that these compounds are present in tea, although at lower concentrations than caffeine, which, as previously mentioned, is particularly soluble in chloroform.
NMR analysis also shows a decreasing trend in caffeine concentration from G1 to food grade (FG). Specifically, caffeine concentrations (mg/100 g) were found to be 472.53 ± 17.76 for G1, 386.6 ± 30.49 for G4, and 260.28 ± 4.24 for FG.
The organic extracts, as expected, showed a higher caffeine content compared to the hydroalcoholic extracts. In general, this secondary metabolite was found to be highly abundant and exhibited a concentration trend opposite to that of other secondary metabolites: specifically, G1 had a higher caffeine content than FG. This finding is supported by literature data. In fact, it is well known that the caffeine content in tea leaves varies depending on several factors, including the cultivar, leaf developmental stage, environmental conditions, harvest season, and degree of oxidation. In general, young leaves contain more caffeine than mature ones, with differences reaching up to 40% [
38].
This pattern can be explained by the biological role of caffeine in the plant. Caffeine acts as a chemical defense compound, deterring phytophagous insects. Young leaves, being more tender and vulnerable, are more reliant on such protective mechanisms. As a result, caffeine biosynthesis is more active in the early stages of leaf development, leading to its greater accumulation in younger tissues. In contrast, as leaves mature and lignify, their dependence on chemical defense lessens, and caffeine levels tend to decline accordingly [
39].
2.3. Organic Acids and Phenols
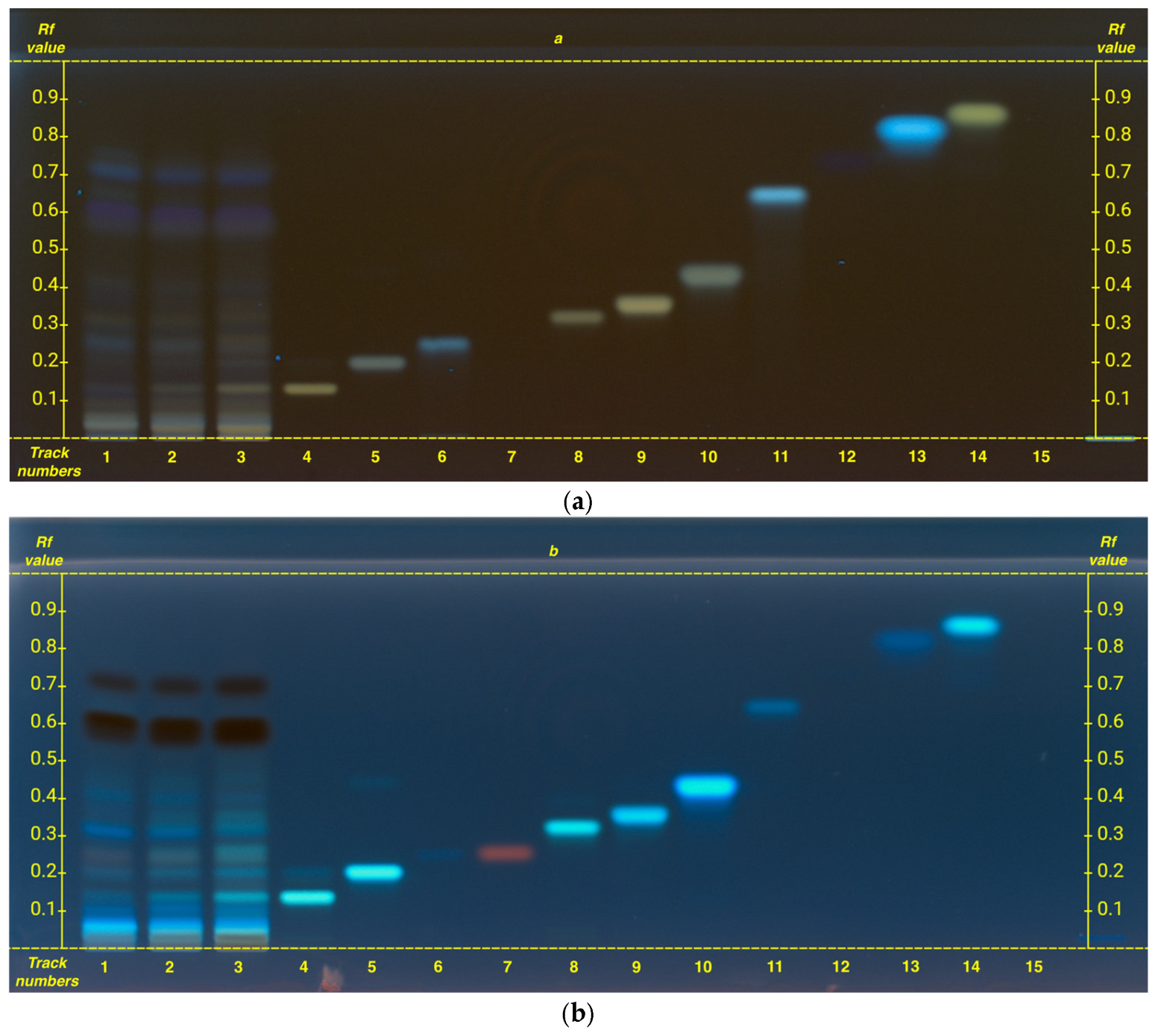
A single analysis was performed for both organic acids and flavonoids, as the selected mobile phase, chosen based on previous experience, enabled clear separation and visualization of both chemical classes under identical conditions, without any overlap issues (
Figure 3).
The analyzed compounds include the following: 3,5-di-caffeoylquinic acid, apigenin, caffeic acid, chlorogenic acid, cinnamic acid, gallic acid, hyperoside, kaempferol, luteolin, luteolin 7-O-glucoside, protocatechuic acid, quercetin, rutin, and shikimic acid.
