Complex Signaling Networks Underlying Blue-Light-Mediated Floral Transition in Plants
Abstract
1. Introduction
2. Photoreceptors and Photosynthetic Pigments
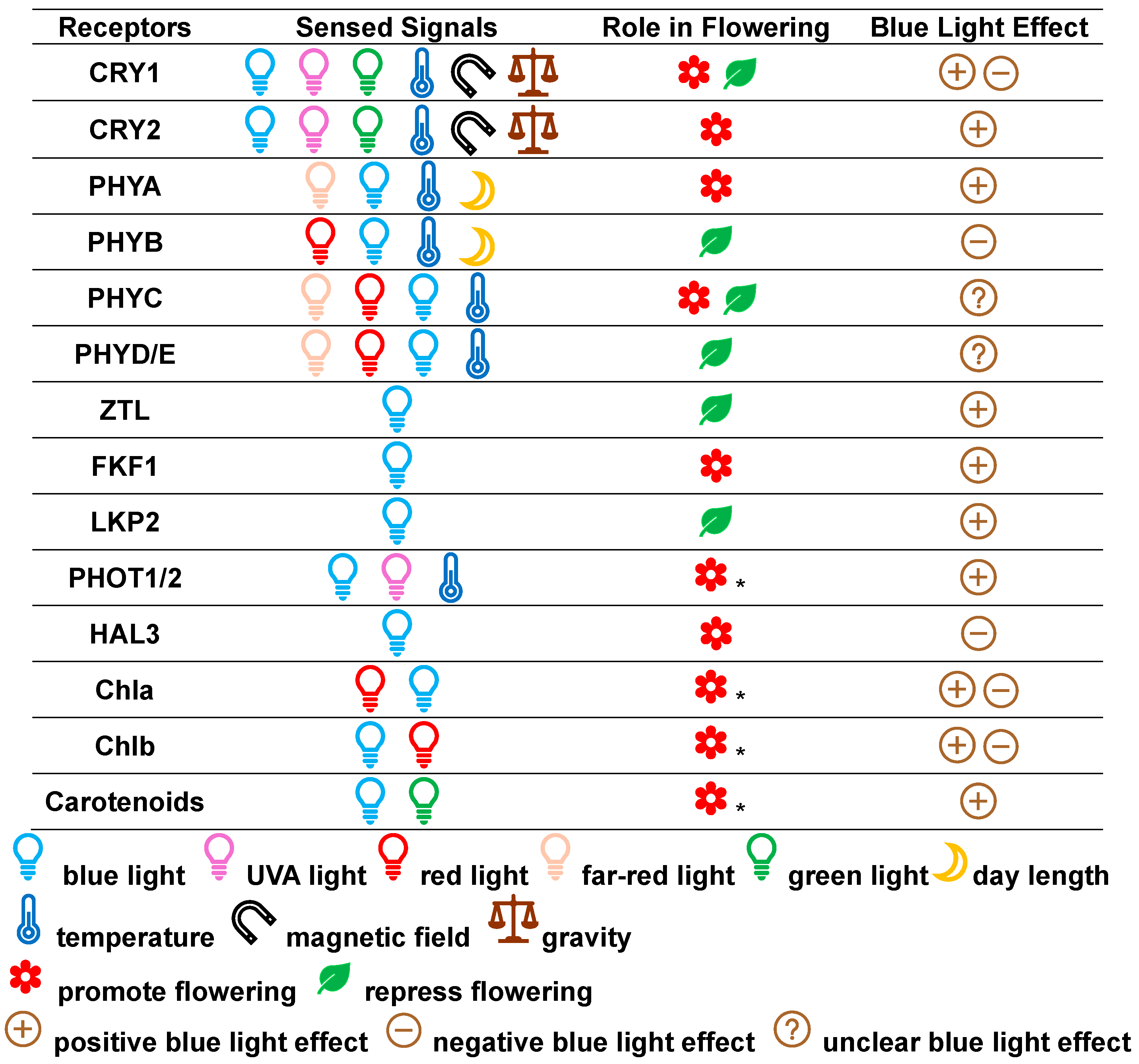
2.1. Photoreceptors
2.1.1. Cryptochromes (CRYs)
2.1.2. Phytochromes (PHYs)
2.1.3. ZEITLUPE (ZTL) Family Members
- (1)
- ZTL
- (2)
- FLAVIN-BINDING, KELCHREPEAT, F-BOX (FKF1)
- (3)
- LOV KELCH PROTEIN2 (LKP2)
2.1.4. Other Photoreceptors
- (1)
- Phototropins (PHOTs)
- (2)
- Halotolerance protein (HAL3)
2.2. Photosynthetic Pigments
2.2.1. Chlorophylls (Chls)
2.2.2. Carotenoids
2.3. Integration of Multiple Photoreceptors and Photosynthetic Pigments
3. Floral Integrator Proteins
3.1. Floral Activators
3.1.1. FLOWERING LOCUS T (FT)
3.1.2. SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1)
3.1.3. Other Floral Activators
3.2. Floral Inhibitors
3.3. Co-Action of Floral Integrator Proteins
4. Signal Transduction Pathways
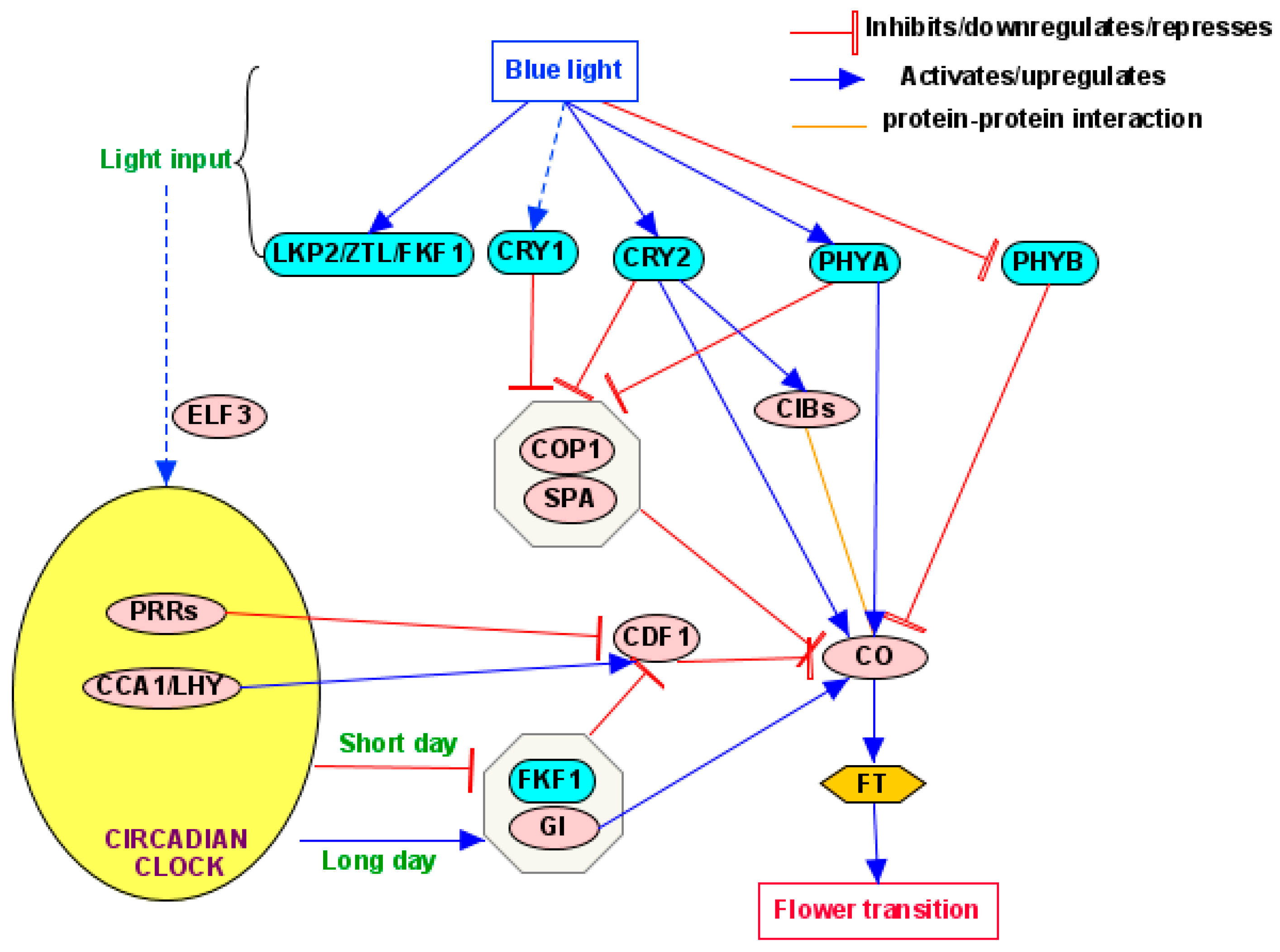
4.1. Photoperiod Pathway
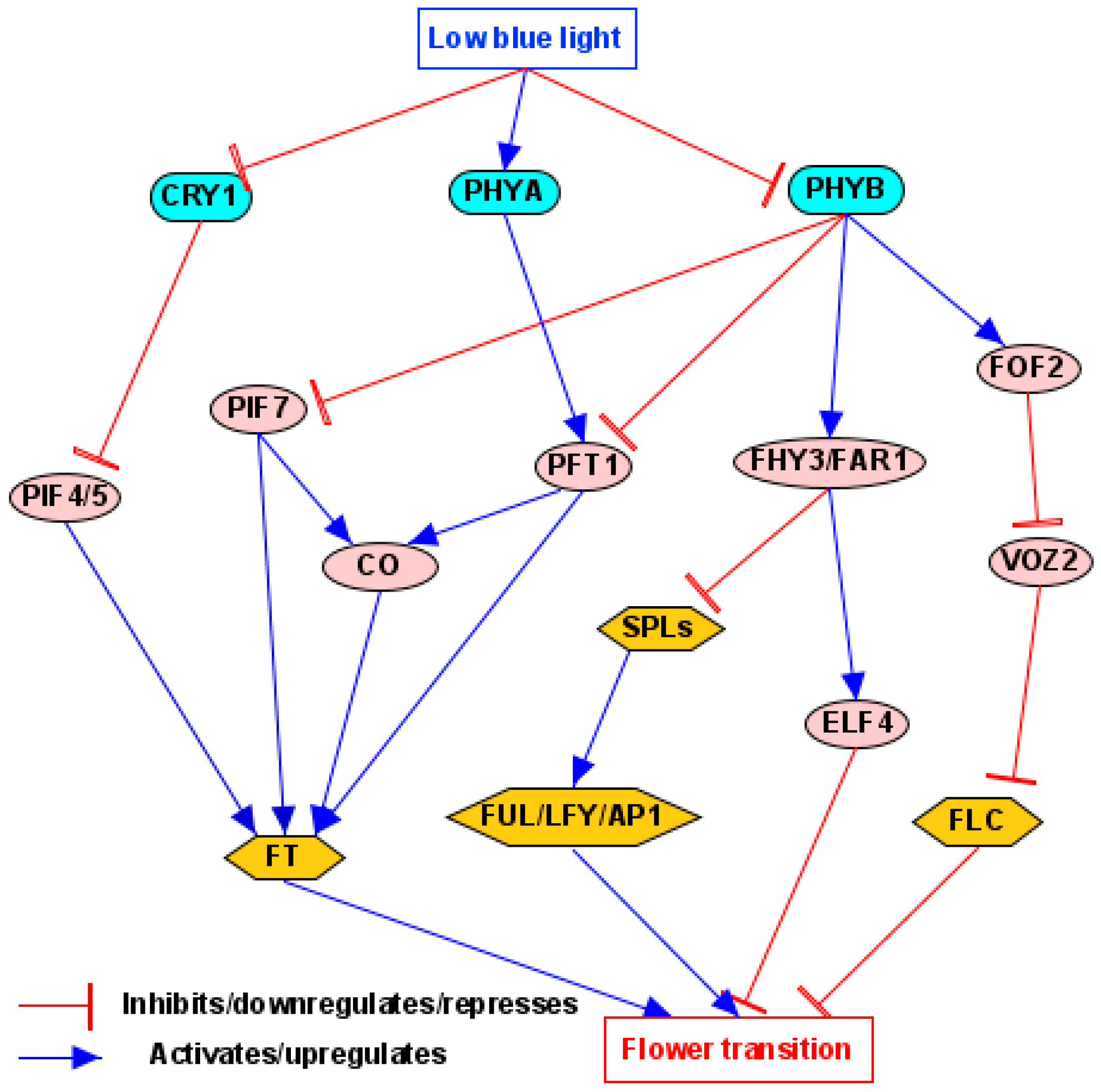
4.2. Light Quality/Shade Pathway
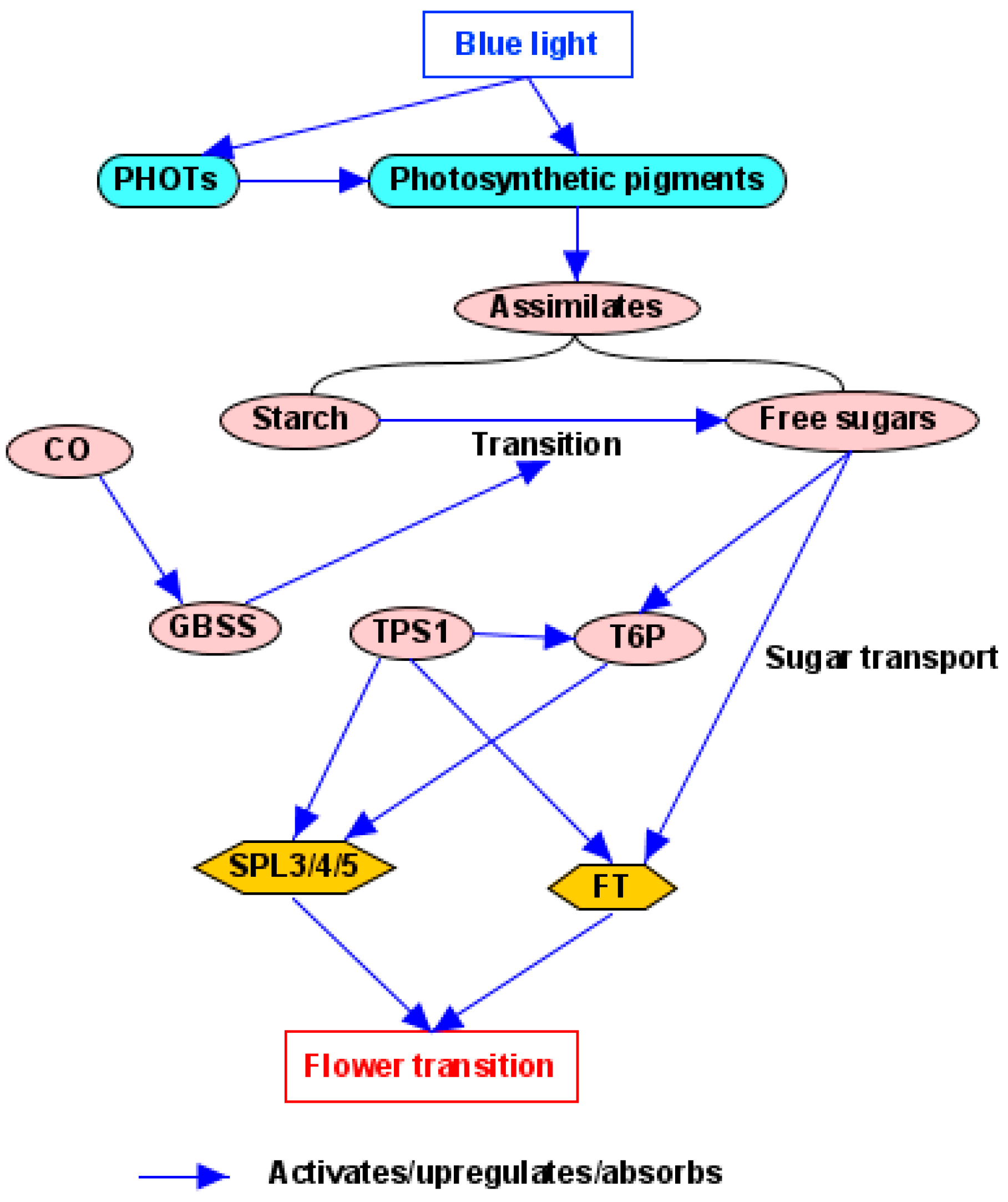
4.3. Light Quantity/Photosynthesis Pathway
5. Key Midstream Pathway Components
5.1. CONSTANS (CO)
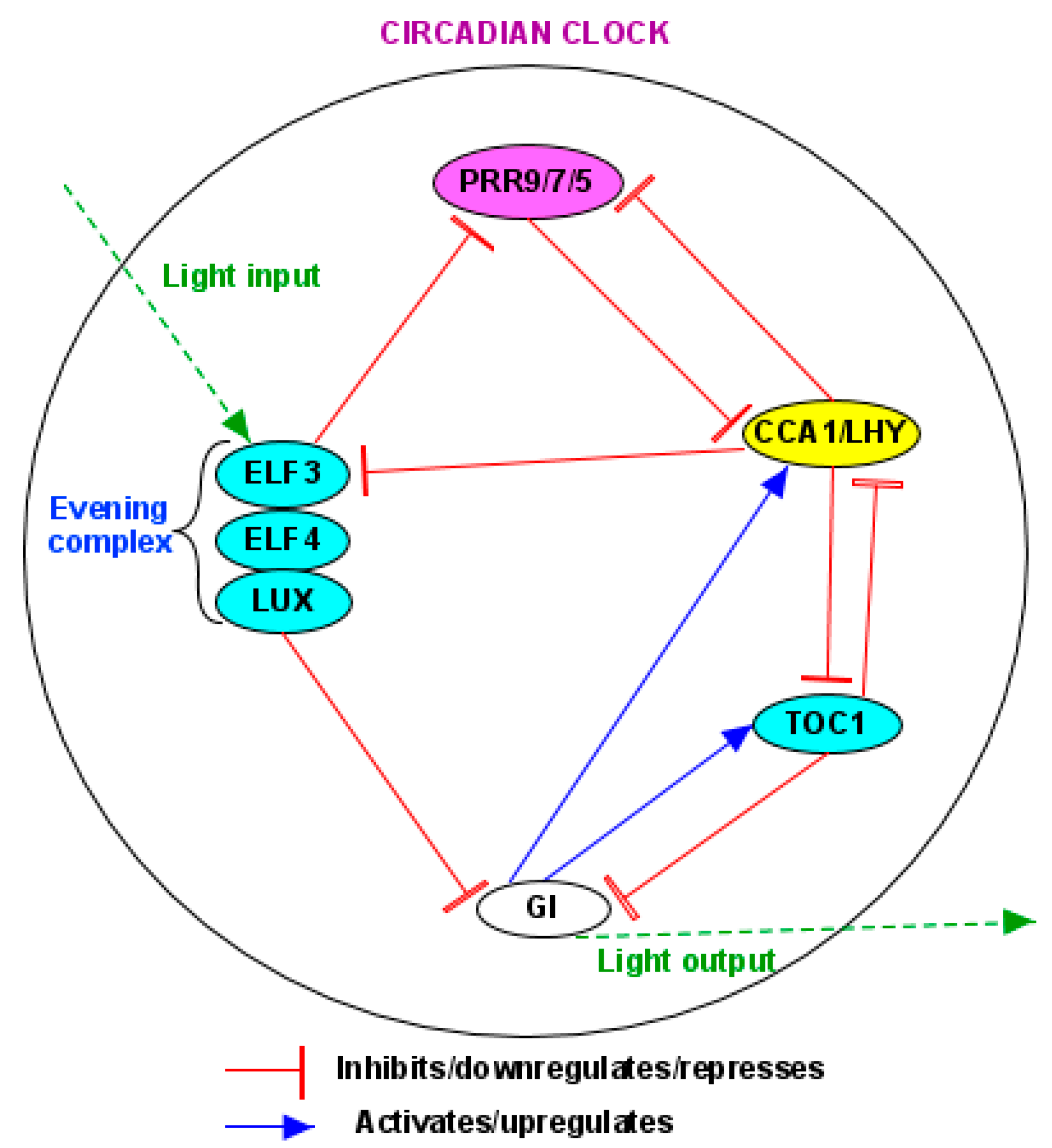
5.2. Circadian Clock
5.2.1. Clock Components
5.2.2. Regulation of Clockwork by BL
5.2.3. Responses of Key Clock Components to BL
- (1)
- BL and GI
- (2)
- BL and PRRs
- (3)
- BL and evening complex (EC)
5.3. Other Key Transcription Factors/Regulators
5.3.1. CONSTITUTIVE PHOTOMORPHOGENIC 1/SUPPRESSOR OF PHYTOCHROME A (COP1/SPA)
5.3.2. CRYPTOCHROME-INTERACTING Basic Helix–Loop–Helix (CIB) Proteins
5.3.3. CYCLING DOF FACTOR (CDF)
5.3.4. PHYTOCHROME INTERACTING FACTORS (PIFs)
5.3.5. ELONGATED HYPOCOTYL 5 (HY5)
5.3.6. Hypersensitive to Red and Blue Protein (HRB1)
5.3.7. TARGET OF EAT1/2/3 (TOE1/2/3)
5.3.8. B-Box Containing Proteins (BBXs)
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jung, C.; Pillen, K.; Staiger, D.; Coupland, G.; Von Korff, M. Recent advances in flowering time control. Front. Plant Sci. 2017, 7, 2011. [Google Scholar] [CrossRef]
- Peer, L.A.; Bhat, M.Y.; Ahmad, N.; Mir, B.A. Floral induction pathways: Decision making and determination in plants to flower—A comprehensive review. J. Appl. Biol. Biotechnol. 2021, 9, 7–17. [Google Scholar]
- Li, L.; Li, X.; Liu, Y.; Liu, H. Flowering responses to light and temperature. Sci. China Life Sci. 2016, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Demotes-Mainard, S.; Peron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huche-Thelier, L.; Boumaza, R. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Huche-Thelier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue-light-promoted elongation and flowering are not artifacts from 24-h lighting: A comparison with red light in four bedding plant species. Acta Hortic 2020, 1296, 659–666. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Blue light associated with low phytochrome activity can promote flowering: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 433–440. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Diverse Flowering Response to Blue Light Manipulation: Application of Electric Lighting in Controlled-Environment Plant Production. Horticulturae 2024, 10, 578. [Google Scholar] [CrossRef]
- Shibuya, T.; Kanayama, Y. Flowering response to blue light and its molecular mechanisms in Arabidopsis and horticultural plants. Adv. Hortic. Sci. 2014, 28, 179–183. [Google Scholar]
- Fukuda, N.; Suenaga, T.; Miura, E.; Tsukamoto, A.; Olsen, J.E. The expression of ELF4-like genes is influenced by light quality in petunia. Agronomy 2020, 10, 1800. [Google Scholar] [CrossRef]
- Shibuya, T.; Murakawa, Y.; Nishidate, K.; Nishiyama, M.; Kanayama, Y. Characterization of flowering-related genes and flowering response in relation to blue light in Gypsophila paniculata. Hortic. J. 2017, 86, 94–104. [Google Scholar] [CrossRef]
- Eskins, K. Light-quality effects on Arabidopsis development. Red, blue and far-red regulation of flowering and morphology. Physiol. Plant. 1992, 86, 439–444. [Google Scholar] [CrossRef]
- Fukuda, N.; Ishii, Y.; Ezura, H.; Olsen, J.E. Effects of light quality under red and blue light emitting diodes on growth and expression of FBP28 in Petunia. Acta Hortic. 2011, 907, 361–366. [Google Scholar] [CrossRef]
- Hori, Y.; Nishidate, K.; Nishiyama, M.; Kanahama, K.; Kanayama, Y. Flowering and expression of flowering-related genes under long-day conditions with light-emitting diodes. Planta 2011, 234, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Jeong, B.R. Night interruption light quality changes morphogenesis, flowering, and gene expression in Dendranthema grandiflorum. Hortic. Environ. Biotechnol. 2019, 60, 167–173. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. How supplementary or night-interrupting low-intensity blue light affects the flower induction in chrysanthemum, a qualitative short-day plant. Plants 2020, 9, 1694. [Google Scholar] [CrossRef]
- Jeong, S.W.; Hogewoning, S.W.; van Ieperen, W. Responses of supplemental blue light on flowering and stem extension growth of cut chrysanthemum. Sci. Hortic. 2014, 165, 69–74. [Google Scholar] [CrossRef]
- Singh, M.C.; van Ieperen, W.; Heuvelink, E.P. Effect of LEDs on flower bud induction in Chrysanthemum morifolium cv. Zembla. HortFlora Res. Spectr. 2013, 2, 185–188. [Google Scholar]
- Meng, Q.; Runkle, E.S. Moderate-intensity blue radiation can regulate flowering, but not extension growth, of several photoperiodic ornamental crops. Environ. Exp. Bot. 2017, 134, 12–20. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Low-intensity blue light in night-interruption lighting does not influence flowering of herbaceous ornamentals. Sci. Hortic. 2015, 186, 230–238. [Google Scholar] [CrossRef]
- Thomas, B. Light signals and flowering. J. Exp. Bot. 2006, 57, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Pardi, S.A.; Nusinow, D.A. Out of the dark and into the light: A new view of phytochrome photobodies. Front. Plant Sci. 2021, 12, 732947. [Google Scholar] [CrossRef]
- Shinomura, T.; Nagatani, A.; Hanzawa, H.; Kubota, M.; Watanabe, M.; Furuya, M. Action spectra for phytochrome A-and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Liu, Z.; Rochaix, J.-D.; Sun, X. Retrograde and anterograde signaling in the crosstalk between chloroplast and nucleus. Front. Plant Sci. 2022, 13, 980237. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Perrella, G.; Vellutini, E.; Zioutopoulou, A.; Patitaki, E.; Headland, L.R.; Kaiserli, E. Let it bloom: Cross-talk between light and flowering signaling in Arabidopsis. Physiol. Plant. 2020, 169, 301–311. [Google Scholar] [CrossRef]
- Xu, C.; Lv, Y.; Chen, C.; Zhang, Y.; Wei, S. Blue light-dependent phosphorylations of cryptochromes are affected by magnetic fields in Arabidopsis. Adv. Space Res. 2014, 53, 1118–1124. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C. Mechanisms of cryptochrome-mediated photoresponses in plants. Annu. Rev. Plant Biol. 2020, 71, 103–129. [Google Scholar] [CrossRef]
- Khudyakova, A.; Kosobryukhov, A.; Pashkovskiy, P.; Kreslavski, V. Cryptochromes and Their Role in the Process of Plant Adaptation. Russ. J. Plant Physiol. 2024, 71, 42. [Google Scholar] [CrossRef]
- Lin, C. Blue light receptors and signal transduction. Plant Cell 2002, 14, S207–S225. [Google Scholar] [CrossRef]
- Perrotta, G.; Ninu, L.; Flamma, F.; Weller, J.L.; Kendrick, R.E.; Nebuloso, E.; Giuliano, G. Tomato contains homologues of Arabidopsis cryptochromes 1 and 2. Plant Mol. Biol. 2000, 42, 765–773. [Google Scholar] [CrossRef]
