Pharmacognostic Evaluation and Antioxidant Profiling of Five Varieties of Ribes nigrum Grown in Romania
Abstract
1. Introduction
2. Results
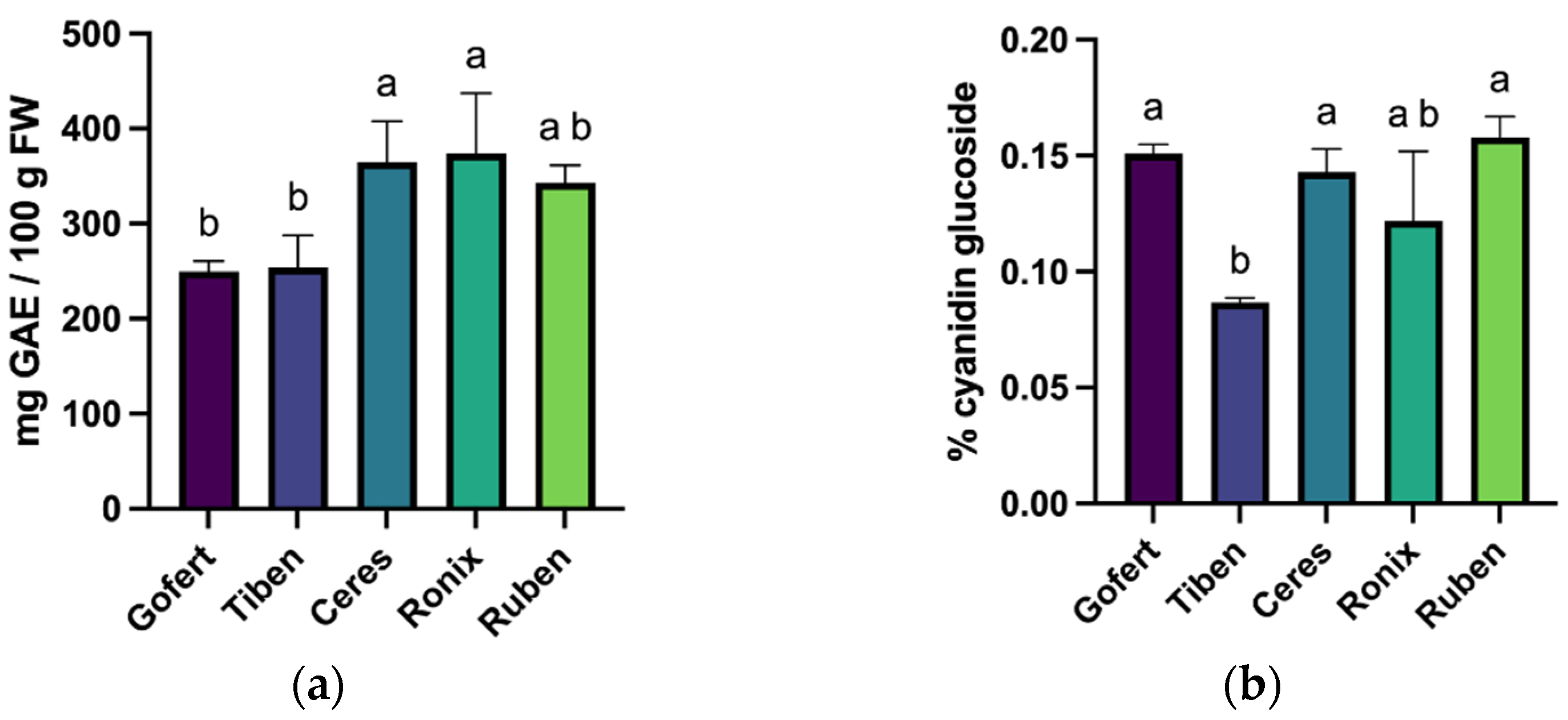
2.1. Total Polyphenol (TPC) and Total Anthocyanin (TA) Content
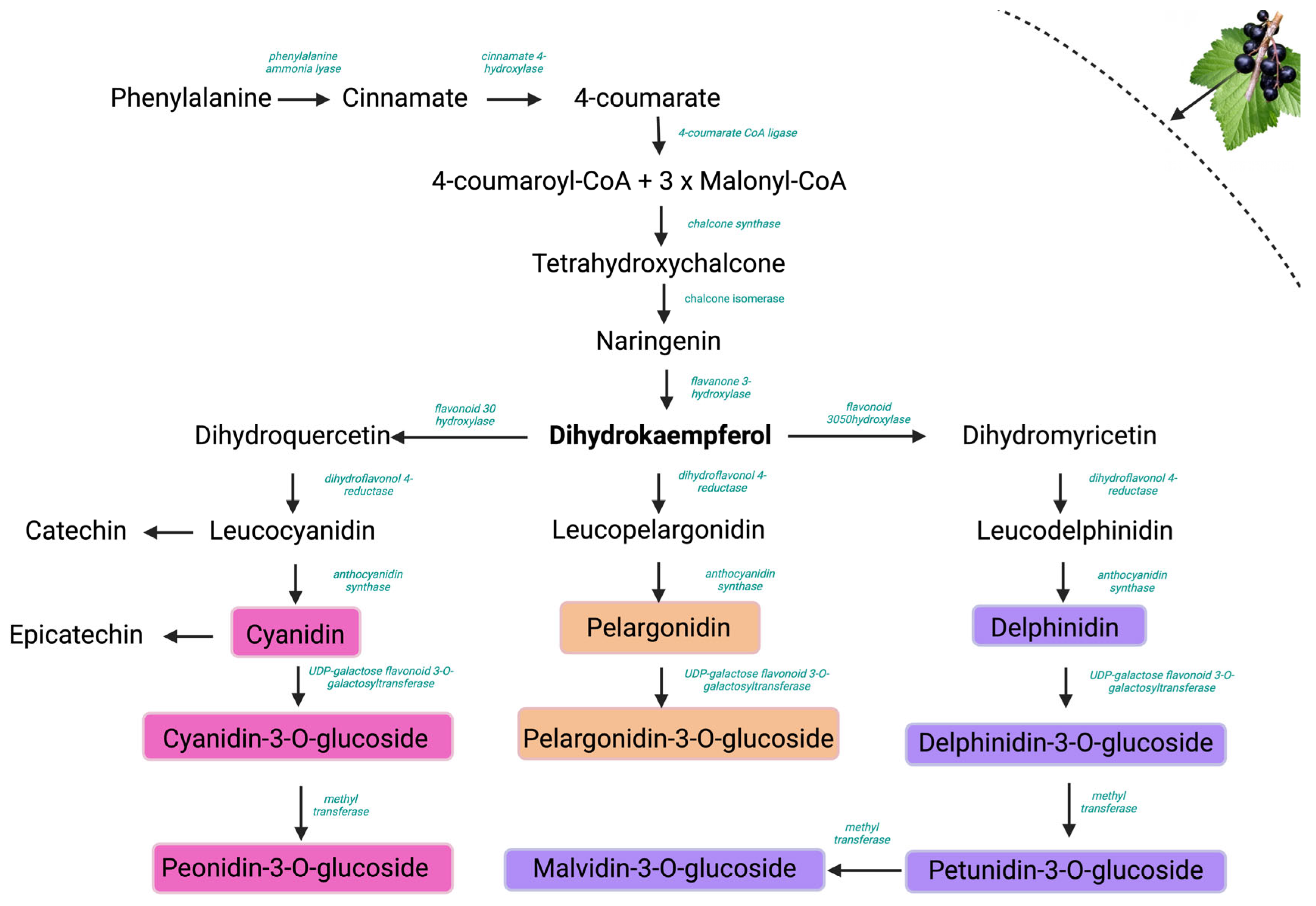
2.2. HPLC-DAD Analysis of Anthocyanins
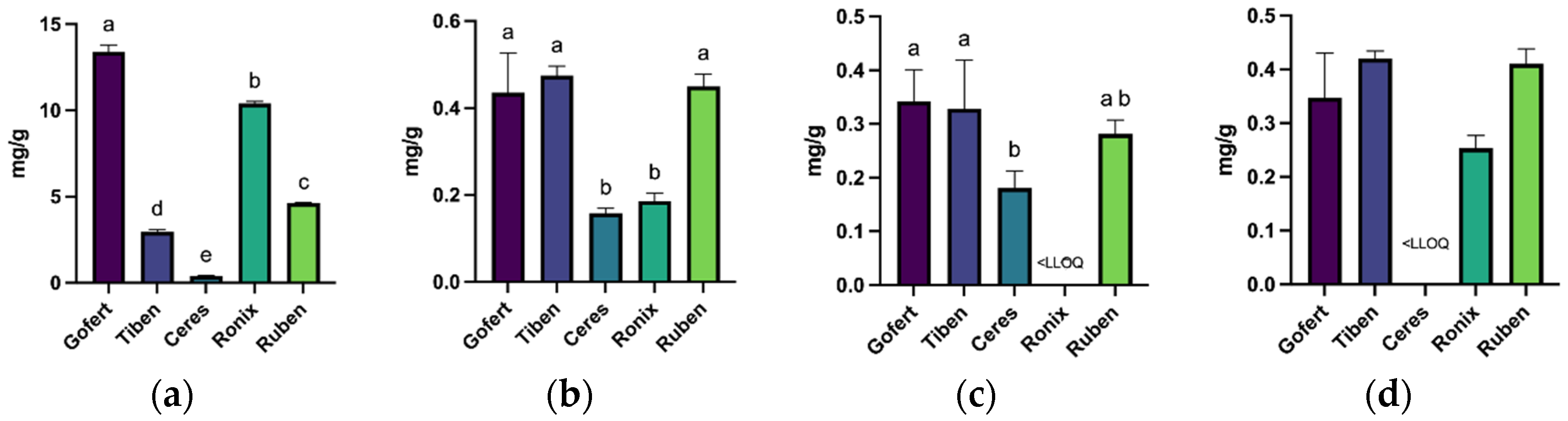
2.3. UHPLC-PDA Analysis of Phenolic Compounds
