The Impact of OsERF34 on Rice Grain-Processing Traits and Appearance Quality
Abstract
1. Introduction
2. Results
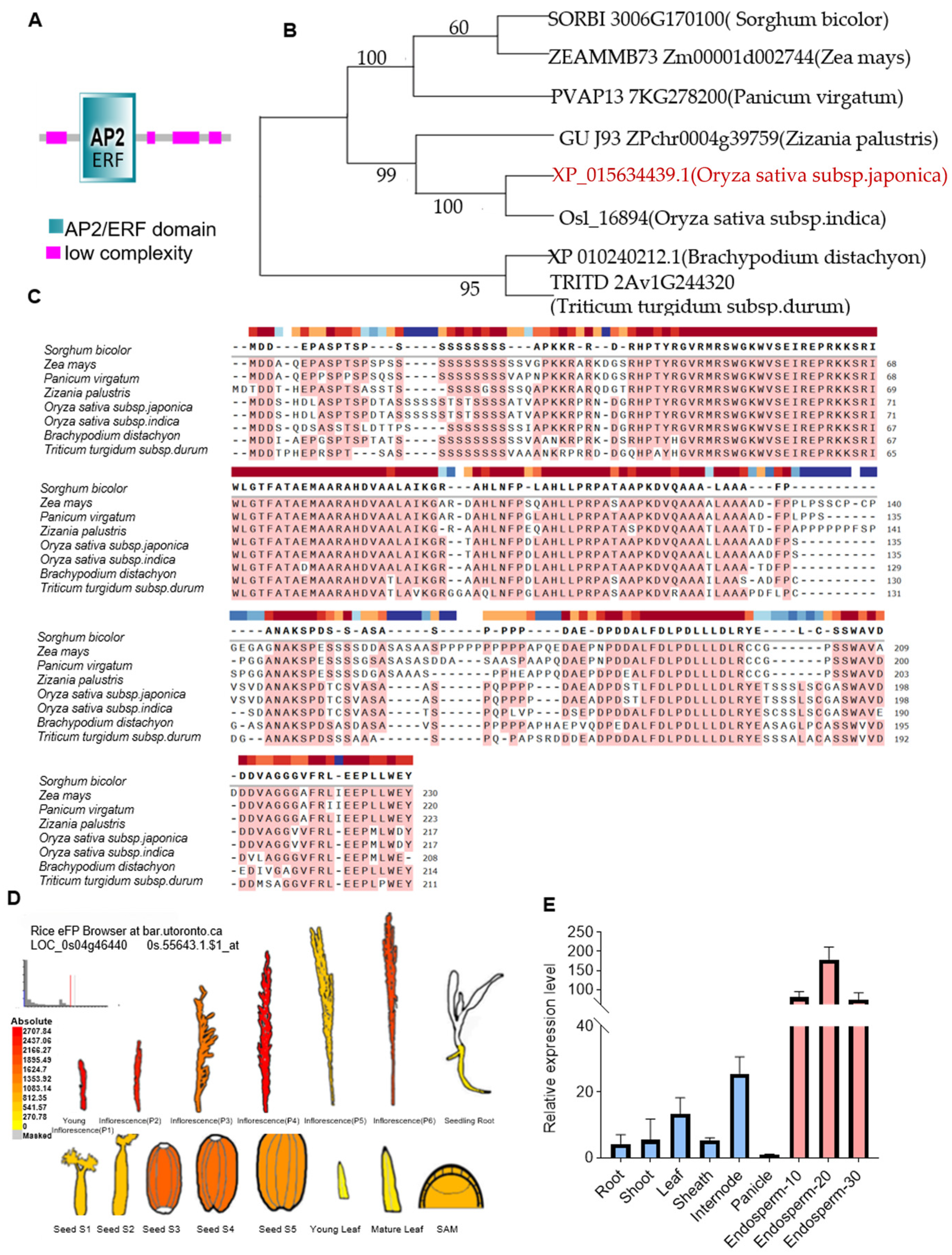
2.1. Structure and Functional Importance of the AP2 Domain
2.2. OsERF34 and Its Impact on Rice Morphology
2.3. Comparison of Chalkiness Phenotype and Starch Granules Morphology Between ZH11 and Oserf34
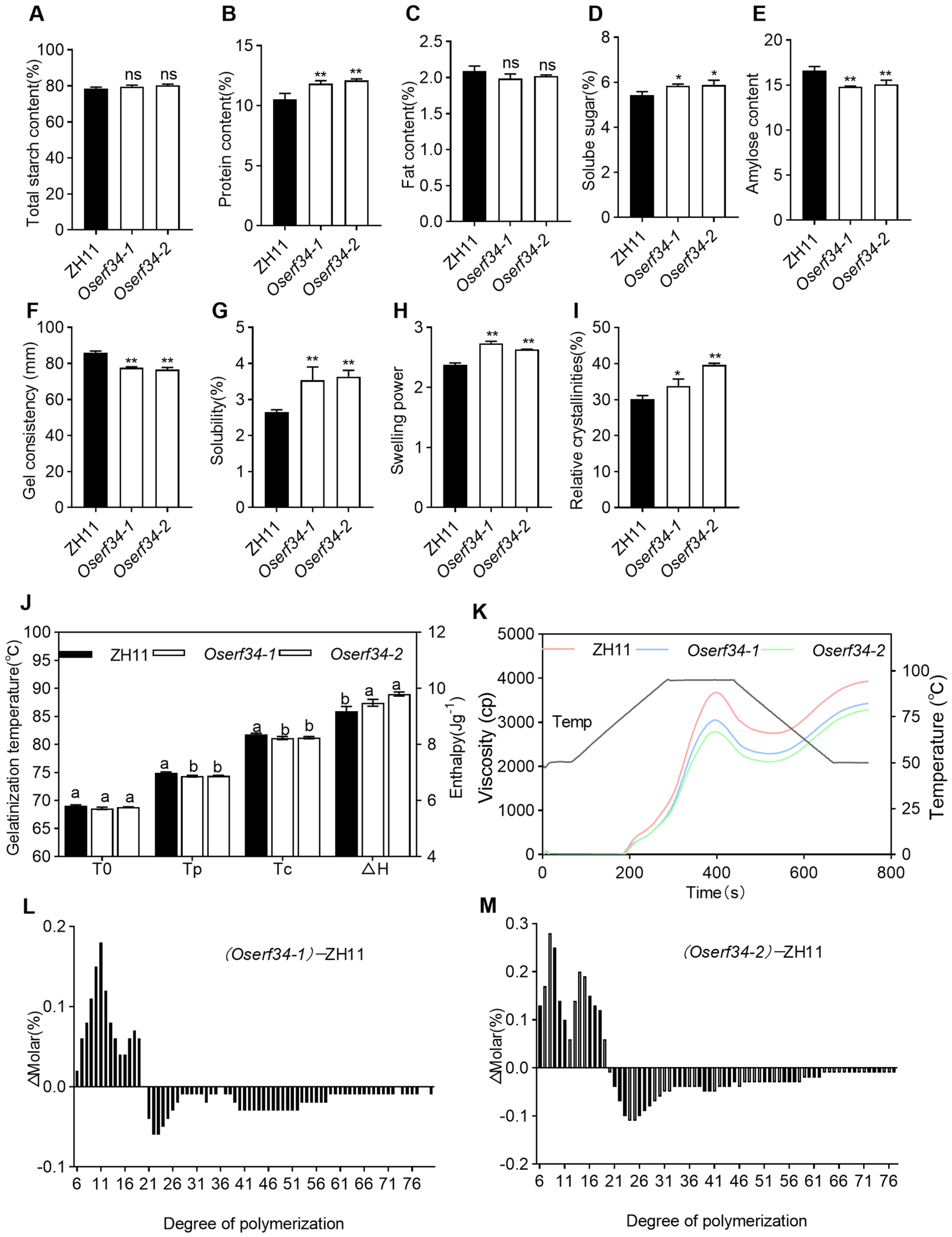
2.4. Comparison of Physiochemical Properties Between ZH11 and Oserf34