- Perrotta, G.; Yahoubyan, G.; Nebuloso, E.; Renzi, L.; Giuliano, G. Tomato and barley contain duplicated copies of cryptochrome 1. Plant Cell Environ. 2001, 24, 991–998. [Google Scholar] [CrossRef]
- Li, Q.H.; Yang, H.Q. Cryptochrome signaling in plants. Photochem. Photobiol. 2007, 83, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Fraikin, G.Y.; Belenikina, N.S.; Rubin, A.B. Molecular bases of signaling processes regulated by cryptochrome sensory photoreceptors in plants. Biochemistry 2023, 88, 770–782. [Google Scholar] [PubMed]
- Yang, Z.; Liu, B.; Su, J.; Liao, J.; Lin, C.; Oka, Y. Cryptochromes orchestrate transcription regulation of diverse blue light responses in plants. Photochem. Photobiol. 2017, 93, 112–127. [Google Scholar] [CrossRef]
- Jia, Q.; Yin, Y.; Gai, S.; Tian, L.; Zhu, Z.; Qin, L.; Wang, Y. Onion cryptochrome 1 (AcCRY1) regulates photomorphogenesis and photoperiod flowering in Arabidopsis and exploration of its functional mechanisms under blue light. Plant Physiol. Biochem. 2024, 206, 108300. [Google Scholar] [CrossRef]
- Kruusvee, V.; Toft, A.M.; Aguida, B.; Ahmad, M.; Wenkel, S. Stop CRYing! Inhibition of cryptochrome function by small proteins. Biochem. Soc. Trans. 2022, 50, 773–782. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Low-activity cryptochrome 1 plays a role in promoting stem elongation and flower initiation of mature Arabidopsis under blue light associated with low phytochrome activity. Can. J. Plant Sci. 2022, 102, 755–759. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Spectral effects of light-emitting diodes on plant growth, visual color quality, and photosynthetic photon efficacy: White versus blue plus red radiation. PLoS ONE 2018, 13, e0202386. [Google Scholar] [CrossRef]
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue radiation interacts with green radiation to influence growth and predominantly controls quality attributes of lettuce. J. Am. Soc. Hortic. Sci. 2020, 145, 75–87. [Google Scholar] [CrossRef]
- Kang, W.H.; Kim, J.; Yoon, H.I.; Son, J.E. Quantification of spectral perception of plants with light absorption of photoreceptors. Plants 2020, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Han, Y.J.; Liu, Q.; Gu, L.; Yang, Z.; Su, J.; Liu, B.; Zuo, Z.; He, W. A CRY–BIC negative-feedback circuitry regulating blue light sensitivity of Arabidopsis. Plant J. 2017, 92, 426–436. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Wang, X.; Gu, L.; Yoshizumi, T.; Yang, Z.; Yang, L.; Liu, Q.; Liu, W.; Han, Y.-J. Photoactivation and inactivation of Arabidopsis cryptochrome 2. Science 2016, 354, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Q.; Wang, X.; Zuo, Z.; Oka, Y.; Lin, C. New insights into the mechanisms of phytochrome–cryptochrome coaction. New Phytol. 2018, 217, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Fantini, E.; Facella, P. Cryptochromes in the field: How blue light influences crop development. Physiol. Plant. 2020, 169, 336–346. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Mishra, S.; Khurana, P.; Khurana, J.P. CRY2 gene of rice (Oryza sativa subsp. indica) encodes a blue light sensory receptor involved in regulating flowering, plant height and partial photomorphogenesis in dark. Plant Cell Rep. 2023, 42, 73–89. [Google Scholar] [CrossRef]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Ahn, J.H.; Weigel, D. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 2003, 33, 168–171. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Li, R.; Hu, R.; Fan, C.; Chen, F.; Wang, Z.; Liu, X.; Fu, Y.; Lin, C. Association of the circadian rhythmic expression of GmCRY1a with a latitudinal cline in photoperiodic flowering of soybean. Proc. Natl. Acad. Sci. USA 2008, 105, 21028–21033. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, L.; Ma, Y.; Gao, J.; Li, W.; Piao, M.; Zeng, B.; Yang, Z.; Bian, M. Cryptochrome 1b from sweet sorghum regulates photoperiodic flowering, photomorphogenesis, and ABA response in transgenic Arabidopsis thaliana. Plant Mol. Biol. Rep. 2018, 36, 13–22. [Google Scholar] [CrossRef]
- Fantini, E.; Sulli, M.; Zhang, L.; Aprea, G.; Jiménez-Gómez, J.M.; Bendahmane, A.; Perrotta, G.; Giuliano, G.; Facella, P. Pivotal roles of cryptochromes 1a and 2 in tomato development and physiology. Plant Physiol. 2019, 179, 732–748. [Google Scholar] [CrossRef]
- Mockler, T.C.; Guo, H.; Yang, H.; Duong, H.; Lin, C. Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 1999, 126, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Liu, B.; Liao, J.; Yang, Z.; Lin, C.; Oka, Y. Coordination of cryptochrome and phytochrome signals in the regulation of plant light responses. Agronomy 2017, 7, 25. [Google Scholar] [CrossRef]
- Klose, C.; Büche, C.; Fernandez, A.P.; Schäfer, E.; Zwick, E.; Kretsch, T. The mediator complex subunit PFT1 interferes with COP1 and HY5 in the regulation of Arabidopsis light signaling. Plant Physiol. 2012, 160, 289–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Legris, M.; Ince, Y.Ç.; Fankhauser, C. Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat. Commun. 2019, 10, 5219. [Google Scholar] [CrossRef]
- Hernando, C.E.; Murcia, M.G.; Pereyra, M.E.; Sellaro, R.; Casal, J.J. Phytochrome B links the environment to transcription. J. Exp. Bot. 2021, 72, 4068–4084. [Google Scholar] [CrossRef]
- Mathews, S. Evolutionary studies illuminate the structural-functional model of plant phytochromes. Plant Cell 2010, 22, 4–16. [Google Scholar] [CrossRef]
- Sheehan, M.J.; Kennedy, L.M.; Costich, D.E.; Brutnell, T.P. Subfunctionalization of PhyB1 and PhyB2 in the control of seedling and mature plant traits in maize. Plant J. 2007, 49, 338–353. [Google Scholar] [CrossRef]
- Lei, Y.; Ma, Q.; Zhang, Y.; Li, J.; Ning, X.; Wang, Y.; Ge, X.; Zhao, H.; Lin, H. Functional dissection of phytochrome A in plants. Front. Plant Sci. 2024, 15, 1340260. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef]
- Cheng, M.-C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Magic Blue Light: A Versatile Mediator of Plant Elongation. Plants 2023, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Schiestel, K.; Zheng, Y. Pure blue light effects on growth and morphology are slightly changed by adding low-level UVA or far-red light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2019, 157, 58–68. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Growth and morphology responses to narrow-band blue light and its co-action with low-level UVB or green light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2020, 178, 104189. [Google Scholar] [CrossRef]
- Junior, C.A.S.; D’Amico-Damião, V.; Carvalho, R.F. Phytochrome type B family: The abiotic stress responses signaller in plants. Ann. Appl. Biol. 2021, 178, 135–148. [Google Scholar] [CrossRef]
- Han, Y.-J.; Kim, S.-H.; Kim, J.-I. Phytochrome phosphorylation in plant light signaling. Front. Plant Sci. 2024, 15, 1259720. [Google Scholar] [CrossRef]
- Van Buskirk, E.K.; Decker, P.V.; Chen, M. Photobodies in light signaling. Plant Physiol. 2012, 158, 52–60. [Google Scholar] [CrossRef]
- Lin, C. Photoreceptors and regulation of flowering time. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Mockler, T.; Yang, H.; Yu, X.; Parikh, D.; Cheng, Y.-C.; Dolan, S.; Lin, C. Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc. Natl. Acad. Sci. USA 2003, 100, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.L.; Beauchamp, N.; Kerckhoffs, L.H.J.; Platten, J.D.; Reid, J.B. Interaction of phytochromes A and B in the control of de-etiolation and flowering in pea. Plant J. 2001, 26, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Li, C.; Hu, W.; Lau, M.Y.; Lin, H.; Rockwell, N.C.; Martin, S.S.; Jernstedt, J.A.; Lagarias, J.C.; Dubcovsky, J. PHYTOCHROME C plays a major role in the acceleration of wheat flowering under long-day photoperiod. Proc. Natl. Acad. Sci. USA 2014, 111, 10037–10044. [Google Scholar] [CrossRef]