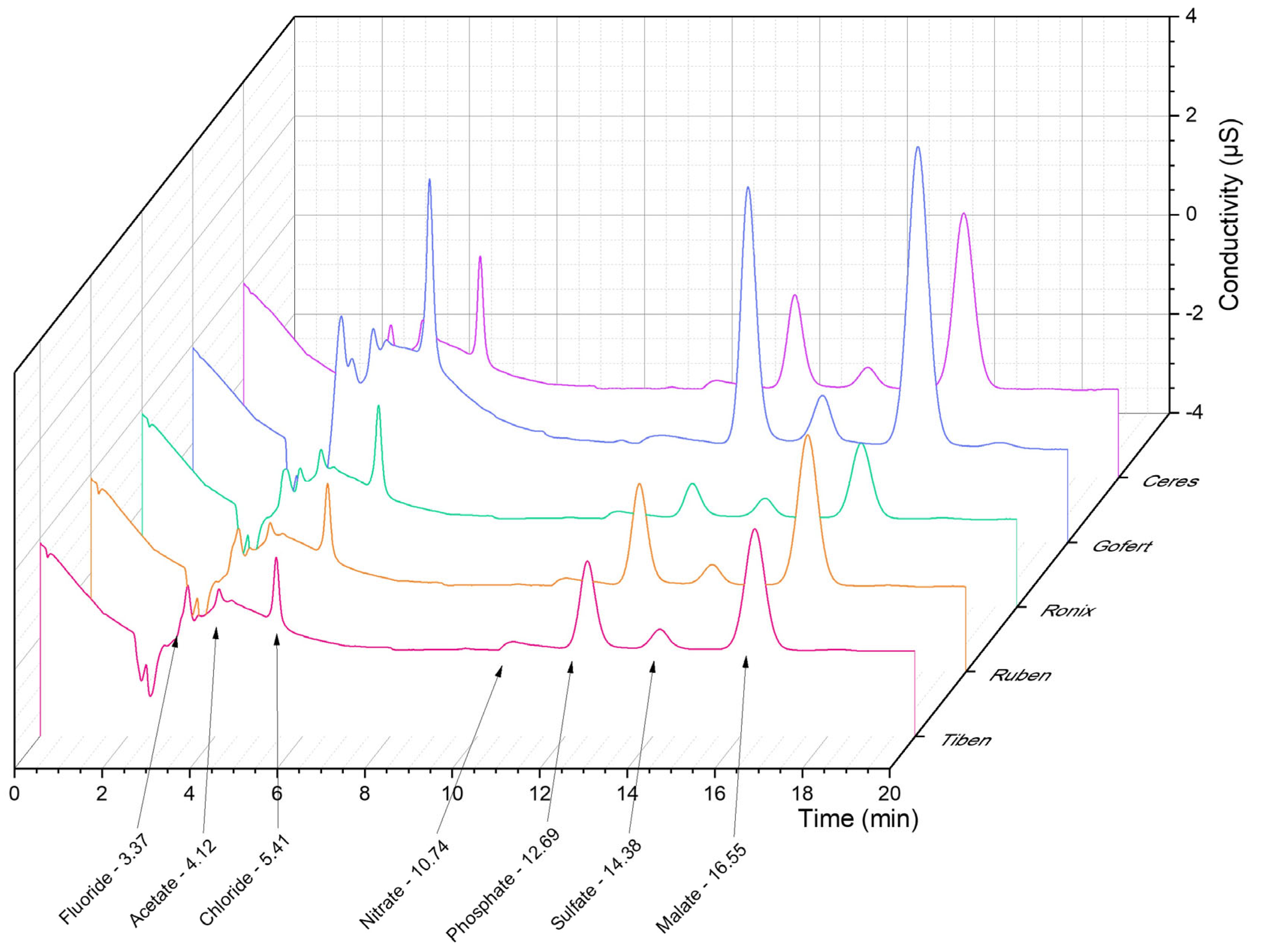
2.4. IC Analysis
2.5. Determination of Antioxidant Activity Through DPPH and ABTS Free Radical Scavenging Assay
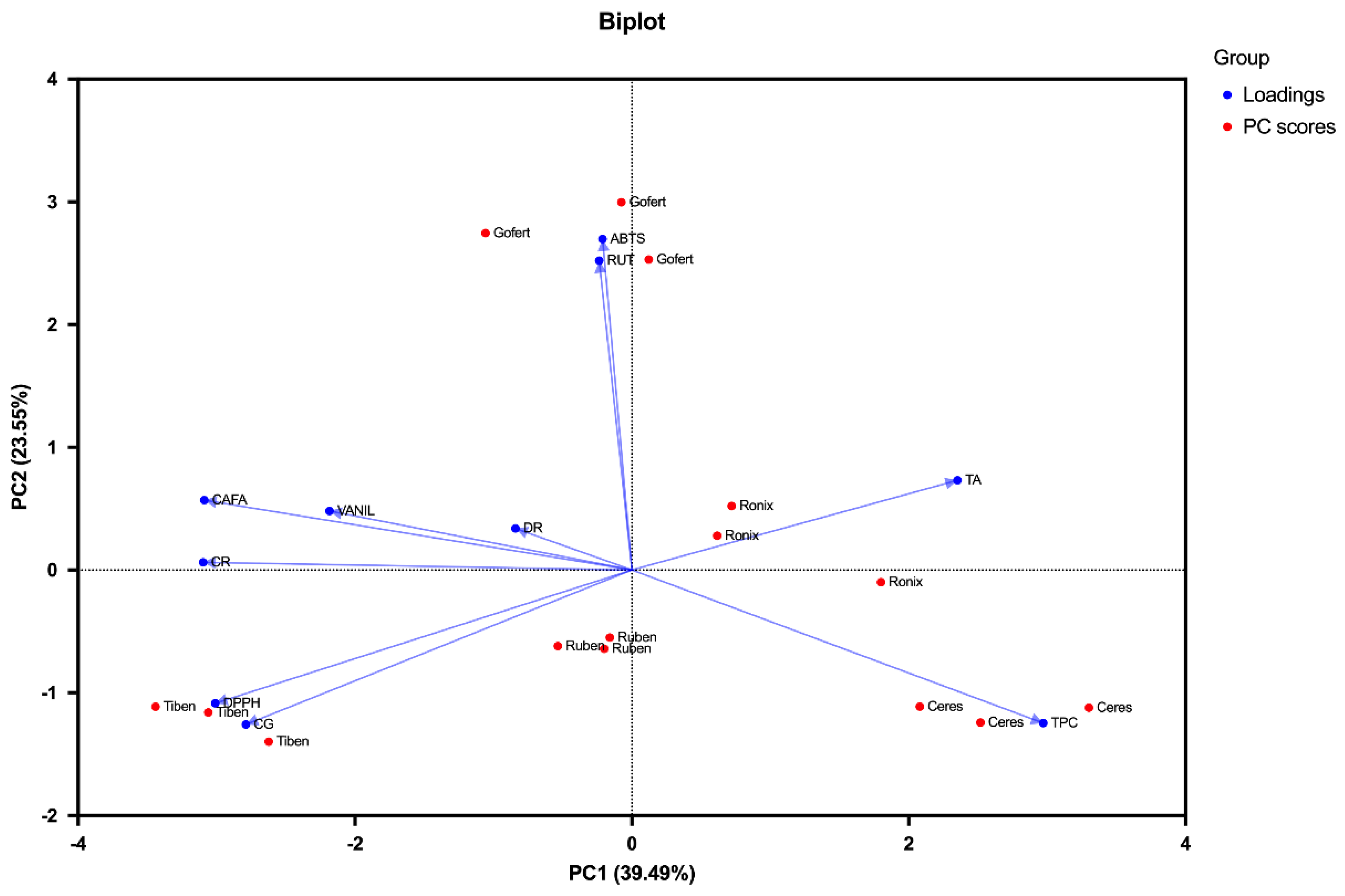
2.6. Pearson’s Correlation and Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Chemicals
4.3. Extracts Preparation
4.4. Total Polyphenol Content (TPC) Determination
4.5. Total Anthocyanin Content (TAC)