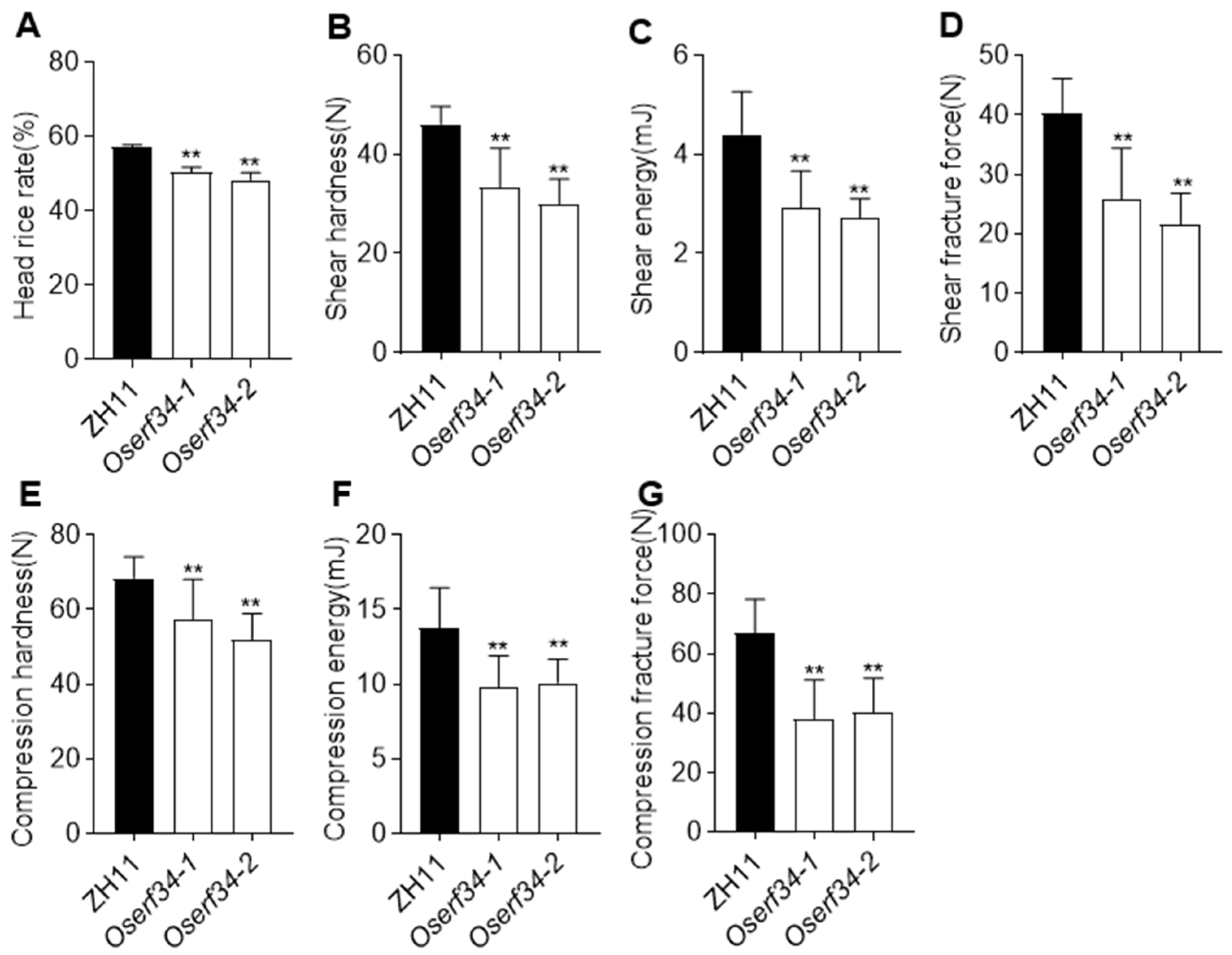
2.5. Processing Quality and Grain Hardness Characters of ZH11 and Oserf34
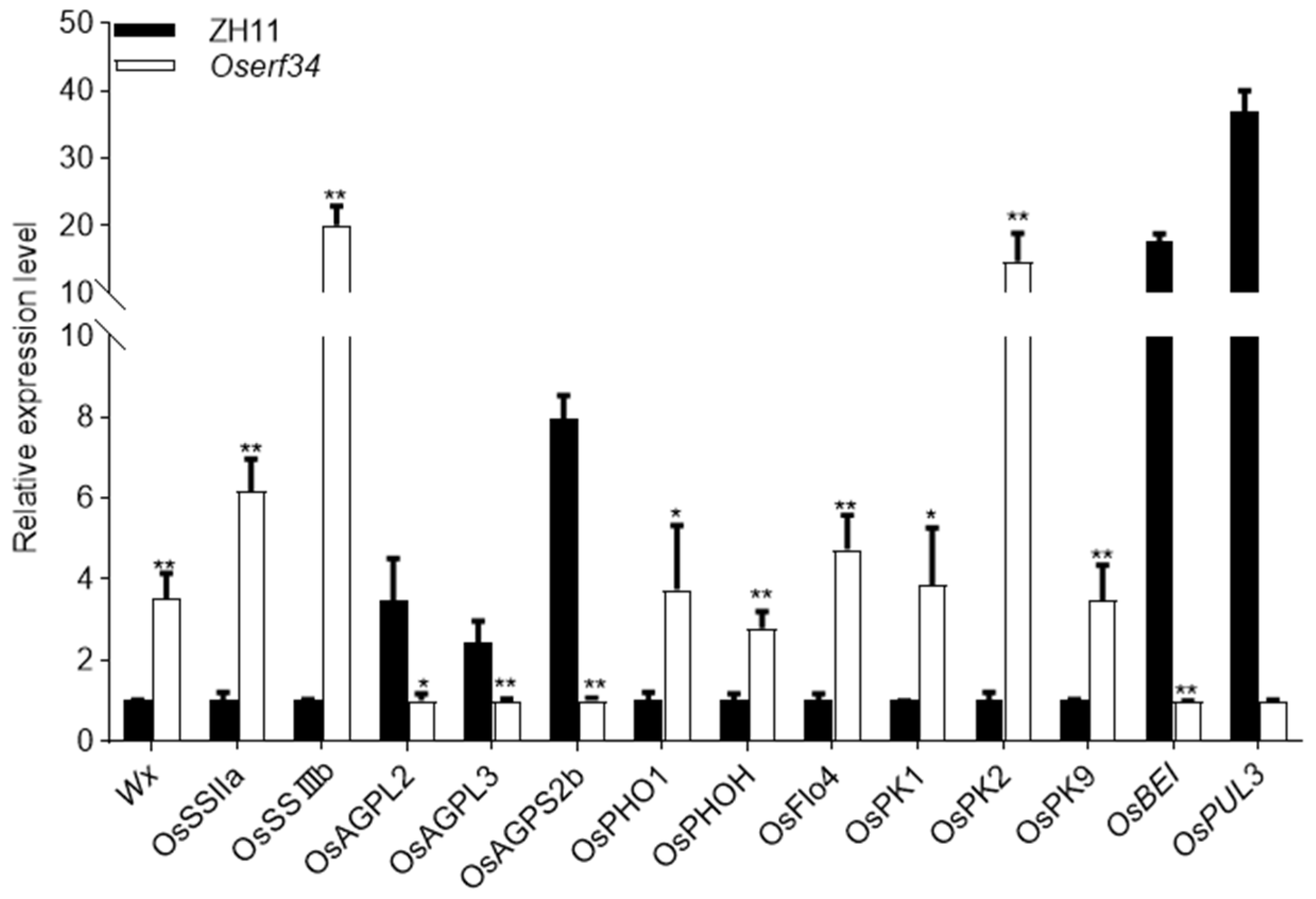
2.6. qRT-PCR Analysis of Starch Synthesis and Grain Morphology Related Genes
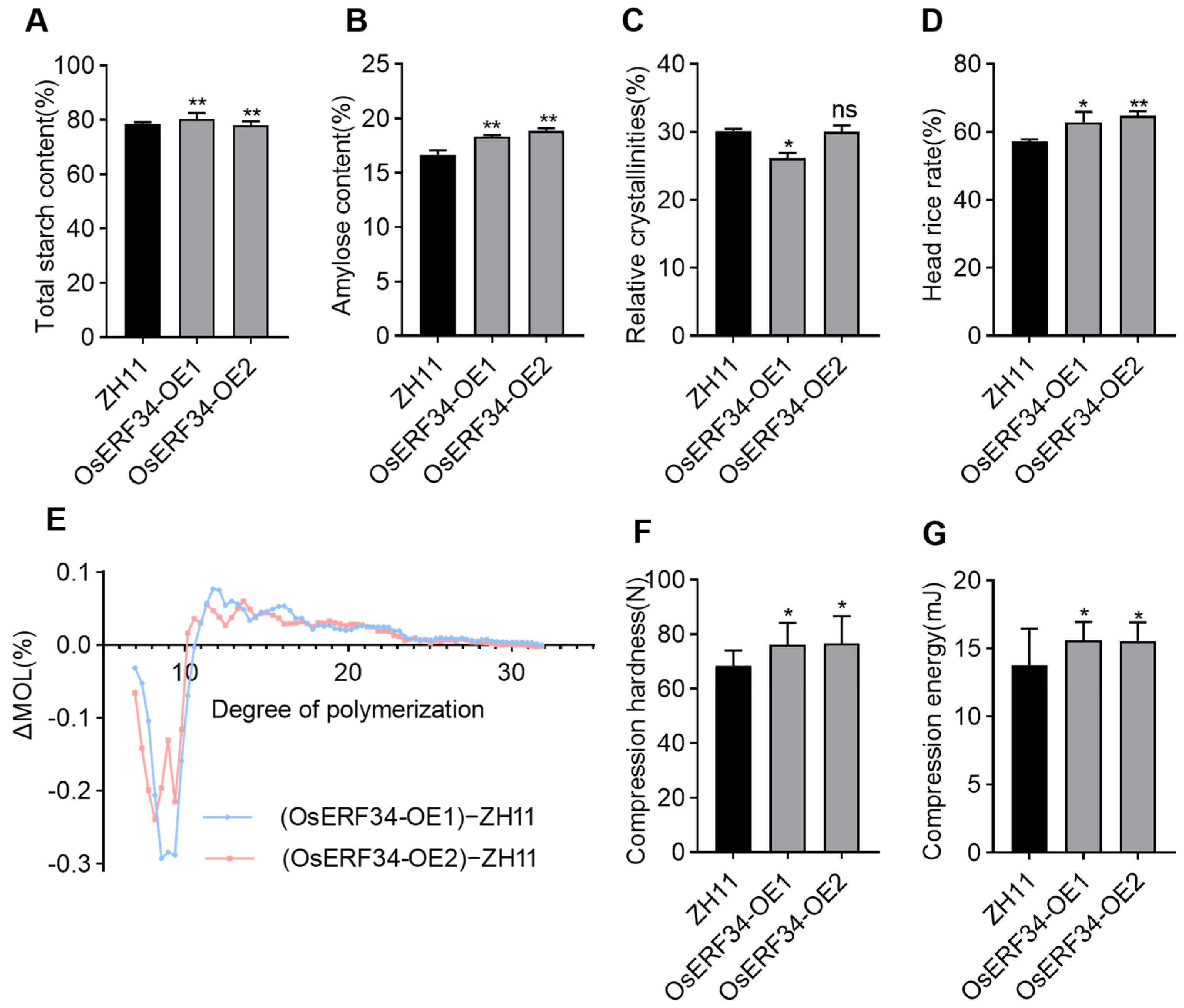
2.7. Comparative Analysis of Key Phenotypes Between ZH11 and OsERF34-OE
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Vectors Construction and Plant Transformation
4.3. Rice Grain and Flour Sample Preparation
4.4. Measurement of Appearance Quality
4.5. Observation of Starch Granules in the Endosperm
4.6. Physicochemical Properties Analysis
4.7. Determination of Solubility and Swelling Capacity
4.8. Processing Quality and Character Analysis
4.9. RNA Extraction and qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fukagawa, N.K.; Ziska, L.H. Rice: Importance for Global Nutrition. J. Nutr. Sci. Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, J.; Soomar, A.M.; Khalid, F.; Abbasi, Y. Assessing rice (Oryza sativa L.) quality: A comprehensive review of current techniques and future directions. J. Agric. Food Res. 2023, 14, 100843. [Google Scholar] [CrossRef]
- China National Rice Research Institute (CNRRI), the National Rice Whole-Industry-Chain Big Data Platform. China Rice Industry Development Report 2024, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 2024; p. 247. (In Chinese) [Google Scholar]
- China National Rice Research Institute (CNRRI), the National Rice Whole-Industry-Chain Big Data Platform. China Rice Industry Development Report 2023, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 2023; p. 238. (In Chinese) [Google Scholar]
- GB/T 21719-2008; Determination of Head Rice Yield of Paddy. National Standard of the People’s Republic of China: Beijing, China, 2008.