- Takano, M.; Inagaki, N.; Xie, X.; Yuzurihara, N.; Hihara, F.; Ishizuka, T.; Yano, M.; Nishimura, M.; Miyao, A.; Hirochika, H. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 2005, 17, 3311–3325. [Google Scholar] [CrossRef]
- Monte, E.; Alonso, J.M.; Ecker, J.R.; Zhang, Y.; Li, X.; Young, J.; Austin-Phillips, S.; Quail, P.H. Isolation and characterization of phyC mutants in Arabidopsis reveals complex crosstalk between phytochrome signaling pathways. Plant Cell 2003, 15, 1962–1980. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Phytochrome contributes to blue-light-mediated stem elongation and flower initiation in mature Arabidopsis thaliana plants. Can. J. Plant Sci. 2021, 102, 449–458. [Google Scholar] [CrossRef]
- Más, P.; Devlin, P.F.; Panda, S.; Kay, S.A. Functional interaction of phytochrome B and cryptochrome 2. Nature 2000, 408, 207–211. [Google Scholar] [CrossRef]
- Banerjee, R.; Batschauer, A. Plant blue-light receptors. Planta 2005, 220, 498–502. [Google Scholar] [CrossRef]
- Teixeira, R.T. Distinct responses to light in plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.E.; Rugnone, M.L.; Kay, S.A. Light perception: A matter of time. Mol. Plant 2020, 13, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Voitsekhovskaja, O. Phytochromes and other (photo)receptors of information in plants. Russ. J. Plant Physiol. 2019, 66, 351–364. [Google Scholar] [CrossRef]
- Pudasaini, A.; Zoltowski, B.D. Zeitlupe senses blue-light fluence to mediate circadian timing in Arabidopsis thaliana. Biochemistry 2013, 52, 7150–7158. [Google Scholar] [CrossRef]
- Christie, J.M.; Blackwood, L.; Petersen, J.; Sullivan, S. Plant flavoprotein photoreceptors. Plant Cell Physiol. 2015, 56, 401–413. [Google Scholar] [CrossRef]
- Christie, J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, N.; Kaur, H. Wake up: It’s time to bloom. Russ. J. Plant Physiol. 2021, 68, 579–595. [Google Scholar] [CrossRef]
- Li, X.; Liang, T.; Liu, H. How plants coordinate their development in response to light and temperature signals. Plant Cell 2022, 34, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Zoltowski, B.D.; Imaizumi, T. Structure and function of the ZTL/FKF1/LKP2 group proteins in Arabidopsis. Enzymes 2014, 35, 213–239. [Google Scholar]
- Zhou, L.; Lu, Y.; Huang, J.; Sha, Z.; Mo, W.; Xue, J.; Ma, S.; Shi, W.; Yang, Z.; Gao, J. Arabidopsis CIB3 regulates photoperiodic flowering in an FKF1-dependent way. Biosci. Biotechnol. Biochem. 2021, 85, 765–774. [Google Scholar] [CrossRef]
- Han, S.H.; Yoo, S.C.; Lee, B.D.; An, G.; Paek, N.C. Rice FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (OsFKF 1) promotes flowering independent of photoperiod. Plant Cell Environ. 2015, 38, 2527–2540. [Google Scholar] [CrossRef] [PubMed]
- Shchennikova, A. Photoperiod-Dependent Mechanisms of Flowering Initiation in Arabidopsis thaliana L. and Zea mays L. Russ. J. Plant Physiol. 2022, 69, 43. [Google Scholar] [CrossRef]
- Kwon, E.; Pathak, D.; Dahal, P.; Tandukar, S.; Jung, H.S.; Kim, W.-Y.; Kim, D.Y. Structural analysis of the regulation of blue-light receptors by GIGANTEA. Cell Rep. 2022, 39, 110700. [Google Scholar] [CrossRef] [PubMed]
- Toman, E.; Käs, M.D.; Taubert, B.; Moritz, M.; Qi, J.; Bolle, C. Photosynthetic and plastid performance effects of photoreceptors. J. Mitochondria Plast. Endosymbiosis 2024, 2, 2330972. [Google Scholar] [CrossRef]
- Ponnu, J. Molecular mechanisms suppressing COP1/SPA E3 ubiquitin ligase activity in blue light. Physiol. Plant. 2020, 169, 418–429. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Phototropin is partly involved in blue-light-mediated stem elongation, flower initiation, and leaf expansion: A comparison of phenotypic responses between wild Arabidopsis and its phototropin mutants. Environ. Exp. Bot. 2020, 171, 103967. [Google Scholar] [CrossRef]
- Okajima, K. Molecular mechanism of phototropin light signaling. J. Plant Res. 2016, 129, 149–157. [Google Scholar] [CrossRef]
- Łabuz, J.; Sztatelman, O.; Banaś, A.K.; Gabryś, H. The expression of phototropins in Arabidopsis leaves: Developmental and light regulation. J. Exp. Bot. 2012, 63, 1763–1771. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Chao, D.-Y.; Li, X.-M.; Shi, M.; Gao, J.-P.; Zhu, M.-Z.; Yang, H.-Q.; Luan, S.; Lin, H.-X. OsHAL3 mediates a new pathway in the light-regulated growth of rice. Nat. Cell Biol. 2009, 11, 845–851. [Google Scholar] [CrossRef]
- Su, L.; Shan, J.-X.; Gao, J.-P.; Lin, H.-X. OsHAL3, a blue light-responsive protein, interacts with the floral regulator Hd1 to activate flowering in rice. Mol. Plant 2016, 9, 233–244. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, Z.; Zhu, M.; Hu, W.; Hu, J.; Chen, M.; Guan, Y. The Significance of Florigen Activation Complex in Controlling Flowering in Rice. Crit. Rev. Plant Sci. 2023, 42, 300–323. [Google Scholar] [CrossRef]
- Chamovitz, D.A.; Deng, X.W.; Lam, E. Light signaling in plants. Crit. Rev. Plant Sci. 1996, 15, 455–478. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Voitsekhovskaja, O.; Tyutereva, E. Chlorophyll b in angiosperms: Functions in photosynthesis, signaling and ontogenetic regulation. J. Plant Physiol. 2015, 189, 51–64. [Google Scholar] [CrossRef]
- Gawarecka, K.; Ahn, J.H. Isoprenoid-derived metabolites and sugars in the regulation of flowering time: Does day length matter? Front. Plant Sci. 2021, 12, 765995. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Koo, Y.; He, J.; Poethig, R.S. Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. eLife 2013, 2, e00260. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Hubbart, S.; Chen, Y.; Peng, S.; Horton, P. Acclimation of rice photosynthesis to irradiance under field conditions. Plant Physiol. 2002, 130, 1999–2010. [Google Scholar] [CrossRef]
- Griffin, J.H.; Toledo-Ortiz, G. Plant photoreceptors and their signalling components in chloroplastic anterograde and retrograde communication. J. Exp. Bot. 2022, 73, 7126–7138. [Google Scholar] [CrossRef]
- Pashkovskiy, P.P.; Kartashov, A.V.; Zlobin, I.E.; Pogosyan, S.I.; Kuznetsov, V.V. Blue light alters miR167 expression and microRNA-targeted auxin response factor genes in Arabidopsis thaliana plants. Plant Physiol. Biochem. 2016, 104, 146–154. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Pradhan, S. Regulation of gene expression by LED lighting. In Light Emitting Diodes for Agriculture: Smart Lighting; Dutta Gupta, S., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2017; pp. 237–258. [Google Scholar]
- Higuchi, Y.; Sumitomo, K.; Oda, A.; Shimizu, H.; Hisamatsu, T. Day light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J. Plant Physiol. 2012, 169, 1789–1796. [Google Scholar] [CrossRef]
- Amaki, W.; Kunii, M. Effects of light quality on the flowering responses in Kalanchoe blossfeldiana. Acta Hortic. 2015, 1107, 279–284. [Google Scholar] [CrossRef]
- Runkle, E. Environmental control of the flowering process of Phalaenopsis orchids. Acta Hortic. 2019, 1262, 7–12. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Jeong, B.R. Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int. J. Mol. Sci. 2015, 16, 16497–16513. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, R.; Shen, S.; Zhao, J. Molecular mechanism of flowering time regulation in Brassica rapa: Similarities and differences with Arabidopsis. Hortic. Plant J. 2024, 10, 615–628. [Google Scholar] [CrossRef]
