Smart Bioinoculants for Arachis hypogaea: Controlled Release of Bradyrhizobium and the Role of Naringin in Symbiosis Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Entrapment Conditions of Bradyrhizobium sp. SEMIA6144
2.3. Viability Characterization of Immobilized Cells
2.4. Determination of the Effect of Naringin on Cellular Respiratory Activity and ROS Production
2.5. Naringin Intracellular Content
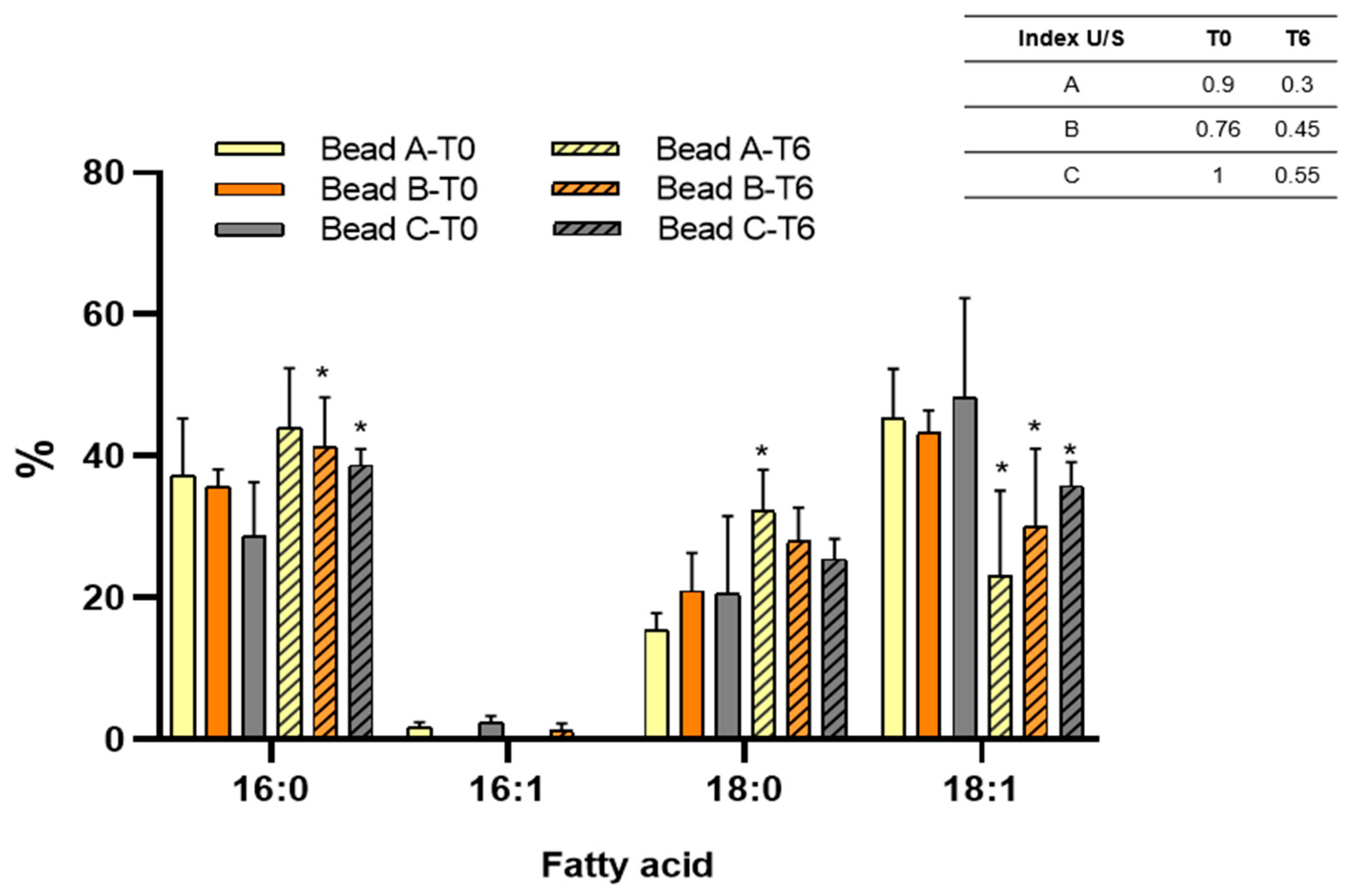
2.6. Membrane Fatty Acids Composition of Immobilized Bacteria
2.7. Chemical Beads Characterization
2.8. Bead Swelling Behavior
2.9. In Vitro Release of Bacteria and Flavonoid from Beads
2.10. Release Mechanism Prediction
2.11. Pot Experiment and Nodulation Kinetics Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Viability and Metabolic Bacteria Activity of SEMIA6144 in New Beads
3.2. Viability of SEMIA6144 in 6-Month Beads
3.3. Physicochemical Characterization of Beads
3.4. Membrane Fatty Acids Composition of Immobilized Bacteria
3.5. Bead Swelling and Release of Bacterial and Naringin
3.6. Release Mechanism Studies
3.7. Bacterial Colonization of Peanut Root and Nodulation Kinetic
3.8. Multivariate Statistical Analysis of Principal Components
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sizenando, C.I.T.; Ramos, J.P.C.; Fernandes-Junior, P.I.; Lima, L.M.; Freire, R.M.M.; Santos, R.C. Agronomic efficiency of Bradyrhizobium in peanut under different environments in Brazilian Northeast. Afr. J. Agric. Res. 2016, 11, 3482–3487. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Sprent, J.I. Evolving ideas of legume evolution and diversity: A taxonomic perspective on the occurrence of nodulation. New Phytol. 2007, 174, 575–581. [Google Scholar] [CrossRef]
- Ibáñez, F.; Fabra, A. Rhizobial Nod factors are required for cortical cell division in the nodule morphogenetic programme of the Aeschynomeneae legume Arachis. Plant Biol. 2011, 13, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, N.M.; Bouwmeester, H.J. Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Lam, P.Y.; Dini-Andreote, F.; Dai, L.; Wei, Z. Multifaceted roles of flavonoids mediating plant-microbe interactions. Microbiome 2022, 10, 233. [Google Scholar] [CrossRef]