- Lyman, N.B.; Jagadish, K.S.V.; Nalley, L.L.; Dixon, B.L.; Siebenmorgen, T. Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS ONE 2013, 8, e72157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, X.; Ma, J.; Li, T.; Zhou, X.; Guo, T. Effects of high air temperature on rice grain quality and yield under field condition. Agron. J. 2013, 105, 446–454. [Google Scholar] [CrossRef]
- Xia, Y.; Qin, J.; Huang, R.; Feng, F.; Zhao, Q.; Song, X. Responses of rice qualities to temperature and light in three different ecological environments in karst regions. J. Cereal Sci. 2024, 118, 103984. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, B.Y.; Zhan, X.C.; Zheng, L.Y.; Zhang, P.J. Development trends and strategies of head rice yield in indica rice in southern China. J. Anhui Agric. Sci. 2021, 49, 38–45. [Google Scholar]
- Gao, J.P.; Sui, Y.H.; Zhang, W.Z.; Yao, C.; Gao, M.C.; Zhao, M.H.; Xu, Z.J. Effects of canopy temperature during grain filling stage on plant physiological traits and rice quality. Chin. J. Rice Sci. 2015, 29, 501–510. [Google Scholar]
- Xu, Z.J.; Chen, W.F.; Ma, D.R.; Lv, Y.N.; Zhou, S.Q.; Liu, L.N. Relationship between grain shape and main quality traits of rice. Acta Agron. Sin. 2004, 30, 894–900. [Google Scholar]
- Zhou, L.; Liang, S.; Ponce, K.; Marundon, S.; Ye, G.; Zhao, X. Factors affecting head rice yield and chalkiness in indica rice. Field Crops Res. 2015, 172, 1–10. [Google Scholar] [CrossRef]
- Shi, C.H.; He, C.X.; Zhu, J. Genetic analysis of main effects and genotype-environment interactions for milling quality traits in rice. Acta Genet. Sin. 1998, 25, 46–53. [Google Scholar]
- Guo, Y.Y.; Zhang, Y.K.; Chen, R.X.; Hu, B.M.; Chen, K.R. Study on variety, location, and their interaction effects for milling quality of early indica rice. Hereditas 1997, 19, 12–16. [Google Scholar]
- Weng, J.F.; Wan, X.Y.; Guo, T.; Jiang, L.; Zhai, H.Q. Stability of QTL expression related to rice milling quality using chromosome segment substitution lines (CSSL) population. Sci. Agric. Sin. 2007, 40, 2128–2135. [Google Scholar]
- Liu, J.F.; Kui, L.M.; Zhu, Z.F.; Tan, L.B.; Wang, G.J.; Li, Q.W.; Shu, J.H.; Sun, C.Q. QTL mapping for milling quality and appearance quality traits in common wild rice (Oryza rufipogon). J. Agric. Biotechnol. 2007, 15, 90–96. [Google Scholar]
- Wang, X.; Pang, Y.; Wang, C.; Chen, K.; Zhu, Y.; Shen, C.; Ali, J.; Xu, J.; Li, Z. New candidate genes affecting rice grain appearance and milling quality detected by genomewide and gene-based association analyses. Front. Plant Sci. 2017, 7, 1998. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.Y. Genetic Dissection of Rice Quality Traits Under Different Environmental Conditions. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2017. [Google Scholar]
- Chen, X.N.; Yuan, Z.K.; Hu, Z.Z.; Zhao, Q.Z.; Sun, H.Z. Mapping QTL for head rice yield in japonica rice using QTL-Seq. Chin. J. Rice Sci. 2021, 35, 449–454. [Google Scholar]
- Li, Y.; Fan, C.; Xing, Y.; Yun, P.; Luo, L.; Yan, B.; Peng, B.; Xie, W.; Wang, G.; Li, X.; et al. Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 2014, 46, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Cheng, Y.; Li, Y.; Wang, W.; Tian, H.; Zhang, N.; Wang, Y.; Yuan, Y.; Hussain, H.; Lin, R.; et al. AtbZIP62 Acts as a Transcription Repressor to Positively Regulate ABA Responses in Arabidopsis. Plants 2022, 11, 3037. [Google Scholar] [CrossRef]
- Jarambasa, T.; Regon, P.; Jyoti, S.Y.; Gupta, D.; Panda, S.K.; Tanti, B. Genome-wide identification and expression analysis of the Pisum sativum (L.) APETALA2/ethylene-responsive factor (AP2/ERF) gene family reveals functions in drought and cold stresses. Genetica 2023, 151, 225–239. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Kim, Y.; Hyeon, D.Y.; Park, H.; Jeong, J.; Jeong, U.; Yoon, Y.S.; You, D.; Kwak, J.; et al. Ethylene responsive factor34 mediates stress-induced leaf senescence by regulating salt stress-responsive genes. Plant Cell Environ. 2022, 45, 1719–1733. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, L.; Jiang, W. Understanding AP2/ERF Transcription Factor Responses and Tolerance to Various Abiotic Stresses in Plants: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 893. [Google Scholar] [CrossRef]