4.6. HPLC-DAD Analysis of Anthocyanins
4.7. UHPLC-PDA Analysis of Phenolic Compounds
4.8. Ion Chromatography Analysis
4.9. Antioxidant Activity Through the DPPH Free Radical Scavenging Assay
4.10. Antioxidant Activity Through the ABTS Free Radical Scavenging Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CAFA | caffeic acid |
| CAT | catechin |
| CG | cyanidin-3-O-glucoside |
| CR | cyanidin-3-O-rutinoside |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DR | delphinidin-3-O-rutinoside |
| FW | fresh weight |
| GAE | gallic acid equivalent |
| IC50 | concentration of inhibitor resulting in 50% inhibition |
| LLOQ | lower limit of quantification |
| LOQ | limit of quantification |
| NO | nitric oxide |
| PCA | principal component analysis |
| RUT | rutin |
| TPC | total polyphenolic content |
| VANIL | vanillic acid |
| XO | xanthine oxidase |
References
- Assessment Report on Ribes nigrum L., Folium. 29. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-ribes-nigrum-l-folium_en.pdf (accessed on 7 February 2025).
- Global-BC-Production-2022-2024.Pdf. Available online: https://www.blackcurrant-iba.com/wp-content/uploads/2024/07/Global-BC-Production-2022-2024.pdf (accessed on 7 February 2025).