- Tsoy, O.; Mushegian, A. Florigen and its homologs of FT/CETS/PEBP/RKIP/YbhB family may be the enzymes of small molecule metabolism: Review of the evidence. BMC Plant Biol. 2022, 22, 56. [Google Scholar] [CrossRef]
- Song, G.-q.; Liu, Z.; Zhong, G.-y. Regulatory frameworks involved in the floral induction, formation and developmental programming of woody horticultural plants: A case study on blueberries. Front. Plant Sci. 2024, 15, 1336892. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, P.; Huang, R.; Zhang, J.; Ouyang, X. A daylength recognition model of photoperiodic flowering. Front. Plant Sci. 2021, 12, 778515. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, T.; Jeffrey Chen, Z. Circadian and photoperiodic regulation of the vegetative to reproductive transition in plants. Commun. Biol. 2024, 7, 579. [Google Scholar] [CrossRef]
- Liu, L.; Xuan, L.; Jiang, Y.; Yu, H. Regulation by FLOWERING LOCUS T and TERMINAL FLOWER 1 in flowering time and plant architecture. Small Struct. 2021, 2, 2000125. [Google Scholar] [CrossRef]
- Oda, A.; Higuchi, Y.; Hisamatsu, T. Constitutive expression of CsGI alters critical night length for flowering by changing the photo-sensitive phase of anti-florigen induction in chrysanthemum. Plant Sci. 2020, 293, 110417. [Google Scholar] [CrossRef]
- Colleoni, P.E.; van Es, S.W.; Winkelmolen, T.; Immink, R.G.; van Esse, G.W. Flowering time genes branching out. J. Exp. Bot. 2024, 75, 4195–4209. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, M.; Dodueva, I.; Gancheva, M.; Tvorogova, V.; Kuznetsova, K.; Lutova, L. The Evolutionary Aspects of Flowering Control: Florigens and Anti-Florigens. Russ. J. Genet. 2020, 56, 1323–1344. [Google Scholar] [CrossRef]
- Komiya, R.; Ikegami, A.; Tamaki, S.; Yokoi, S.; Shimamoto, K. Hd3a and RFT1 are essential for flowering in rice. Development 2008, 135, 767–774. [Google Scholar] [CrossRef]
- Tamaki, S.; Matsuo, S.; Wong, H.L.; Yokoi, S.; Shimamoto, K. Hd3a protein is a mobile flowering signal in rice. Science 2007, 316, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef]
- Prisca, M.; Maarten, V.; Bart, N.; Wouter, S.; Timo, H. Blue and far-red light control flowering time of woodland strawberry (Fragaria vesca) distinctively via CONSTANS (CO) and FLOWERING LOCUS T1 (FT1) in the background of sunlight mimicking radiation. Environ. Exp. Bot. 2022, 198, 104866. [Google Scholar] [CrossRef]
- Magar, Y.; Ohyama, K.; Noguchi, A.; Amaki, W.; Furufuji, S. Effects of light quality during supplemental lighting on the flowering in an everbearing strawberry. Acta Hortic. 2018, 1206, 279–284. [Google Scholar] [CrossRef]
- Nie, W.; Li, Y.; Chen, Y.; Zhou, Y.; Yu, T.; Zhou, Y.; Yang, Y. Spectral light quality regulates the morphogenesis, architecture, and flowering in pepper (Capsicum annuum L.). J. Photochem. Photobiol. B Biol. 2023, 241, 112673. [Google Scholar] [CrossRef]
- Ishikawa, R.; Shinomura, T.; Takano, M.; Shimamoto, K. Phytochrome dependent quantitative control of Hd3a transcription is the basis of the night break effect in rice flowering. Genes Genet. Syst. 2009, 84, 179–184. [Google Scholar] [CrossRef]
- Itoh, H.; Nonoue, Y.; Yano, M.; Izawa, T. A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat. Genet. 2010, 42, 635–638. [Google Scholar] [CrossRef]
- Tsukamoto, A.; Hirai, T.; Chin, D.P.; Mii, M.; Mizoguchi, T.; Mizuta, D.; Yoshida, H.; Olsen, J.E.; Ezura, H.; Fukuda, N. The FT-like gene PehFT in petunia responds to photoperiod and light quality but is not the main gene promoting light quality-associated flowering. Plant Biotechnol. 2016, 33, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Marcelis, L.F.; Kong, F.; Zhu, Y. Flowering time control in agricultural and horticultural crops. Front. Plant Sci. 2023, 14, 1116197. [Google Scholar]
- Sohail, A. Genetic and signaling pathways of flowering regulation in rice (Oryza sativa L.). Braz. J. Bot. 2023, 46, 599–608. [Google Scholar] [CrossRef]
- Shibuya, T.; Takahashi, T.; Hashimoto, S.; Nishiyama, M.; Kanayama, Y. Effects of overnight radiation with monochromatic far-red and blue light on flower budding and expression of flowering-related and light quality-responsive genes in Eustoma grandiflorum. J. Agric. Meteorol. 2019, 75, 160–165. [Google Scholar] [CrossRef]
- Preston, J.C.; Hileman, L.C. Functional evolution in the plant SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) gene family. Front. Plant Sci. 2013, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod control of plant growth: Flowering time genes beyond flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Wang, Y.; Severing, E.I.; Koornneef, M.; Aarts, M.G. FLC and SVP are key regulators of flowering time in the biennial/perennial species Noccaea caerulescens. Front. Plant Sci. 2020, 11, 582577. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Vasupalli, N.; Hou, D.; Lin, X. Ectopic expression of a bamboo SVP-like gene alters flowering time and floral organs in Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2022, 150, 721–732. [Google Scholar] [CrossRef]
- Higuchi, Y. Florigen and anti-florigen: Flowering regulation in horticultural crops. Breed. Sci. 2018, 68, 109–118. [Google Scholar] [CrossRef]
- Higuchi, Y.; Narumi, T.; Oda, A.; Nakano, Y.; Sumitomo, K.; Fukai, S.; Hisamatsu, T. The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in chrysanthemums. Proc. Natl. Acad. Sci. USA 2013, 110, 17137–17142. [Google Scholar] [CrossRef]
- Rantanen, M.; Kurokura, T.; Mouhu, K.; Pinho, P.; Tetri, E.; Halonen, L.; Palonen, P.; Elomaa, P.; Hytönen, T. Light quality regulates flowering in FvFT1/FvTFL1 dependent manner in the woodland strawberry Fragaria vesca. Front. Plant Sci. 2014, 5, 271. [Google Scholar] [CrossRef]
- Zhu, Y.; Klasfeld, S.; Wagner, D. Molecular regulation of plant developmental transitions and plant architecture via PEPB family proteins: An update on mechanism of action. J. Exp. Bot. 2021, 72, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Kaneko-Suzuki, M.; Kurihara-Ishikawa, R.; Okushita-Terakawa, C.; Kojima, C.; Nagano-Fujiwara, M.; Ohki, I.; Tsuji, H.; Shimamoto, K.; Taoka, K.-I. TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. Plant Cell Physiol. 2018, 59, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Chen, F.; Jiang, J. Functional diversification and molecular mechanisms of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in horticultural plants. Mol. Hortic. 2022, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, J.; Jeong, B.R. Flowering and runnering of seasonal strawberry under different photoperiods are affected by intensity of supplemental or night-interrupting blue light. Plants 2024, 13, 375. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Low-intensity blue light supplemented during photoperiod in controlled environment induces flowering and antioxidant production in kalanchoe. Antioxidants 2022, 11, 811. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Park, Y.G.; Jeong, B.R. Both the Positioned Supplemental or Night-Interruptional Blue Light and the Age of Leaves (or Tissues) Are Important for Flowering and Vegetative Growth in Chrysanthemum. Plants 2024, 13, 2874. [Google Scholar] [CrossRef]
- YAMAZAKI, K.; ISHII, Y.; MATSUI, S.; TANAKA, I. Effects of light quality, daylength and growing temperature on flowering in morning glory (Pharbitis nil Choisy). Environ. Control Biol. 2003, 41, 211–219. [Google Scholar] [CrossRef]
- Yuan, J.; Ott, T.; Hiltbrunner, A. Phytochromes and flowering: Legumes do it another way. Trends Plant Sci. 2023, 28, 379–381. [Google Scholar] [CrossRef]