- Wang, K.; Guo, H.; Yin, Y. AP2/ERF transcription factors and their functions in Arabidopsis responses to abiotic stresses. Environ. Exp. Bot. 2024, 222, 105763. [Google Scholar] [CrossRef]
- Srivastava, R.; Kumar, R. The expanding roles of APETALA2/Ethylene Responsive Factors and their potential applications in crop improvement. Brief Funct. Genom. 2019, 18, 240–254. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Tang, Y.-J.; Ma, Q.-B.; Yang, C.-Y.; Mu, Y.-H.; Suo, H.-C.; Luo, L.-H.; Nian, H. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PLoS ONE 2013, 8, e83011. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Sakamoto, S.; Mitsuda, N.; Ren, A.; Persson, S.; Zhang, D. ETHYLENE RESPONSE FACTOR 34 promotes secondary cell wall thickening and strength of rice peduncles. Plant Physiol. 2022, 190, 1806–1820. [Google Scholar] [CrossRef]
- Cai, J.; Man, J.; Huang, J.; Liu, Q.; Wei, W.; Wei, C. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohydr. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Chibbar, R.N. Amylopectin small chain glucans form structure fingerprint that determines botanical origin of starch. Carbohydr. Polym. 2017, 158, 112–123. [Google Scholar] [CrossRef]
- Nishi, A.; Nakamura, Y.; Tanaka, N.; Satoh, H. Biochemical and Genetic Analysis of the Effects ofAmylose-Extender Mutation in Rice Endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar] [CrossRef]
- Kang, H.; Park, S.; Matsuoka, M.; An, G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J. 2005, 42, 901–911. [Google Scholar] [CrossRef]
- Schmidt, R.; Schippers, J.H.; Mieulet, D.; Watanabe, M.; Hoefgen, R.; Guiderdoni, E.; Mueller-Roeber, B. SALT-RESPONSIVE ERF1 Is a Negative Regulator of Grain Filling and Gibberellin-Mediated Seedling Establishment in Rice. Mol. Plant 2014, 7, 404–421. [Google Scholar] [CrossRef]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Shomura, A.; Izawa, T.; Ebana, K.; Ebitani, T.; Kanegae, H.; Konishi, S.; Yano, M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 2008, 40, 1023–1028. [Google Scholar] [CrossRef]
- Fu, F.F.; Xue, H.W. Coexpression Analysis Identifies Rice Starch Regulator1, a Rice AP2/EREBP Family Transcription Factor, as a Novel Rice Starch Biosynthesis Regulator. Plant Physiol. 2010, 154, 927–938. [Google Scholar] [CrossRef]
- Nakagawa, H.; Tanaka, A.; Tanabata, T.; Ohtake, M.; Fujioka, S.; Nakamura, H.; Ichikawa, H.; Mori, M. Decreases Organ Elongation and Brassinosteroid Response in Rice. Plant Physiol. 2012, 158, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Somssich, M.; Nakata, M.T.; Unda, F.; Atsuzawa, K.; Kaneko, Y.; Wang, T.; Bågman, A.-M.; Gaudinier, A.; Yoshida, K.; et al. Complete substitution of a secondary cell wall with a primary cell wall in. Nat. Plants 2018, 4, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wessels, B.; Seyfferth, C.; Escamez, S.; Vain, T.; Antos, K.; Vahala, J.; Delhomme, N.; Kangasjärvi, J.; Eder, M.; Felten, J.; et al. An AP2/ERF transcription factor ERF139 coordinates xylem cell expansion and secondary cell wall deposition. New Phytol. 2019, 224, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Jin, L.; Miao, Y.; He, X.; Hu, Q.; Guo, K.; Zhu, L.; Zhang, X. An ethylene response-related factor, GbERF1-like, from improves resistance to via activating lignin synthesis. Plant Mol. Biol. 2016, 91, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhang, X.F.; Xue, H.W. Rice aleurone layer specific OsNF-YB1 regulates grain filling and endosperm development by interacting with an ERF transcription factor. J. Exp. Bot. 2016, 67, 6399–6411. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Chen, S.; Fan, X.; Li, Q.; Lu, Y.; Wang, M.; Yu, H.; Yi, C.; Tang, S.; et al. Wx, the Ancestral Allele of Rice Gene. Mol. Plant 2019, 12, 1157–1166. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Jiao, G.; Yu, J.; Zhao, R.; Lu, A.; Zhou, W.; Cao, N.; Wu, J.; Hu, S.; et al. The elite eating quality alleles Wx(b) and ALK(b) are regulated by OsDOF18 and coordinately improve head rice yield. Plant Biotechnol. J. 2024, 22, 1582–1595. [Google Scholar] [CrossRef]