- Nayik, G.A.; Gull, A. (Eds.) Antioxidants in Fruits: Properties and Health Benefits; Springer: Singapore, 2020; ISBN 9789811572845. [Google Scholar]
- Lee, Y.; Lee, J.-Y. Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. Nutrients 2019, 11, 975. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Ştefănescu, R.; Vari, C.; Imre, S.; Huţanu, A.; Fogarasi, E.; Todea, T.; Groşan, A.; Eşianu, S.; Laczkó-Zöld, E.; Dogaru, M. Vaccinium Extracts as Modulators in Experimental Type 1 Diabetes. J. Med. Food 2018, 21, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Molonia, M.S.; Muscarà, C.; Cristani, M.; Salamone, F.L.; Saija, A.; Cimino, F. An Overview on the Cellular Mechanisms of Anthocyanins in Maintaining Intestinal Integrity and Function. Fitoterapia 2024, 175, 105953. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.F.; Monteiro, V.V.S.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action Mechanism and Cardiovascular Effect of Anthocyanins: A Systematic Review of Animal and Human Studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Pluta, S.; Żurawicz, E. The Last Twenty Years Of Blackcurrant (Ribes nigrum L.) Breeding Work In Poland. Acta Hortic. 2009, 814, 309–314. [Google Scholar] [CrossRef]
- Pluta, S.; Zurawicz, E. “Tiben” And “Tisel”—New Blackcurrant Cultivars Released In Poland. Acta Hortic. 2002, 585, 221–223. [Google Scholar] [CrossRef]
- Pluta, S.; Żurawicz, E. Production Value And Suitability Of New Polish Blackcurrant Cultivars For Mechanical Fruit Harvesting. Acta Hortic. 2008, 777, 447–452. [Google Scholar] [CrossRef]
- Lanham, P.G.; Brennan, R.M.; Hackett, C.; McNicol, R.J. RAPD Fingerprinting of Blackcurrant (Ribes nigrum L.) Cultivars. Theoret. Appl. Genet. 1995, 90, 166–172. [Google Scholar] [CrossRef]
- Braniște, N. Soiuri de pomi, arbuști fructiferi și căpșuni create în România. Paralela 2007, 45, 476. [Google Scholar]
- Pluta, S.; Żurawicz, E. “Ores” And “Ruben”—New Blackcurrant Cultivars Bred In Poland. Acta Hortic. 2004, 663, 927–930. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; Volume 1. [Google Scholar]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Untea, A.E.; Oancea, A.-G.; Vlaicu, P.A.; Varzaru, I.; Saracila, M. Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization before and after In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation. Foods 2024, 13, 1514. [Google Scholar] [CrossRef]
- Laczkó-Zöld, E.; Komlósi, A.; Ülkei, T.; Fogarasi, E.; Croitoru, M.; Fülöp, I.; Domokos, E.; Ştefănescu, R.; Varga, E. Extractability of Polyphenols from Black Currant, Red Currant and Gooseberry and Their Antioxidant Activity. Acta Biol. Hung. 2018, 69, 156–169. [Google Scholar] [CrossRef]
- Karaklajic-Stajic, Z.; Tomic, J.; Pesakovic, M.; Paunovic, S.M.; Stampar, F.; Mikulic-Petkovsek, M.; Grohar, M.C.; Hudina, M.; Jakopic, J. Black Queens of Fruits: Chemical Composition of Blackberry (Rubus Subg. Rubus Watson) and Black Currant (Ribes nigrum L.) Cultivars Selected in Serbia. Foods 2023, 12, 2775. [Google Scholar] [CrossRef] [PubMed]
- Kierońska, E.; Skoczylas, J.; Dziadek, K.; Pomietło, U.; Piątkowska, E.; Kopeć, A. Basic Chemical Composition, Selected Polyphenolic Profile and Antioxidant Activity in Various Types of Currant (Ribes spp.) Fruits. Appl. Sci. 2024, 14, 8882. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from Black Currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef]