- Sawa, M.; Kay, S.A.; Imaizumi, T. Photoperiodic flowering occurs under internal and external coincidence. Plant Signal. Behav. 2008, 3, 269–271. [Google Scholar] [CrossRef]
- Lin, X.; Dong, L.; Tang, Y.; Li, H.; Cheng, Q.; Li, H.; Zhang, T.; Ma, L.; Xiang, H.; Chen, L. Novel and multifaceted regulations of photoperiodic flowering by phytochrome A in soybean. Proc. Natl. Acad. Sci. USA 2022, 119, e2208708119. [Google Scholar] [CrossRef]
- Yu, J.-W.; Rubio, V.; Lee, N.-Y.; Bai, S.; Lee, S.-Y.; Kim, S.-S.; Liu, L.; Zhang, Y.; Irigoyen, M.L.; Sullivan, J.A. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol. Cell 2008, 32, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The cryptochrome blue light receptors. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Li, K.; Liu, H.; Lin, C. Multiple bHLH proteins form heterodimers to mediate CRY2-dependent regulation of flowering-time in Arabidopsis. PLoS Genet. 2013, 9, e1003861. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Hempton, A.K.; Imaizumi, T. Photoperiodic flowering in Arabidopsis: Multilayered regulatory mechanisms of CONSTANS and the florigen FLOWERING LOCUS T. Plant Commun. 2023, 4, 100552. [Google Scholar] [CrossRef]
- Romero, J.M.; Serrano-Bueno, G.; Camacho-Fernández, C.; Vicente, M.H.; Ruiz, M.T.; Pérez-Castiñeira, J.R.; Pérez-Hormaeche, J.; Nogueira, F.T.; Valverde, F. CONSTANS, a HUB for all seasons: How photoperiod pervades plant physiology regulatory circuits. Plant Cell 2024, 36, 2086–2102. [Google Scholar] [CrossRef]
- Ni, M. Integration of light signaling with photoperiodic flowering and circadian rhythm. Cell Res. 2005, 15, 559–566. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Carrillo, L.; Dominguez-Figueroa, J.; Vicente-Carbajosa, J.; Molina, R.V.; Nebauer, S.G.; Medina, J. CDF transcription factors: Plant regulators to deal with extreme environmental conditions. J. Exp. Bot. 2020, 71, 3803–3815. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Y.; Li, X.; Chen, Q.; Zhang, Y.; Luo, Y.; Liu, Z.; Wang, Y.; Lin, Y.; Zhang, Y. Transcriptome profile analysis of strawberry leaves reveals flowering regulation under blue light treatment. Int. J. Genom. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Goralogia, G.S.; Liu, T.K.; Zhao, L.; Panipinto, P.M.; Groover, E.D.; Bains, Y.S.; Imaizumi, T. CYCLING DOF FACTOR 1 represses transcription through the TOPLESS co-repressor to control photoperiodic flowering in Arabidopsis. Plant J. 2017, 92, 244–262. [Google Scholar] [CrossRef]
- Liu, L.; Xie, Y.; Yahaya, B.S.; Wu, F. GIGANTEA Unveiled: Exploring Its Diverse Roles and Mechanisms. Genes 2024, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, W.; Xu, Y.; Xie, B.; Yu, B.; Chen, L.; Huang, W. Flowering-time regulation by the circadian clock: From Arabidopsis to crops. Crop J. 2024, 12, 17–27. [Google Scholar] [CrossRef]
- Jung, J.-H.; Seo, Y.-H.; Seo, P.J.; Reyes, J.L.; Yun, J.; Chua, N.-H.; Park, C.-M. The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 2007, 19, 2736–2748. [Google Scholar] [CrossRef] [PubMed]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef]
- Hayama, R.; Sarid-Krebs, L.; Richter, R.; Fernández, V.; Jang, S.; Coupland, G. PSEUDO RESPONSE REGULATORs stabilize CONSTANS protein to promote flowering in response to day length. EMBO J. 2017, 36, 904–918. [Google Scholar] [CrossRef]
- Toda, Y.; Kudo, T.; Kinoshita, T.; Nakamichi, N. Evolutionary insight into the clock-associated PRR5 transcriptional network of flowering plants. Sci. Rep. 2019, 9, 2983. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H. Revisiting the role and mechanism of ELF3 in circadian clock modulation. Gene 2024, 913, 148378. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Kay, S.A. Photoperiodic control of flowering: Not only by coincidence. Trends Plant Sci. 2006, 11, 550–558. [Google Scholar] [CrossRef]
- Oakenfull, R.J.; Davis, S.J. Shining a light on the Arabidopsis circadian clock. Plant Cell Environ. 2017, 40, 2571–2585. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, D.; Tian, T.; Kong, F.; Lin, K.; Gan, S.; Zhang, H.; Li, G. Molecular and functional dissection of EARLY-FLOWERING 3 (ELF3) and ELF4 in Arabidopsis. Plant Sci. 2021, 303, 110786. [Google Scholar] [CrossRef]
- Ponnu, J.; Hoecker, U. Illuminating the COP1/SPA ubiquitin ligase: Fresh insights into its structure and functions during plant photomorphogenesis. Front. Plant Sci. 2021, 12, 662793. [Google Scholar] [CrossRef]
- Song, Y.H.; Estrada, D.A.; Johnson, R.S.; Kim, S.K.; Lee, S.Y.; MacCoss, M.J.; Imaizumi, T. Distinct roles of FKF1, GIGANTEA, and ZEITLUPE proteins in the regulation of CONSTANS stability in Arabidopsis photoperiodic flowering. Proc. Natl. Acad. Sci. USA 2014, 111, 17672–17677. [Google Scholar] [CrossRef]
- Song, Y.H.; Smith, R.W.; To, B.J.; Millar, A.J.; Imaizumi, T. FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering. Science 2012, 336, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Seaton, D.D.; Toledo-Ortiz, G.; Ganpudi, A.; Kubota, A.; Imaizumi, T.; Halliday, K.J. Dawn and photoperiod sensing by phytochrome A. Proc. Natl. Acad. Sci. USA 2018, 115, 10523–10528. [Google Scholar] [CrossRef] [PubMed]
- Pedmale, U.V.; Huang, S.-s.C.; Zander, M.; Cole, B.J.; Hetzel, J.; Ljung, K.; Reis, P.A.; Sridevi, P.; Nito, K.; Nery, J.R. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 2016, 164, 233–245. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, C.; Jiang, Y.; Li, L. A PIF7-CONSTANS-centered molecular regulatory network underlying shade-accelerated flowering. Mol. Plant 2019, 12, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 2011, 62, 4087–4100. [Google Scholar] [CrossRef]
- Buti, S.; Hayes, S.; Pierik, R. The bHLH network underlying plant shade-avoidance. Physiol. Plant. 2020, 169, 312–324. [Google Scholar] [CrossRef]
- Schwartz, C.; Lee, J.; Amasino, R. Variation in shade-induced flowering in Arabidopsis thaliana results from FLOWERING LOCUS T allelic variation. PLoS ONE 2017, 12, e0187768. [Google Scholar] [CrossRef]
- Thomson, B.; Wellmer, F. Molecular regulation of flower development. Curr. Top. Dev. Biol. 2019, 131, 185–210. [Google Scholar]
- Liu, Y.; Jafari, F.; Wang, H. Integration of light and hormone signaling pathways in the regulation of plant shade avoidance syndrome. Abiotech 2021, 2, 131–145. [Google Scholar] [CrossRef]
- Freytes, S.N.; Canelo, M.; Cerdán, P.D. Regulation of flowering time: When and where? Curr. Opin. Plant Biol. 2021, 63, 102049. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Q.; Zha, P.; Lin, R. The chromatin-remodelling factor PICKLE interacts with CONSTANS to promote flowering in Arabidopsis. Plant Cell Environ. 2019, 42, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Cerdán, P.D.; Chory, J. Regulation of flowering time by light quality. Nature 2003, 423, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Iñigo, S.; Alvarez, M.J.; Strasser, B.; Califano, A.; Cerdán, P.D. PFT1, the MED25 subunit of the plant Mediator complex, promotes flowering through CONSTANS dependent and independent mechanisms in Arabidopsis. Plant J. 2012, 69, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Khan, S.; Rhodes, B.M.; Devlin, P.F. FHY3 and FAR1 act downstream of light stable phytochromes. Front. Plant Sci. 2016, 7, 175. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, Q.; Zhao, Y.; Li, Q.; Liu, Y.; Ma, M.; Wang, B.; Shen, R.; Zheng, Z.; Wang, H. FHY3 and FAR1 integrate light signals with the miR156-SPL module-mediated aging pathway to regulate Arabidopsis flowering. Mol. Plant 2020, 13, 483–498. [Google Scholar] [CrossRef]