- Wang, J.-C.; Xu, H.; Zhu, Y.; Liu, Q.-Q.; Cai, X.-L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013, 64, 3453–3466. [Google Scholar] [CrossRef]
- Cruz, N.D.; Khush, G.S. Rice Grain Quality Evaluation Procedures. In Aromatic Rices; Singh, R.K., Singh, U.S., Khush, G.S., Eds.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 2000; pp. 15–28. [Google Scholar]
- Wickramasinghe, H.A.M.; Noda, T. Physicochemical properties of starches from Sri Lankan rice varieties. Food Sci. Technol. Res. 2008, 14, 49–54. [Google Scholar] [CrossRef][Green Version]
- Rajapakse, D.; Benthota, A.P.; Wijesundara, S.M.; Premakumara, G.A.S.; Herath, T. Properties of Some Traditional Rice Varieties of Sri Lanka; Industrial Technology Institute and Department of Agriculture: Colombo, Sri Lanka, 2011; Volume 61. [Google Scholar]
- Rohilla, R.; Singh, V.P.; Singh, U.S.; Singh, R.K.; Khush, G.S. Crop husbandry and environmental factors affecting aroma and other quality traits. In Aromatic Rices; Singh, R.K., Singh, U.S., Khush, G.S., Eds.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 2000; pp. 201–216. [Google Scholar]
- Li, P.; Chen, Y.-H.; Lu, J.; Zhang, C.-Q.; Liu, Q.-Q.; Li, Q.-F. Genes and Their Molecular Functions Determining Seed Structure, Components, and Quality of Rice. Rice 2022, 15, 18. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Yamamoto, M.P.; Touno, S.M.; Yasuda, H.; Takaiwa, F. Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. Plant J. 2009, 59, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, S.; Ren, X.; Lu, Y.; Liu, D.; Cai, X.; Li, Q.; Gao, J.; Liu, Q. Molecular Structure and Physicochemical Properties of Starches from Rice with Different Amylose Contents Resulting from Modification of OsGBSSI Activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jia, M.; Luo, S.; Liu, Y.; Zhang, Y.; Zhu, K.; Wang, W.; Xu, Y.; Gu, J.; Zhang, H.; et al. Controlled soil drying mitigates the effects of high-temperature stress on rice quality by enhancing starch accumulation and stabilizing starch structure and functional properties. Carbohydr. Polym. 2025, 362, 123688. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Huang, W.; Li, Z.; Fang, Z. Amino acid transporter OsATL13 coordinately regulates rice yield and quality by transporting phenylalanine and methionine. Plant Sci. 2025, 353, 112398. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, S.; Ling, H.Q. An efficient regeneration system and Agrobacterium-mediated transformation of Chinese upland rice cultivar Handao297. Plant Cell Tissue Organ Cult. 2011, 106, 475. [Google Scholar] [CrossRef]
- Qin, F.; Man, J.; Xu, B.; Hu, M.; Gu, M.; Liu, Q.; Wei, C. Structural properties of hydrolyzed high-amylose rice starch by alpha-amylase from Bacillus licheniformis. J. Agric. Food Chem. 2011, 59, 12667–12673. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Jia, Y.; Hu, P.; Xu, H.; Jiao, G.; Tang, S. The Impact of OsERF34 on Rice Grain-Processing Traits and Appearance Quality. Plants 2025, 14, 1633. https://doi.org/10.3390/plants14111633
Du Z, Jia Y, Hu P, Xu H, Jiao G, Tang S. The Impact of OsERF34 on Rice Grain-Processing Traits and Appearance Quality. Plants. 2025; 14(11):1633. https://doi.org/10.3390/plants14111633
Chicago/Turabian StyleDu, Zhimin, Yinan Jia, Peisong Hu, Hai Xu, Guiai Jiao, and Shaoqing Tang. 2025. "The Impact of OsERF34 on Rice Grain-Processing Traits and Appearance Quality" Plants 14, no. 11: 1633. https://doi.org/10.3390/plants14111633
APA StyleDu, Z., Jia, Y., Hu, P., Xu, H., Jiao, G., & Tang, S. (2025). The Impact of OsERF34 on Rice Grain-Processing Traits and Appearance Quality. Plants, 14(11), 1633. https://doi.org/10.3390/plants14111633