- Paunović, S.M.; Mašković, P.; Nikolić, M.; Miletić, R. Bioactive Compounds and Antimicrobial Activity of Black Currant (Ribes nigrum L.) Berries and Leaves Extract Obtained by Different Soil Management System. Sci. Hortic. 2017, 222, 69–75. [Google Scholar] [CrossRef]
- Maatta, K.; Kamal-Eldin, A.; Törrönen, R. Phenolic Compounds in Berries of Black, Red, Green, and White Currants (Ribes sp.). Antioxid. Redox Signal. 2001, 3, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Šimerdová, B.; Bobríková, M.; Lhotská, I.; Kaplan, J.; Křenová, A.; Šatínský, D. Evaluation of Anthocyanin Profiles in Various Blackcurrant Cultivars over a Three-Year Period Using a Fast HPLC-DAD Method. Foods 2021, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.-M.; Veteläinen, M. High Variability in Flavonoid Contents and Composition between Different North-European Currant (Ribes spp.) Varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef]
- Kikas, A.; Rätsep, R.; Kaldmäe, H.; Aluvee, A.; Libek, A.-V. Comparison of Polyphenols and Anthocyanin Content of Different Blackcurrant (Ribes nigrum L.) Cultivars at the Polli Horticultural Research Centre in Estonia. Agron. Res. 2020, 18, 2715–2726. [Google Scholar]
- Tian, Y.; Laaksonen, O.; Haikonen, H.; Vanag, A.; Ejaz, H.; Linderborg, K.; Karhu, S.; Yang, B. Compositional Diversity among Blackcurrant (Ribes nigrum) Cultivars Originating from European Countries. J. Agric. Food Chem. 2019, 67, 5621–5633. [Google Scholar] [CrossRef]
- Jaakola, L. New Insights into the Regulation of Anthocyanin Biosynthesis in Fruits: Trends in Plant Science. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef]
- Parkar, S.G.; Redgate, E.L.; McGhie, T.K.; Hurst, R.D. In Vitro Studies of Modulation of Pathogenic and Probiotic Bacterial Proliferation and Adhesion to Intestinal Cells by Blackcurrant Juices. J. Funct. Foods 2014, 8, 35–44. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Rachtan-Janicka, J.; Ponder, A.; Hallmann, E. The Effect of Organic and Conventional Cultivations on Antioxidants Content in Blackcurrant (Ribes nigrum L.) Species. Appl. Sci. 2021, 11, 5113. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Rescic, J.; Schmitzer, V.; Stampar, F.; Slatnar, A.; Koron, D.; Veberic, R. Changes in Fruit Quality Parameters of Four Ribes Species during Ripening. Food Chem. 2015, 173, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Kumar, D.; Sasmal, D.; Mukhopadhyay, K. Antioxidant and DNA Damage Protective Properties of Anthocyanin-Rich Extracts from Hibiscus and Ocimum: A Comparative Study. Nat. Prod. Res. 2014, 28, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 107–115. ISBN 978-1-119-13538-8. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Babotă, M.; Nișca, A.; Nicolescu, A.; Ștefănescu, R.; Mocan, A.; Farczadi, L.; Mare, A.D.; Ciurea, C.N.; Man, A. Potential Use of Quercus dalechampii Ten. and Q. frainetto Ten. Barks Extracts as Antimicrobial, Enzyme Inhibitory, Antioxidant and Cytotoxic Agents. Pharmaceutics 2023, 15, 343. [Google Scholar] [CrossRef]


| Variety | Parents and Origin | Characteristics | References |
|---|---|---|---|
| Gofert | Golubka × Fertodi-1 Origin: Poland | It is an early-maturing and high-yielding variety, exhibiting enhanced resistance to pests. The fruits are of medium size and are characterized by low acidity, while both anthocyanin and vitamin C levels are high. | [9] |