- Bolaños-Vásquez, M.C.; Werner, D. Effects of Rhizobium tropici, R. etli, and R. leguminosarum bv. phaseoli on nod gene-inducing flavonoids in root exudates of Phaseolus vulgaris. Mol. Plant-Microbe Interact. 1997, 10, 339–346. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat.Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Bhattacharyya, S.; Kumar, R.; Kumar, A.; Ibañez, F.; Wang, J.; Pandey, M.K. Molecular basis of root nodule symbiosis between Bradyrhizobium and ‘crack-entry’legume groundnut (Arachis hypogaea L.). MDPI Plants 2020, 9, 276. [Google Scholar] [CrossRef]
- D’Aoust, F.; Begum, A.; Zhang, H.; Smith, D.; Driscoll, B.; Charles, T. Bradyrhizobium japonicum mutants with enhanced sensitivity to genistein resulting in altered nod gene regulation. Mol. Plant-Microbe Interact. 2001, 14, 1404–1410. [Google Scholar] [CrossRef]
- Zhang, F.; Lynch, D.H.; Smith, D.L. Impact of low root zone temperatures in soybean [Glycine max (L.) Merr] on nodulation and nitrogen fixation. Environ. Exp. Bot. 1995, 35, 279–285. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.K.; Jain, V.; Nainawate, H.S. Nitrate alters the flavonoid profile and nodulation in pea (Pisum sativum L.). Biol. Fert. Soils 1996, 21, 189–192. [Google Scholar] [CrossRef]
- Recourt, K.; van Brussel, A.A.; Driessen, A.J.; Lugtenberg, B.J. Accumulation of a nod gene inducer, the flavonoid naringenin, in the cytoplasmic membrane of Rhizobium leguminosarum biovar viciae is caused by the pH-dependent hydrophobicity of naringenin. J. Bacteriol. 1989, 171, 4370–4377. [Google Scholar] [CrossRef] [PubMed]
- Cesari, A.; Paulucci, N.; López-Gómez, M.; Hidalgo-Castellanos, J.; Plá, C.L.; Dardanelli, M.S. Restrictive water condition modifies the root exudates composition during peanut-PGPR interaction and conditions early events, reversing the negative effects on plant growth. Plant Physiol. Biochem. 2019, 142, 519–527. [Google Scholar] [CrossRef]
- Barto, E.; Cipollini, D. Half-lives and field soil concentrations of Alliaria petiolata secondary metabolites. Chemosphere 2009, 76, 71–75. [Google Scholar] [CrossRef]
- Guo, X.; Yue, Y.; Tang, F.; Wang, J.; Yao, X.; Sun, J. A comparison of C-glycosidic flavonoid isomers by electrospray ionization quadrupole time-of-flight tandem mass spectrometry in negative and positive ion mode. Int. J. Mass. Spectrom. 2013, 333, 59–66. [Google Scholar] [CrossRef]
- Ghorbani, M.; Kahrizi, D.; Arkan, E.; Aghaz, F. Enhancing rooting tobacco (Nicotiana tabacum) plant by loaded indole-3-butyric acid in alginate/chitosan nanocapsule. Cell. Mol. Biol. 2024, 70, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.; do ES Pereira, A.; Aleksieienko, I.; Do Carmo, G.C.; Gohari, G.; Santaella, C.; Oliveira, H.C. Encapsulated plant growth regulators and associative microorganisms: Nature-based solutions to mitigate the effects of climate change on plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef]