- Li, G.; Siddiqui, H.; Teng, Y.; Lin, R.; Wan, X.-y.; Li, J.; Lau, O.-S.; Ouyang, X.; Dai, M.; Wan, J. Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat. Cell Biol. 2011, 13, 616–622. [Google Scholar] [CrossRef]
- Qu, L.; Zhong, M.; Duan, F.; Li, X.; Yang, J.; Zhou, Q.; Tang, D.; He, R.; Liu, X.; Zhao, X. The PHYB–FOF2–VOZ2 module functions to fine-tune flowering in response to changes in light quality by modulating FLC expression in Arabidopsis. Plant Commun. 2024, 5, 100922. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Gao, J.; Lei, Y.; Zhang, J.; Zou, J.; Lu, Z.; Li, S.; Lei, N.; Dhungana, D. Genetic Regulatory Pathways of Plant Flowering Time Affected by Abiotic Stress. Plant Stress 2025, 15, 100747. [Google Scholar] [CrossRef]
- Adams, S.; Pearson, S.; Hadley, P.; Patefield, W. The Effects of Temperature and Light Integral on the Phases of Photoperiod Sensitivity inPetunia× hybrida. Ann. Bot. 1999, 83, 263–269. [Google Scholar] [CrossRef]
- Bernier, G.; Périlleux, C. A physiological overview of the genetics of flowering time control. Plant Biotechnol. J. 2005, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, G.; Dong, Y.; Qian, X.; Li, J.; Xu, X.; Huang, H.; Xu, L.; Li, L. Screening of key proteins affecting floral initiation of saffron under cold stress using iTRAQ-based proteomics. Front. Plant Sci. 2021, 12, 644934. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Wingler, A. Transitioning to the next phase: The role of sugar signaling throughout the plant life cycle. Plant Physiol. 2018, 176, 1075–1084. [Google Scholar] [CrossRef]
- Modarelli, G.C.; Arena, C.; Pesce, G.; Dell’Aversana, E.; Fusco, G.M.; Carillo, P.; De Pascale, S.; Paradiso, R. The role of light quality of photoperiodic lighting on photosynthesis, flowering and metabolic profiling in Ranunculus asiaticus L. Physiol. Plant. 2020, 170, 187–201. [Google Scholar] [CrossRef]
- Lejeune, P.; Bernier, G.; Requier, M.-C.; Kinet, J.-M. Sucrose increase during floral induction in the phloem sap collected at the apical part of the shoot of the long-day plant Sinapis alba L. Planta 1993, 190, 71–74. [Google Scholar] [CrossRef]
- Duplat-Bermúdez, L.; Ruiz-Medrano, R.; Landsman, D.; Mariño-Ramírez, L.; Xoconostle-Cázares, B. Transcriptomic analysis of Arabidopsis overexpressing flowering locus T driven by a meristem-specific promoter that induces early flowering. Gene 2016, 587, 120–131. [Google Scholar] [CrossRef]
- Kolbe, A.; Tiessen, A.; Schluepmann, H.; Paul, M.; Ulrich, S.; Geigenberger, P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA 2005, 102, 11118–11123. [Google Scholar] [CrossRef]
- Wahl, V.; Ponnu, J.; Schlereth, A.; Arrivault, S.; Langenecker, T.; Franke, A.; Feil, R.; Lunn, J.E.; Stitt, M.; Schmid, M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 2013, 339, 704–707. [Google Scholar] [CrossRef]
- Ortiz-Marchena, M.I.; Albi, T.; Lucas-Reina, E.; Said, F.E.; Romero-Campero, F.J.; Cano, B.; Ruiz, M.T.; Romero, J.M.; Valverde, F. Photoperiodic control of carbon distribution during the floral transition in Arabidopsis. Plant Cell 2014, 26, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Marchena, M.I.; Romero, J.M.; Valverde, F. Photoperiodic control of sugar release during the floral transition: What is the role of sugars in the florigenic signal? Plant Signal. Behav. 2015, 10, e1017168. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, J.; Jeong, B.R. The flowering of SDP chrysanthemum in response to intensity of supplemental or night-interruptional blue light is modulated by both photosynthetic carbon assimilation and photoreceptor-mediated regulation. Front. Plant Sci. 2022, 13, 981143. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, H.; Ren, L.; Chen, S.; Chen, F.; Jiang, J. CmFTL2 is involved in the photoperiod-and sucrose-mediated control of flowering time in chrysanthemum. Hortic. Res. 2017, 4, 17001. [Google Scholar] [CrossRef]
- Luccioni, L.; Krzymuski, M.; Sánchez-Lamas, M.; Karayekov, E.; Cerdán, P.D.; Casal, J.J. CONSTANS delays Arabidopsis flowering under short days. Plant J. 2019, 97, 923–932. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Liu, X.; Xue, J.; Ren, X.; Zhai, Y.; Zhang, X. The red/blue light ratios from light-emitting diodes affect growth and flower quality of hippeastrum hybridum ‘red lion’. Front. Plant Sci. 2022, 13, 1048770. [Google Scholar] [CrossRef]
- Lee, B.-D.; Kim, M.R.; Kang, M.-Y.; Cha, J.-Y.; Han, S.-H.; Nawkar, G.M.; Sakuraba, Y.; Lee, S.Y.; Imaizumi, T.; McClung, C.R. The F-box protein FKF1 inhibits dimerization of COP1 in the control of photoperiodic flowering. Nat. Commun. 2017, 8, 2259. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zeng, L.; Zhang, C.; Ma, H. Arabidopsis TOE proteins convey a photoperiodic signal to antagonize CONSTANS and regulate flowering time. Genes Dev. 2015, 29, 975–987. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef]
- Hajdu, A.; Dobos, O.; Domijan, M.; Bálint, B.; Nagy, I.; Nagy, F.; Kozma-Bognár, L. ELONGATED HYPOCOTYL 5 mediates blue light signalling to the Arabidopsis circadian clock. Plant J. 2018, 96, 1242–1254. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, X.; Feng, C.-H.; Niu, M.-X.; Liu, C.; Wang, H.-L.; Yin, W.; Xia, X. Light and Light Signals Regulate Growth and Development in Woody Plants. Forests 2024, 15, 523. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Quiroz, L.F.; Spillane, C.; Wu, R.; Mattoo, A.K.; Ortiz, R. Unlocking allelic variation in circadian clock genes to develop environmentally robust and productive crops. Planta 2024, 259, 72. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, Y.; Wang, X.; Qin, Y.; Su, C.; Wang, L. Aschoff’s rule on circadian rhythms orchestrated by blue light sensor CRY2 and clock component PRR9. Nat. Commun. 2022, 13, 5869. [Google Scholar] [CrossRef]
- Putterill, J. Flowering in time: Genes controlling photoperiodic flowering in Arabidopsis. Philos. Trans. R. Soc. London Ser. B 2001, 356, 1761. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, D.; Lu, S.X.; Hu, X.; Huang, R.; Liang, T.; Xu, T.; Tobin, E.M.; Liu, H. Blue light-and low temperature-regulated COR27 and COR28 play roles in the Arabidopsis circadian clock. Plant Cell 2016, 28, 2755–2769. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Fujiwara, S.; Suh, S.-S.; Kim, J.; Kim, Y.; Han, L.; David, K.; Putterill, J.; Nam, H.G.; Somers, D.E. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 2007, 449, 356–360. [Google Scholar] [CrossRef]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The photoperiod: Handling and causing stress in plants. Front. Plant Sci. 2022, 12, 781988. [Google Scholar] [CrossRef]
- Sawa, M.; Nusinow, D.A.; Kay, S.A.; Imaizumi, T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 2007, 318, 261–265. [Google Scholar] [CrossRef]
- Fujiwara, S.; Oda, A.; Yoshida, R.; Niinuma, K.; Miyata, K.; Tomozoe, Y.; Tajima, T.; Nakagawa, M.; Hayashi, K.; Coupland, G. Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell 2008, 20, 2960–2971. [Google Scholar] [CrossRef]
- Izawa, T. What is going on with the hormonal control of flowering in plants? Plant J. 2021, 105, 431–445. [Google Scholar] [CrossRef]
- Farré, E.M.; Harmer, S.L.; Harmon, F.G.; Yanovsky, M.J.; Kay, S.A. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 2005, 15, 47–54. [Google Scholar] [CrossRef]
- Covington, M.F.; Panda, S.; Liu, X.L.; Strayer, C.A.; Wagner, D.R.; Kay, S.A. ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 2001, 13, 1305–1316. [Google Scholar] [CrossRef]
