Transcriptomic and Metabolic Analysis Reveal Potential Mechanism of Starch Accumulation in Spirodela polyrhiza Under Nutrient Stress
Abstract
:1. Introduction
2. Results
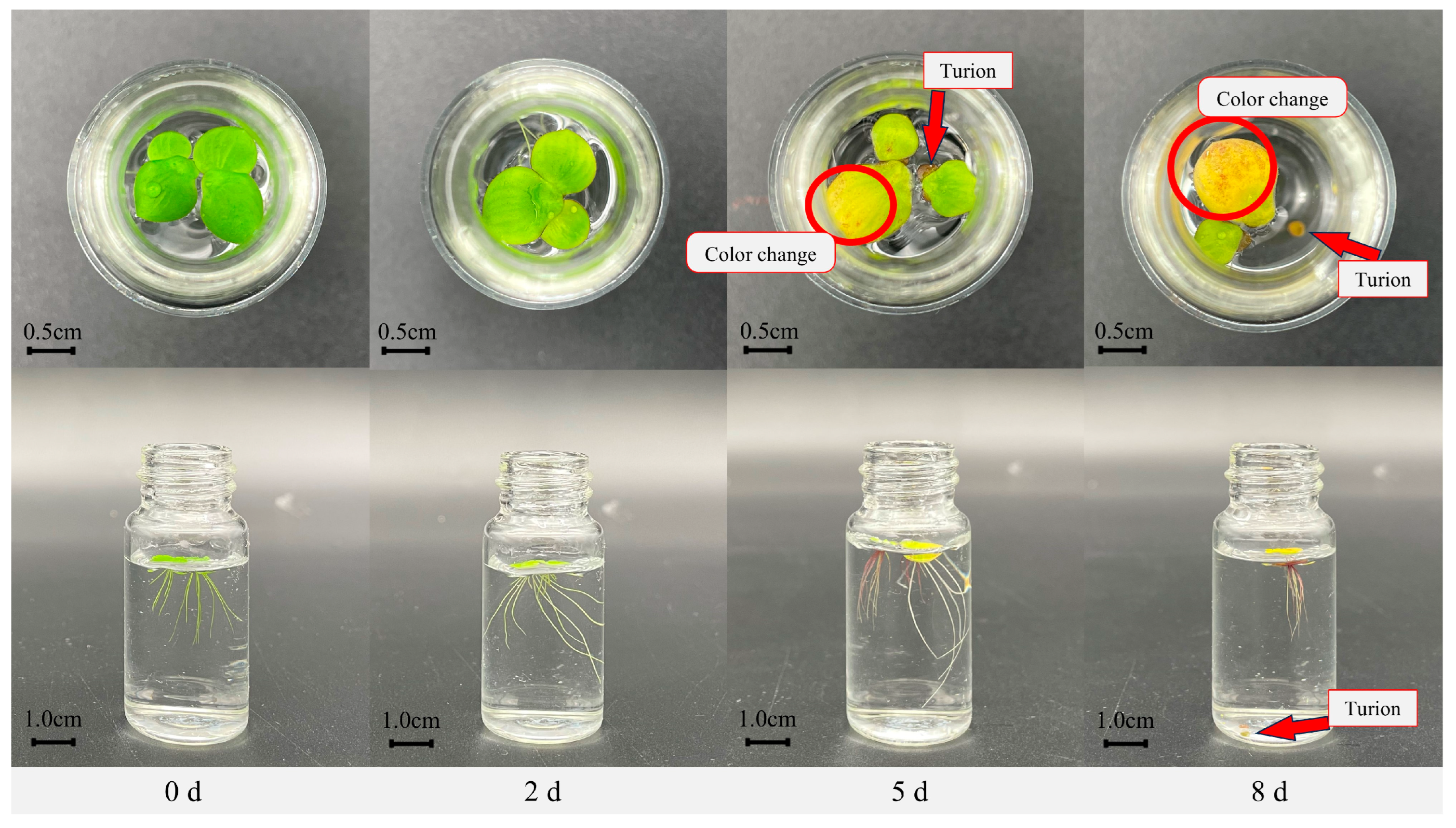
2.1. Growth Characteristics in ZH0196 Frond Under Nutrient Stress
2.1.1. Morphological Changes in Fronds Under Nutrient Stress
2.1.2. Starch Granule Changes in Frond Under Nutrient Stress
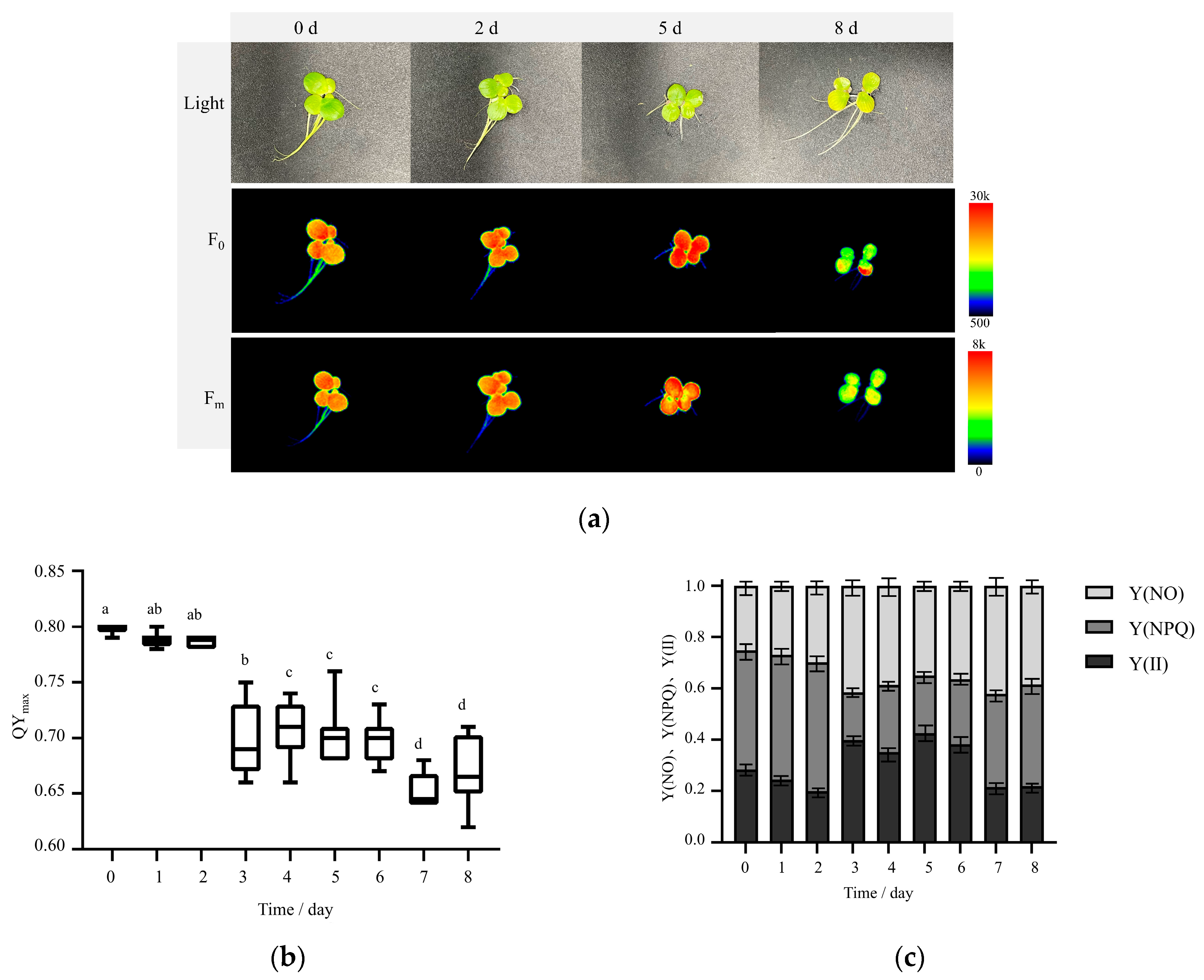
2.2. Photosystem II (PS II) Activity
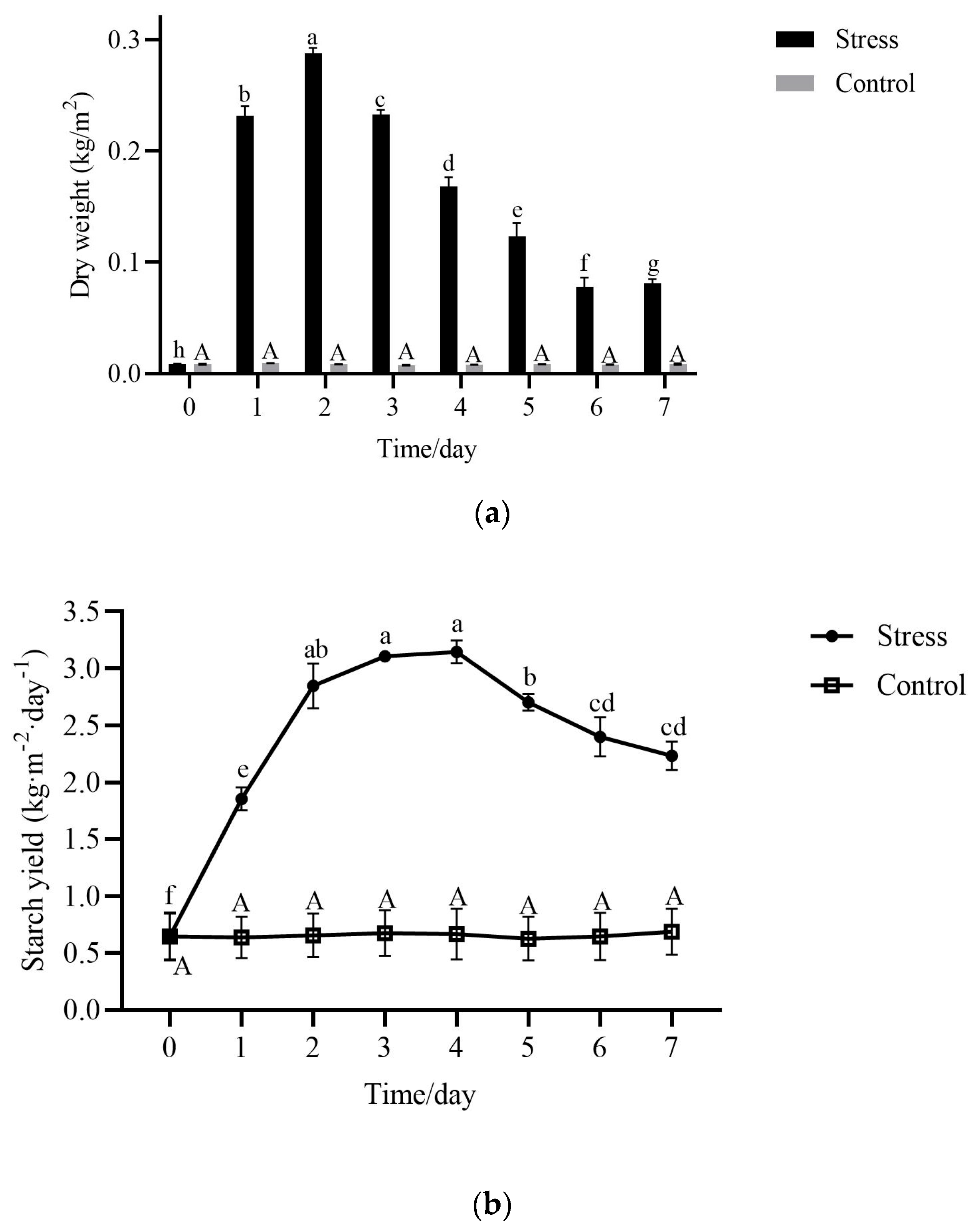
2.3. Starch Yield in Fronds During Nutrient Stress
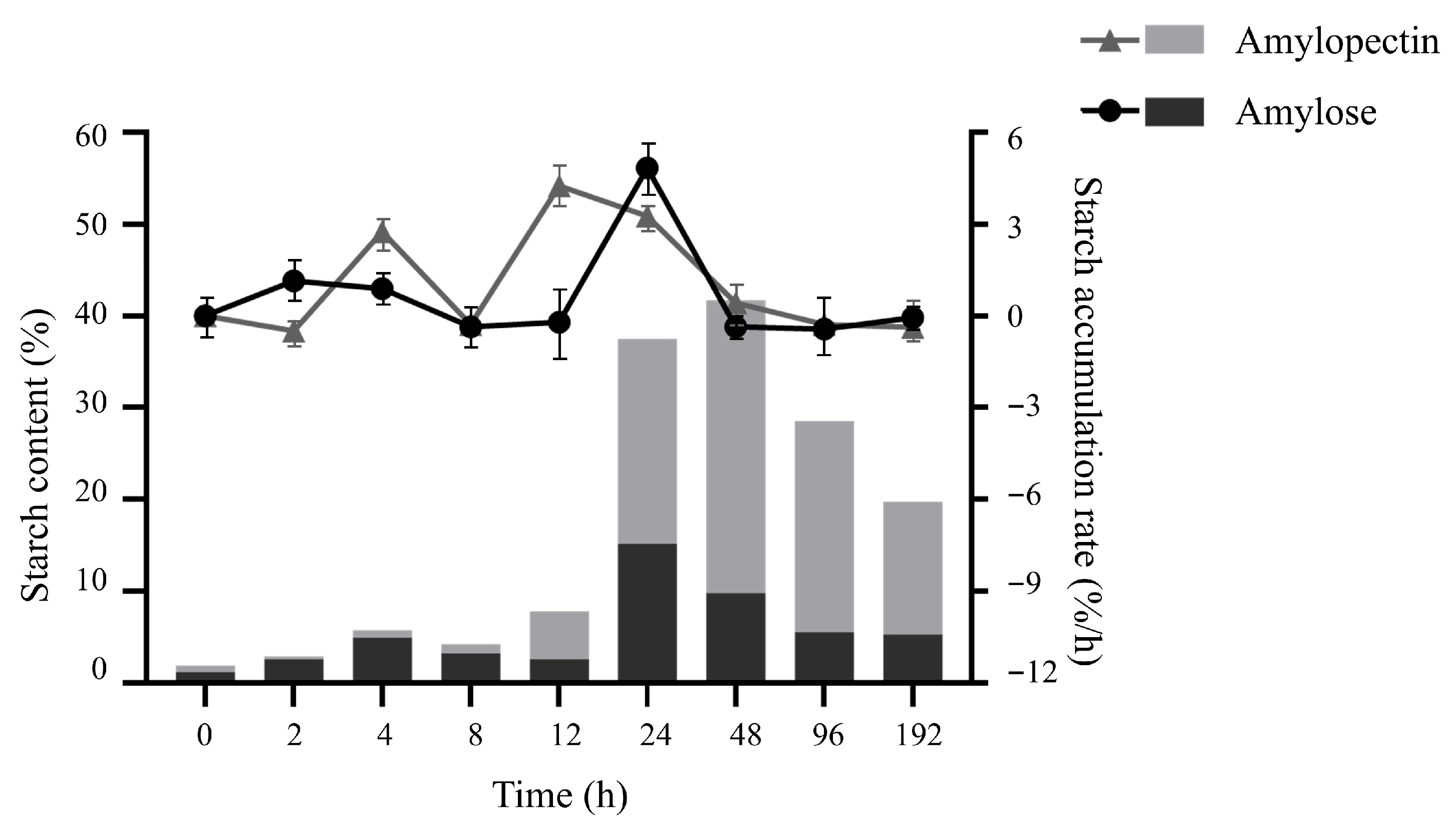
2.4. Starch Composition and Accumulation Rate
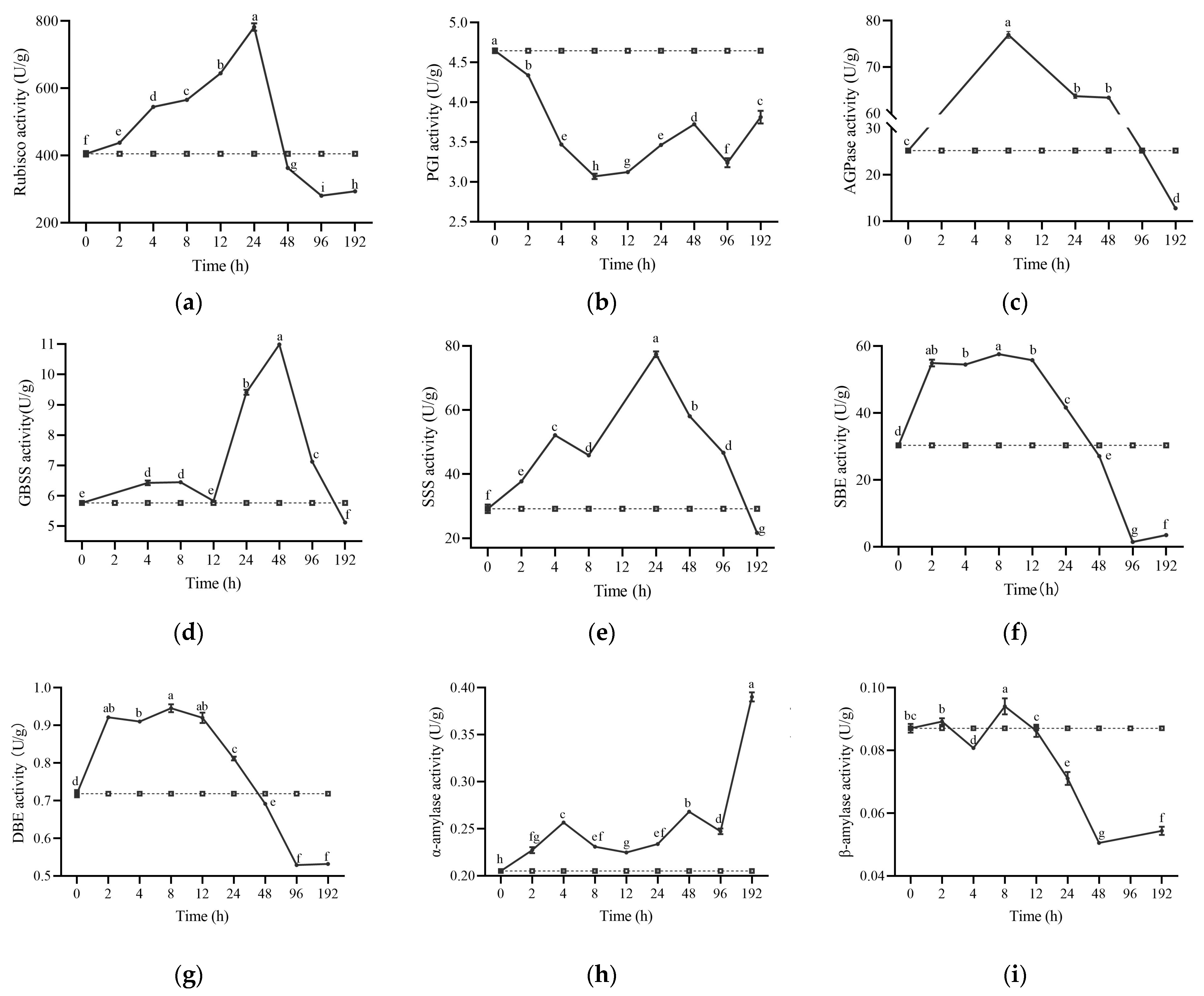
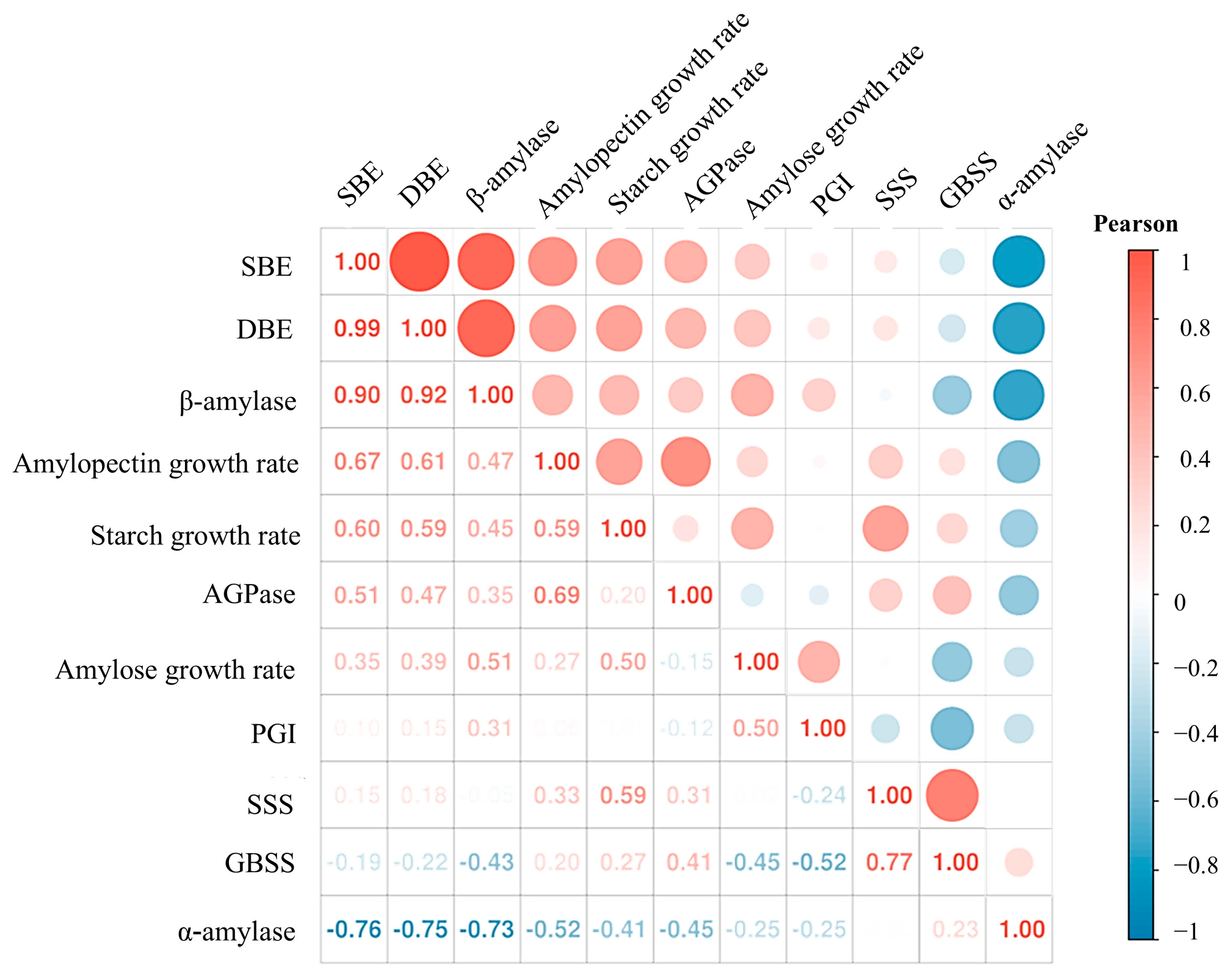
2.5. Enzyme Activity Related to Starch Metabolism
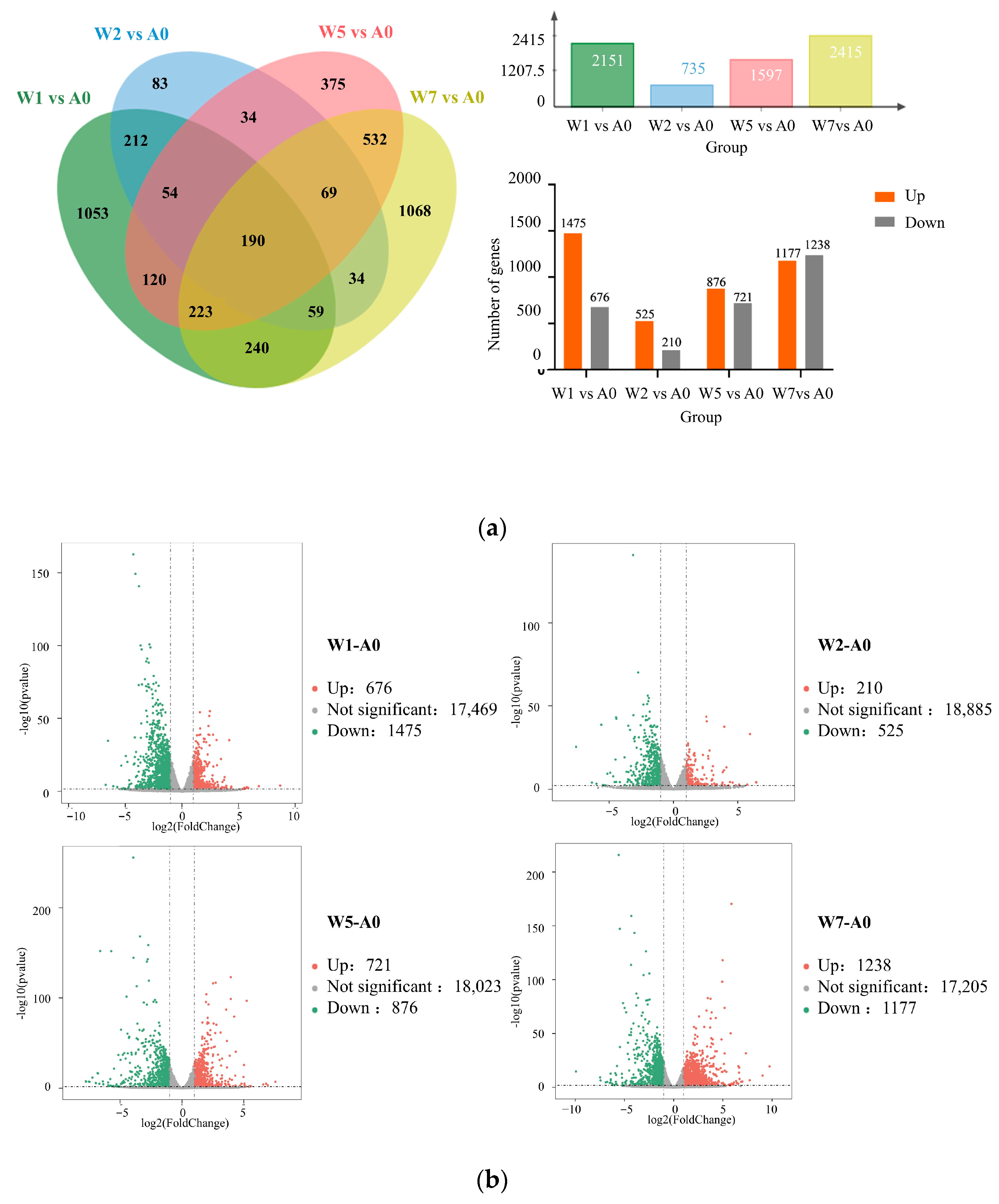
2.6. Differentially Expressed Gene
2.7. Gene Ontology Enrichment Analysis
2.8. Effects of Nutritional Stress on Starch Metabolic Processes
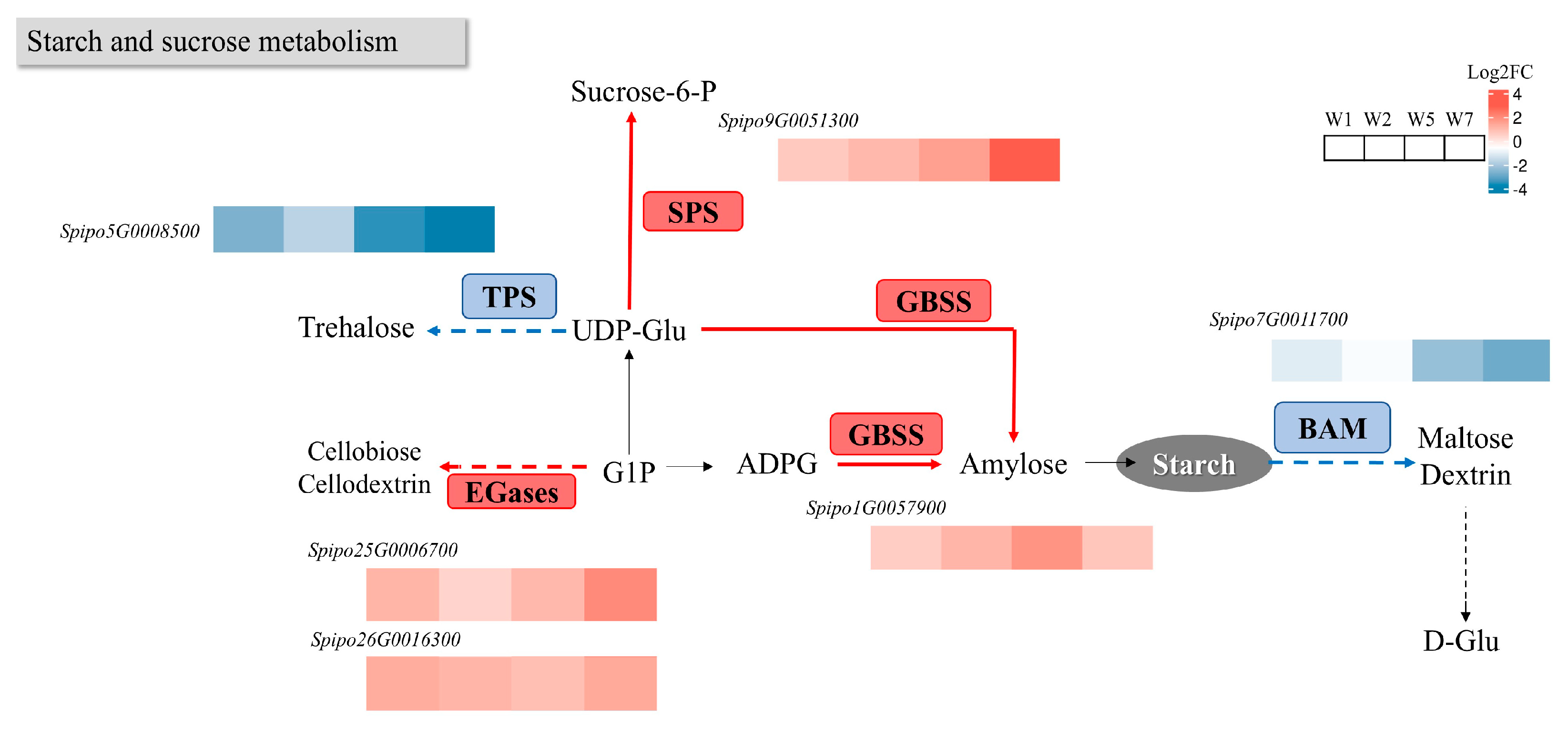
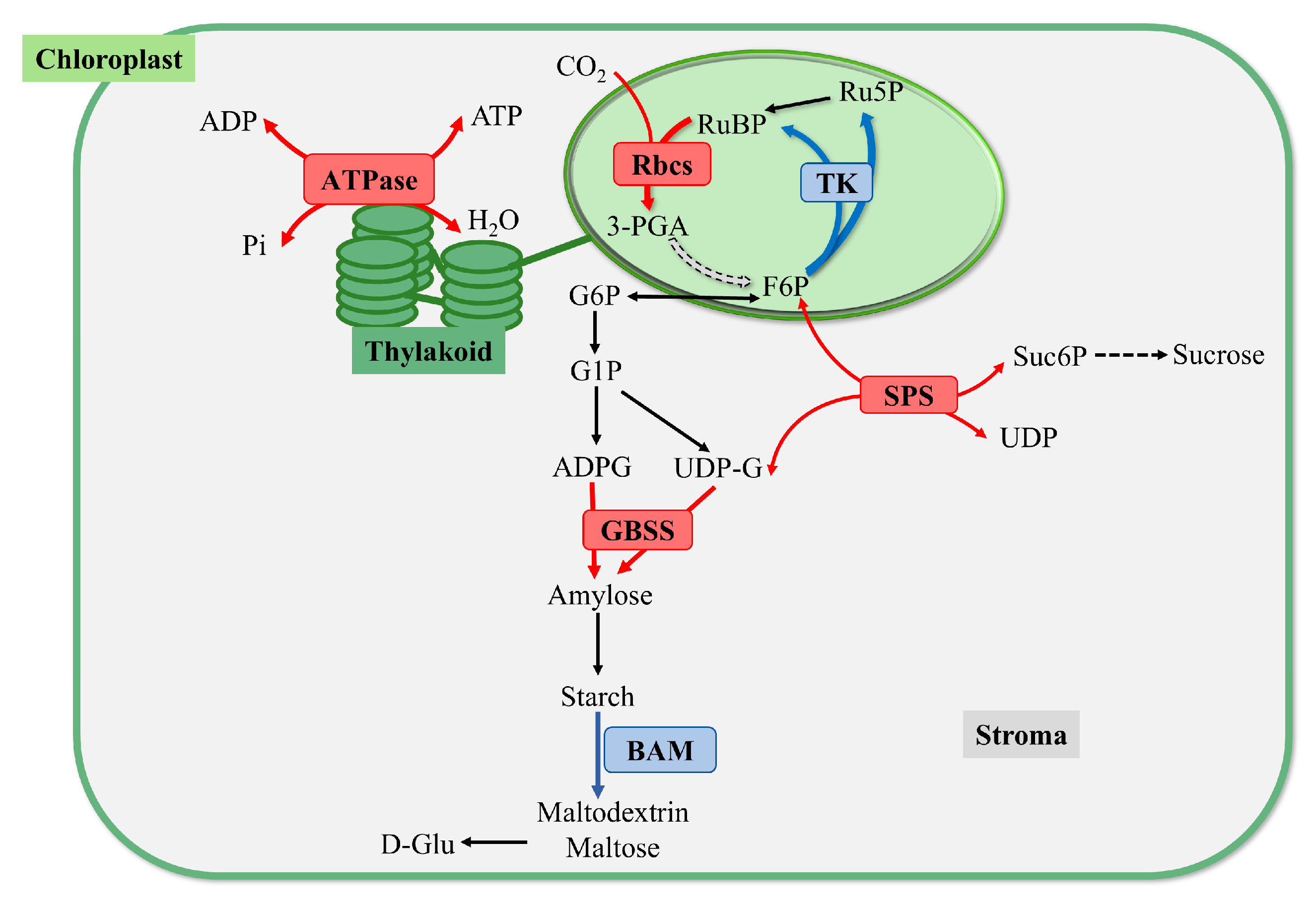
2.8.1. Effects on Starch and Sucrose Metabolism Pathway
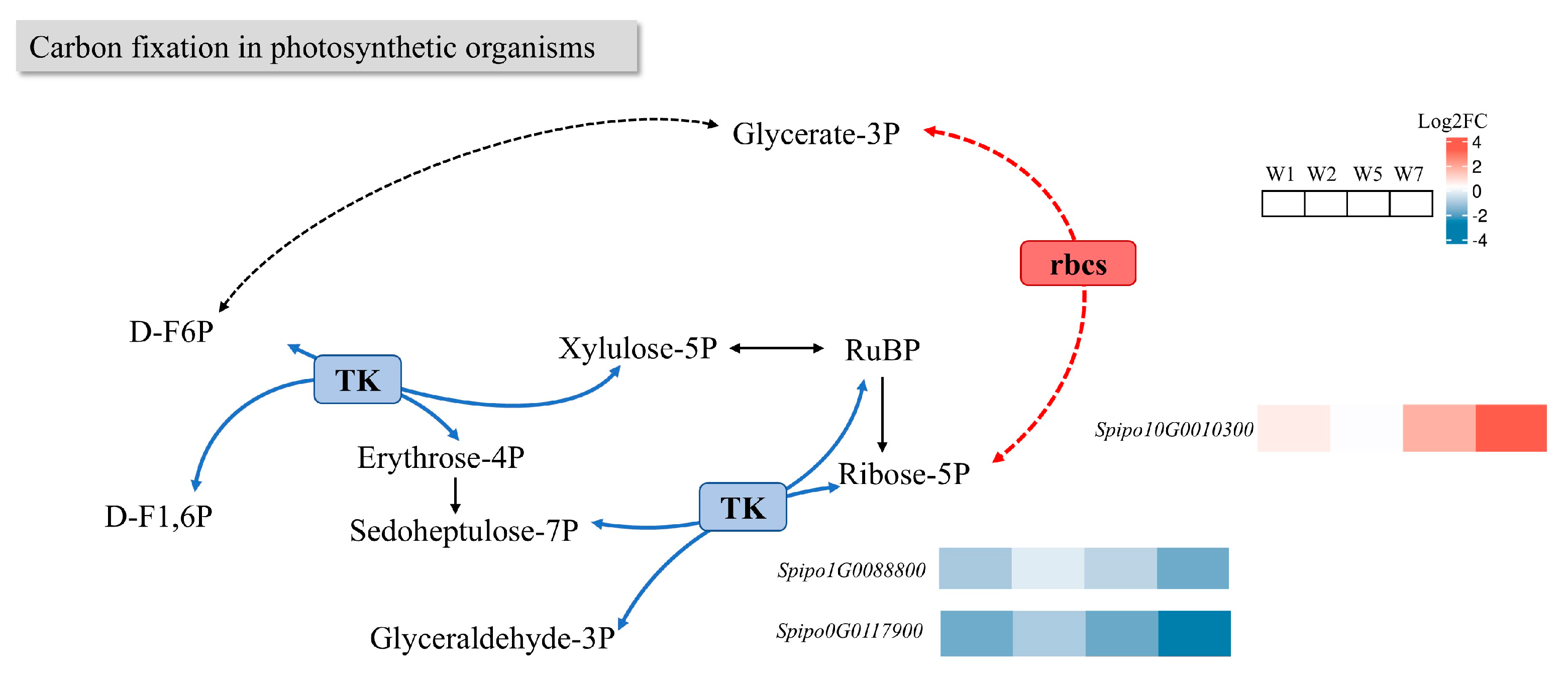
2.8.2. Effects on Carbon Fixation in Photosynthetic Organism Pathway
3. Discussion
4. Materials and Methods
4.1. Duckweed Species and Culture Conditions
4.2. Photosystem II (PS II) Activity Measurement
4.3. Growth Measurement
4.4. Determination and Yield of the Total Starch Content
4.5. Determination and Calculation of Growth Rate of Amylose/Amylopectin Content
4.6. Light Microscopy Starch Observations
4.7. Determination of Starch Metabolism and Photosynthesis-Related Enzyme Activities
4.8. Total RNA Isolation, cDNA Library Construction, and RNA Sequencing
4.9. Differential Gene Expression and Enrichment Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADPG | Adenosine 5′-Diphosphoglucose |
| AGPase | ADP-glucose pyrophosphorylase |
| BAM | Arabidopsis β-amylase |
| DBE | Starch debranching enzyme |
| DEGs | Differentially expressed genes |
| EGase | Endo-1,4-β-D-glucanohydrolase |
| F0 | Minimal Fluorescence |
| F6P | Fructose-6-phosphate |
| Fm | Maximal fluorescence |
| Fv | Variable fluorescence |
| G1P | Glucose 1-phosphate |
| G6P | Glucose 6-phosphate |
| GBSS | Granule-bound starch synthase |