- Cesari, A.B.; Paulucci, N.S.; Yslas, E.I.; Dardanelli, M.S. Immobilization of Bradyrhizobium and Azospirillum in alginate matrix for long time of storage maintains cell viability and interaction with peanut. Appl. Microbiol. Biotechnol. 2020, 104, 10145–10164. [Google Scholar] [CrossRef]
- Liffourrena, A.S.; Lucchesi, G.I. Alginate-perlite encapsulated Pseudomonas putida A (ATCC 12633) cells: Preparation, characterization and potential use as plant inoculants. J. Biotechnol. 2018, 278, 28–33. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, L.; Lang, D.; Zhang, X.; Ma, X.; Li, X.; Zhang, X. Eco-friendly bio-encapsulation from sodium alginate-trehalose-kaolin and its performance evaluation in improving plant growth under salt or/and drought conditions. Int. J. Biol. Macromol. 2023, 225, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Cesari, A.B.; Fernandez, M.; Paulucci, N.S.; Dardanelli, M.S. Long-Life Inoculant: Bradyrhizobium Stored in Biodegradable Beads for Four Years Shows Optimal Cell Vitality, Interacts with Peanut Roots, and Promotes Early Growth. Plants 2024, 13, 2983. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert. Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in Legume-Rhizobium Technology; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Schoebitz, M.; Lopez Belch, M.D. Encapsulation techniques for plant growth-promoting rhizobacteria. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 251–265. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Garms, B.C.; Poli, H.; Baggley, D.; Han, F.Y.; Whittaker, A.K.; Grøndahl, L. Evaluating the effect of synthesis, isolation, and characterisation variables on reported particle size and dispersity of drug loaded PLGA nanoparticles. Mater. Adv. 2021, 2, 5657–5671. [Google Scholar] [CrossRef]
- da Silva Romeiro, R. Tecnica de Microgota para Contagem de Celulas Bacterianas Viaveisem Uma Suspensao. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 1996. [Google Scholar]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.; Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef]