- Huang, H.; Alvarez, S.; Bindbeutel, R.; Shen, Z.; Naldrett, M.J.; Evans, B.S.; Briggs, S.P.; Hicks, L.M.; Kay, S.A.; Nusinow, D.A. Identification of evening complex associated proteins in Arabidopsis by affinity purification and mass spectrometry. Mol. Cell. Proteom. 2016, 15, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Ronald, J. Extrinsic and Intrinsic Factors Regulate the Spatio-Temporal Localisation of the Arabidopsis Circadian Component ELF3. Doctoral Thesis, University of York, York, UK, 2021. [Google Scholar]
- Balcerowicz, M.; Kerner, K.; Schenkel, C.; Hoecker, U. SPA proteins affect the subcellular localization of COP1 in the COP1/SPA ubiquitin ligase complex during photomorphogenesis. Plant Physiol. 2017, 174, 1314–1321. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Su, S.; Peng, H.; Li, S.; Li, K.; Ji, L.; Xing, Y.; Zhang, J.; Du, X. Functions of COP1/SPA E3 ubiquitin ligase mediated by MpCRY in the liverwort Marchantia polymorpha under blue light. Int. J. Mol. Sci. 2021, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Ma, D.; Chen, Z.; Wang, J.W.; Liu, H. CIB 1 and CO interact to mediate CRY 2-dependent regulation of flowering. EMBO Rep. 2018, 19, e45762. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems integrators in plant development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef]
- Sharma, A.; Samtani, H.; Sahu, K.; Sharma, A.K.; Khurana, J.P.; Khurana, P. Functions of Phytochrome-Interacting Factors (PIFs) in the regulation of plant growth and development: A comprehensive review. Int. J. Biol. Macromol. 2023, 244, 125234. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, E.-Y.; Han, S.-H.; Piao, W.; An, G.; Todaka, D.; Yamaguchi-Shinozaki, K.; Paek, N.-C. Rice Phytochrome-Interacting Factor-Like1 (OsPIL1) is involved in the promotion of chlorophyll biosynthesis through feed-forward regulatory loops. J. Exp. Bot. 2017, 68, 4103–4114. [Google Scholar] [CrossRef]
- Ammari, M.; Maseh, K.; Zander, M. PIF transcription factors-versatile plant epigenome landscapers. Front. Epigenet. Epigenom. 2024, 2, 1404958. [Google Scholar] [CrossRef]
- Wu, M.; Liu, D.; Abdul, W.; Upreti, S.; Liu, Y.; Song, G.; Wu, J.; Liu, B.; Gan, Y. PIL5 represses floral transition in Arabidopsis under long day conditions. Biochem. Biophys. Res. Commun. 2018, 499, 513–518. [Google Scholar] [CrossRef]
- Castillon, A.; Shen, H.; Huq, E. Blue light induces degradation of the negative regulator phytochrome interacting factor 1 to promote photomorphogenic development of Arabidopsis seedlings. Genetics 2009, 182, 161–171. [Google Scholar] [CrossRef]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Singh, S.; Khurana, J.P.; Burman, N. HY5-COP1: The central module of light signaling pathway. J. Plant Biochem. Biotechnol. 2020, 29, 590–610. [Google Scholar] [CrossRef]
- Liu, S.; He, M.; Lin, X.; Kong, F. Epigenetic regulation of photoperiodic flowering in plants. Plant Genome 2023, 16, e20320. [Google Scholar] [CrossRef]
- Chu, L.; Yang, C.; Zhuang, F.; Gao, Y.; Luo, M. The HDA9-HY5 module epigenetically regulates flowering time in Arabidopsis thaliana. J. Cell. Physiol. 2022, 237, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhou, Y.; Sun, X.; Ni, M. Hypersensitive to red and blue 1 and its C-terminal regulatory function control flowering locus T expression. Plant J. 2007, 52, 937–948. [Google Scholar] [CrossRef]
- Du, S.; Li, L.; Li, L.; Wei, X.; Xu, F.; Xu, P.; Wang, W.; Xu, P.; Cao, X.; Miao, L. Photoexcited cryptochrome2 interacts directly with TOE1 and TOE2 in flowering regulation. Plant Physiol. 2020, 184, 487–505. [Google Scholar] [CrossRef]
- Song, Z.; Bian, Y.; Xiao, Y.; Xu, D. B-BOX proteins: Multi-layered roles of molecular cogs in light-mediated growth and development in plants. J. Plant Physiol. 2024, 299, 154265. [Google Scholar] [CrossRef]
- Xu, X.; Xu, J.; Yuan, C.; Chen, Q.; Liu, Q.; Wang, X.; Qin, C. BBX17 interacts with CO and negatively regulates flowering time in Arabidopsis thaliana. Plant Cell Physiol. 2022, 63, 401–409. [Google Scholar] [CrossRef]
- Graeff, M.; Straub, D.; Eguen, T.; Dolde, U.; Rodrigues, V.; Brandt, R.; Wenkel, S. MicroProtein-mediated recruitment of CONSTANS into a TOPLESS trimeric complex represses flowering in Arabidopsis. PLoS Genet. 2016, 12, e1005959. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2–COP1–HY5–BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef]
- Jung, C.; Müller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Blue light supplemented at intervals in long-day conditions intervenes in photoperiodic flowering, photosynthesis, and antioxidant properties in chrysanthemums. Antioxidants 2022, 11, 2310. [Google Scholar] [CrossRef]
- Kohler, A.E.; Birtell, E.M.; Runkle, E.S.; Meng, Q. Day-extension Blue Light Inhibits Flowering of Chrysanthemum When the Short Main Photoperiod Includes Far-red Light. J. Am. Soc. Hortic. Sci. 2023, 148, 89–98. [Google Scholar] [CrossRef]
- SharathKumar, M.; Luo, J.; Xi, Y.; van Ieperen, W.; Marcelis, L.F.; Heuvelink, E. Several short-day species can flower under blue-extended long days, but this response is not universal. Sci. Hortic. 2024, 325, 112657. [Google Scholar] [CrossRef]
- Fukuda, N.; Yoshida, T.; Olsen, J.; Senaha, C.; Jikumaru, Y.; Kamiya, Y. Short main shoot length and inhibition of floral bud development under red light can be recovered by application of gibberellin and cytokinin. Acta Hortic. 2012, 956, 215–222. [Google Scholar] [CrossRef]
- Spaninks, K.; Van Lieshout, J.; Van Ieperen, W.; Offringa, R. Regulation of early plant development by red and blue light: A comparative analysis between Arabidopsis thaliana and Solanum lycopersicum. Front. Plant Sci. 2020, 11, 599982. [Google Scholar] [CrossRef]


| Common Name | Scientific Name | Main Results | Reference(s) |
|---|---|---|---|
| 1. Horticultural crops | |||
| Chrysanthemum | Chrysanthemum morifolium | Blue light (BL) did not inhibit floral transition under long day (LD), despite increased expression of both PHYA and PHYB. | [16] |
| Shifts in the sequence of blue and other LEDs for night interruption (NI) lighting caused varying flowering responses associated with different expressions of related genes, such as PHYA, CRY1, PHYB, AFT, and FTL. | [114] | ||
| At least two distinct phytochrome responses were involved in the flowering response; daytime light quality affects the light quality required for effective NI lighting due to different effects on FLOWERING LOCUS T (CmFTL3). | [111] | ||
| An anti-florigen gene, AFT, was identified to contribute to the phytochrome (PHY)-mediated response to light and to determine the obligate photoperiodic flowering response. | [141] | ||
| Flowering-promotion responses by long day BL treatments resulted from balanced expression of a series of genes, such as CmFTL3, APETALA1 (CDM111), FRUITFULL (CmAFL1), LEAFY (CmFL), CmPHYA, CmCRY1, CmAFT, and CmPHYB. Also, the youngest leaf showed greater sensitivity to BL. | [148] | ||
| BL-promoted flowering might be due to the co-regulation of photosynthetic carbon assimilation and differential photoreceptors in flowering. | [204] | ||
| Increased photosynthesis, carbohydrate accumulation, and antioxidant production contributed to BL-promoted flowering. | [246] | ||
| Exogenous sucrose induced a high expression of CmFTLs, and it flowered early regardless of the photoperiod. | [205] | ||
| CsGI controlled photoperiodic flowering by gating light-induced CsAFT. | [121] | ||
| A high daytime phytochrome photoequilibrium prevented plants from perceiving subsequent BL as a long day. | [247] | ||
| Plant flowering under long-day BL treatment was not observed in all short-day (SD) plants. | [248] | ||