| GBSS | Granule-bound Starch Synthase |
| PGI | Phosphoglucose isomerase |
| PSII | Photosystem II |
| QYmax | Maximum Quantum Yield of Photosystem II Photochemistry |
| rbcs | Small Subunits of Ribulose1,5-bisphosphate Carboxylase/oxygenase |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| RuBP | Ribulose 1,5-bisphosphate |
| SBE | Starch branching enzyme |
| SPS | Sucrose Phosphate Synthase |
| S. polyrhiza | Spirodela polyrhiza |
| SSS | Starch synthase enzyme |
| TK | Transketolase |
| TPS | Trehalose-6-phosphate synthase |
References
- Said, M.Z.M.; Culley, D.D.; Standifer, L.C.; Epps, E.A.; Myers, R.W.; Boney, S.A. Effect of Harvest Rate, Waste Loading, and Stocking Density on the Yield of Duckweeds. Proc. World Maric. Soc. 1979, 10, 769–780. [Google Scholar] [CrossRef]
- Sharma, J.; Clark, W.D.; Shrivastav, A.K.; Goswami, R.K.; Tocher, D.R.; Chakrabarti, R. Production Potential of Greater Duckweed Spirodela polyrhiza (L. Schleiden) and Its Biochemical Composition Evaluation. Aquaculture 2019, 513, 734419. [Google Scholar] [CrossRef]
- Guo, L.; Fang, Y.; Wang, S.; Xiao, Y.; Ding, Y.; Jin, Y.; Tian, X.; Du, A.; Liao, Z.; He, K.; et al. Duckweed: A Starch-Hyperaccumulating Plant under Cultivation with a Combination of Nutrient Limitation and Elevated CO2. Front. Plant Sci. 2025, 16, 1531849. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-L.; Fang, Y.; Li, Q.; Yang, G.-L.; Guo, L.; Chen, G.-K.; Tan, L.; He, K.-Z.; Jin, Y.; Zhao, H. Turion, an Innovative Duckweed-Based Starch Production System for Economical Biofuel Manufacture. Ind. Crops Prod. 2018, 124, 108–114. [Google Scholar] [CrossRef]
- Farid, M.; Sajjad, A.; Asam, Z.U.Z.; Zubair, M.; Rizwan, M.; Abbas, M.; Farid, S.; Ali, S.; Alharby, H.F.; Alzahrani, Y.M.; et al. Phytoremediation of Contaminated Industrial Wastewater by Duckweed (Lemna minor L.): Growth and Physiological Response under Acetic Acid Application. Chemosphere 2022, 304, 135262. [Google Scholar] [CrossRef] [PubMed]
- Körner, S.; Vermaat, J.E.; Veenstra, S. The Capacity of Duckweed to Treat Wastewater: Ecological Considerations for a Sound Design. J. Environ. Qual. 2003, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Calicioglu, O.; Richard, T.L.; Brennan, R.A. Anaerobic Bioprocessing of Wastewater-Derived Duckweed: Maximizing Product Yields in a Biorefinery Value Cascade. Bioresour. Technol. 2019, 289, 121716. [Google Scholar] [CrossRef]