- Lin, M.; Liang, S.Z.; Shi, J.; Niu, L.Z.; Chen, J.B.; Zhang, M.J.; Xu, K.C. Circulating tumor cell as a biomarker for evaluating allogenic NK cell immunotherapy on stage IV non-small cell lung cancer. Immunol. Lett. 2017, 191, 10–15. [Google Scholar] [CrossRef]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlaginger, G.J. A microplate assay for the detection of oxidative products using 2’, 7’-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Hubac, C.; Ferran, J.; Guerrier, D.; Tremolieres, A.; Kondorosi, A. Luteolin absorption in Rhizobium meliloti wild-type and mutant strains. Microbiology 1993, 139, 1571–1578. [Google Scholar] [CrossRef][Green Version]
- Bligh, E.; Dyer, W. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Saarai, A.; Sedlacek, T.; Kasparkova, V.; Kitano, T.; Saha, P. On the characterization of sodium alginate/gelatine-based hydrogels for wound dressing. J. Appl. Polym. Sci 2012, 126, E79–E88. [Google Scholar] [CrossRef]
- Hoagland, D.; Arnon, D. The water-culture method for growing plants without soil. Circ. Calif. Univ. Agric. Exp. Stn. 1938, 347, 1–39. [Google Scholar]
- Young, C.; Rekha, P.; Wei-An, L.; Arun, A. Encapsulation of plant growth-promoting bacteria in alginate beads enriched with humic acid. Biotechnol. Bioeng. 2006, 95, 76–83. [Google Scholar] [CrossRef]
- Jay, S.M.; Saltzman, W.M. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J. Control. Release 2009, 134, 26–34. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Gupta, A. A study towards release dynamics of Thiram fungicide from starch—Alginate beads to control environmental and health hazards. J. Hazard. Mater. 2009, 161, 208–216. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.G.; Chen, X.S. Controlled release of urea encapsulated by starch-g-poly (g). Carbohyd. Polym. 2008, 72, 342–348. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Guha, S.; Molla, F.; Sarkar, M.; Ibañez, F.; Fabra, A.; DasGupta, M. Nod factor-independent ‘crack-entry’ symbiosis in dalbergoid legume Arachis hypogaea. Environ. Microbiol. 2022, 24, 2732–2746. [Google Scholar] [CrossRef]
- Vincent, J.M. A manual for the practical study of the root-nodule bacteria, International Biological Program. In IBP Handbook; Blackwell Scientific Publications: Oxford, UK, 1970. [Google Scholar]
- Albareda, M.; Dardanelli, M.; Sousa, C.; Megías, M.; Temprano, F.; Rodriguez-Navarro, D.N. Factors affecting the attachment of rhizospheric bacteria to bean and soybean roots. FEMS Microbiol. Lett 2006, 259, 68–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Dal Piaz, F. Interactions with microbial proteins driving the antibacterial activity of flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef]