| Tiben | Titania × Ben Nevis Origin: Poland | The shrubs reach medium size, as do the fruits, which are also of moderate dimensions. This variety demonstrates strong resistance to diseases. The vitamin C content is modest. | [10,11] |
| Ceres | Pavlinka × Pilot Origin: Poland | This variety is characterized by medium-sized shrubs and large fruits, with enhanced resistance to frost. The fruit harvesting period is late, typically occurring in mid-July. The ‘Ceres’ variety is distinguished by its low acidity. | [12] |
| Ronix | Tsema × Kantata Origin: Romania | The shrub exhibits vigorous growth and large dimensions, while the berries are resistant to cracking. This semi-early variety shows good disease resistance and is notable for its high vitamin C content as well as high acidity. | [13] |
| Ruben | Bieloruskoja Slodkaja × Ben Lomond Origin: Poland | It is a high-yielding variety, characterized by medium to large fruits, with notable resistance to various diseases and tolerance to harsh winter conditions. | [11,14] |
| Variety | IC50 DPPH (µg/mL) | IC50 ABTS (µg/mL) |
|---|---|---|
| Gofert | 1.95 ± 0.26 b | 3.06 ± 0.14 a |
| Tiben | 2.78 ± 0.07 a | 0.71 ± 0.10 c |
| Ceres | 1.89 ± 0.21 b | 0.58 ± 0.10 c |
| Ronix | 1.96 ± 0.25 b | 1.39 ± 0.37 b |
| Ruben | 1.98 ± 0.12 b | 0.56 ± 0.07 c |
| Origin of the Analyzed Samples | TPC (mg GAE/100 g FW) | TAC (%) | References |
|---|---|---|---|
| Finland | - | 0.27–0.67 | [27] |
| Romania | 110–195 | 0.18–0.32 | [18] |
| Serbia | - | 0.20–0.31 | [24] |
| Serbia | 164 | 0.13 | [19] |
| Belarus | 354–408 | 0.23–0.27 | [28] |
| Estonia | 324–506 | 0.19–0.35 | [28] |
| Finland | 428–534 | 0.27–0.36 | [28] |
| Latvia | 358 | 0.24 | [28] |
| Lithuania | 328–470 | 0.20–0.32 | [28] |
| Norway | 290–634 | 0.18–0.47 | [28] |
| Poland | 362–515 | 0.24–0.35 | [28] |
| Russia | 460 | 0.31 | [28] |
| Scotland | 469–613 | 0.30–0.44 | [28] |
| Sweden | 343–529 | 0.22–0.37 | [28] |
| Ukraine | 424 | 0.29 | [28] |
| Italy | 530–888 | 0.15–0.28 | [33] |
| Poland | 163–195 * | 0.14–0.17 * | [34] |
| Slovenia | 452–662 | - | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefănescu, R.; Boda, F.; Sebestyen, M.; Râșteiu, I.; Laczkó-Zöld, E.; Farczádi, L. Pharmacognostic Evaluation and Antioxidant Profiling of Five Varieties of Ribes nigrum Grown in Romania. Plants 2025, 14, 1604. https://doi.org/10.3390/plants14111604
Ștefănescu R, Boda F, Sebestyen M, Râșteiu I, Laczkó-Zöld E, Farczádi L. Pharmacognostic Evaluation and Antioxidant Profiling of Five Varieties of Ribes nigrum Grown in Romania. Plants. 2025; 14(11):1604. https://doi.org/10.3390/plants14111604
Chicago/Turabian StyleȘtefănescu, Ruxandra, Francisc Boda, Monica Sebestyen, Ioana Râșteiu, Eszter Laczkó-Zöld, and Lénárd Farczádi. 2025. "Pharmacognostic Evaluation and Antioxidant Profiling of Five Varieties of Ribes nigrum Grown in Romania" Plants 14, no. 11: 1604. https://doi.org/10.3390/plants14111604
APA StyleȘtefănescu, R., Boda, F., Sebestyen, M., Râșteiu, I., Laczkó-Zöld, E., & Farczádi, L. (2025). Pharmacognostic Evaluation and Antioxidant Profiling of Five Varieties of Ribes nigrum Grown in Romania. Plants, 14(11), 1604. https://doi.org/10.3390/plants14111604