- Sree, K.S.; Dahse, H.-M.; Chandran, J.N.; Schneider, B.; Jahreis, G.; Appenroth, K.J. Duckweed for Human Nutrition: No Cytotoxic and No Anti-Proliferative Effects on Human Cell Lines. Plant Foods Hum. Nutr. 2019, 74, 223–224. [Google Scholar] [CrossRef]
- Zelicha, H.; Kaplan, A.; Yaskolka Meir, A.; Tsaban, G.; Rinott, E.; Shelef, I.; Tirosh, A.; Brikner, D.; Pupkin, E.; Qi, L.; et al. The Effect of Wolffia Globosa Mankai, a Green Aquatic Plant, on Postprandial Glycemic Response: A Randomized Crossover Controlled Trial. Diabetes Care 2019, 42, 1162–1169. [Google Scholar] [CrossRef]
- De Beukelaar, M.F.A.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R.H. Duckweed as Human Food. The Influence of Meal Context and Information on Duckweed Acceptability of Dutch Consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Fang, Y.; Huang, M.; Chen, X.; Zhang, Y.; Zhao, H. The Effects of Photoperiod and Nutrition on Duckweed (Landoltia punctata) Growth and Starch Accumulation. Ind. Crops Prod. 2018, 115, 243–249. [Google Scholar] [CrossRef]
- Yin, Y.; Yu, C.; Yu, L.; Zhao, J.; Sun, C.; Ma, Y.; Zhou, G. The Influence of Light Intensity and Photoperiod on Duckweed Biomass and Starch Accumulation for Bioethanol Production. Bioresour. Technol. 2015, 187, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, L.; Ma, Z.; Du, X. Physiological Responses and Transcriptome Analysis of Spirodela polyrhiza under Red, Blue, and White Light. Planta 2022, 255, 11. [Google Scholar] [CrossRef]
- Shao, J.; Liu, Z.; Ding, Y.; Wang, J.; Li, X.; Yang, Y. Biosynthesis of the Starch Is Improved by the Supplement of Nickel (Ni2+) in Duckweed (Landoltia punctata). J. Plant Res. 2020, 133, 587–596. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, X.; Fang, Y.; Huang, M.; Guo, L.; Zhang, Y.; Zhao, H. Improving Biomass and Starch Accumulation of Bioenergy Crop Duckweed (Landoltia punctata) by Abscisic Acid Application. Sci. Rep. 2018, 8, 9544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef]