- Fathima, N. Association of Peroxisomes, Reactive Oxygen Species (ROS) and Antioxidants: Insights from Preclinical and Clinical Evaluations; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250. [Google Scholar] [CrossRef]
- Ferreira, S.; Silva, F.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Resveratrol against Arcobacter butzleri and Arcobacter cryaerophilus: Activity and effect on cellular functions. Int. J. Food Microbiol. 2014, 180, 62–68. [Google Scholar] [CrossRef]
- Caldeira, E.; Piskin, E.; Granadeiro, L.; Silva, F.; Gouveia, I.C. Biofunctionalization of cellulosic fibres with l-cysteine: Assessment of antibacterial properties and mechanism of action against Staphylococcus aureus and Klebsiella pneumoniae. J. Biotechnol. 2013, 168, 426–435. [Google Scholar] [CrossRef]
- Morishige, Y.; Fujimori, K.; Amano, F. Use of flow cytometry for quantitative analysis of metabolism of VBNC (Viable But Non-Culturable) Salmonella. Biol. Pharm. Bull. 2015, 38, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, E.J.; Jeong, K.W.; Kim, Y.M. Antimicrobial flavonoid, 3, 6-dihydroxyflavone, have dual inhibitory activity against KAS III and KAS I. Bull. Korean Chem. Soc. 2011, 32, 3219–3222. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A. Recovery of plant growth-promoting rhizobacteria from sodium alginate beads after 3 years following storage at 4 °C. J. Ind. Microbiol. Biotechnol. 2008, 35, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.F.; Saidi, N.B.; Vadamalai, G.; The, C.Y.; Zulperi, D. Effect of bioformulations on the biocontrol efficacy, microbial viability and storage stability of a consortium of biocontrol agents against Fusarium wilt of Banana. J. Appl. Microbiol 2019, 127, 544–555. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, L.; Qin, S.; Li, C. Encapsulation of R. planticola Rs-2 from alginate-starch-bentonite and its controlled release and swelling behavior under simulated soil conditions. Arch. Microbiol. 2012, 39, 317–327. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Kozłowska, J.; Baczyńska, D.; Krzyżek, P. Ether Derivatives of Naringenin and Their Oximes as Factors Modulating Bacterial Adhesion. Antibiotics 2023, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Wongverawattanakul, C.; Suklaew, P.O.; Chusak, C.; Adisakwattana, S.; Thilavech, T. Encapsulation of Mesona chinensis benth extract in alginate beads enhances the stability and antioxidant activity of polyphenols under simulated gastrointestinal digestion. Foods 2022, 11, 2378. [Google Scholar] [CrossRef]