Among the flavonoids, the presence of rutin and kaempferol was identified, with a concentration increasing from G1 to FG.
Conversely, among the organic acids, only chlorogenic acid and shikimic acid were identified, showing an opposite trend compared to flavonoids.
In this analysis, as well as in preliminary analyses, none of the following compounds were detected: 3,5-di-caffeoylquinic acid, apigenin, caffeic acid, cinnamic acid, gallic acid, hyperoside, luteolin, luteolin 7-O-glucoside, protocatechuic acid, and quercetin.
Flavonoids and organic acids contents are compared in
Table 3.
In other studies, some of these compounds were detected [
40,
41]. This observed discrepancy can likely be attributed to variations in the analytical conditions, with a particular emphasis on the extraction methodology employed. Differences in metabolite recovery and composition are known to arise based on the choice of extraction protocol, solvent polarity, and sample preparation techniques. In our case, we utilized the Bligh–Dyer method, a widely recognized biphasic extraction technique that employs short volumes of a chloroform–methanol–water system, which could be suboptimal for low solubility compounds. Another possible major factor could be the tea cultivar as assessed by Monobe et al. [
40]. Indeed, in this work, the concentrations of tea polyphenols were very heavily dependent on plant cultivar and pedoclimatic conditions.
Concurrently, NMR analysis allowed the identification of the following organic acids: 4-hydroxybenzoic acid, cinnamic acid quinic ester, protocatechuic acid, gallic acid quinic ester, total chlorogenic acids, and quinic acid. The corresponding concentrations are reported in
Table 4.
Univariate analysis showed, among organic acids metabolites, a decrease in concentration from G1 to G4 and FG of gallic acid quinic ester, quinic acid, and protocatechuic acid (which has not been detected in G4 and FG), where the total content of chlorogenic acids and cinnamic acid quinic ester is higher in FG and 4-hydroxybenzoic acid in G4.