- Guo, L.; Jin, Y.; Xiao, Y.; Tan, L.; Tian, X.; Ding, Y.; He, K.; Du, A.; Li, J.; Yi, Z.; et al. Energy-Efficient and Environmentally Friendly Production of Starch-Rich Duckweed Biomass Using Nitrogen-Limited Cultivation. J. Clean. Prod. 2020, 251, 119726. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, W.; Zhao, X.; Chen, Y.; Yang, J.; Xu, S.; Hou, H. Sulfur Limitation Boosts More Starch Accumulation than Nitrogen or Phosphorus Limitation in Duckweed (Spirodela polyrhiza). Ind. Crops Prod. 2022, 185, 115098. [Google Scholar] [CrossRef]
- Rossi, M.; Bermudez, L.; Carrari, F. Crop Yield: Challenges from a Metabolic Perspective. Curr. Opin. Plant Biol. 2015, 25, 79–89. [Google Scholar] [CrossRef]
- Taghipour, E.; Bog, M.; Frootan, F.; Shojaei, S.; Rad, N.; Arezoumandi, M.; Jafari, M.; Salmanian, A.H. DNA Barcoding and Biomass Accumulation Rates of Native Iranian Duckweed Species for Biotechnological Applications. Front. Plant Sci. 2022, 13, 1034238. [Google Scholar] [CrossRef]
- Li, L.; Wu, K.; Zhang, J. Study on Turion Formation in Spirodela polyrhiza. Mol. Plant Breed. 2016, 14, 3554–3560. [Google Scholar] [CrossRef]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Kromdijk, J.; Bardal, V.; Stern, D.B. Overexpression of Rubisco Subunits with RAF1 Increases Rubisco Content in Maize. Nat. Plants 2018, 4, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Dong, X.; Wu, G.; Wang, M.; Ai, X. Decreased TK Activity Alters Growth, Yield and Tolerance to Low Temperature and Low Light Intensity in Transgenic Cucumber Plants. Plant Cell Rep. 2015, 34, 345–354. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Gao, X.; Sun, J.; Ji, X.; Feng, G.; Shen, G.; Xiang, B.; Wang, Y. Molecular Mechanism Underlying the Effect of Maleic Hydrazide Treatment on Starch Accumulation in S. polyrrhiza 7498 Fronds. Biotechnol. Biofuels 2021, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Nakao, E.; Takeuchi, M.; Tanimura, A.; Ando, A.; Kishino, S.; Kikukawa, H.; Shima, J.; Ogawa, J.; Shimizu, S. Characterization of Starch-Accumulating Duckweeds, Wolffia globosa, as Renewable Carbon Source for Bioethanol Production. Biocatal. Agric. Biotechnol. 2016, 6, 123–127. [Google Scholar] [CrossRef]
- Guo, L.; Fang, Y.; Jin, Y.; He, K.; Zhao, H. High Starch Duckweed Biomass Production and Its Highly-Efficient Conversion to Bioethanol. Environ. Technol. Innov. 2023, 32, 103296. [Google Scholar] [CrossRef]