- Fathi, F.; Saberi-Riseh, R.; Khodaygan, P. Survivability and controlled release of alginate-microencapsulated Pseudomonas fluorescens VUPF506 and their effects on biocontrol of Rhizoctonia solani on potato. Int. J. Biol. Macromol. 2021, 183, 627–634. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Chapter 3: Nuclear magnetic resonance: Part one: Basic concept. In Pavia-Introduction to Spectroscopy, 3rd ed; Thomson Learning Academic Resource Center: Irvine, CA, USA, 2001; pp. 152–154. [Google Scholar]
- Puri, M.; Kaur, A.; Schwarz, W.H.; Singh, S.; Kennedy, J.F. Molecular characterization and enzymatic hydrolysis of naringin extracted from kinnow peel waste. Int. J. Biol. Macromol. 2011, 48, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Murínová, S.; Dercová, K. Response Mechanisms of Bacterial Degraders to Environmental Contaminants on the Level of Cell Walls and Cytoplasmic Membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef]
- Wang, L.H.; Zeng, X.A.; Wang, M.S.; Brennan, C.S.; Gong, D. Modification of membrane properties and fatty acids biosynthesis-related genes in Escherichia coli and Staphylococcus aureus: Implications for the antibacterial mechanism of naringenin. BBA-Biomembr. 2018, 1860, 481–490. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Linden, M.; Flegler, A.; Feuereisen, M.M.; Weber, F.; Lipski, A.; Schieber, A. Effects of flavonoids on membrane adaptation of food-associated bacteria. Biochim. Biophys. Acta-Biomembr. 2023, 1865, 184137. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Khademi, Z.; Jones, D.L.; Malakouti, M.J.; Asadi, F.; Ardebili, M. Organic acid mediated nutrient extraction efficiency in three calcareous soils. Aust. J. Soil. Res. 2009, 47, 213–220. [Google Scholar] [CrossRef]
- Ćujić, N.; Bugarski, B.; Ibrić, S.; Pljevljakušić, D.; Šavikin, K. Chokeberry (Aronia melanocarpa L.) extract loaded in alginate and alginate/inulin system. Ind. Crop. Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R.; Orts, W.J.; Mattoso, L.H.C. Polyacrylamide and methylcellulose hydrogel as delivery vehicles for the controlled release of paraquat pesticide. J. Mater. Sci. 2010, 45, 4977–4985. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Lee, J.; Li, Y.; Dong, H. Controlled release of herbicide acetochlor from clay/carboxymethylcellulose gel formulations. J. Agric. Food Chem. 2008, 56, 1336–1342. [Google Scholar] [CrossRef]
- Mbengue, M.D.; Hervé, C.; Debellé, F. Nod factor signaling in symbiotic nodulation. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 1–39. [Google Scholar] [CrossRef]