Flavonoid and organic acid profiling revealed the presence of kaempferol, rutin, chlorogenic acid, and shikimic acid. Notably, rutin and kaempferol were most abundant in FG, with their concentrations progressively decreasing toward G1. Conversely, organic acids, particularly shikimic acid, exhibited an opposite trend, increasing from FG to G1.
This inverse correlation aligns with the known role of shikimic acid as a key intermediate in the shikimate pathway, which produces phenolic compounds such as flavonoids, and other secondary metabolites important for plant defense and pigmentation. The higher accumulation of shikimic acid in G1 likely indicates a slower conversion into flavonoids, leading to lower levels of final products. In contrast, the elevated levels of rutin and kaempferol in FG suggest a more active flavonoid biosynthesis, possibly due to increased enzyme activity or gene expression in the earlier stage. Overall, these results highlight a coordinated relationship between precursor levels and the production of secondary metabolites during plant development [
25].
HPTLC analyses did not detect the presence of protocatechuic acid, whereas NMR spectra showed peaks that could be assigned to this compound. Since the sensitivity of HPTLC is higher than that of NMR and direct comparison with standards yielded negative results, it is possible for this molecule to be an ester or a glycoside rather than its free form.
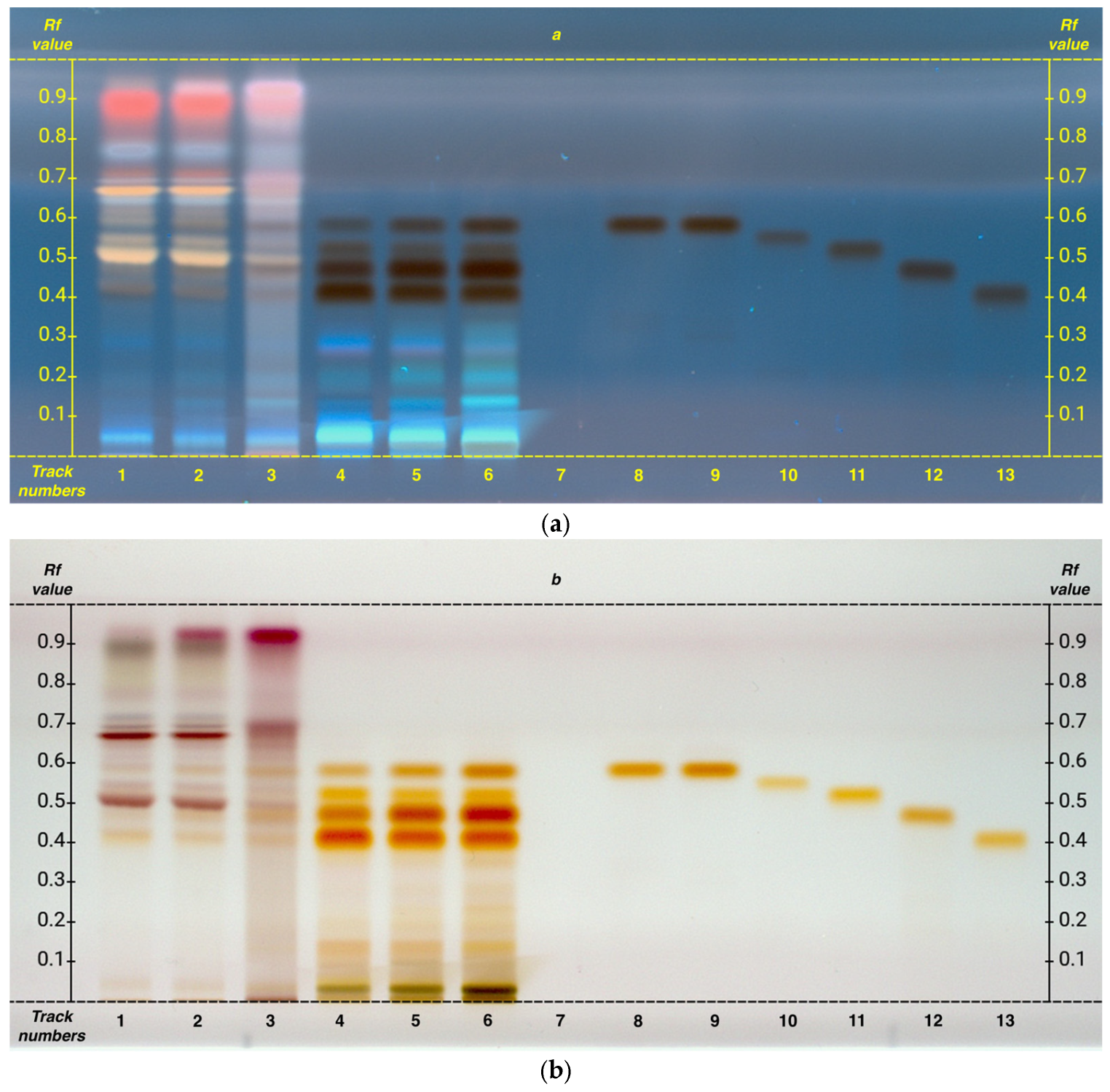
A separate analysis was conducted for the study of catechins. The HPTLC analysis also allowed the identification of catechin and its derivatives. The catechins analyzed were as follows: catechin, epicatechin, catechin gallate, epicatechin gallate, epigallocatechin, and epigallocatechin gallate.
All the analyzed compounds were found to be present in abundant concentrations. Additionally, as clearly shown in
Figure 4, a gradual increase in these secondary metabolites is evident, following an upward trend from G1 to FG. It can be observed that among these metabolites, epigallocatechin is the most abundant (R
f 0.46), followed by epigallocatechin gallate (R
f 0.41), epicatechin (R
f 0.55), and finally epicatechin gallate (R
f 0.51).
Catechin gallate is the only compound not detected in the samples, while catechin and epicatechin exhibit very similar Rf values. Therefore, as previously noted for the amino acids leucine and isoleucine, it is difficult to determine whether both compounds are present or only one of them.