- Dragone, G.; Fernandes, B.D.; Abreu, A.P.; Vicente, A.A.; Teixeira, J.A. Nutrient Limitation as a Strategy for Increasing Starch Accumulation in Microalgae. Appl. Energy 2011, 88, 3331–3335. [Google Scholar] [CrossRef]
- Wang, L.; Kuang, Y.; Zheng, S.; Tong, Y.; Zhu, Y.; Wang, Y. Overexpression of the Phosphoserine Phosphatase-Encoding Gene (AtPSP1) Promotes Starch Accumulation in Lemna turionifera 5511 under Sulfur Deficiency. Plants 2023, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Juergens, M.T.; Disbrow, B.; Shachar-Hill, Y. The Relationship of Triacylglycerol and Starch Accumulation to Carbon and Energy Flows during Nutrient Deprivation in Chlamydomonas Reinhardtii. Plant Physiol. 2016, 171, 2445–2457. [Google Scholar] [CrossRef]
- Li, J.; Du, A.; Liu, P.; Tian, X.; Jin, Y.; Yi, Z.; He, K.; Fang, Y.; Zhao, H. High Starch Accumulation Mechanism and Phosphorus Utilization Efficiency of Duckweed (Landoltia punctata) under Phosphate Starvation. Ind. Crops Prod. 2021, 167, 113529. [Google Scholar] [CrossRef]
- Muradov, N.; Fidalgo, B.; Gujar, A.C.; T-Raissi, A. Pyrolysis of Fast-Growing Aquatic Biomass -Lemna Minor (Duckweed): Characterization of Pyrolysis Products. Bioresour. Technol. 2010, 101, 8424–8428. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.P.; Ernst, E.; Hartwick, N.; Chu, P.; Bryant, D.; Gilbert, S.; Ortleb, S.; Baggs, E.L.; Sree, K.S.; Appenroth, K.J.; et al. Genome and Time-of-Day Transcriptome of Wolffia australiana Link Morphological Minimization with Gene Loss and Less Growth Control. Genome Res. 2021, 31, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Romero-Vergel, A.P. TURION: A Physiological Crop Model for Yield Prediction of Asparagus Using Sentinel-1 Data. Eur. J. Agron. 2023, 143, 126690. [Google Scholar] [CrossRef]
- Ritchie, G.A. Chlorophyll Fluorescence: What Is It and What Do the Numbers Mean? USDA Forest Service Proceedings: Washington, DC, USA, 2006. [Google Scholar]
- Kumar, P.; Eriksen, R.L.; Simko, I.; Mou, B. Molecular Mapping of Water-Stress Responsive Genomic Loci in Lettuce (Lactuca spp.) Using Kinetics Chlorophyll Fluorescence, Hyperspectral Imaging and Machine Learning. Front. Genet. 2021, 12, 634554. [Google Scholar] [CrossRef]