- Taboada, H.; Dunn, M.F.; Meneses, N.; Vargas-Lagunas, C.; Buchs, N.; Andrade-Domínguez, A.; Encarnación, S. Qualitative changes in proteins contained in outer membrane vesicles produced by Rhizobium etli grown in the presence of the nod gene inducer naringenin. Arch. Microbiol. 2019, 201, 1173–1194. [Google Scholar] [CrossRef]
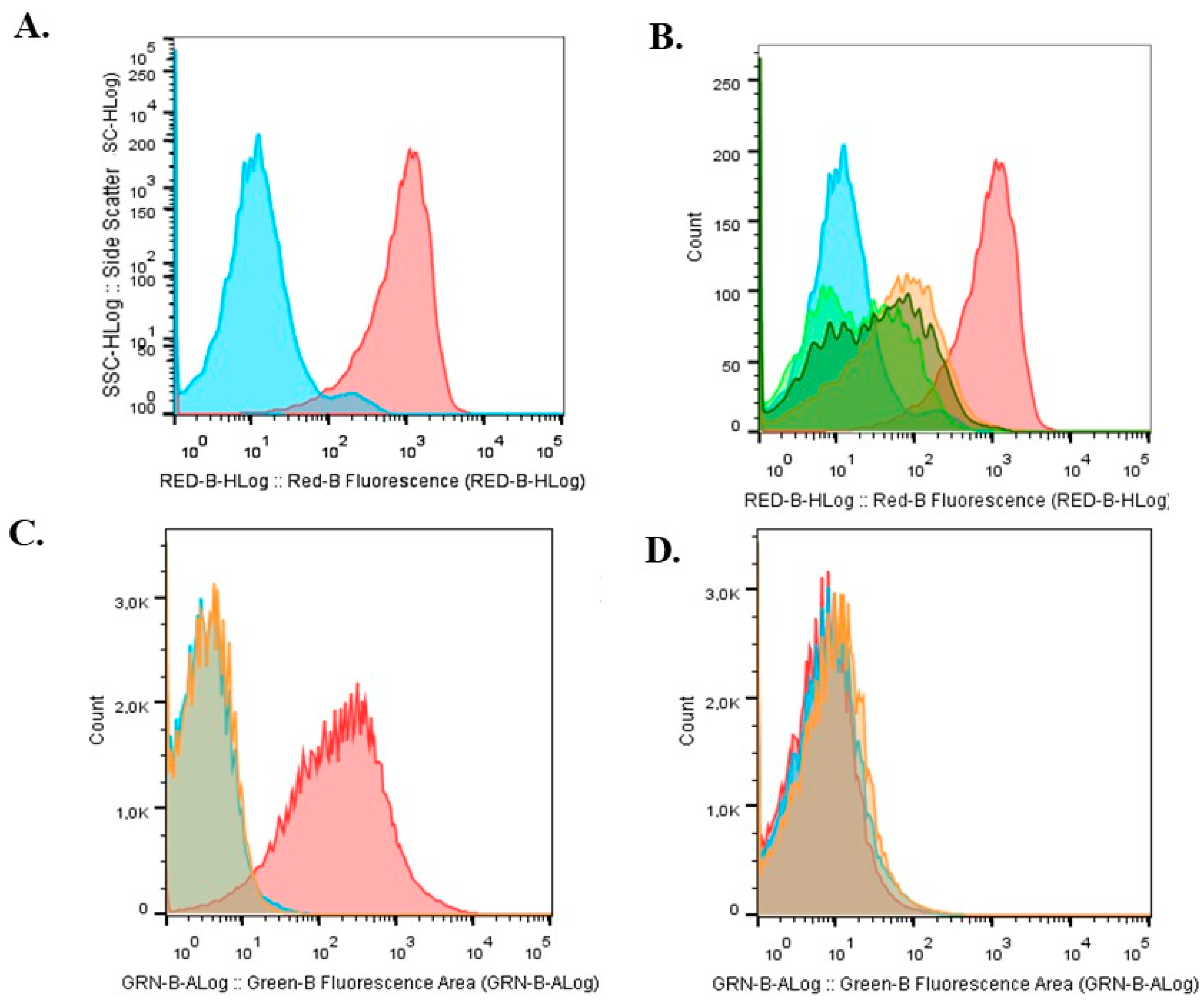





| Condition | Yield (%) | T0 | T6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nar (µg·µg−1) | Viability (CFU·mL−1) | Live % | Dead % | Nar (µg·µg−1) | Viability (CFU/L) | Live % | Dead % | ||
| Bead A | 79.7 ± 5 | 0 | 4.8 × 108 a | 96 ± 3 a | 0.57 ± 0.1 a | 0 | 7.8 × 107 a | 95.7 ± 3 a | 2.1 ± 0.8 a |
| Bead B | 76.1 ± 4 | 0.4 ± 0.02 a | 1.1 × 109 a | 96.6 ± 3 a | 1.08 ± 0.2 b | 0.6 ± 0.1 a | 1.5 × 108 a | 96 ± 1 a | 3.1 ± 0.9 b |
| Bead C | 74.8 ± 6 | 0.7 ± 0.2 b | 1.8 × 109 a | 97 ± 2.8 a | 1.41 ± 0.2 c | 0.6 ± 0.2 a | 8.6 × 107 a | 97 ± 2 a | 1.09 ± 0.3 b |
| Model Parameters | |||
|---|---|---|---|
| Bacterial release in PS | n | k | r |
| Bead A | 0.12 | 0.74 | 0.95 |
| Bead B | 0.11 | 0.74 | 0.94 |
| Bead C | 0.18 | 0.64 | 0.97 |
| Bacterial release in RE | n | k | r |
| Bead A | 0.07 | 0.85 | 0.95 |
| Bead B | 0.06 | 0.86 | 0.94 |
| Bead C | 0.10 | 0.78 | 0.96 |
| Naringin release | n | k | r |
| PS | 0.45 | 0.37 | 0.97 |
| RE | 0.53 | 0.3 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesari, A.B.; Paulucci, N.S.; Dardanelli, M.S. Smart Bioinoculants for Arachis hypogaea: Controlled Release of Bradyrhizobium and the Role of Naringin in Symbiosis Enhancement. Plants 2025, 14, 1601. https://doi.org/10.3390/plants14111601
Cesari AB, Paulucci NS, Dardanelli MS. Smart Bioinoculants for Arachis hypogaea: Controlled Release of Bradyrhizobium and the Role of Naringin in Symbiosis Enhancement. Plants. 2025; 14(11):1601. https://doi.org/10.3390/plants14111601
Chicago/Turabian StyleCesari, Adriana Belén, Natalia Soledad Paulucci, and Marta Susana Dardanelli. 2025. "Smart Bioinoculants for Arachis hypogaea: Controlled Release of Bradyrhizobium and the Role of Naringin in Symbiosis Enhancement" Plants 14, no. 11: 1601. https://doi.org/10.3390/plants14111601
APA StyleCesari, A. B., Paulucci, N. S., & Dardanelli, M. S. (2025). Smart Bioinoculants for Arachis hypogaea: Controlled Release of Bradyrhizobium and the Role of Naringin in Symbiosis Enhancement. Plants, 14(11), 1601. https://doi.org/10.3390/plants14111601