| Baby’s Breath | Gypsophila paniculata | BL did not induce FT and SOC1 expression and had weaker flowering promotion than far-red (FR) light. | [14] |
| Genotype variation in the flowering response to blue light was associated with GpFT and GpSOC1, rather than GpFKF1 and GpGI. | [11] | ||
| Kalanchoe | Kalanchoe blossfeldiana | BL was not perceived as a photoperiod signal to regulate flowering, which was mainly controlled by red light. | [112] |
| The BL signal at the end of day increased flower bud formation regardless of the photoperiod, which was associated with higher expression of flowering promoter genes (KfPHYA, KfCRY1, KfFT, and KfFPF-1) and lower expression of the flowering suppressor gene (KfPHYB). | [147] | ||
| Lisianthus | Eustoma grandiflorum | Prolonged photoperiod lighting with BL promoted flowering under SD, associated with increased expression of EgFTL and EgSOC1L, but there was weaker promotion compared to FR light. | [135] |
| Marigold | Tagetes erecta | Twenty-four-hour sole-source lighting with pure BL promoted flowering compared with red light; however, impure BL containing a low level of red light failed to induce flowering, and adding a low level of far-red light restored the flowering-promoting effect. | [6,7] |
| Petunia | Petunia × hybrida | BL influenced PehFT expression but not the main gene promoting flowering. | [132] |
| Blue vs. red LED light increased the expression of FBP28, a SOC1-like gene, which transmitted the BL signal from the FT protein to induce flowering. | [10,249] | ||
| Two ELF4-like genes, PhELF4-1 and PhELF4-2, were identified to act in signal transduction from one or more BL photoreceptors. | [10] | ||
| Lower irradiances prolonged the juvenile phase. | [192] | ||
| Twenty-four-hour sole-source lighting with pure BL promoted flowering compared with red light; however, impure BL containing a low level of red light failed to induce flowering, and adding a low level of far-red light restored the flowering-promoting effect. | [6,7] | ||
| Phalaenopsis orchid | Phalaenopsis spp. | Flowering response depended on active PHY levels under supplemental lighting. | [113] |
| Strawberry | Fragaria × ananassa | Sole-source lighting with blue vs. white LEDs promoted flowering associated with altered expression of PHYB, PIFs, HY5, FKF1, CCA1, LHY, and CO. The downregulated FaBBX29, an identified BBX protein, played an important role in BL-promoted flowering. | [160] |
| Both blue and FR light promoted flowering in day-neutral accessions through FvFT1, but BL acted partially independent of FvCO, and FR light was completely independent of FvCO. Also, BL induced the expression of FvFT1 exclusively in veins of older leaves. | [127] | ||
| Blue LED light increased the number of flower clusters and final yield in an everbearing variety. | [128] | ||
| End-of-day lighting with BL or FR light could induce a higher expression of FvTFL1, the repressor of floral induction, in an SD variety. | [142] | ||
| Blue LED light promoted the flowering of an SD variety in LD conditions, associated with increased expression of FaFT1 and decreased expression of FaTFL1, as well as enhanced photosynthesis and carbohydrate production. | [146] | ||
| Sweet pepper | Capsicum annuum | Sole-source lighting with BL induced the expression of CaFT1 and CaFT2 compared to white light. | [129] |
| Tomato | Solanum lycopersicum | BL sensitivity differs from Arabidopsis; indifferent flowering response found. | [250] |
| Both CRY2 and CRY1a function to repress tomato flowering: knockout of CRY2 or CRY1a does not affect flowering time, but the simultaneous knockout of both CRY1a and CRY2 promotes flowering. | [46,52] | ||
| This species has at least three CRY genes, CRY1a, CRY1b, and CRY2. | [31,32] | ||
| Under LD conditions, CRY2 overexpression in this species delayed flowering. | [48] | ||
| 2. Field/agronomic crops | |||
| Barley | Hordeum vulgare | CRY1a and CRY2a were identified, and CRY1a was a major regulator of photoperiodic flowering. | [32] |
| Maize | Zea mays | PHYB2 and, to a lesser extent, PHYB1 mediated photoperiodic flowering, and the sub-functionalization might contribute to flowering variation among varieties. | [59] |
| Onion | Allium cepa | Overexpression of AcCRY1 accelerated flowering; BL promoted cytoplasmic localization. | [36] |
| Rice | Oryza sativa | Knockdown of CRY2, but not CRY1, delayed flowering both in long- and short-day conditions. | [46] |
| Overexpression of CRY2 in plants with a photoperiod-insensitive genetic background did not affect flowering time. | [47] | ||
| PHYC functioned as a flowering repressor under noninductive photoperiods. | [76] | ||
| FKF1 had a similar role in photoperiod-mediated flowering relative to Arabidopsis and promoted flowering independent of the photoperiod. | [91] | ||
| OsHAL3 was identified as a new BL sensor, which was structurally inactivated by light, especially BL. | [99] | ||
| OsHAL3 was a positive regulator of flowering by directly binding to the promoter of Hd3a and forming a complex with Hd1 under SD conditions. | [100] | ||
| Hd3a and RFT1 are essential for promoting rice flowering under SD conditions, while RFT1 functions as a floral activator under LD conditions. | [119] | ||
| Both Hd3a and RFT1 are expressed in leaves and move to the SAM, where they enhance the expression of floral meristem identity genes and trigger flowering. | [119,124,125] | ||
| NI lighting with BL suppressed Hd3a expression and delayed flowering. | [130] | ||
| RCN (a TFL1 homolog) inhibited flowering by competing with Hd3a (a FT homolog) for 14-3-3 binding to form a florigen repression complex. | [118,144] | ||
| There are two important COL transcription factors. These are Heading date1 (Hd1), an ortholog of the Arabidopsis CO, and Early heading date1 (Ehd1) which is unique in rice. | [119] | ||
| Ehd1 always acts as an inducer of florigen genes (Hd3a in SD conditions or RFT1 in LD conditions), and its expression is upregulated by BL in the morning; however, Hd1 acts as a repressor in noninductive LD. | [157] | ||
| OsGI plays a critical gatekeeper role in BL induction of Ehd1, a CO-like protein, resulting in early flowering under SD conditions. | [131,221] | ||
| OsPIL13 might regulate floral development; ospil13, one of the putative PIF4 homologs, mutants headed earlier compared to the wild type. | [230,231] | ||
| Sorghum | Sorghum bicolor | CRY2 is a major regulator of photoperiodic flowering; CRY1b can rescue the late-flowering phenotype in Arabidopsis cry1/cry2 double mutant. | [46,51] |
| Soybean | Glycine max | CRY1a rather than CRY2a is a major regulator of photoperiodic flowering. | [46,50] |
| The regulation of photoperiodic flowering through PHYA-LUXE1-FT is different from the PHYB-CO-FT flowering pathway in many nonlegume plants. | [150,152] | ||
| Wheat | Triticum aestivum | PHYC promoted flowering under inductive photoperiods. | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Zheng, Y. Complex Signaling Networks Underlying Blue-Light-Mediated Floral Transition in Plants. Plants 2025, 14, 1533. https://doi.org/10.3390/plants14101533
Kong Y, Zheng Y. Complex Signaling Networks Underlying Blue-Light-Mediated Floral Transition in Plants. Plants. 2025; 14(10):1533. https://doi.org/10.3390/plants14101533
Chicago/Turabian StyleKong, Yun, and Youbin Zheng. 2025. "Complex Signaling Networks Underlying Blue-Light-Mediated Floral Transition in Plants" Plants 14, no. 10: 1533. https://doi.org/10.3390/plants14101533
APA StyleKong, Y., & Zheng, Y. (2025). Complex Signaling Networks Underlying Blue-Light-Mediated Floral Transition in Plants. Plants, 14(10), 1533. https://doi.org/10.3390/plants14101533