- Adhikari, N.D.; Simko, I.; Mou, B. Phenomic and Physiological Analysis of Salinity Effects on Lettuce. Sensors 2019, 19, 4814. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Zhao, J.; Li, C.; Wu, H.; Zhao, H.; Wu, Q. Granule-Bound Starch Synthase in Plants: Towards an Understanding of Their Evolution, Regulatory Mechanisms, Applications, and Perspectives. Plant Sci. 2023, 336, 111843. [Google Scholar] [CrossRef]
- Bouvier, J.W.; Emms, D.M.; Kelly, S. Rubisco Is Evolving for Improved Catalytic Efficiency and CO2 Assimilation in Plants. Proc. Natl. Acad. Sci. USA 2024, 121, e2321050121. [Google Scholar] [CrossRef]
- Bahaji, A.; Sánchez-López, Á.M.; De Diego, N.; Muñoz, F.J.; Baroja-Fernández, E.; Li, J.; Ricarte-Bermejo, A.; Baslam, M.; Aranjuelo, I.; Almagro, G.; et al. Plastidic Phosphoglucose Isomerase Is an Important Determinant of Starch Accumulation in Mesophyll Cells, Growth, Photosynthetic Capacity, and Biosynthesis of Plastidic Cytokinins in Arabidopsis. PLoS ONE 2015, 10, e0119641. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, H.; Zhang, W.; Wang, N.; Zhang, S.; Chu, C.; Liu, C. Engineering of the Cytosolic Form of Phosphoglucose Isomerase into Chloroplasts Improves Plant Photosynthesis and Biomass. New Phytol. 2021, 231, 315–325. [Google Scholar] [CrossRef]
- Kunz, H.-H.; Zamani-Nour, S.; Häusler, R.E.; Ludewig, K.; Schroeder, J.I.; Malinova, I.; Fettke, J.; Flügge, U.-I.; Gierth, M. Loss of Cytosolic Phosphoglucose Isomerase Affects Carbohydrate Metabolism in Leaves and Is Essential for Fertility of Arabidopsis. Plant Physiol. 2014, 166, 753–765. [Google Scholar] [CrossRef]
- Azoulay-Shemer, T.; Schwankl, N.; Rog, I.; Moshelion, M.; Schroeder, J.I. Starch Biosynthesis by AGPase, but Not Starch Degradation by BAM1/3 and SEX1, Is Rate-Limiting for CO2-Regulated Stomatal Movements under Short-Day Conditions. FEBS Lett. 2018, 592, 2739–2759. [Google Scholar] [CrossRef]
- Roberts, M.E.; Bhatia, M.P.; Rowland, E.; White, P.L.; Waterman, S.; Cavaco, M.A.; Williams, P.; Young, J.N.; Spence, J.S.; Tremblay, J.; et al. Rubisco in High Arctic Tidewater Glacier-marine Systems: A New Window into Phytoplankton Dynamics. Limnol. Oceanogr. 2024, 69, 802–817. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. Expansion of the Gene Ontology Knowledgebase and Resources. Nucleic Acids Res. 2017, 45, D331–D338. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hua, Y.; Luo, T.; Liu, C.; Zhang, B.; Zhang, X.; Liu, Y.; Zhu, Z.; Tao, Y.; Zhu, Z.; et al. Generating Waxy Rice Starch with Target Type of Amylopectin Fine Structure and Gelatinization Temperature by Waxy Gene Editing. Carbohydr. Polym. 2023, 306, 120595. [Google Scholar] [CrossRef]
- Huang, L.; Sreenivasulu, N.; Liu, Q. Waxy Editing: Old Meets New. Trends Plant Sci. 2020, 25, 963–966. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, T.; Ma, X.; Wang, B.; Zheng, Z.; Zhang, Y.; Xie, X.; Yang, B.; Zhao, Z.; Zhu, Q.; et al. Quantitative Regulation of Waxy Expression by CRISPR/Cas9-Based Promoter and 5′UTR-Intron Editing Improves Grain Quality in Rice. Plant Biotechnol. J. 2020, 18, 2385–2387. [Google Scholar] [CrossRef]
- Gao, H.; Gadlage, M.J.; Lafitte, H.R.; Lenderts, B.; Yang, M.; Schroder, M.; Farrell, J.; Snopek, K.; Peterson, D.; Feigenbutz, L.; et al. Superior Field Performance of Waxy Corn Engineered Using CRISPR–Cas9. Nat. Biotechnol. 2020, 38, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Coiro, M.; Meier, T.; Wicker, T.; Zeeman, S.C.; Santelia, D. The Evolution of Functional Complexity within the β-Amylase Gene Family in Land Plants. BMC Evol. Biol. 2019, 19, 66. [Google Scholar] [CrossRef]
- Monroe, J.D.; Breault, J.S.; Pope, L.E.; Torres, C.E.; Gebrejesus, T.B.; Berndsen, C.E.; Storm, A.R. Arabidopsis β-Amylase2 Is a K+-Requiring, Catalytic Tetramer with Sigmoidal Kinetics. Plant Physiol. 2017, 175, 1525–1535. [Google Scholar] [CrossRef]
- Funfgeld, M.M.F.F.; Wang, W.; Ishihara, H.; Arrivault, S.; Feil, R.; Smith, A.M.; Stitt, M.; Lunn, J.E.; Niittyla, T. Sucrose Synthases Are Not Involved in Starch Synthesis in Arabidopsis Leaves. Nat. Plants 2022, 8, 574–582. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Z.; Zhou, Q.; Zhao, J.; Zhao, Y.; Zhao, M.; Ma, S.; Fan, Y.; Huang, Z.; Zhang, W. An Integrated Physiology and Proteomics Analysis Reveals the Response of Wheat Grain to Low Temperature Stress during Booting. J. Integr. Agric. 2025, 24, 114–131. [Google Scholar] [CrossRef]