NMR analysis allowed the identification of EC, ECG, EGC, and EGCG (
Table 5). Univariate analysis of these compounds showed that EC and EGC levels are lower in G1 compared to G4 and FG, while an opposite trend was observed for the gallate derivatives ECG and EGCG.
The catechins confidently identified were ECG, EGC, and EGCG. Due to overlapping Rf values, catechin and epicatechin could not be reliably distinguished, and catechin was not quantified via NMR.
Despite these limitations, our results showed that the total catechin content was remarkably high, ranging between 5 and 6 g per 100 g of matrix. This is consistent with the well-established fact that green tea contains higher levels of catechins compared to other types of tea, such as black tea [
15].
These findings agree with other studies; in fact, other works have shown that high-grade teas tend to contain lower levels of total catechins compared to lower-grade ones. Specifically, Horie et al. (2017) observed that EC and EGC contents were higher in lower-grade teas, while no clear correlation was found between tea grade and EGCG or ECG levels [
5].
The higher catechin content in lower-grade teas is typically associated with the use of older, more senescent leaves, which accumulate greater amounts of these metabolites. Conversely, younger leaves, used in premium-grade teas, naturally contain lower levels of EC and EGC. Furthermore, shading treatments, which are common in the cultivation of matcha, are known to reduce the concentration of EGC and EC, contributing to the generally lower catechin levels found in high-grade products.
About food-grade matcha, drawing clear conclusions is more challenging, as these products are often blends of powders differing in leaf age, harvest time, and shading conditions. As a result, the biological variability of the samples significantly hinders the identification of consistent trends [
5,
42].
In addition to cultivation method, leaf age, and tea type, it is important to consider other factors that may influence the polyphenol content, such as catechins. Among these, brewing time and temperature are known to play a significant role in modulating the extractability and final concentration of these compounds [
15,
16].