- Morabito, C.; Secchi, F.; Schubert, A. Grapevine TPS (Trehalose-6-Phosphate Synthase) Family Genes Are Differentially Regulated during Development, upon Sugar Treatment and Drought Stress. Plant Physiol. Biochem. 2021, 164, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]



| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
|---|---|---|---|---|---|---|---|
| Starch content/% | 1.95 ± 0.05 e | 37.54 ± 0.86 c | 41.71 ± 0.51 a | 39.18 ± 0.38 b | 40.18 ± 0.81 a | 39.06 ± 1.19 ab | 33.77 ± 0.81 d |
| Starch growth ratio | - | 18.28 | 0.11 | −0.06 | 0.03 | −0.03 | −0.12 |
| 0 h | 2 h | 4 h | 8 h | 12 h | 24 h | 48 h | 96 h | 192 h | |
|---|---|---|---|---|---|---|---|---|---|
| Starch Content/% | 1.95 ± 0.05 i | 2.95 ± 0.13 h | 5.78 ± 0.04 f | 4.26 ± 0.06 g | 7.84 ± 0.10 e | 37.55 ± 0.87 a | 41.71 ± 0.51 b | 28.58 ± 0.44 c | 19.78 ± 0.62 d |
| Amylose Content in Total Starch/% | 63.37 ± 3.12 c | 89.65 ± 3.03 a | 77.39 ± 4.41 b | 76.62 ± 3.79 b | 33.30 ± 0.82 e | 40.60 ± 1.12 d | 23.50 ± 3.84 fg | 19.50 ± 2.55 g | 26.90 ± 2.61 f |
| Amylopectin Content in Total Starch/% | 36.63 ± 3.12 e | 10.34 ± 3.03 g | 22.61 ± 4.40 f | 23.37 ± 3.79 f | 66.68 ± 0.82 c | 59.40 ± 1.12 d | 76.51 ± 3.84 ab | 80.49 ± 2.54 a | 73.37 ± 2.61 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Hong, Y.; Fang, Y.; Cheng, L.; Li, Z.; Li, C.; Ban, X. Transcriptomic and Metabolic Analysis Reveal Potential Mechanism of Starch Accumulation in Spirodela polyrhiza Under Nutrient Stress. Plants 2025, 14, 1617. https://doi.org/10.3390/plants14111617
Fang X, Hong Y, Fang Y, Cheng L, Li Z, Li C, Ban X. Transcriptomic and Metabolic Analysis Reveal Potential Mechanism of Starch Accumulation in Spirodela polyrhiza Under Nutrient Stress. Plants. 2025; 14(11):1617. https://doi.org/10.3390/plants14111617
Chicago/Turabian StyleFang, Xin, Yan Hong, Yang Fang, Li Cheng, Zhaofeng Li, Caiming Li, and Xiaofeng Ban. 2025. "Transcriptomic and Metabolic Analysis Reveal Potential Mechanism of Starch Accumulation in Spirodela polyrhiza Under Nutrient Stress" Plants 14, no. 11: 1617. https://doi.org/10.3390/plants14111617
APA StyleFang, X., Hong, Y., Fang, Y., Cheng, L., Li, Z., Li, C., & Ban, X. (2025). Transcriptomic and Metabolic Analysis Reveal Potential Mechanism of Starch Accumulation in Spirodela polyrhiza Under Nutrient Stress. Plants, 14(11), 1617. https://doi.org/10.3390/plants14111617








